Abstract
The function of the kidney, filtering blood and concentrating metabolic waste into urine, takes place in an intricate and functionally elegant structure called the renal glomerulus. Normal glomerular function retains circulating cells and valuable macromolecular components of plasma in blood, resulting in urine with just trace amounts of proteins. Endothelial cells of glomerular capillaries, the podocytes wrapped around them, and the fused extracellular matrix these cells form altogether comprise the glomerular filtration barrier, a dynamic and highly selective filter that sieves on the basis of molecular size and electrical charge. Current understanding of the structural organization and the cellular and molecular basis of renal filtration draws from studies of human glomerular diseases and animal models of glomerular dysfunction.
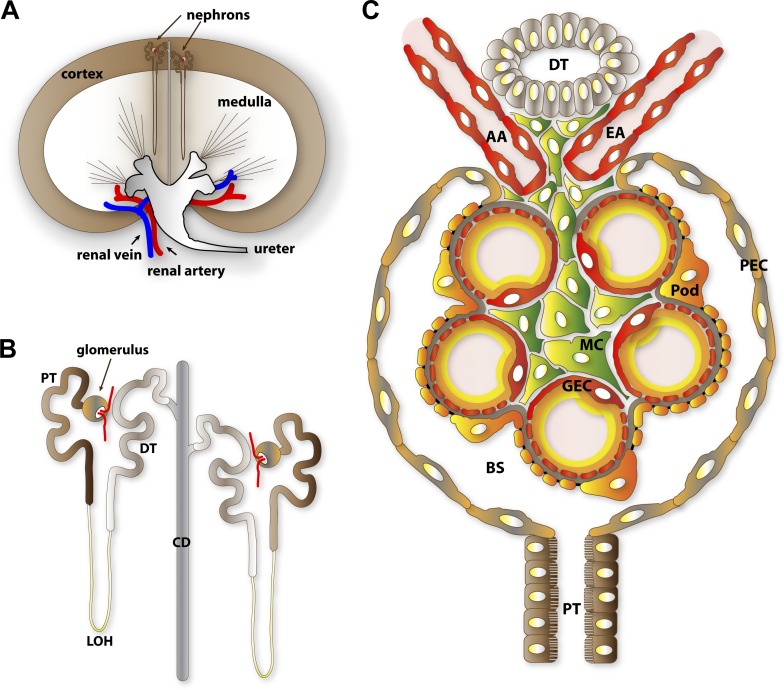
The mammalian kidney orchestrates the excretion of metabolic wastes found in blood, a function intimately related to its essential roles in general fluid homeostasis and osmoregulation. It is also important in the control of blood pressure, synthesis of vitamin D, bone mineralization, and the promotion of erythrocyte development. Despite its modest size (each is approximately the size of a human fist), a mammalian kidney is highly vascularized. A pair of kidneys receives and filters a remarkable volume of blood, estimated to be the equivalent of roughly 20% of total cardiac output (Stein and Fadem, 1978; Munger et al., 2011). In humans, blood filtration by the kidneys generates on average 1 liter of urine per day. Urine is produced and concentrated along the length of nephrons, the basic unit of kidneys (Fig. 1 A). An adult human kidney is known to contain an average of 1 million and up to as many as 2.5 million nephrons (Puelles et al., 2011).
Figure 1.
Anatomical overview of renal filtration. (A) Diagrammatic representation of nephron distribution in the kidney. Glomeruli, the filtration compartments of nephrons, are found within the kidney cortex. (B) Segmental structure of nephrons. The vascularized glomerulus is found at the proximal end and is connected through a series of renal tubules where urinary filtrate composition is refined through resorption and secretion. (C) Cellular organization of the glomeruli. GEC, glomerular endothelial cell; AA, afferent arteriole; EA, efferent arteriole; Pod, podocyte; MC, mesangial cell; PEC, parietal epithelial cell; PT, proximal tubule; DT, distal tubule; LOH, loop of Henle; CD, collecting duct; BS, Bowman’s space.
A nephron is functionally subdivided into a filtration unit called the renal corpuscle or glomerulus and a segmented tubular resorption compartment (Fig. 1 B). The glomerulus is an assembly of four different cells: the glomerular endothelial cells (GECs), podocytes, mesangial cells (MCs), and parietal epithelial cells (PECs; Figs. 1 C and 2). The word glomerulus is a reference to its intricately tortuous inner capillary tuft formed by GECs, after the Latin word glomus for a ball of yarn (Fig. 2 C). Podocytes are specialized perivascular cells aptly named for their elaborate projections, known as foot processes (FPs) or pedicels, that are intimately wrapped around the exterior of glomerular capillaries (Fig. 2, A and B). GECs and podocytes share a common ECM known as the glomerular basement membrane (GBM). The GECs, the podocytes, and the GBM in between constitute the three distinctive layers of the glomerular filtration barrier (GFB; Fig. 2 E), an elegant sieve that selectively filters blood components, generating a dilute primary urinary filtrate. The mesangium, a stalk-like aggregate of MCs and their ECM called the mesangial matrix, provides the structural reinforcement for the glomerular vasculature. The PECs forms a watertight cuplike enclosure called the Bowman’s capsule. Primary urinary filtrate collects within the Bowman’s capsule and empties through a connected series of epithelial tubules starting from the proximal tubules, the loop of Henle, the distal tubules, and a final collecting duct. The renal tubules of the nephrons and the collecting ducts express various ion and water channels, as well as transporters that help concentrate and adjust the composition of the urinary filtrate by resorption and secretion. This last step is vital for fluid conservation, maintenance of electrolyte balance, and resorption of glucose.
Figure 2.
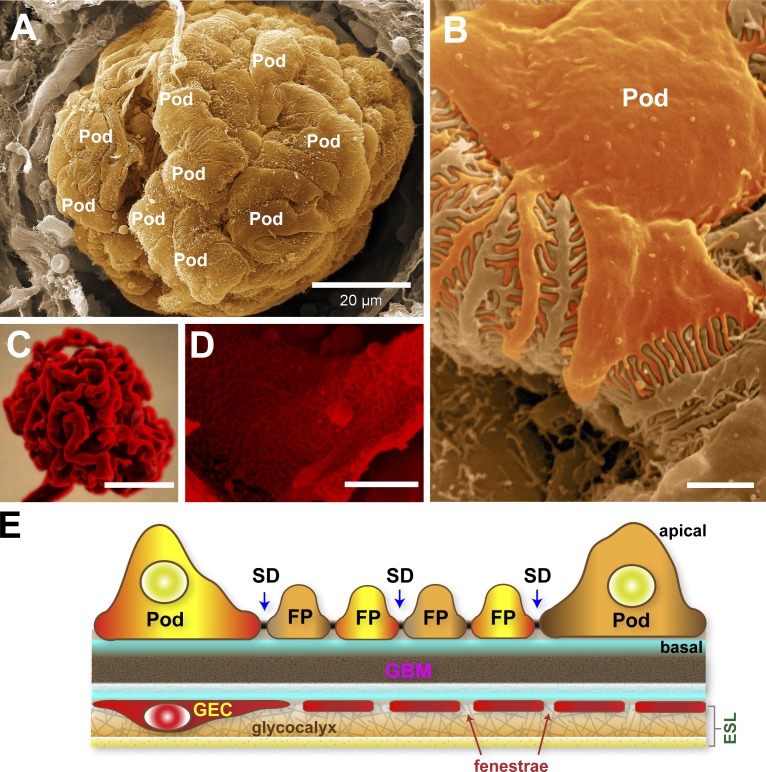
An ultrastructural overview of podocytes and the glomerular endothelium. (A) Scanning electron micrograph of an exposed glomerulus. In this image, the Bowman’s capsule is broken, permitting a striking view of podocytes (Pod) completely wrapped around the glomerular capillaries. (B) Higher magnification of a podocyte within the glomerulus revealing the interdigitated FPs. (C) A resin cast of the glomerular capillary tuft with the cells corroded to reveal its highly convoluted shape. Image courtesy of F. Hossler (East Tennessee State University, Johnson City, TN). (D) Scanning electron micrograph of an exposed glomerular capillary and its numerous perforations (fenestrae). (E) Simplified diagram of the GFB. The GEC and its fenestrae are lined by a filamentous glycocalyx enriched in negatively charged proteoglycans. The glycocalyx and adsorbed plasma components form the thicker ESL. The GBM is a stratified ECM in between podocytes and GECs. Podocytes form the final layer of the GFB. The interdigitating FPs of podocytes are linked by porous SDs where primary urinary filtrate passes through. Bars: (A) 20 µm; (B and D) 1 µm; (C) 50 µm.
Selective permeability in renal filtration
Water and small solutes (e.g., urea, glucose, amino acids, mineral ions) in blood plasma freely traverse the GFB while circulating cells such as erythrocytes and high-molecular-weight plasma components such as albumin are selectively retained in blood. Intriguingly, the glomerular permeability of proteins, particularly negatively charged proteins such as albumin, is well exceeded by those of neutral dextrans of comparable or even larger sizes (Chang et al., 1975). Additionally, the GFB strongly restricts passage of anionic macromolecules (Thomson and Blantz, 2010). Size and charge selectivity thus makes the GFB a formidable barrier for the bulk of plasma proteins and results in a urinary product that is virtually protein-free. While the role of tubular reuptake of proteins leaked into urine is well recognized, recent intravital imaging studies with fluorescent albumin conjugates validate the predominant role of the GFB in ensuring minimal loss of albumin in urine (Peti-Peterdi and Sipos, 2010).
The diagnostic hallmark of a compromised GFB is the incidence of protein in urine, a condition called proteinuria, or more specifically albuminuria if measured in terms of urinary albumin content. Proteinuria manifests in a host of ailments ranging from congenital nephropathy, hypertension, and diabetes to chronic kidney diseases. Over the last two decades, multidisciplinary studies combining genetics, cell biology, physiology, and signal transduction analysis including extensive studies on proteinuric disease models have provided us with valuable insights regarding the physiological importance of each of the three distinctive layers of the GFB and the intricacies of how they may function as an integral unit. In this review, we summarize our current understanding of the cell and molecular basis of renal filtration, highlighting the development, organization, and properties of each compartment of the GFB, and how they contribute to selective permeability.
Fenestrated capillaries as primary portals of renal filtration
The glomerular vasculature consists of afferent and efferent arterioles and the glomerular capillary tuft (Fig. 1 C). Blood enters and exits the glomerulus via the afferent and efferent arterioles, respectively. Inside the glomerulus, the afferent arteriole immediately branches into the elaborate glomerular capillary tuft, a specialized region where blood filters through. Unlike the afferent and efferent arterioles, the glomerular capillaries are heavily perforated with transcellular pores and are not surrounded by smooth muscles. These glomerular capillary pores, known as fenestrae (the plural of fenestra, which means window in Latin), are 60–100 nm wide and comprise ∼20% of the endothelial surface, making glomerular capillaries efficient portals for the rapid passage of high volumes of fluid characteristic of renal filtration (Fig. 2 D; Levick and Smaje, 1987).
The idea that the glomerular capillary is a bona fide filtering compartment was previously contentious due to the size of its fenestrae, which are seemingly wide enough to accommodate albumin with a molecular dimension of 8 × 8 × 3 nm (Sugio et al., 1999). Nevertheless, biophysical studies demonstrate that fenestrated and nonfenestrated capillaries have comparable permeabilities to macromolecules (Sarin, 2010). Recent studies indicate that the glomerular endothelium plays an active role in renal filtration on the basis of its negatively charged surface. The lumen of the glomerular capillaries and the fenestral surfaces are lined with a fibrous lattice of negatively charged glycoproteins called the glycocalyx (Fig. 2 E; Rostgaard and Qvortrup, 2002; Curry and Adamson, 2012). Additionally, plasma components are adsorbed within the glycocalyx, forming a broader coat >200 nm thick called the endothelial surface layer (ESL; Hjalmarsson et al., 2004). The filamentous structure and strongly negative charge of the ESL thus effectively make fenestrae narrower and more restrictive. Enzymatic destruction of different ESL components resulted in elevated albumin excretion accompanied by diminished ESL depth and loss of anionic sites on the endothelial surface (Gelberg et al., 1996; Jeansson and Haraldsson, 2003; Jeansson and Haraldsson, 2006; Meuwese et al., 2010; Dane et al., 2013). Similar loss of ESL charge density and increased passage of albumin across the GFB was observed when adsorbed ESL components are eluted by salt perfusion (Fridén et al., 2011).
The role of the ESL in glomerular filtration has also been examined in proteinuric disease models. Renal perfusion of adriamycin, a drug used to induce proteinuria in mice, disrupted synthesis of glomerular proteoglycans and dramatically shriveled the glomerular ESL, impairing the size selectivity and charge density of the GFB (Jeansson et al., 2009). In rats, ageing-related proteinuria correlated with the loss of glomerular ESL (Salmon et al., 2012). In both animal models and in human patients, diabetes-induced proteinuria has also been strongly correlated with damage to the ESL (Salmon and Satchell, 2012). It has been proposed that the ESL could serve as a mechanosensor of fluid flow, an argument consistent with the loss of vasodilation upon removal of the ESL (Curry and Adamson, 2012; Fu and Tarbell, 2013). Altogether these findings align with the notion that the ESL is an essential feature of the glomerular endothelium and a crucial determinant of glomerular permeability.
The establishment of a functional GFB is contingent on the proper development of the glomerular endothelium. Nascent podocytes secrete VEGFA, a potent chemoattractant and trophic factor for migratory angioblasts that become that glomerular endothelium. VEGFA binds the receptors VEGFR1 (Flt1), VEGFR2 (Flk1/Kdr), and neuropilin-1, which are expressed by these angioblasts (Robert et al., 2000). Homozygous ablation of Vegfa from podocytes results in arrest of glomerular development and failure to form a GFB. Haploinsufficiency for Vegfa, however, causes a latent and progressive hypertrophy of GECs with a concomitant disappearance of fenestrae, a phenomenon called endotheliosis (Eremina et al., 2003, 2006). This breakdown of the glomerular endothelium is seen when Vegfa ablation is induced in adult mouse podocytes or when its receptor is absent in GECs, which indicates that VEGFA acts in a paracrine manner via VEGFR2 (Eremina et al., 2008; Sison et al., 2010). These corroborate earlier findings showing that inhibition of VEGFA function causes rapid onset of endotheliosis and proteinuria (Sugimoto et al., 2003). Additionally, compound loss of the phospholipid-binding ATPases EHD3 and EHD4, which are expressed exclusively by GECs, strikingly resembles Vegfa haploinsufficiency and VEGFR2 deficiency (George et al., 2011). Their importance in vesicle trafficking suggests that EHD3 and EHD4 likely regulate the recycling of VEGFR2 based on the altered cell surface distribution of VEGFR2 in their absence.
Interestingly, podocyte-specific overexpression of VEGFA164, the predominant VEGFA isoform in the kidney, causes global collapse of the glomerular tuft, rapid depletion of GECs, and massive proteinuria (Eremina et al., 2003). Inducible overexpression of moderate levels of VEGFA164 in postnatal and adult podocytes, however, causes a reversible disruption of glomerular structure and function (Veron et al., 2010a,b). As diabetic patients are known to have elevated levels of circulating VEGFA, these overexpression studies suggest that excessive VEGFA signaling could contribute to the progression of diabetic nephropathy (Chiarelli et al., 2000; Hovind et al., 2000). These findings further indicate that a delicately balanced dosage of VEGFA is necessary to coordinate the development and maintenance of the glomerular vasculature and the GFB.
Signaling via secreted glycoproteins called angiopoietins intersects with the VEGFA-dependent pathway to balance stabilization and remodeling of renal and systemic vasculature (Augustin et al., 2009). The angiopoietin Angpt1 is produced by podocytes and MCs, whereas its cognate receptor Tie2 is expressed by GECs (Kolatsi-Joannou et al., 2001; Satchell et al., 2002). Inducible knockout of Angpt1 at mid-gestation (from mouse embryonic day 10.5) results in simplified and enlarged glomerular capillary tufts, and the delamination of GECs (Jeansson et al., 2011). Late gestation (embryonic day 16.5) deletion of Angpt1 does not cause overt glomerular maldevelopment but increased susceptibility to diabetic nephropathy. One factor that could contribute to impairment of the GFB is the loss of glomerular endothelial glycocalyx, which is caused by diabetic nephropathy and likely exacerbated by Angpt1 deficiency. In systemic vasculature, Angpt1 promotes barrier property and reduced permeability to albumin by stimulating the synthesis of glycocalyx and thickening of the ESL (Satchell et al., 2004; Salmon et al., 2009). Altogether, these studies underscore the importance of GECs and the ESL in renal filtration.
The GBM: A highly ordered ECM and filtration bed
The GBM derives from the fusion of the respective basement membranes of both podocytes and GECs (Abrahamson, 2012; Miner, 2012). Ultrastructure imaging by electron microscopy reveals a fibrous and stratified lattice with heterogeneous pores. Proteomic analysis identified 144 distinct proteins in purified human glomerular ECM including the GBM (Byron et al., 2014; Lennon et al., 2014), with the most abundant being collagens (types I, IV, VI, and XVIIII subunits), laminins (α5, β2, and γ1), nidogen-1, heparan sulfate proteoglycans (HSPGs, agrin, and perlecan), and tubulointerstitial nephritis antigen-like (TINAGL1) protein. The GBM is an integral component of the GFB acting as an intermediary sieving matrix. The GBM may also function as a sink for pro-angiogenic ligands and secreted factors that mediate cellular communication between podocytes and GECs. Lastly, the GBM cements podocytes and GEC in place by cell–ECM adhesive interactions, thus effectively stabilizing the GFB. Among the abundant components of the GBM, type IV collagens and laminins are the most indispensable.
Alport syndrome is a hereditary disorder that targets the GBM, causing mild proteinuria during adolescence and progressing to end-stage renal failure. This ailment is linked to mutations in the genes COL4A3, COL4A4, and COL4A5, which encode the type IV collagen subunits α3, α4, and α5, respectively. Maturation of the GBM involves the substitution of the α1α1α2 (IV) collagen with the α3α4α5 (IV) collagen trimers as the predominant collagen complex, a developmental change that has been inferred to strengthen the GBM (Miner and Sanes, 1994). Mutations in Alport syndrome disrupt the assembly of α3α4α5 (IV) collagen trimers, leading to the persistent prominence of α1α1α2 (IV) collagen complexes. As α3α4α5 (IV) collagen trimers represent half the total proteins of a mature GBM (Candiello et al., 2010), it comes as no surprise that Alport GBMs are grossly perturbed in composition and are morphologically distorted. The importance of the collagen IV complex in the GBM is further highlighted by Goodpasture’s disease, an autoimmune disorder whereby self-reactive antibodies target the α3 subunit of collagen IV, resulting in glomerulonephritis (Cui and Zhao, 2011).
Pierson syndrome is an autosomal recessive disorder presenting with congenital proteinuria and neuromuscular maldevelopment. Mutations linked to Pierson syndrome map to the gene LAMB2, which encodes the laminin β2, impairing the assembly of the laminin complex LM-521 (a heterotrimer formed among laminin-α5, -β2, and -γ1 subunits; Zenker et al., 2004; Matejas et al., 2010). Mice lacking Lamb2 also show the abnormal renal and neuromuscular phenotype of Pierson syndrome, and reveal a distinctive splitting of the GBM (Noakes et al., 1995). In mice, loss of Lama5 in podocytes or the expression of a hypomorphic allele of Lama5 (causing attenuated expression of laminin-α5) results in progressive proteinuria and ultrastructural deformation of the GBM (Kikkawa and Miner, 2006; Shannon et al., 2006; Goldberg et al., 2010). Loss of LM-521 in Pierson syndrome causes other laminin complexes (LM-111, -211, -332, and -511) to become more prevalent, although this apparent compensation is insufficient to restore normal GBM structure and GFB function.
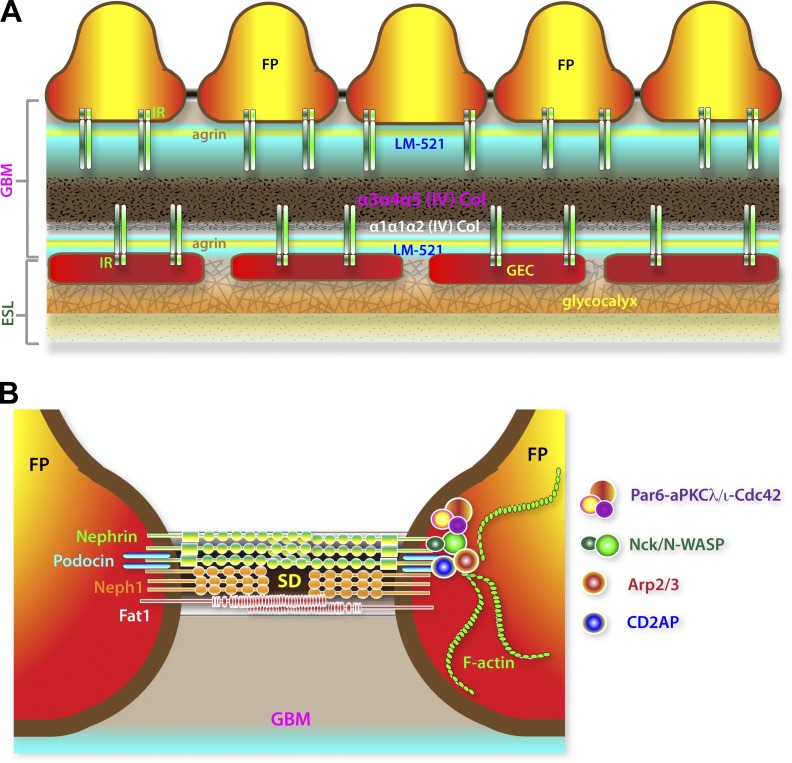
Scanning electron microscopy reveals that the GBM is not amorphous, but is rather a highly organized labyrinth of interconnected polygonal fibrils of varying thickness ranging from 4 to 10 nm (Kubosawa and Kondo, 1985; Hironaka et al., 1993). The fibrils are most densely packed within the core and have heterogeneous pores averaging 10 nm in diameter. In a proteinuric nephritis disease model in rodents, it was observed that the GBM fibril network was more loosely packed and had enlarged pores as big as 40 nm (Hironaka et al., 1996). Imaging analysis combining stochastic optical reconstruction microscopy (STORM) and correlative electron microscopy has revealed nanometer-resolution details of the highly stratified organization of the GBM, delineating the location and orientation of epitopes of major GBM components relative to the adhesion receptor integrin-β1 expressed by GECs and podocytes (Fig. 3 A; Suleiman et al., 2013). Collagen IV and nidogen-1 both map within the central region of the GBM. In contrast, laminin-α5, agrin, and integrin-β1 bimodally align within two distinct layers. Interestingly, the α3α4α5 and α1α1α2 (IV) collagen networks are particularly concentrated at the core, closer to the endothelial side, a distribution that is unexpectedly too distant from the extracellular domains of integrin-β1 at the surface of podocytes. This indicates that the physiologically important ligands of podocyte integrin-β1 are the agrin and laminin complexes. Remarkably, this imaging analysis correlates well with established domain–domain interactions of these GBM components.
Figure 3.
Molecular organization of the GBM and the SD. (A) Highly stratified assembly of GBM components. Laminin LM-521 and agrin are bimodally distributed, whereas collagen IV complexes are concentrated at the core of the GBM. The minor α1α1α2 collagen (Col) is notably biased toward the glomerular endothelium. Both the predominant α3α4α5 and the less abundant α1α1α2 type IV collagens are normally too distant from β1–integrin receptor (IR) complexes on the podocyte side. This suggests that LM-521 and agrin but not type IV collagens are the normal physiological ligands of IR complexes expressed by podocytes. (B) Simplified representation of major adhesion receptors (nephrin, Neph1, and Fat1) found in the SD. Lipid-raft localization of the SD is dependent on the cholesterol-binding podocin. The SD is coupled to both F-actin regulatory (Nck–N-WASP–Arp2/3 and CD2AP–Arp2/3) and cell polarity (Par6–aPKCλ/ι–Cdc42) complexes.
Application of correlative STORM imaging to kidneys of Alport mice (Col4a3 mutant mice) demonstrates the dramatic redistribution of agrin and α1α1α2 (IV) collagen into a diffuse pattern throughout the width of the GBM. A likely implication of this is that podocytes might be inappropriately exposed to type IV collagens, thereby inducing a pathological transformation as observed in Alport disease. These imaging analyses bolster the argument that the ultrafine pore structure of the GBM is key to normal filtration, and that proteinuria results from perturbing the molecular and structural organization of the GBM.
The abundance of HSPGs such as agrin, perlecan, and collagen XVIII confers a net negative charge to the GBM, which prompted a long-held assumption that the GBM is a critical determinant of the charge selectivity of the GFB (Rennke et al., 1975; Rennke and Venkatachalam, 1977; Harvey et al., 2007; van den Hoven et al., 2008; Goldberg et al., 2009). Genetic studies in mice aimed at minimizing the net negative charge of the GBM have disputed this argument and failed to result in overt proteinuria (Rossi et al., 2003; Harvey et al., 2007; Chen et al., 2008; Goldberg et al., 2009; Hamano et al., 2010). Similarly, treatment of the GBM with heparanase in order to strip glycosaminoglycan-associated anionic charges did not cause overt changes in glomerular morphology or induce proteinuria (van den Hoven et al., 2008). In light of these findings it is tempting to speculate that charge repulsion of circulating macromolecules in the GFB is primarily established within the glomerular compartment instead of the GBM.
The final gatekeepers: Renal podocytes and their slit diaphragms (SDs)
The defining feature of normal fully differentiated podocytes is their elaborate cytoarchitecture, which resembles the stellate body shape of an octopus, characterized by an arborized cell body with multiple projections subdivided into larger major processes and finer pedicels or FPs (Fig. 2, A and B). Major processes are reinforced by microtubules and intermediate filaments while FPs are actin-rich projections anchored to the GBM via focal adhesions (Ichimura et al., 2003). The podocyte cell bodies and their FPs wrap around the glomerular capillaries in a strikingly elaborate interdigitating pattern. Neighboring podocytes are physically adjoined through their FPs via unique intercellular junctions called the SD (Fig. 3 B). Unlike tight junctions, the SD lacks E-cadherin and is structurally porous (Tassin et al., 1994). The SD thereby serves as the exit port for primary urinary filtrate and is now well recognized as essential in the selective retention of high-molecular-weight plasma components. The seminal discoveries of the proteins nephrin and podocin as integral components of the SD are instrumental in proving that podocytes and their structural integrity are of key importance in the establishment and maintenance of the GFB (Kestilä et al., 1998; Holzman et al., 1999; Ruotsalainen et al., 1999; Boute et al., 2000; Schwarz et al., 2001; Huber et al., 2003). Inactivating mutations of NPHS1 and NPHS2, the respective genes encoding for nephrin and podocin, lead to congenital nephropathy characterized by the collapse of FPs and the absence of SDs (Kestilä et al., 1998; Boute et al., 2000). This stereotypical pathological transformation of podocytes called effacement is a distinctive hallmark of podocyte injury and is strongly correlated with the onset of proteinuria.
Several genes, apart from NPHS1 and NPHS2, that encode for podocyte-specific proteins are strongly associated with the onset of proteinuric diseases including CD2AP (Shih et al., 1999; Kim et al., 2003), Kirrel/Neph1 (Donoviel et al., 2001), Fat1 (Ciani et al., 2003), TRPC6 (Reiser et al., 2005; Winn et al., 2005), ACTN4 (Kaplan et al., 2000), MYO1E (Krendel et al., 2009; Mele et al., 2011), ARHGAP24 (Akilesh et al., 2011), ARHGDIA (Togawa et al., 1999; Gee et al., 2013; Gupta et al., 2013), INF2 (Brown et al., 2010), COQ2 (Diomedi-Camassei et al., 2007), COQ6 (Heeringa et al., 2011), PLCE1 (Sadl et al., 2002; Hinkes et al., 2006), ANLN (Gbadegesin et al., 2014), PTPRO (Wharram et al., 2000; Ozaltin et al., 2011), and ADCK4 (Ashraf et al., 2013). Most of these genes encode for intrinsic SD components or their respective interacting partners while the rest encode for proteins needed for the survival, differentiation, cytoskeletal dynamics, and unique morphology of podocytes. The consequences of mutations of these genes highlight the important relationship between podocyte dysfunction and the disruption of the GFB.
While many of the molecular constituents of the SDs have been identified, their topological assembly into a functional complex is poorly understood. The ectodomains of several adhesion receptors in the SD likely organize the bridge linking juxtaposed FPs via a combination of homophilic and heterophilic receptor–receptor interactions. By virtue of their large ectodomains, nephrin and Fat1 are excellent candidates to associate in trans to connect opposing FPs (Inoue et al., 2001; Ciani et al., 2003; Khoshnoodi et al., 2003; Wartiovaara et al., 2004). The smaller adhesion receptors such as Neph1 and Neph3, however, may interact in cis with nephrin and Fat1 (Gerke et al., 2003; Heikkilä et al., 2011). Consistent with its anatomical appearance as a junction between differentiated podocytes, other components of the SD are key molecules associated with adherens and tight junctions including ZO-1, CASK, spectrins, MAGI-2, JAMA-A, occludin, cingulin, and IQGAP1 (Lehtonen et al., 2005; Fukasawa et al., 2009). The huge scaffold protein ZO-1 is essential for the normal interdigitation of FPs and the formation of the SD (Itoh et al., 2014). Lack of ZO-1 triggers early onset proteinuria with podocyte effacement and the progressive scarring of the glomerulus (glomerulosclerosis). ZO-1 appears to be required to maintain the expression and correct spatial distribution of nephrin and podocin. Consistently, ZO-1 expression is significantly diminished in models of diabetic nephropathy.
The distinctive morphology of podocytes underscores the importance of cell polarity signaling in podocyte biology. The SD marks the boundary between the apical and basolateral membrane domains of podocytes. Nephrin and Neph1 are known to interact with polarity proteins such as Par3, Par6, and aPKCλ/ι (Hartleben et al., 2008). The deletion of aPKCλ/ι and the small GTPase Cdc42, which regulates the activation of the Par3-Par6-aPKCλ/ι polarity complex, causes proteinuria and the formation of aberrant junctions between effaced FPs (Hirose et al., 2009; Huber et al., 2009; Scott et al., 2012; Blattner et al., 2013). Loss of aPKCλ/ι has been shown to interfere with cell surface localization of nephrin, podocin, and Neph1 (Satoh et al., 2014). Furthermore, studies in zebrafish demonstrate that the Crumbs (Crb) protein family member Crb2b is required for the differentiation of pronephric podocytes, whereas mutations in the human orthologue CRB2 have been linked to proteinuric disease (Ebarasi et al., 2009, 2015; Slavotinek et al., 2015). Crb proteins are part of the Crb–Patj–Pals1 polarity complex, which works alongside the Par3–Par6–aPKCλ/ι complex in directing the apical localization of particular membrane-bound proteins. Crb2b suppression in zebrafish leads to loss of polarized distribution of nephrin and the disruption of SD assembly (Ebarasi et al., 2009). In contrast, podocyte-specific ablation of Scribble, a determinant of basolateral trafficking, did not inhibit proper SD formation (Hartleben et al., 2012). These studies suggest that apical sorting predominates over basolateral sorting mechanisms to specify the polarized and dynamic assembly of the SD complex.
Parallel bundles of actin filaments and a network of cortical actin form the backbone of terminal FPs, and the perturbation of this cytoskeletal assembly is thought to underlie FP effacement and the dismantling of the SD (Ichimura et al., 2003). Compound ablation of the adaptor molecules Nck1 and Nck2 in podocytes causes proteinuria, which demonstrates that the SD is intimately and dynamically coupled to the actin cytoskeleton (Jones et al., 2006). In vitro, oligomerized nephrin interacts with Nck adaptors, leading to the recruitment of N-WASP and Arp2/3 complex that mediates localized polymerization of actin filaments (Jones et al., 2006; Verma et al., 2006). Nck adaptors are not only required during podocyte maturation but are also needed to maintain preformed FPs (Jones et al., 2009). Since Nck proteins also interact with the PINCH–ILK–integrin complex, these adaptors could also help anchor podocytes to the GBM via actin-linked focal contacts (Tu et al., 1999; Dai et al., 2006; El-Aouni et al., 2006). Similarly, the Rho GTPase Cdc42 is required to mediate the linkage between the actin cytoskeleton and nephrin complexes (Scott et al., 2012). The cytoskeletal tethering of the SD is also dependent on the scaffold protein CD2AP, which promotes the stability of actin microfilament network of podocytes (Shih et al., 1999; Kim et al., 2003; Yaddanapudi et al., 2011; Tang and Brieher, 2013).
Equally important to the assembly of the podocyte actin cytoskeleton are opposing events counteracting actin filament polymerization. Slit1-Robo2 signaling antagonizes nephrin-dependent actin polymerization yet is required to establish a normal pattern of FP interdigitation (Fan et al., 2012). Additionally, depletion of the actin-severing factor cofilin-1 has been shown to cause late-onset proteinuria and ultrastructural defects in podocytes in a manner akin to specific loss of Robo2 in podocytes (Ashworth et al., 2010; Garg et al., 2010). Mutations in ACTN4 (Kaplan et al., 2000), ARHGDIA (Togawa et al., 1999; Gee et al., 2013; Gupta et al., 2013), ARHGAP24 (Akilesh et al., 2011), INF2 (Brown et al., 2010), MYO1E (Krendel et al., 2009; Mele et al., 2011), and ANLN (Gbadegesin et al., 2014), which encode for known regulators of the actin cytoskeleton, have all been implicated in the etiology of proteinuric diseases.
The cytoskeletal dynamics and structural plasticity of podocytes are also regulated by calcium signaling, lipid–protein interactions at the SD, and endocytosis. In podocytes, the ion channels Trpc5 and Trpc6 mediate distinctive calcium influx in response to angiotensin II, eliciting the reorganization of the actin cytoskeleton via modulation of the Rho GTPases Rac1 and RhoA (Tian et al., 2010). Gain-of-function mutations of TRPC6 are known to cause proteinuria in humans, whereas genetic loss of Trpc5 or Trpc6 prevents podocyte injury (Reiser et al., 2005; Winn et al., 2005; Schaldecker et al., 2013). These findings suggest that unbalanced elevation of intracellular calcium mitigates podocyte dysfunction and provide an explanation as to the protective benefits of blockade of angiotensin signaling in the progression of proteinuric renal diseases such as glomerulosclerosis and diabetic nephropathy. Interestingly, Trpc6 interacts with podocin, which suggests how intimately calcium signaling is coupled to the SD complex (Huber et al., 2007; Schurek et al., 2014). Lipid-dependent autocrine signaling in podocytes involving sFlt1 is required in the regulation of actin dynamics and the proper formation of FPs and the SDs (Jin et al., 2012). Specifically, sFlt1 secreted by podocytes binds to the glycosphingolipid GM3 at the podocyte surface, promoting cell adhesion, nephrin phosphorylation, and consequent remodeling of the cytoskeleton. Defective endocytosis in podocytes has also been shown to impair renal filtration. FP effacement and proteinuria ensues in the absence of endocytosis-related lipid-binding proteins, specifically dynamins, endophilins, and synaptojanin-1 (Soda et al., 2012). It has been postulated that clathrin-mediated endocytosis could dynamically sculpt FPs by regulating the turnover of SD components (Soda and Ishibe, 2013).
Podocytes also play multiple important functions in maintaining the GFB independent of the formation of the SDs. Podocytes have a vital role in promoting the proliferation, survival, and development of endothelial cells. The pro-angiogenic factors VEGFA, Angpt1, and SDF1 are secreted by podocytes and are essential for the normal development of the glomerular endothelium (Simon et al., 1998; Yuan et al., 1999; Satchell et al., 2002; Takabatake et al., 2009; Haege et al., 2012). Podocytes together with the glomerular endothelium also collaborate in building the GBM (Byron et al., 2014). Whereas α1α1α2 (IV) collagen is produced jointly by endothelial cells and podocytes, the α3α4α5 (IV) collagen network is derived primarily from podocytes, as seen in vivo (Abrahamson et al., 2009). Macromolecules and proteins that traverse the GBM can be sequestered by podocytes via endocytosis, a mechanism that likely prevents the GFB from clogging (Eyre et al., 2007; Akilesh et al., 2008). Megalin and cubilin, which form a multifunctional endocytic receptor complex commonly found in absorptive epithelia, are coexpressed in podocytes and could mediate the retrieval of urinary albumin by podocytes (Yamazaki et al., 2004; Prabakaran et al., 2012). Overwhelming genetic evidence undeniably underscores the fact that the podocyte is an essential component of the GFB.
The importance of cell adhesion to the GBM
Biophysical studies demonstrate that GBM compression reduces permeability to albumin and the polysaccharide Ficoll (Robinson and Walton, 1989; Fissell et al., 2009). It is therefore tempting to speculate based on this that GECs and podocytes physically constrain and mitigate compression of the GBM. Consistent with this supposition are genetic studies showing that loss of Itga3 (Kreidberg et al., 1996), Itgb1 (Pozzi et al., 2008), Cd151 (Karamatic Crew et al., 2004; Sachs et al., 2006), Ddr1 (Gross et al., 2004), Ilk (Dai et al., 2006; El-Aouni et al., 2006), Tln1 (Tian et al., 2014), and Rap1a/b (Potla et al., 2014), genes encoding for proteins implicated in the adhesion of podocytes to GBM components such as collagens and laminins, results in impairment of the GFB. Intrinsic structural reorganization of the GBM such as in Alport and Pierson syndrome may also perturb the normal anchorage of podocytes and endothelial cells, cumulatively altering the compressibility of the GBM. This is not at all far-fetched, as ECM stiffness, by way of mechanotransduction via adhesion molecules, is known to influence a diverse range of cellular behavior including restructuring of the actin cytoskeleton, contractility, motility, gene expression, proliferation, and overall differentiation (DuFort et al., 2011).
Effaced podocyte FPs could very well be indicative of not just the remodeling of cell–cell junctions or SDs but also of a maladaptive response to reestablish weakening focal contacts on the GBM. In fact, unfastening of podocytes and denuded GBM are common in the progression of diabetic nephropathy and chronic kidney disease (Toyoda et al., 2007; Weil et al., 2012; Kriz and Lemley, 2015), whereas endothelial detachment has been observed in Vegfa-null mutant mice (Eremina et al., 2003). Interestingly, comparison of the elastic properties of purified glomeruli by atomic force microscopy reveals that glomerular rigidity is reduced by as much as 30% in mouse models of Alport syndrome (Col4a3 knockout) and HIV-induced nephropathy (HIVAN) before the onset of overt pathological histology (Wyss et al., 2011). Increased glomerular deformability correlating with increased permeability of the GFB is likely symptomatic of an aberrant interaction between the GBM and the cells attached to it.
Streaming potential and charge selectivity in renal filtration
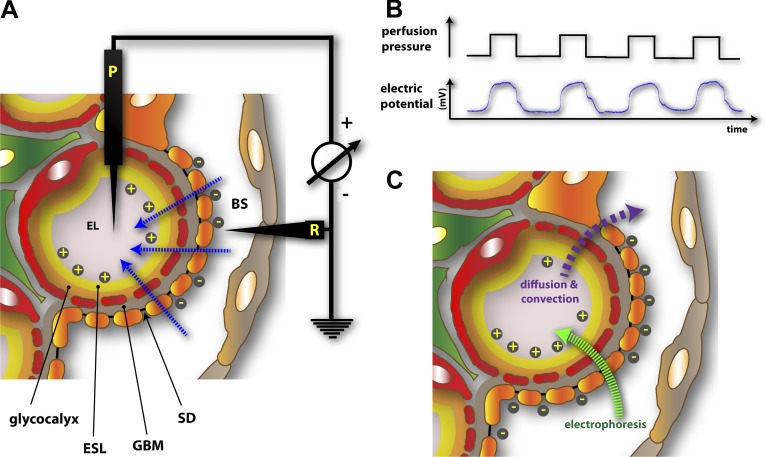
An attractive hypothesis based on electrokinetic principles has been proposed to account for the charge selectivity in renal filtration. Micropuncture measurements on salamander (Necturus maculosus) glomeruli demonstrate that filtration pressure establishes a distinctive streaming potential or charge difference across the GFB, with the Bowman’s space being more negative than the endothelial lumen (Fig. 4, A and B; Hausmann et al., 2010). The phenomenon of streaming potentials arises when electrolytes are forced by a pressure gradient across porous media or a channel carrying a permanent charge. In essence, the electrokinetic model posits that when small cations traverse the GFB, they bind and counterbalance the negatively charged surfaces within the GFB, reaching a threshold at which net ionic movement of small cations lags behind that of small anions (Hausmann et al., 2012; Moeller and Tenten, 2013). This differential advance of oppositely charged small ions thereby establishes a net charge separation and a measurable electrical field that polarizes the GFB. The streaming potential hypothesis therefore predicts that larger anions such as native albumin would encounter a retrograde electrophoretic field running opposite to the direction of hydraulic flux (Fig. 4 C). Given the minor importance of negative charges within the GBM, it can be inferred that the streaming potential is largely initiated from the highly charged ESL. The finding that neutral albumin traverses the GFB independent of the glomerular filtration rate whereas native anionic albumin passage becomes increasingly more restricted with increasing glomerular pressure is congruent with an electrokinetic model of the GFB (Lund et al., 2003). Similarly, albumin readily diffuses and equilibrates across the GFB once plasma flow is halted (Ryan and Karnovsky, 1976).
Figure 4.
Electrokinetic model of renal filtration. (A) Experimental setup used to demonstrate the existence of a flow-dependent electrical potential (streaming potential) across the GFB in salamander (N. maculosus) glomeruli (Hausmann et al., 2010). P, potential electrode; R, reference electrode. Small ions, due to differential interaction with the negatively charged GFB, create a net gradient of charges measurable as a streaming potential (blue arrows), making the endothelial lumen (EL) more positive than the Bowman’s space (BS). (B) Filtration pressure dependence of glomerular streaming potential. (C) Retrograde electrophoretic field created by streaming potentials. Due to streaming potentials, macromolecules encounter a dynamic electrophoretic field (green arrow) that is opposite to that of diffusive and convective fluxes (purple arrow). Albumin, a negatively charged macromolecule, would not only encounter size-dependent exclusion by the GFB but would effectively be electrophoresed away from the GFB during the course of active filtration.
The hypothesis also predicts that podocyte FP effacement can be detrimental to the generation of a streaming potential as more rapidly advancing small anions bounce back upon encountering the broadened FPs, causing the electrical field across the GFB to be short-circuited (Hausmann et al., 2010, 2012). Hence, normal podocytes with their elaborate and regular network of FPs and SDs guarantees that streaming potential and filtration occur uniformly across the GFB. Further proof regarding this prediction is needed and should ideally be based on recording glomerular streaming potentials in the context of proteinuric disease models in mice. Nevertheless, in salamander glomeruli, streaming potentials are reversibly blocked by protamine, a polycationic protein that neutralizes the negative charge of the GFB and is well-known to induce proteinuria (Hausmann et al., 2010).
Conclusions
The robustness of the GFB depends on the plasticity and the dynamic signaling between its distinctive layers. Vigorous investigations on this subject over the years have shown that targeted damage to any one layer can lead to collapse of the GFB, and that in many cases compromising one layer has inevitable deleterious repercussions for the other layers. These highlight an emerging theme that the GFB, despite being multilayered, consists of components with dynamically intertwined roles, no single one of which is more important than the others, that harmonize together into one functionally elegant ensemble. Continued efforts to refine our understanding of the mechanism of renal filtration and the biology of the GFB are invaluable for the development of better therapeutic strategies to alleviate the burden of proteinuric diseases.
Acknowledgments
This work was funded by the National Institutes of Health (grant R01HL124120).
The authors declare no competing financial interests.
Footnotes
Abbreviations used in this paper:
- ESL
- endothelial surface layer
- FP
- foot process
- GBM
- glomerular basement membrane
- GEC
- glomerular endothelial cell
- GFB
- glomerular filtration barrier
- MC
- mesangial cell
- PEC
- parietal epithelial cell
- SD
- slit diaphragm
References
- Abrahamson D.R.2012. Role of the podocyte (and glomerular endothelium) in building the GBM. Semin. Nephrol. 32:342–349 10.1016/j.semnephrol.2012.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrahamson D.R., Hudson B.G., Stroganova L., Borza D.B., and St. John P.L.. 2009. Cellular origins of type IV collagen networks in developing glomeruli. J. Am. Soc. Nephrol. 20:1471–1479 10.1681/ASN.2008101086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akilesh S., Huber T.B., Wu H., Wang G., Hartleben B., Kopp J.B., Miner J.H., Roopenian D.C., Unanue E.R., and Shaw A.S.. 2008. Podocytes use FcRn to clear IgG from the glomerular basement membrane. Proc. Natl. Acad. Sci. USA. 105:967–972 10.1073/pnas.0711515105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akilesh S., Suleiman H., Yu H., Stander M.C., Lavin P., Gbadegesin R., Antignac C., Pollak M., Kopp J.B., Winn M.P., and Shaw A.S.. 2011. Arhgap24 inactivates Rac1 in mouse podocytes, and a mutant form is associated with familial focal segmental glomerulosclerosis. J. Clin. Invest. 121:4127–4137 10.1172/JCI46458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashraf S., Gee H.Y., Woerner S., Xie L.X., Vega-Warner V., Lovric S., Fang H., Song X., Cattran D.C., Avila-Casado C., et al. 2013. ADCK4 mutations promote steroid-resistant nephrotic syndrome through CoQ10 biosynthesis disruption. J. Clin. Invest. 123:5179–5189 10.1172/JCI69000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashworth S., Teng B., Kaufeld J., Miller E., Tossidou I., Englert C., Bollig F., Staggs L., Roberts I.S., Park J.K., et al. 2010. Cofilin-1 inactivation leads to proteinuria—studies in zebrafish, mice and humans. PLoS ONE. 5:e12626 10.1371/journal.pone.0012626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustin H.G., Koh G.Y., Thurston G., and Alitalo K.. 2009. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat. Rev. Mol. Cell Biol. 10:165–177 10.1038/nrm2639 [DOI] [PubMed] [Google Scholar]
- Blattner S.M., Hodgin J.B., Nishio M., Wylie S.A., Saha J., Soofi A.A., Vining C., Randolph A., Herbach N., Wanke R., et al. 2013. Divergent functions of the Rho GTPases Rac1 and Cdc42 in podocyte injury. Kidney Int. 84:920–930 10.1038/ki.2013.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boute N., Gribouval O., Roselli S., Benessy F., Lee H., Fuchshuber A., Dahan K., Gubler M.C., Niaudet P., and Antignac C.. 2000. NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat. Genet. 24:349–354 10.1038/74166 [DOI] [PubMed] [Google Scholar]
- Brown E.J., Schlöndorff J.S., Becker D.J., Tsukaguchi H., Tonna S.J., Uscinski A.L., Higgs H.N., Henderson J.M., and Pollak M.R.. 2010. Mutations in the formin gene INF2 cause focal segmental glomerulosclerosis. Nat. Genet. 42:72–76 (published erratum appears in Nat. Genet. 42:361) 10.1038/ng.505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byron A., Randles M.J., Humphries J.D., Mironov A., Hamidi H., Harris S., Mathieson P.W., Saleem M.A., Satchell S.C., Zent R., et al. 2014. Glomerular cell cross-talk influences composition and assembly of extracellular matrix. J. Am. Soc. Nephrol. 25:953–966 10.1681/ASN.2013070795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candiello J., Cole G.J., and Halfter W.. 2010. Age-dependent changes in the structure, composition and biophysical properties of a human basement membrane. Matrix Biol. 29:402–410 10.1016/j.matbio.2010.03.004 [DOI] [PubMed] [Google Scholar]
- Chang R.L., Deen W.M., Robertson C.R., and Brenner B.M.. 1975. Permselectivity of the glomerular capillary wall: III. Restricted transport of polyanions. Kidney Int. 8:212–218 10.1038/ki.1975.104 [DOI] [PubMed] [Google Scholar]
- Chen S., Wassenhove-McCarthy D.J., Yamaguchi Y., Holzman L.B., van Kuppevelt T.H., Jenniskens G.J., Wijnhoven T.J., Woods A.C., and McCarthy K.J.. 2008. Loss of heparan sulfate glycosaminoglycan assembly in podocytes does not lead to proteinuria. Kidney Int. 74:289–299 10.1038/ki.2008.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiarelli F., Spagnoli A., Basciani F., Tumini S., Mezzetti A., Cipollone F., Cuccurullo F., Morgese G., and Verrotti A.. 2000. Vascular endothelial growth factor (VEGF) in children, adolescents and young adults with Type 1 diabetes mellitus: relation to glycaemic control and microvascular complications. Diabet. Med. 17:650–656 10.1046/j.1464-5491.2000.00350.x [DOI] [PubMed] [Google Scholar]
- Ciani L., Patel A., Allen N.D., and ffrench-Constant C.. 2003. Mice lacking the giant protocadherin mFAT1 exhibit renal slit junction abnormalities and a partially penetrant cyclopia and anophthalmia phenotype. Mol. Cell. Biol. 23:3575–3582 10.1128/MCB.23.10.3575-3582.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Z., and Zhao M.H.. 2011. Advances in human antiglomerular basement membrane disease. Nat. Rev. Nephrol. 7:697–705 10.1038/nrneph.2011.89 [DOI] [PubMed] [Google Scholar]
- Curry F.E., and Adamson R.H.. 2012. Endothelial glycocalyx: permeability barrier and mechanosensor. Ann. Biomed. Eng. 40:828–839 10.1007/s10439-011-0429-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai C., Stolz D.B., Bastacky S.I., St-Arnaud R., Wu C., Dedhar S., and Liu Y.. 2006. Essential role of integrin-linked kinase in podocyte biology: Bridging the integrin and slit diaphragm signaling. J. Am. Soc. Nephrol. 17:2164–2175 10.1681/ASN.2006010033 [DOI] [PubMed] [Google Scholar]
- Dane M.J., van den Berg B.M., Avramut M.C., Faas F.G., van der Vlag J., Rops A.L., Ravelli R.B., Koster B.J., van Zonneveld A.J., Vink H., and Rabelink T.J.. 2013. Glomerular endothelial surface layer acts as a barrier against albumin filtration. Am. J. Pathol. 182:1532–1540 10.1016/j.ajpath.2013.01.049 [DOI] [PubMed] [Google Scholar]
- Diomedi-Camassei F., Di Giandomenico S., Santorelli F.M., Caridi G., Piemonte F., Montini G., Ghiggeri G.M., Murer L., Barisoni L., Pastore A., et al. 2007. COQ2 nephropathy: a newly described inherited mitochondriopathy with primary renal involvement. J. Am. Soc. Nephrol. 18:2773–2780 10.1681/ASN.2006080833 [DOI] [PubMed] [Google Scholar]
- Donoviel D.B., Freed D.D., Vogel H., Potter D.G., Hawkins E., Barrish J.P., Mathur B.N., Turner C.A., Geske R., Montgomery C.A., et al. 2001. Proteinuria and perinatal lethality in mice lacking NEPH1, a novel protein with homology to NEPHRIN. Mol. Cell. Biol. 21:4829–4836 10.1128/MCB.21.14.4829-4836.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuFort C.C., Paszek M.J., and Weaver V.M.. 2011. Balancing forces: architectural control of mechanotransduction. Nat. Rev. Mol. Cell Biol. 12:308–319 10.1038/nrm3112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebarasi L., He L., Hultenby K., Takemoto M., Betsholtz C., Tryggvason K., and Majumdar A.. 2009. A reverse genetic screen in the zebrafish identifies crb2b as a regulator of the glomerular filtration barrier. Dev. Biol. 334:1–9 10.1016/j.ydbio.2009.04.017 [DOI] [PubMed] [Google Scholar]
- Ebarasi L., Ashraf S., Bierzynska A., Gee H.Y., McCarthy H.J., Lovric S., Sadowski C.E., Pabst W., Vega-Warner V., Fang H., et al. 2015. Defects of CRB2 cause steroid-resistant nephrotic syndrome. Am. J. Hum. Genet. 96:153–161 10.1016/j.ajhg.2014.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Aouni C., Herbach N., Blattner S.M., Henger A., Rastaldi M.P., Jarad G., Miner J.H., Moeller M.J., St-Arnaud R., Dedhar S., et al. 2006. Podocyte-specific deletion of integrin-linked kinase results in severe glomerular basement membrane alterations and progressive glomerulosclerosis. J. Am. Soc. Nephrol. 17:1334–1344 10.1681/ASN.2005090921 [DOI] [PubMed] [Google Scholar]
- Eremina V., Sood M., Haigh J., Nagy A., Lajoie G., Ferrara N., Gerber H.P., Kikkawa Y., Miner J.H., and Quaggin S.E.. 2003. Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J. Clin. Invest. 111:707–716 10.1172/JCI17423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eremina V., Cui S., Gerber H., Ferrara N., Haigh J., Nagy A., Ema M., Rossant J., Jothy S., Miner J.H., and Quaggin S.E.. 2006. Vascular endothelial growth factor a signaling in the podocyte-endothelial compartment is required for mesangial cell migration and survival. J. Am. Soc. Nephrol. 17:724–735 10.1681/ASN.2005080810 [DOI] [PubMed] [Google Scholar]
- Eremina V., Jefferson J.A., Kowalewska J., Hochster H., Haas M., Weisstuch J., Richardson C., Kopp J.B., Kabir M.G., Backx P.H., et al. 2008. VEGF inhibition and renal thrombotic microangiopathy. N. Engl. J. Med. 358:1129–1136 10.1056/NEJMoa0707330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyre J., Ioannou K., Grubb B.D., Saleem M.A., Mathieson P.W., Brunskill N.J., Christensen E.I., and Topham P.S.. 2007. Statin-sensitive endocytosis of albumin by glomerular podocytes. Am. J. Physiol. Renal Physiol. 292:F674–F681 10.1152/ajprenal.00272.2006 [DOI] [PubMed] [Google Scholar]
- Fan X., Li Q., Pisarek-Horowitz A., Rasouly H.M., Wang X., Bonegio R.G., Wang H., McLaughlin M., Mangos S., Kalluri R., et al. 2012. Inhibitory effects of Robo2 on nephrin: a crosstalk between positive and negative signals regulating podocyte structure. Cell Reports. 2:52–61 10.1016/j.celrep.2012.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fissell W.H., Hofmann C.L., Ferrell N., Schnell L., Dubnisheva A., Zydney A.L., Yurchenco P.D., and Roy S.. 2009. Solute partitioning and filtration by extracellular matrices. Am. J. Physiol. Renal Physiol. 297:F1092–F1100 10.1152/ajprenal.00162.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridén V., Oveland E., Tenstad O., Ebefors K., Nyström J., Nilsson U.A., and Haraldsson B.. 2011. The glomerular endothelial cell coat is essential for glomerular filtration. Kidney Int. 79:1322–1330 10.1038/ki.2011.58 [DOI] [PubMed] [Google Scholar]
- Fu B.M., and Tarbell J.M.. 2013. Mechano-sensing and transduction by endothelial surface glycocalyx: composition, structure, and function. Wiley Interdiscip Rev. Syst. Biol. Med. 5:381–390 10.1002/wsbm.1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukasawa H., Bornheimer S., Kudlicka K., and Farquhar M.G.. 2009. Slit diaphragms contain tight junction proteins. J. Am. Soc. Nephrol. 20:1491–1503 10.1681/ASN.2008101117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg P., Verma R., Cook L., Soofi A., Venkatareddy M., George B., Mizuno K., Gurniak C., Witke W., and Holzman L.B.. 2010. Actin-depolymerizing factor cofilin-1 is necessary in maintaining mature podocyte architecture. J. Biol. Chem. 285:22676–22688 10.1074/jbc.M110.122929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gbadegesin R.A., Hall G., Adeyemo A., Hanke N., Tossidou I., Burchette J., Wu G., Homstad A., Sparks M.A., Gomez J., et al. 2014. Mutations in the gene that encodes the F-actin binding protein anillin cause FSGS. J. Am. Soc. Nephrol. 25:1991–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee H.Y., Saisawat P., Ashraf S., Hurd T.W., Vega-Warner V., Fang H., Beck B.B., Gribouval O., Zhou W., Diaz K.A., et al. 2013. ARHGDIA mutations cause nephrotic syndrome via defective RHO GTPase signaling. J. Clin. Invest. 123:3243–3253 10.1172/JCI69134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelberg H., Healy L., Whiteley H., Miller L.A., and Vimr E.. 1996. In vivo enzymatic removal of alpha 2-->6-linked sialic acid from the glomerular filtration barrier results in podocyte charge alteration and glomerular injury. Lab. Invest. 74:907–920. [PubMed] [Google Scholar]
- George M., Rainey M.A., Naramura M., Foster K.W., Holzapfel M.S., Willoughby L.L., Ying G., Goswami R.M., Gurumurthy C.B., Band V., et al. 2011. Renal thrombotic microangiopathy in mice with combined deletion of endocytic recycling regulators EHD3 and EHD4. PLoS ONE. 6:e17838 10.1371/journal.pone.0017838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerke P., Huber T.B., Sellin L., Benzing T., and Walz G.. 2003. Homodimerization and heterodimerization of the glomerular podocyte proteins nephrin and NEPH1. J. Am. Soc. Nephrol. 14:918–926 10.1097/01.ASN.0000057853.05686.89 [DOI] [PubMed] [Google Scholar]
- Goldberg S., Harvey S.J., Cunningham J., Tryggvason K., and Miner J.H.. 2009. Glomerular filtration is normal in the absence of both agrin and perlecan-heparan sulfate from the glomerular basement membrane. Nephrol. Dial. Transplant. 24:2044–2051 10.1093/ndt/gfn758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg S., Adair-Kirk T.L., Senior R.M., and Miner J.H.. 2010. Maintenance of glomerular filtration barrier integrity requires laminin α5. J. Am. Soc. Nephrol. 21:579–586 10.1681/ASN.2009091004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross O., Beirowski B., Harvey S.J., McFadden C., Chen D., Tam S., Thorner P.S., Smyth N., Addicks K., Bloch W., et al. 2004. DDR1-deficient mice show localized subepithelial GBM thickening with focal loss of slit diaphragms and proteinuria. Kidney Int. 66:102–111 10.1111/j.1523-1755.2004.00712.x [DOI] [PubMed] [Google Scholar]
- Gupta I.R., Baldwin C., Auguste D., Ha K.C., El Andalousi J., Fahiminiya S., Bitzan M., Bernard C., Akbari M.R., Narod S.A., et al. 2013. ARHGDIA: a novel gene implicated in nephrotic syndrome. J. Med. Genet. 50:330–338 10.1136/jmedgenet-2012-101442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haege S., Einer C., Thiele S., Mueller W., Nietzsche S., Lupp A., Mackay F., Schulz S., and Stumm R.. 2012. CXC chemokine receptor 7 (CXCR7) regulates CXCR4 protein expression and capillary tuft development in mouse kidney. PLoS ONE. 7:e42814 10.1371/journal.pone.0042814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamano Y., Okude T., Shirai R., Sato I., Kimura R., Ogawa M., Ueda Y., Yokosuka O., Kalluri R., and Ueda S.. 2010. Lack of collagen XVIII/endostatin exacerbates immune-mediated glomerulonephritis. J. Am. Soc. Nephrol. 21:1445–1455 10.1681/ASN.2009050492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartleben B., Schweizer H., Lübben P., Bartram M.P., Möller C.C., Herr R., Wei C., Neumann-Haefelin E., Schermer B., Zentgraf H., et al. 2008. Neph-Nephrin proteins bind the Par3-Par6-atypical protein kinase C (aPKC) complex to regulate podocyte cell polarity. J. Biol. Chem. 283:23033–23038 10.1074/jbc.M803143200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartleben B., Widmeier E., Wanner N., Schmidts M., Kim S.T., Schneider L., Mayer B., Kerjaschki D., Miner J.H., Walz G., and Huber T.B.. 2012. Role of the polarity protein Scribble for podocyte differentiation and maintenance. PLoS ONE. 7:e36705 10.1371/journal.pone.0036705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey S.J., Jarad G., Cunningham J., Rops A.L., van der Vlag J., Berden J.H., Moeller M.J., Holzman L.B., Burgess R.W., and Miner J.H.. 2007. Disruption of glomerular basement membrane charge through podocyte-specific mutation of agrin does not alter glomerular permselectivity. Am. J. Pathol. 171:139–152 10.2353/ajpath.2007.061116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausmann R., Kuppe C., Egger H., Schweda F., Knecht V., Elger M., Menzel S., Somers D., Braun G., Fuss A., et al. 2010. Electrical forces determine glomerular permeability. J. Am. Soc. Nephrol. 21:2053–2058 10.1681/ASN.2010030303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausmann R., Grepl M., Knecht V., and Moeller M.J.. 2012. The glomerular filtration barrier function: new concepts. Curr. Opin. Nephrol. Hypertens. 21:441–449 10.1097/MNH.0b013e328354a28e [DOI] [PubMed] [Google Scholar]
- Heeringa S.F., Chernin G., Chaki M., Zhou W., Sloan A.J., Ji Z., Xie L.X., Salviati L., Hurd T.W., Vega-Warner V., et al. 2011. COQ6 mutations in human patients produce nephrotic syndrome with sensorineural deafness. J. Clin. Invest. 121:2013–2024 10.1172/JCI45693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkilä E., Ristola M., Havana M., Jones N., Holthöfer H., and Lehtonen S.. 2011. Trans-interaction of nephrin and Neph1/Neph3 induces cell adhesion that associates with decreased tyrosine phosphorylation of nephrin. Biochem. J. 435:619–628 10.1042/BJ20101599 [DOI] [PubMed] [Google Scholar]
- Hinkes B., Wiggins R.C., Gbadegesin R., Vlangos C.N., Seelow D., Nürnberg G., Garg P., Verma R., Chaib H., Hoskins B.E., et al. 2006. Positional cloning uncovers mutations in PLCE1 responsible for a nephrotic syndrome variant that may be reversible. Nat. Genet. 38:1397–1405 10.1038/ng1918 [DOI] [PubMed] [Google Scholar]
- Hironaka K., Makino H., Yamasaki Y., and Ota Z.. 1993. Pores in the glomerular basement membrane revealed by ultrahigh-resolution scanning electron microscopy. Nephron. 64:647–649 10.1159/000187418 [DOI] [PubMed] [Google Scholar]
- Hironaka K., Makino H., Onbe T., Yamasaki Y., Shikata K., Kamata K., and Ota Z.. 1996. Ultrastructural change of the glomerular basement membrane in rats with Heymann nephritis revealed by ultrahigh resolution scanning electron microscopy. J. Pathol. 179:112–120 [DOI] [PubMed] [Google Scholar]
- Hirose T., Satoh D., Kurihara H., Kusaka C., Hirose H., Akimoto K., Matsusaka T., Ichikawa I., Noda T., and Ohno S.. 2009. An essential role of the universal polarity protein, aPKCλ, on the maintenance of podocyte slit diaphragms. PLoS ONE. 4:e4194 10.1371/journal.pone.0004194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjalmarsson C., Johansson B.R., and Haraldsson B.. 2004. Electron microscopic evaluation of the endothelial surface layer of glomerular capillaries. Microvasc. Res. 67:9–17 10.1016/j.mvr.2003.10.001 [DOI] [PubMed] [Google Scholar]
- Holzman L.B., St John P.L., Kovari I.A., Verma R., Holthofer H., and Abrahamson D.R.. 1999. Nephrin localizes to the slit pore of the glomerular epithelial cell. Kidney Int. 56:1481–1491 10.1046/j.1523-1755.1999.00719.x [DOI] [PubMed] [Google Scholar]
- Hovind P., Tarnow L., Oestergaard P.B., and Parving H.H.. 2000. Elevated vascular endothelial growth factor in type 1 diabetic patients with diabetic nephropathy. Kidney Int. Suppl. 57:S56–S61 10.1046/j.1523-1755.2000.07504.x [DOI] [PubMed] [Google Scholar]
- Huber T.B., Simons M., Hartleben B., Sernetz L., Schmidts M., Gundlach E., Saleem M.A., Walz G., and Benzing T.. 2003. Molecular basis of the functional podocin-nephrin complex: mutations in the NPHS2 gene disrupt nephrin targeting to lipid raft microdomains. Hum. Mol. Genet. 12:3397–3405 10.1093/hmg/ddg360 [DOI] [PubMed] [Google Scholar]
- Huber T.B., Schermer B., and Benzing T.. 2007. Podocin organizes ion channel-lipid supercomplexes: implications for mechanosensation at the slit diaphragm. Nephron Exp. Nephrol. 106:e27–e31 10.1159/000101789 [DOI] [PubMed] [Google Scholar]
- Huber T.B., Hartleben B., Winkelmann K., Schneider L., Becker J.U., Leitges M., Walz G., Haller H., and Schiffer M.. 2009. Loss of podocyte aPKCλ/ι causes polarity defects and nephrotic syndrome. J. Am. Soc. Nephrol. 20:798–806 10.1681/ASN.2008080871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimura K., Kurihara H., and Sakai T.. 2003. Actin filament organization of foot processes in rat podocytes. J. Histochem. Cytochem. 51:1589–1600 10.1177/002215540305101203 [DOI] [PubMed] [Google Scholar]
- Inoue T., Yaoita E., Kurihara H., Shimizu F., Sakai T., Kobayashi T., Ohshiro K., Kawachi H., Okada H., Suzuki H., et al. 2001. FAT is a component of glomerular slit diaphragms. Kidney Int. 59:1003–1012 10.1046/j.1523-1755.2001.0590031003.x [DOI] [PubMed] [Google Scholar]
- Itoh M., Nakadate K., Horibata Y., Matsusaka T., Xu J., Hunziker W., and Sugimoto H.. 2014. The structural and functional organization of the podocyte filtration slits is regulated by Tjp1/ZO-1. PLoS ONE. 9:e106621 10.1371/journal.pone.0106621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeansson M., and Haraldsson B.. 2003. Glomerular size and charge selectivity in the mouse after exposure to glucosaminoglycan-degrading enzymes. J. Am. Soc. Nephrol. 14:1756–1765 10.1097/01.ASN.0000072742.02714.6E [DOI] [PubMed] [Google Scholar]
- Jeansson M., and Haraldsson B.. 2006. Morphological and functional evidence for an important role of the endothelial cell glycocalyx in the glomerular barrier. Am. J. Physiol. Renal Physiol. 290:F111–F116 10.1152/ajprenal.00173.2005 [DOI] [PubMed] [Google Scholar]
- Jeansson M., Björck K., Tenstad O., and Haraldsson B.. 2009. Adriamycin alters glomerular endothelium to induce proteinuria. J. Am. Soc. Nephrol. 20:114–122 10.1681/ASN.2007111205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeansson M., Gawlik A., Anderson G., Li C., Kerjaschki D., Henkelman M., and Quaggin S.E.. 2011. Angiopoietin-1 is essential in mouse vasculature during development and in response to injury. J. Clin. Invest. 121:2278–2289 10.1172/JCI46322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J., Sison K., Li C., Tian R., Wnuk M., Sung H.K., Jeansson M., Zhang C., Tucholska M., Jones N., et al. 2012. Soluble FLT1 binds lipid microdomains in podocytes to control cell morphology and glomerular barrier function. Cell. 151:384–399 10.1016/j.cell.2012.08.037 [DOI] [PubMed] [Google Scholar]
- Jones N., Blasutig I.M., Eremina V., Ruston J.M., Bladt F., Li H., Huang H., Larose L., Li S.S., Takano T., et al. 2006. Nck adaptor proteins link nephrin to the actin cytoskeleton of kidney podocytes. Nature. 440:818–823 10.1038/nature04662 [DOI] [PubMed] [Google Scholar]
- Jones N., New L.A., Fortino M.A., Eremina V., Ruston J., Blasutig I.M., Aoudjit L., Zou Y., Liu X., Yu G.L., et al. 2009. Nck proteins maintain the adult glomerular filtration barrier. J. Am. Soc. Nephrol. 20:1533–1543 10.1681/ASN.2009010056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan J.M., Kim S.H., North K.N., Rennke H., Correia L.A., Tong H.Q., Mathis B.J., Rodríguez-Pérez J.C., Allen P.G., Beggs A.H., and Pollak M.R.. 2000. Mutations in ACTN4, encoding α-actinin-4, cause familial focal segmental glomerulosclerosis. Nat. Genet. 24:251–256 10.1038/73456 [DOI] [PubMed] [Google Scholar]
- Karamatic Crew V., Burton N., Kagan A., Green C.A., Levene C., Flinter F., Brady R.L., Daniels G., and Anstee D.J.. 2004. CD151, the first member of the tetraspanin (TM4) superfamily detected on erythrocytes, is essential for the correct assembly of human basement membranes in kidney and skin. Blood. 104:2217–2223 10.1182/blood-2004-04-1512 [DOI] [PubMed] [Google Scholar]
- Kestilä M., Lenkkeri U., Männikkö M., Lamerdin J., McCready P., Putaala H., Ruotsalainen V., Morita T., Nissinen M., Herva R., et al. 1998. Positionally cloned gene for a novel glomerular protein—nephrin—is mutated in congenital nephrotic syndrome. Mol. Cell. 1:575–582 10.1016/S1097-2765(00)80057-X [DOI] [PubMed] [Google Scholar]
- Khoshnoodi J., Sigmundsson K., Ofverstedt L.G., Skoglund U., Obrink B., Wartiovaara J., and Tryggvason K.. 2003. Nephrin promotes cell-cell adhesion through homophilic interactions. Am. J. Pathol. 163:2337–2346 10.1016/S0002-9440(10)63590-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikkawa Y., and Miner J.H.. 2006. Molecular dissection of laminin alpha 5 in vivo reveals separable domain-specific roles in embryonic development and kidney function. Dev. Biol. 296:265–277 10.1016/j.ydbio.2006.04.463 [DOI] [PubMed] [Google Scholar]
- Kim J.M., Wu H., Green G., Winkler C.A., Kopp J.B., Miner J.H., Unanue E.R., and Shaw A.S.. 2003. CD2-associated protein haploinsufficiency is linked to glomerular disease susceptibility. Science. 300:1298–1300 10.1126/science.1081068 [DOI] [PubMed] [Google Scholar]
- Kolatsi-Joannou M., Li X.Z., Suda T., Yuan H.T., and Woolf A.S.. 2001. Expression and potential role of angiopoietins and Tie-2 in early development of the mouse metanephros. Dev. Dyn. 222:120–126 10.1002/dvdy.1170 [DOI] [PubMed] [Google Scholar]
- Kreidberg J.A., Donovan M.J., Goldstein S.L., Rennke H., Shepherd K., Jones R.C., and Jaenisch R.. 1996. Alpha 3 beta 1 integrin has a crucial role in kidney and lung organogenesis. Development. 122:3537–3547. [DOI] [PubMed] [Google Scholar]
- Krendel M., Kim S.V., Willinger T., Wang T., Kashgarian M., Flavell R.A., and Mooseker M.S.. 2009. Disruption of Myosin 1e promotes podocyte injury. J. Am. Soc. Nephrol. 20:86–94 10.1681/ASN.2007111172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriz W., and Lemley K.V.. 2015. A potential role for mechanical forces in the detachment of podocytes and the progression of CKD. J. Am. Soc. Nephrol. 26:258–269 10.1681/ASN.2014030278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubosawa H., and Kondo Y.. 1985. Ultrastructural organization of the glomerular basement membrane as revealed by a deep-etch replica method. Cell Tissue Res. 242:33–39 10.1007/BF00225560 [DOI] [PubMed] [Google Scholar]
- Lehtonen S., Ryan J.J., Kudlicka K., Iino N., Zhou H., and Farquhar M.G.. 2005. Cell junction-associated proteins IQGAP1, MAGI-2, CASK, spectrins, and α-actinin are components of the nephrin multiprotein complex. Proc. Natl. Acad. Sci. USA. 102:9814–9819 10.1073/pnas.0504166102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennon R., Byron A., Humphries J.D., Randles M.J., Carisey A., Murphy S., Knight D., Brenchley P.E., Zent R., and Humphries M.J.. 2014. Global analysis reveals the complexity of the human glomerular extracellular matrix. J. Am. Soc. Nephrol. 25:939–951 10.1681/ASN.2013030233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levick J.R., and Smaje L.H.. 1987. An analysis of the permeability of a fenestra. Microvasc. Res. 33:233–256 10.1016/0026-2862(87)90020-3 [DOI] [PubMed] [Google Scholar]
- Lund U., Rippe A., Venturoli D., Tenstad O., Grubb A., and Rippe B.. 2003. Glomerular filtration rate dependence of sieving of albumin and some neutral proteins in rat kidneys. Am. J. Physiol. Renal Physiol. 284:F1226–F1234 10.1152/ajprenal.00316.2002 [DOI] [PubMed] [Google Scholar]
- Matejas V., Hinkes B., Alkandari F., Al-Gazali L., Annexstad E., Aytac M.B., Barrow M., Bláhová K., Bockenhauer D., Cheong H.I., et al. 2010. Mutations in the human laminin β2 (LAMB2) gene and the associated phenotypic spectrum. Hum. Mutat. 31:992–1002 10.1002/humu.21304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mele C., Iatropoulos P., Donadelli R., Calabria A., Maranta R., Cassis P., Buelli S., Tomasoni S., Piras R., Krendel M., et al. PodoNet Consortium. 2011. MYO1E mutations and childhood familial focal segmental glomerulosclerosis. N. Engl. J. Med. 365:295–306 10.1056/NEJMoa1101273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuwese M.C., Broekhuizen L.N., Kuikhoven M., Heeneman S., Lutgens E., Gijbels M.J., Nieuwdorp M., Peutz C.J., Stroes E.S., Vink H., and van den Berg B.M.. 2010. Endothelial surface layer degradation by chronic hyaluronidase infusion induces proteinuria in apolipoprotein E-deficient mice. PLoS ONE. 5:e14262 10.1371/journal.pone.0014262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner J.H.2012. The glomerular basement membrane. Exp. Cell Res. 318:973–978 10.1016/j.yexcr.2012.02.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner J.H., and Sanes J.R.. 1994. Collagen IV alpha 3, alpha 4, and alpha 5 chains in rodent basal laminae: sequence, distribution, association with laminins, and developmental switches. J. Cell Biol. 127:879–891 10.1083/jcb.127.3.879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller M.J., and Tenten V.. 2013. Renal albumin filtration: alternative models to the standard physical barriers. Nat. Rev. Nephrol. 9:266–277 10.1038/nrneph.2013.58 [DOI] [PubMed] [Google Scholar]
- Munger K.A., Kost C.K., Brenner B.M., and Maddox D.A.. 2011. The renal circulations and glomerular ultrafiltration. Brenner and Rector’s The Kidney. Vol. 1 Taal M.W., Chertow G.M., Marsden P.A., Skorecki K., Yu A.S.L., and Brenner B.M., Elsevier, Inc., Philadelphia, PA: 95–137. [Google Scholar]
- Noakes P.G., Miner J.H., Gautam M., Cunningham J.M., Sanes J.R., and Merlie J.P.. 1995. The renal glomerulus of mice lacking s-laminin/laminin β2: nephrosis despite molecular compensation by laminin β1. Nat. Genet. 10:400–406 10.1038/ng0895-400 [DOI] [PubMed] [Google Scholar]
- Ozaltin F., Ibsirlioglu T., Taskiran E.Z., Baydar D.E., Kaymaz F., Buyukcelik M., Kilic B.D., Balat A., Iatropoulos P., Asan E., et al. PodoNet Consortium. 2011. Disruption of PTPRO causes childhood-onset nephrotic syndrome. Am. J. Hum. Genet. 89:139–147 10.1016/j.ajhg.2011.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peti-Peterdi J., and Sipos A.. 2010. A high-powered view of the filtration barrier. J. Am. Soc. Nephrol. 21:1835–1841 10.1681/ASN.2010040378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potla U., Ni J., Vadaparampil J., Yang G., Leventhal J.S., Campbell K.N., Chuang P.Y., Morozov A., He J.C., D’Agati V.D., et al. 2014. Podocyte-specific RAP1GAP expression contributes to focal segmental glomerulosclerosis-associated glomerular injury. J. Clin. Invest. 124:1757–1769 10.1172/JCI67846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzi A., Jarad G., Moeckel G.W., Coffa S., Zhang X., Gewin L., Eremina V., Hudson B.G., Borza D.B., Harris R.C., et al. 2008. Beta1 integrin expression by podocytes is required to maintain glomerular structural integrity. Dev. Biol. 316:288–301 10.1016/j.ydbio.2008.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabakaran T., Christensen E.I., Nielsen R., and Verroust P.J.. 2012. Cubilin is expressed in rat and human glomerular podocytes. Nephrol. Dial. Transplant. 27:3156–3159 10.1093/ndt/gfr794 [DOI] [PubMed] [Google Scholar]
- Puelles V.G., Hoy W.E., Hughson M.D., Diouf B., Douglas-Denton R.N., and Bertram J.F.. 2011. Glomerular number and size variability and risk for kidney disease. Curr. Opin. Nephrol. Hypertens. 20:7–15 10.1097/MNH.0b013e3283410a7d [DOI] [PubMed] [Google Scholar]
- Reiser J., Polu K.R., Möller C.C., Kenlan P., Altintas M.M., Wei C., Faul C., Herbert S., Villegas I., Avila-Casado C., et al. 2005. TRPC6 is a glomerular slit diaphragm-associated channel required for normal renal function. Nat. Genet. 37:739–744 10.1038/ng1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennke H.G., and Venkatachalam M.A.. 1977. Glomerular permeability: in vivo tracer studies with polyanionic and polycationic ferritins. Kidney Int. 11:44–53 10.1038/ki.1977.6 [DOI] [PubMed] [Google Scholar]
- Rennke H.G., Cotran R.S., and Venkatachalam M.A.. 1975. Role of molecular charge in glomerular permeability. Tracer studies with cationized ferritins. J. Cell Biol. 67:638–646 10.1083/jcb.67.3.638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert B., Zhao X., and Abrahamson D.R.. 2000. Coexpression of neuropilin-1, Flk1, and VEGF(164) in developing and mature mouse kidney glomeruli. Am. J. Physiol. Renal Physiol. 279:F275–F282. [DOI] [PubMed] [Google Scholar]
- Robinson G.B., and Walton H.A.. 1989. Glomerular basement membrane as a compressible ultrafilter. Microvasc. Res. 38:36–48 10.1016/0026-2862(89)90015-0 [DOI] [PubMed] [Google Scholar]
- Rossi M., Morita H., Sormunen R., Airenne S., Kreivi M., Wang L., Fukai N., Olsen B.R., Tryggvason K., and Soininen R.. 2003. Heparan sulfate chains of perlecan are indispensable in the lens capsule but not in the kidney. EMBO J. 22:236–245 10.1093/emboj/cdg019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostgaard J., and Qvortrup K.. 2002. Sieve plugs in fenestrae of glomerular capillaries—site of the filtration barrier? Cells Tissues Organs (Print). 170:132–138 10.1159/000046186 [DOI] [PubMed] [Google Scholar]
- Ruotsalainen V., Ljungberg P., Wartiovaara J., Lenkkeri U., Kestilä M., Jalanko H., Holmberg C., and Tryggvason K.. 1999. Nephrin is specifically located at the slit diaphragm of glomerular podocytes. Proc. Natl. Acad. Sci. USA. 96:7962–7967 10.1073/pnas.96.14.7962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan G.B., and Karnovsky M.J.. 1976. Distribution of endogenous albumin in the rat glomerulus: role of hemodynamic factors in glomerular barrier function. Kidney Int. 9:36–45 10.1038/ki.1976.5 [DOI] [PubMed] [Google Scholar]
- Sachs N., Kreft M., van den Bergh Weerman M.A., Beynon A.J., Peters T.A., Weening J.J., and Sonnenberg A.. 2006. Kidney failure in mice lacking the tetraspanin CD151. J. Cell Biol. 175:33–39 10.1083/jcb.200603073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadl V., Jin F., Yu J., Cui S., Holmyard D., Quaggin S., Barsh G., and Cordes S.. 2002. The mouse Kreisler (Krml1/MafB) segmentation gene is required for differentiation of glomerular visceral epithelial cells. Dev. Biol. 249:16–29 10.1006/dbio.2002.0751 [DOI] [PubMed] [Google Scholar]
- Salmon A.H., and Satchell S.C.. 2012. Endothelial glycocalyx dysfunction in disease: albuminuria and increased microvascular permeability. J. Pathol. 226:562–574 10.1002/path.3964 [DOI] [PubMed] [Google Scholar]
- Salmon A.H., Neal C.R., Sage L.M., Glass C.A., Harper S.J., and Bates D.O.. 2009. Angiopoietin-1 alters microvascular permeability coefficients in vivo via modification of endothelial glycocalyx. Cardiovasc. Res. 83:24–33 10.1093/cvr/cvp093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon A.H., Ferguson J.K., Burford J.L., Gevorgyan H., Nakano D., Harper S.J., Bates D.O., and Peti-Peterdi J.. 2012. Loss of the endothelial glycocalyx links albuminuria and vascular dysfunction. J. Am. Soc. Nephrol. 23:1339–1350 10.1681/ASN.2012010017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarin H.2010. Physiologic upper limits of pore size of different blood capillary types and another perspective on the dual pore theory of microvascular permeability. J Angiogenes Res. 2:14 10.1186/2040-2384-2-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satchell S.C., Harper S.J., Tooke J.E., Kerjaschki D., Saleem M.A., and Mathieson P.W.. 2002. Human podocytes express angiopoietin 1, a potential regulator of glomerular vascular endothelial growth factor. J. Am. Soc. Nephrol. 13:544–550. [DOI] [PubMed] [Google Scholar]
- Satchell S.C., Anderson K.L., and Mathieson P.W.. 2004. Angiopoietin 1 and vascular endothelial growth factor modulate human glomerular endothelial cell barrier properties. J. Am. Soc. Nephrol. 15:566–574 10.1097/01.ASN.0000115397.22519.03 [DOI] [PubMed] [Google Scholar]
- Satoh D., Hirose T., Harita Y., Daimon C., Harada T., Kurihara H., Yamashita A., and Ohno S.. 2014. aPKCλ maintains the integrity of the glomerular slit diaphragm through trafficking of nephrin to the cell surface. J. Biochem. 156:115–128 10.1093/jb/mvu022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaldecker T., Kim S., Tarabanis C., Tian D., Hakroush S., Castonguay P., Ahn W., Wallentin H., Heid H., Hopkins C.R., et al. 2013. Inhibition of the TRPC5 ion channel protects the kidney filter. J. Clin. Invest. 123:5298–5309 10.1172/JCI71165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurek E.M., Völker L.A., Tax J., Lamkemeyer T., Rinschen M.M., Ungrue D., Kratz J.E. III, Sirianant L., Kunzelmann K., Chalfie M., et al. 2014. A disease-causing mutation illuminates the protein membrane topology of the kidney-expressed prohibitin homology (PHB) domain protein podocin. J. Biol. Chem. 289:11262–11271 10.1074/jbc.M113.521773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz K., Simons M., Reiser J., Saleem M.A., Faul C., Kriz W., Shaw A.S., Holzman L.B., and Mundel P.. 2001. Podocin, a raft-associated component of the glomerular slit diaphragm, interacts with CD2AP and nephrin. J. Clin. Invest. 108:1621–1629 10.1172/JCI200112849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott R.P., Hawley S.P., Ruston J., Du J., Brakebusch C., Jones N., and Pawson T.. 2012. Podocyte-specific loss of Cdc42 leads to congenital nephropathy. J. Am. Soc. Nephrol. 23:1149–1154 10.1681/ASN.2011121206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon M.B., Patton B.L., Harvey S.J., and Miner J.H.. 2006. A hypomorphic mutation in the mouse laminin α5 gene causes polycystic kidney disease. J. Am. Soc. Nephrol. 17:1913–1922 10.1681/ASN.2005121298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih N.Y., Li J., Karpitskii V., Nguyen A., Dustin M.L., Kanagawa O., Miner J.H., and Shaw A.S.. 1999. Congenital nephrotic syndrome in mice lacking CD2-associated protein. Science. 286:312–315 10.1126/science.286.5438.312 [DOI] [PubMed] [Google Scholar]
- Simon M., Röckl W., Hornig C., Gröne E.F., Theis H., Weich H.A., Fuchs E., Yayon A., and Gröne H.J.. 1998. Receptors of vascular endothelial growth factor/vascular permeability factor (VEGF/VPF) in fetal and adult human kidney: localization and [125I]VEGF binding sites. J. Am. Soc. Nephrol. 9:1032–1044. [DOI] [PubMed] [Google Scholar]
- Sison K., Eremina V., Baelde H., Min W., Hirashima M., Fantus I.G., and Quaggin S.E.. 2010. Glomerular structure and function require paracrine, not autocrine, VEGF-VEGFR-2 signaling. J. Am. Soc. Nephrol. 21:1691–1701 10.1681/ASN.2010030295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavotinek A., Kaylor J., Pierce H., Cahr M., DeWard S.J., Schneidman-Duhovny D., Alsadah A., Salem F., Schmajuk G., and Mehta L.. 2015. CRB2 mutations produce a phenotype resembling congenital nephrosis, Finnish type, with cerebral ventriculomegaly and raised alpha-fetoprotein. Am. J. Hum. Genet. 96:162–169 10.1016/j.ajhg.2014.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soda K., and Ishibe S.. 2013. The function of endocytosis in podocytes. Curr. Opin. Nephrol. Hypertens. 22:432–438 10.1097/MNH.0b013e3283624820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soda K., Balkin D.M., Ferguson S.M., Paradise S., Milosevic I., Giovedi S., Volpicelli-Daley L., Tian X., Wu Y., Ma H., et al. 2012. Role of dynamin, synaptojanin, and endophilin in podocyte foot processes. J. Clin. Invest. 122:4401–4411 10.1172/JCI65289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein J.H., and Fadem S.Z.. 1978. The renal circulation. JAMA. 239:1308–1312 10.1001/jama.1978.03280400048019 [DOI] [PubMed] [Google Scholar]
- Sugimoto H., Hamano Y., Charytan D., Cosgrove D., Kieran M., Sudhakar A., and Kalluri R.. 2003. Neutralization of circulating vascular endothelial growth factor (VEGF) by anti-VEGF antibodies and soluble VEGF receptor 1 (sFlt-1) induces proteinuria. J. Biol. Chem. 278:12605–12608 10.1074/jbc.C300012200 [DOI] [PubMed] [Google Scholar]
- Sugio S., Kashima A., Mochizuki S., Noda M., and Kobayashi K.. 1999. Crystal structure of human serum albumin at 2.5 A resolution. Protein Eng. 12:439–446 10.1093/protein/12.6.439 [DOI] [PubMed] [Google Scholar]
- Suleiman H., Zhang L., Roth R., Heuser J.E., Miner J.H., Shaw A.S., and Dani A.. 2013. Nanoscale protein architecture of the kidney glomerular basement membrane. eLife. 2:e01149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takabatake Y., Sugiyama T., Kohara H., Matsusaka T., Kurihara H., Koni P.A., Nagasawa Y., Hamano T., Matsui I., Kawada N., et al. 2009. The CXCL12 (SDF-1)/CXCR4 axis is essential for the development of renal vasculature. J. Am. Soc. Nephrol. 20:1714–1723 10.1681/ASN.2008060640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang V.W., and Brieher W.M.. 2013. FSGS3/CD2AP is a barbed-end capping protein that stabilizes actin and strengthens adherens junctions. J. Cell Biol. 203:815–833 10.1083/jcb.201304143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassin M.T., Beziau A., Gubler M.C., and Boyer B.. 1994. Spatiotemporal expression of molecules associated with junctional complexes during the in vivo maturation of renal podocytes. Int. J. Dev. Biol. 38:45–54. [PubMed] [Google Scholar]
- Thomson S.C., and Blantz R.C.. 2010. A new role for charge of the glomerular capillary membrane. J. Am. Soc. Nephrol. 21:2011–2013 10.1681/ASN.2010101089 [DOI] [PubMed] [Google Scholar]
- Tian D., Jacobo S.M., Billing D., Rozkalne A., Gage S.D., Anagnostou T., Pavenstädt H., Hsu H.H., Schlondorff J., Ramos A., and Greka A.. 2010. Antagonistic regulation of actin dynamics and cell motility by TRPC5 and TRPC6 channels. Sci. Signal. 3:ra77 (published erratum appears in Sci. Signal 3:er11) 10.1126/scisignal.2001200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian X., Kim J.J., Monkley S.M., Gotoh N., Nandez R., Soda K., Inoue K., Balkin D.M., Hassan H., Son S.H., et al. 2014. Podocyte-associated talin1 is critical for glomerular filtration barrier maintenance. J. Clin. Invest. 124:1098–1113 10.1172/JCI69778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togawa A., Miyoshi J., Ishizaki H., Tanaka M., Takakura A., Nishioka H., Yoshida H., Doi T., Mizoguchi A., Matsuura N., et al. 1999. Progressive impairment of kidneys and reproductive organs in mice lacking Rho GDIα. Oncogene. 18:5373–5380 10.1038/sj.onc.1202921 [DOI] [PubMed] [Google Scholar]
- Toyoda M., Najafian B., Kim Y., Caramori M.L., and Mauer M.. 2007. Podocyte detachment and reduced glomerular capillary endothelial fenestration in human type 1 diabetic nephropathy. Diabetes. 56:2155–2160 10.2337/db07-0019 [DOI] [PubMed] [Google Scholar]
- Tu Y., Li F., Goicoechea S., and Wu C.. 1999. The LIM-only protein PINCH directly interacts with integrin-linked kinase and is recruited to integrin-rich sites in spreading cells. Mol. Cell. Biol. 19:2425–2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Hoven M.J., Wijnhoven T.J., Li J.P., Zcharia E., Dijkman H.B., Wismans R.G., Rops A.L., Lensen J.F., van den Heuvel L.P., van Kuppevelt T.H., et al. 2008. Reduction of anionic sites in the glomerular basement membrane by heparanase does not lead to proteinuria. Kidney Int. 73:278–287 10.1038/sj.ki.5002706 [DOI] [PubMed] [Google Scholar]
- Verma R., Kovari I., Soofi A., Nihalani D., Patrie K., and Holzman L.B.. 2006. Nephrin ectodomain engagement results in Src kinase activation, nephrin phosphorylation, Nck recruitment, and actin polymerization. J. Clin. Invest. 116:1346–1359 10.1172/JCI27414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veron D., Reidy K., Marlier A., Bertuccio C., Villegas G., Jimenez J., Kashgarian M., and Tufro A.. 2010a. Induction of podocyte VEGF164 overexpression at different stages of development causes congenital nephrosis or steroid-resistant nephrotic syndrome. Am. J. Pathol. 177:2225–2233 10.2353/ajpath.2010.091146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veron D., Reidy K.J., Bertuccio C., Teichman J., Villegas G., Jimenez J., Shen W., Kopp J.B., Thomas D.B., and Tufro A.. 2010b. Overexpression of VEGF-A in podocytes of adult mice causes glomerular disease. Kidney Int. 77:989–999 10.1038/ki.2010.64 [DOI] [PubMed] [Google Scholar]
- Wartiovaara J., Ofverstedt L.G., Khoshnoodi J., Zhang J., Mäkelä E., Sandin S., Ruotsalainen V., Cheng R.H., Jalanko H., Skoglund U., and Tryggvason K.. 2004. Nephrin strands contribute to a porous slit diaphragm scaffold as revealed by electron tomography. J. Clin. Invest. 114:1475–1483 10.1172/JCI22562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil E.J., Lemley K.V., Mason C.C., Yee B., Jones L.I., Blouch K., Lovato T., Richardson M., Myers B.D., and Nelson R.G.. 2012. Podocyte detachment and reduced glomerular capillary endothelial fenestration promote kidney disease in type 2 diabetic nephropathy. Kidney Int. 82:1010–1017 10.1038/ki.2012.234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wharram B.L., Goyal M., Gillespie P.J., Wiggins J.E., Kershaw D.B., Holzman L.B., Dysko R.C., Saunders T.L., Samuelson L.C., and Wiggins R.C.. 2000. Altered podocyte structure in GLEPP1 (Ptpro)-deficient mice associated with hypertension and low glomerular filtration rate. J. Clin. Invest. 106:1281–1290 10.1172/JCI7236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winn M.P., Conlon P.J., Lynn K.L., Farrington M.K., Creazzo T., Hawkins A.F., Daskalakis N., Kwan S.Y., Ebersviller S., Burchette J.L., et al. 2005. A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science. 308:1801–1804 10.1126/science.1106215 [DOI] [PubMed] [Google Scholar]
- Wyss H.M., Henderson J.M., Byfield F.J., Bruggeman L.A., Ding Y., Huang C., Suh J.H., Franke T., Mele E., Pollak M.R., et al. 2011. Biophysical properties of normal and diseased renal glomeruli. Am. J. Physiol. Cell Physiol. 300:C397–C405 10.1152/ajpcell.00438.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaddanapudi S., Altintas M.M., Kistler A.D., Fernandez I., Möller C.C., Wei C., Peev V., Flesche J.B., Forst A.L., Li J., et al. 2011. CD2AP in mouse and human podocytes controls a proteolytic program that regulates cytoskeletal structure and cellular survival. J. Clin. Invest. 121:3965–3980 10.1172/JCI58552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki H., Saito A., Ooi H., Kobayashi N., Mundel P., and Gejyo F.. 2004. Differentiation-induced cultured podocytes express endocytically active megalin, a heymann nephritis antigen. Nephron Exp. Nephrol. 96:e52–e58 10.1159/000076404 [DOI] [PubMed] [Google Scholar]
- Yuan H.T., Suri C., Yancopoulos G.D., and Woolf A.S.. 1999. Expression of angiopoietin-1, angiopoietin-2, and the Tie-2 receptor tyrosine kinase during mouse kidney maturation. J. Am. Soc. Nephrol. 10:1722–1736. [DOI] [PubMed] [Google Scholar]
- Zenker M., Aigner T., Wendler O., Tralau T., Müntefering H., Fenski R., Pitz S., Schumacher V., Royer-Pokora B., Wühl E., et al. 2004. Human laminin β2 deficiency causes congenital nephrosis with mesangial sclerosis and distinct eye abnormalities. Hum. Mol. Genet. 13:2625–2632 10.1093/hmg/ddh284 [DOI] [PubMed] [Google Scholar]