Abstract
Thermal stress has a negative effect on the cognitive performance of military personnel and industry workers exposed to extreme environments. However, no studies have investigated the effects of environmental thermal stress on the cognitive functions of older adults. We carried out a controlled trial with 68 healthy older adults (mean age 73.3 years, 69 % female), each of whom has been assessed twice on the same day with selected tests from the Cambridge Neuropsychological Test Automated Battery (CANTAB). Repeated sessions were conducted with air temperatures set at 24 °C and 32 °C in a balanced order. Our primary analyses did not show significant differences when comparing the cognitive performance of the total sample under the two experimental temperatures. However, interaction analysis has shown that humidity levels modify the effect of temperature on cognitive outcomes. The subgroup exposed to relative humidity greater than the median value (57.8 %) presented worse cognitive performance in the heat session when compared to the control session. Reported exercising frequency explained individual vulnerability to heat stress. Volunteers with lower levels of physical activity (<4 times per week) were more likely to present worsened cognitive performance under heat stress. In a fully adjusted linear regression model, the performance under heat stress remained associated with relative humidity (β = −0.21; p = 0.007) and frequency of exercising (β = 0.18; p = 0.020). Our results indicate that heat stress may have detrimental effects on the cognitive functioning of some subgroups of older adults and under particular circumstances. Further research is needed for exploring a variety of potentially influential factors.
Keywords: Environmental exposure, Hot temperature, Heat stress, Cognitive manifestations, Neuropsychological test, Aged
Introduction
The human body tends to maintain its core temperature within relatively narrow limits under a wide range of environmental temperatures (Wunderlich 1869). However, even when the core temperature is preserved, exposure to environmental temperatures above a certain comfortable zone may produce undesired effects in cognitive performance (Hancock et al. 2007).
Differences in methodology and inconsistencies in results across studies make it difficult to determine under what specific environmental and physiological circumstances heat exposure adversely affects cognitive performance. It is generally agreed that the magnitude of the cognitive impairment resulting from heat stress is related to the intensity of the stressing condition and to the complexity of the tasks evaluated (Gaoua 2010).
Most studies investigating the effects of thermal stress on cognitive performance have recruited young adults to ascertain how heat stress may affect abilities that are crucial for military personnel (Radakovic et al. 2007; Hocking et al. 2001) and industry workers exposed to extreme environments (Sun et al. 2013; Simmons et al. 2008; Gaoua 2010). However, to the best of our knowledge, no studies have directly investigated the effects of hot environments on the cognitive functioning of older adults.
Population aging is an unprecedented worldwide phenomenon. The proportion of people 60 years and over was estimated to be 12 % of the world population in 2011 and it is expected to reach 22 % by 2050 (UNFPA and Help Age International 2012). Simultaneously, concerns about the impact of climate change have increased (Tong et al. 2008). “Each of the last three decades has been successively warmer at the Earth’s surface than any preceding decade since 1850” (IPCC 2013). This trend towards warmer climate worldwide raises concerns about the health of elderly populations, as aging leads to some changes of temperature control mechanisms.
Adaptive responses to heat such as cutaneous vasodilatation, sweating, and increase in cardiac output are progressively attenuated with aging (Holowatz and Kenney 2010; Inoue et al. 2004; Blatteis 2012; Minson et al. 1998). In addition to diminished thermoregulatory mechanisms, normal aging is associated with subtle and progressive decline in some cognitive functions such as processing speed, complex attention, executive functions, working memory, learning, and episodic memory (Drag and Bieliauskas 2010).
We have hypothesized that some cognitive functions of older adults can be affected by environmental temperatures that are being increasingly recorded in many regions of the world. Such effects would have significant impact in instrumental activities and quality of life of older populations, representing a potentially relevant issue in public health.
We have carried out a controlled trial to evaluate whether an environment with an air temperature set at 32 °C would have detrimental effects on cognitive performance and to investigate which specific functions would be more sensitive to heat stress. Moreover, we tried to identify factors that would explain variations in susceptibility to heat stress.
Methods
Subjects
A convenience sample of volunteers was recruited between January and August 2013 from community and clinical settings. As required by eligibility criteria, participants were aged 60 years or older, had ability to speak fluent Portuguese, had at least 4 years of formal education, and presented general good health, with no neurological or psychiatric illness. Apart from that, the 15-item Geriatric Depression Scale (GDS-15; Yesavage et al. 1983) and the Mini-Mental State Examination (MMSE; Folstein et al. 1975) were used in the initial visit to screen for depression and cognitive impairment, respectively. None of the participants scored > 5 on the GDS-15 or < 24 on the MMSE.
Sixty nine subjects completed both trials at 24 °C and 32 °C. One subject was excluded because the mean temperature achieved in the heat trial was considerably below the target (30.7 °C for a target of 32 °C). Thus, our final sample consisted of 68 older volunteers with generally good health, 69.1 % of whom were female. The mean age of the studied sample was 73.3 years (±6.6), ranging from 61 to 88 years. Regarding ethnicity, 79.4 % were Caucasian, 17.6 % were Asian, and 2.9 % were African American. The average years of formal schooling was 11.5 (±3.8). The mean MMSE score was 28.3 (±1.6). Most of the subjects (64.7 %) were physically active, i.e., exercised at least twice a week. Mean body surface area was 1.70 m2 (±0.14) and mean body mass index was 25.19 kg/m2 (±2.65).
The study protocol was approved by the Research Ethics Committee of the Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo. All participants were given a full explanation of the study procedures and provided written informed consent.
General procedure
In a preliminary stage, all volunteers were requested to undergo a clinical evaluation and collection of socio-demographic data. On the same day, a training session was performed to make subjects familiar with the neuropsychological tests and adapt them to use the computer. Furthermore, the training session has been used to decrease practice effects, as improvements between two successive administrations tend to decrease with repetitions and the acquisition of performance strategies occurs mainly on the first trial (Falleti et al. 2006). Results of the training sessions were not analyzed in the present study. The mean time between the training session and the actual study procedures was 7.9 (±3.3) days.
Participants were assigned to complete the cognitive battery on the first session at 24 °C (control) or 32 °C (heat). Those who initiated the study at 24 °C were subsequently tested at 32 °C and vice versa. Assignment to initiate the protocol at 24 or 32 °C was based on a computer-generated allocation sequence in a 1:1 proportion. The allocation sequence has been prepared in advance and was not concealed from the researchers enrolling and evaluating patients.
Environments with air temperature of 24 °C are generally considered comfortable for light activity (Campbell and Norman 1998; ISO 9241). The temperature of the heat trial (32 °C) was chosen to represent indoor environments that can be encountered in the summer of most urban areas of the world (Nedel et al. 2009; Mavrogianni et al. 2012).
Upon arrival, volunteers rested seated for 30 minutes in a waiting room at 24 °C. This procedure was carried out to minimize potential effects of the outdoor temperature. In this period, they mainly read magazines and had informal chats with the researcher. The same procedure was conducted between the first and the second session.
Both sessions were carried out on the same day, always in the morning. All the subjects rested again in a seated position, now in the test room, at the experimental temperature for an exposure period of 20 minutes before starting the neuropsychological battery. Volunteers wore their own clothes. During the whole course of testing procedures, they were not allowed to put on or take off any piece of clothing, so they were wearing the same outfit in both sessions.
A 9.5 m3 test room had its temperature controlled by two 30,000 BTUs air conditioners both of which equipped with automatic control of temperature, but not of humidity. The environment temperature and relative humidity (RH) were recorded every 60 seconds by an automated thermohygrometer (Data Logger Perceptec DHT-2260) and the representative measures for each session were obtained by arithmetic means. In order to generate a standardized measure of thermal sensation, the mean effective temperature (ET) of each session was calculated by using the formula proposed by Missenard (1933): ET = Air Temperature – 0.4 x (1 – 0.01RH) x (Air Temperature – 10).
In the control trial the mean temperature and relative humidity were 23.8 °C (±0.22) and 72.5 % (±11.62) respectively. In the heat trial the mean temperature was 32.1 °C (±0.30) and the mean relative humidity was 57.4 % (±11.13). The mean effective temperature was 22.3 °C (±0.53) in the control and 28.3 °C (±0.92) in the heat sessions.
Before and after each session we obtained measures of axillary temperature (AT), tympanic temperature (TT), blood pressure (BP), heart rate (HR), and body mass (BM). At the end of each session, volunteers were requested to answer the following question: “Regarding the temperature, do you consider the environment comfortable or uncomfortable?” The total time to complete the entire procedure, including both testing sessions, was about 3 hours.
Cognitive testing
Selected tests from the Cambridge Neuropsychological Test Automated Battery (CANTAB) Eclipse Version 3.0 were administered using a Windows-based computer with a touch sensitive screen and a press pad supplied by the company Cambridge Cognition Ltd, Bottisham, Cambridge, UK. The CANTAB consists of over 20 tests that can be combined to create customizable batteries. Tests employ language-independent abstract stimuli, require non-verbal responses, and thus are deemed to be culturally independent. CANTAB has been validated and cited in more than 600 peer-reviewed articles, including studies with older adults (Lowe and Rabbitt 1998; Jager et al. 2002).
Five tests from the CANTAB were administered to measure different aspects of cognitive functioning while focusing on memory, attention and processing speed. The Spatial Span (SSP) is a computerized version of the Corsi Blocks used to assess working memory. Squares change color in a variable sequence and the participant must touch them in the same order. The Paired-Associates Learning (PAL) is a learning and episodic memory task. The subject is presented different shapes within eight boxes differently located on the screen. The shapes are then displayed in the middle of the screen, one at a time, and the participant must touch the box where the pattern was originally located. The Pattern Recognition Memory (PRM) is a recognition memory task which uses a two-choice forced discrimination paradigm. Subjects are presented with a series of visual patterns, one at a time. In the recognition phase, they are requested to choose between a pattern they have already seen and a novel pattern. The Reaction Time (RTI) assesses motor and mental speeds. The task has five stages which require increasingly complex chains of responses as soon as a yellow dot appears. The Rapid Visual Information Processing (RVP) is a measure of sustained attention. Digits appear in a pseudo-random order at the rate of 40 digits per minute. Participants are requested to detect target sequences of digits (e.g., 2-4-6, 3-5-7) and to register their occurrence using the press pad.
The tests were administered by the same trained professional (BMT) in accordance with the instruction manual. To ensure compliance with test instructions, we have used a written instruction manual during all sessions. The tests were completed in a variable pre-planned order to counterbalance differential effects that might be caused by the rising of body temperatures throughout the session. Completion time of the entire test battery was approximately 50 minutes. CANTAB can report many outcome measures for each test, describing different aspects of each function. We have chosen to use measures deemed the most representative of the corresponding functions and those that convey overall performance scores.
Statistical analysis
Visual inspection of Q-Q plots was used to confirm normality and potential outliers were screened using Tukey’s criteria for far-out values. Primary analyses were carried out by comparing parameters of cognitive performance under the two experimental temperatures. Due to the fact that variables generally met reasonable assumptions for normality and no significant outliers were detected, paired t-tests were performed to compare interval data between the two sessions.
In order to develop composite scores for domains, raw data from ten selected outcomes (two from each test) were transformed using the Box-Cox technique to improve distribution patterns. Z-scores were obtained using the mean of the total sample as the reference and composite scores were calculated by arithmetic means. Composite scores were then scaled so that the average performance was assigned 100 and the standard deviation was established at 15. Tests in which lower scores indicate better performance had the signs reversed so that, in all the derived measures, higher scores indicate better performance. The global composite score was obtained by the same methods, but in this case, we have used only one measure from each of the 5 tests, defined a priori as those which best represent the cognitive function evaluated: PAL total number of errors adjusted, PRM number of correct, RTI five-choice reaction time, RVP A’ and SSP longest span length.
We have carried out interaction analyses using repeated measures analysis of variance (ANOVA) in order to check whether the effect of temperature on cognition, assessed by the global composite score, was modified by demographic characteristics (age, gender, education, race), frequency of physical activity or relative humidity registered during the heat protocol. For the interaction analyses interval variables were stratified into two levels using the median value.
A multiple linear regression model has been fitted to identify independent variables that would explain susceptibility to heat stress. In this model, the dependent variable was the global cognitive score at 32 °C. Independent variables entered simultaneously into the model were age, gender, education, race, relative humidity, and exercise frequency. Adjustment covariates were cognitive performance at 24 °C and order of the testing sessions.
Since the regression model has included correlated variables, we have investigated multicollinearity by assessing the variance inflation factor (VIF). Linearity assumptions of the adjusted model were checked for each continuous predictor with component-plus-residual plots. The adjusted coefficient of determination has been used to assess the goodness-of-fit of the model.
The sample size was calculated based on the parameters reported by Hancock et al. (2007), who carried out a meta-analysis to evaluate differences in cognitive performance under thermal stress and comfort temperatures in younger populations. In that meta-analysis, the overall effect size of thermal stress on cognitive performance was about one third of a standard deviation (Cohen’s d = 0.34). Using a two-tailed t-test for matched samples and assuming an alpha error of 5 %, we have calculated that a sample with 68 participants would have an 80 % power for detecting differences with effect sizes of 0.34 between the two assessments.
Analyses were performed by using the software Stata version 13.1 (StataCorp, College Station, TX). All statistical tests were two-tailed and an alpha level of less than 0.05 was used to indicate statistical significance.
Results
Tympanic and axillary temperatures were unchanged after the control trial, but increased significantly after the heat trial, with differences of 0.55 °C and 0.43 °C respectively (both with p < 0.001). Heart rate decreased after the control trial and, conversely, increased after the heat trial. No significant changes in body weight were detected (Table 1).
Table 1.
Physiological measures of the participants before and after each trial
| Before Trial | After Trial | Mean Difference | P value* | Cohen’s d | |
|---|---|---|---|---|---|
| Control Trial | |||||
| Tympanic Temperature | 36.01 (0.94) | 36.04 (0.92) | 0.03 (0.47) | 0.580 | 0.03 |
| Axillary Temperature | 35.59 (0.35) | 35.54 (0.28) | −0.05 (0.48) | 0.376 | −0.09 |
| Body weight | 65.11 (8.7) | 65.14 (8.66) | 0.03 (0.29) | 0.365 | 0.01 |
| Systolic Blood Pressure | 127.32 (14.49) | 132.85 (16.01) | 5.53 (14.63) | 0.003 | 0.36 |
| Heart rate | 67.69 (9.62) | 65.8 (8.97) | −1.88 (5.33) | 0.005 | −0.20 |
| Heat Trial | |||||
| Tympanic Temperature | 35.88 (0.86) | 36.44 (0.81) | 0.56 (0.44) | <0.001 | 0.67 |
| Axillary Temperature | 35.47 (0.57) | 35.89 (0.54) | 0.43 (0.45) | <0.001 | 0.77 |
| Body weight | 65.40 (8.49) | 65.35 (8.52) | −0.04 (0.29) | 0.421 | 0.01 |
| Systolic Blood Pressure | 128.62 (15.81) | 126.44 (19.75) | −2.18 (17.2) | 0.300 | −0.12 |
| Heart rate | 66.52 (9.67) | 69.37 (11.10) | 2.86 (6.17) | <0.001 | 0.27 |
Results are presented in mean (standard deviation); *Paired t test
Answers to the question regarding thermal comfort were obtained from 46 volunteers, among whom 11 (23.9 %) considered the control environment uncomfortable and 30 (65.2 %) considered the heated environment uncomfortable.
We have not found significant differences for any individual measures or composite scores when comparing cognitive performance under the two experimental temperatures (Table 2).
Table 2.
Comparisons between repeated measures under two experimental temperatures
| n | 24 °C | 32 °C | P value* | |
|---|---|---|---|---|
| Global | 66 | 100.47 (9.98) | 99.63 (9.43) | 0.280 |
| PRM Composite Score | 67 | 100.91 (13.11) | 99.09 (11.12) | 0.132 |
| PRM Number of Correct | 67 | 20.16 (2.94) | 20.04 (2.60) | 0.710 |
| PRM Latency | 67 | 3035.22 (899.30) | 3137.67 (862.29) | 0.191 |
| PAL Composite Score | 66 | 100.35 (14.83) | 99.65 (14.15) | 0.610 |
| PAL First Trial Memory Score | 66 | 8.80 (3.83) | 8.42 (3.66) | 0.376 |
| PAL Total Errors Adjusted | 66 | 34.12 (23.33) | 33.58 (2.74) | 0.793 |
| RTI Composite Score | 68 | 99.87 (11.41) | 100.13 (12.20) | 0.820 |
| 5-choice Reaction Time | 68 | 395.01 (58.71) | 397.85 (54.42) | 0.586 |
| 5-choice Movement Time | 68 | 490.68 (89.09) | 486.45 (99.41) | 0.682 |
| SSP Composite Score | 68 | 101.03 (11.30) | 98.97 (12.51) | 0.140 |
| SSP Span Length | 68 | 5.32 (0.80) | 5.25 (0.89) | 0.439 |
| SSP Time to Last Response | 68 | 4487.29 (532.61) | 4383.59 (481.54) | 0.100 |
| RVP Composite Score | 68 | 100.49 (12.82) | 99.51 (11.07) | 0.410 |
| RVP A’ | 68 | 0.95 (0.05) | 0.95 (0.04) | 0.627 |
| RVP Mean Latency | 68 | 547.08 (98.83) | 555.79 (104.23) | 0.436 |
Results are presented in mean (standard deviation). *Paired t test. Abbreviations: PRM, Pattern Recognition Memory; PAL, Paired Associated Learning; RTI, Five-Choice Reaction Time (RTI); SSP, Spatial Span (SSP); RVP, Rapid Visual Processing
In interaction analyses, the effects of heat exposure on cognitive performance were not modified by age (p = 0.684), gender (p = 0.179), education (p = 0.053), race (p = 0.735) or subjective perception of comfort (p = 0.893).
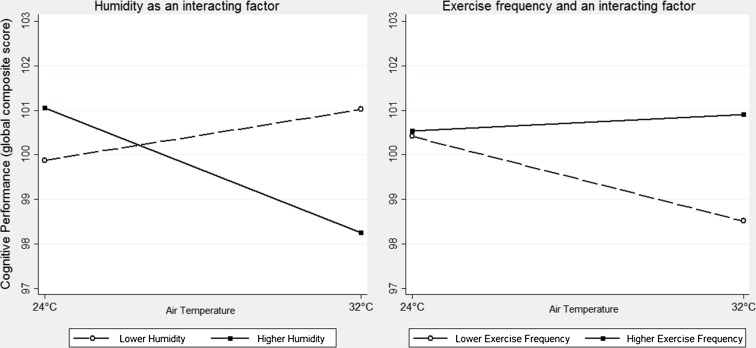
Reliable measures of relative humidity were obtained for 65 subjects. Humidity levels significantly modified the effect of temperature on the global composite score (p = 0.002; Fig. 1). Indeed, the subgroup of 32 subjects exposed to humidity greater than the median value (57.8 %) presented worse cognitive performance under heat stress on the global composite score, as well as on the PAL, SSP, and RTI composite scores (Table 3). No significant differences were observed on the subgroup of 33 subjects exposed to humidity lower than the median value (data not shown).
Fig. 1.
Adjusted predictions using humidity and exercise frequency as factors that modulate the effect of heat stress on cognitive performance
Table 3.
CANTAB global and domain composite scores for subgroups with higher humidity and lower levels of physical activity
| 24 °C | 32 °C | P value* | Cohen’s d | |
|---|---|---|---|---|
| Higher Humidity | ||||
| Global Composite Score | 101.22 (11.24) | 97.72 (10.34) | 0.002 | −0.32 |
| PRM Composite Score | 100.93 (13.68) | 98.56 (10.92) | 0.182 | −0.19 |
| PAL Composite Score | 102.00 (15.85) | 96.66 (15.21) | 0.016 | −0.34 |
| RTI Composite Score | 102.13 (11.72) | 99.33 (12.7) | 0.041 | −0.23 |
| SSP Composite Score | 100.84 (12.69) | 96.78 (12.66) | 0.045 | −0.320 |
| RVP Composite Score | 100.28 (14.7) | 98.81 (11.34) | 0.371 | −0.11 |
| Lower Level of Physical Activity | ||||
| Global Composite Score | 100.41 (9.75) | 98.51 (8.82) | 0.052 | −0.20 |
| PRM Composite Score | 100.51 (12.56) | 97.91 (11.17) | 0.089 | −0.220 |
| PAL Composite Score | 98.09 (14.25) | 97.36 (12.53) | 0.672 | −0.05 |
| RTI Composite Score | 100.66 (13.00) | 100.54 (14.57) | 0.942 | −0.01 |
| SSP Composite Score | 102.40 (11.44) | 90.05 (12.49) | 0.085 | 0.28 |
| RVP Composite Score | 102.23 (11.58) | 99.37 (9.98) | 0.030 | −0.26 |
Results are presented in mean (standard deviation). *Paired t test. Abbreviations: PRM, Pattern Recognition Memory; PAL, Paired Associated Learning; RTI, Five-Choice Reaction Time (RTI); SSP, Spatial Span (SSP); RVP, Rapid Visual Processing
Information about exercising was obtained from 63 volunteers, 42.9 % of whom reported a frequency of four or more times a week, 27.0 % between two and three, 9.5 % once a week, and 20.6 % were completely sedentary. The frequency of exercising has also modified the effect of temperature on the global composite score (p = 0.014). Accordingly, the subgroup of 36 volunteers with lower levels of physical activity (<4 times per week) presented worse cognitive performance under heat stress on the RVP, with a corresponding trend on the global score, PRM, and SSP composite scores (Table 3). No significant differences were observed on the subgroup of volunteers who exercised four or more times a week (data not shown).
In a fully adjusted linear regression model, the cognitive performance under heat stress was negatively associated with relative humidity, with a β of −0.21 (p = 0.007). This means that, with all other variables held constant, a one-standard deviation (SD) increase in relative humidity is associated with a 0.21 SD decrease on the global cognitive score. Among the participants characteristics, the only associated predictor was the frequency of exercise (β = 0.18; p = 0.020). This means that, under heat stress, physically active individuals presented a mean global cognitive score 0.18 SD higher when compared to inactive individuals (Table 4).
Table 4.
Predictors of susceptibility to heat stress in a fully adjusted linear regression model
| β | B | SE | t | P value | |
|---|---|---|---|---|---|
| Relative humidity (%) | −0.21 | −0.18 | 0.06 | −2.82 | 0.007 |
| Physically active (y/n)* | 0.18 | 3.48 | 1.44 | 2.41 | 0.020 |
| Age (years) | −0.12 | −0.17 | 0.11 | −1.54 | 0.131 |
| Gender (male) | 0.06 | 1.34 | 1.62 | 0.83 | 0.413 |
| Education (years) | −0.07 | −0.17 | 0.20 | −0.87 | 0.390 |
| Race (white) | 0.02 | 0.43 | 1.81 | 0.24 | 0.814 |
| Global score at 24 °C | 0.81 | 0.78 | 0.07 | 10.6 | <0.001 |
| Order of the sessions (32 °C first) | −0.02 | −0.38 | 1.46 | −0.26 | 0.797 |
Abbreviations: β, standardized beta coefficient, B unstandardized beta coefficient, SE standard error
* Defined as those who exercise at least four times per week
The maximum VIF was 1.30 and mean VIF was 1.23, indicating that multicollinearity was not a problem. Linearity assumptions were met for all independent variables. The adjusted coefficient of determination was 0.74, indicating appropriate fit of the model to the data.
Discussion
To our knowledge, this is the first study designed to investigate the effects of environmental heat exposure on the cognitive performance of older adults. Global results of the primary analysis have shown that, even for more demanding tasks, healthy older individuals managed to maintain cognitive performance preserved at 32 °C. Although we observed a trend for worse performance at 32 °C when compared to 24 °C in most measures, no statistically significant differences were encountered. A post hoc analysis was conducted based on a paired t-test for detecting differences in the global score with 80 % power. If the differences encountered between 24 and 32 °C were maintained (Cohen’s d = 0.09), a sample of 425 participants would be required for the comparison to reach statistical significance. The practical implications of such a small effect size would be questionable.
Some features of the sample and of the experimental methods may explain the results. First, the temperature to which volunteers were exposed in the heat trial was lower than that used in preceding studies with younger adults, some of which reached 50 °C (Racinais et al. 2008; Gaoua et al. 2012). In our study, the heat trial temperature (32 °C) was chosen to represent a typical summer day compatible with the weather in most urban areas of the world, rather than extreme temperatures observed in particular circumstances and places.
Another feature that may have influenced our results was the physical fitness of the participants. About two-thirds of the volunteers practiced physical exercises at least twice a week. It has been hypothesized that part of the age-related limitations in thermoregulation is due to decline in maximal oxygen uptake (VO2max), so that elderly people with good physical fitness could be able to keep their thermoregulation capacity similar to that of a younger adult (Kenney and Munce 2003). Although we have not obtained specific measures of aerobic capacity, we could observe that the effect of heat on cognition was modified by reported exercise frequency, with less active participants being more vulnerable to the effects of heat, particularly on sustained attention. That association was further confirmed in a fully adjusted regression model.
Even though our study did not control for humidity, we have adequately registered this variable and were able to detect an important interaction effect. The subgroup exposed to greater air humidity has suffered deleterious effects of heat on cognitive performance, particularly on memory and psychomotor speed. Relative air humidity influences the thermal sensation and thermoregulation capacity. Evaporation of perspiration depends on the pressure gradient between the skin surface and air humidity (vapor partial pressure). The higher the relative humidity, the lower the heat transfer by perspiration evaporation. Therefore, hot and humid environments impose greater thermal strain by declining evaporative heat losses (Moyen et al. 2014).
We hypothesized that heat would cause a greater cognitive deterioration in subjects with lower levels education, a marker of cognitive reserve. The concept of cognitive reserve was originally proposed to explain discrepancies between the degree of brain pathology and cognitive outcomes (Stern 2002). Recently, this concept has been extrapolated to the context of transient insults, such as those observed in delirium (Jones et al. 2010). However, our findings did not confirm such hypothesis. Education did not modify the effects of heat exposure on cognitive performance and it has not been a significant predictor of susceptibility to heat stress. That issue needs to be further investigated with more specific markers of cognitive reserve.
A non-negligible proportion of the participants (34.8 %) has considered 32 °C a comfortable temperature. It has been proposed that the effect of thermal stimuli on cognition could be mediated by the unpleasant sensation that they can cause (Gaoua 2010). Aging seems to be associated with a decreased thermal sensitivity and the absence of thermal discomfort could have influenced the results (Guergova and Dufour 2011). In fact, thermal sensitivity did not modulate the association between thermal stress and cognitive performance, with a P value of 0.89 for interaction.
Previous studies have suggested that cognitive impairment resulting from heat exposure depends on task complexity (Gaoua 2010; Gaoua et al. 2011; Pilcher et al. 2002), with tasks that place lower demands on integrative processing showing less decrement during heat exposure than more demanding tasks. According to that rationale, low-complexity tasks such as reaction time (RTI) and those relatively passive such as recognition memory (PRM) would be expected to be relatively unaffected by heat exposure. In contrast, more complex tasks such as learning and episodic memory (PAL), working memory (SSP), and sustained attention (RVP) would be the most affected by heat exposure. Nevertheless, in this study we have not observed any trends to confirm that hypothesis.
Some methodological limitations are noteworthy. First, although the sample was larger than most previous studies, it may not have been large enough to demonstrate differences in performance with small effect sizes. Second, humidity was not controlled and varied greatly between the experiments, causing a potential loss of statistical power. Third, although we have implemented a 30-minute interval between the two sessions and a 20-minute period of exposure before testing, it is not possible to assure that the effect of the temperature of the first session was completely vanished at the beginning of the second session. Some important strengths should also be noted. Temperature was tightly controlled, the order of exposures was balanced, and the neuropsychological assessment was based on well-standardized tests chosen to evaluate a variety of cognitive functions.
In conclusion, a general sample of healthy elderly managed to maintain a relatively preserved cognitive performance when exposed to an environment with the air temperature set at 32 °C.However, we have found some significant influential factors. Namely, humidity levels modified the effect of temperature on cognitive outcomes and reported exercising frequency explained individual vulnerability to heat stress. Results of these secondary analyses must be confirmed in future studies with controlled air humidity and more accurate assessments of physical fitness and aerobic capacity. Our findings indicate the necessity for further research on a variety of potentially influential factors.
Acknowledgments
Disclosure
The authors declare that they have no competing interests.
Funding
This study received financial support from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP; grant number 2008-57717-6) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq; grant number 573813/2008-6).
References
- Blatteis CM. Age-dependent changes in temperature regulation. Gerontology. 2012;58(4):289–295. doi: 10.1159/000333148. [DOI] [PubMed] [Google Scholar]
- Campbell GC, Norman JM. An introduction to environmental biophysics. New York: Springer-Verlag; 1998. [Google Scholar]
- Drag LL, Bieliauskas LA. Contemporary review 2009: cognitive aging. J Geriatr Psychiatry Neurol. 2010;23(2):75–93. doi: 10.1177/0891988709358590. [DOI] [PubMed] [Google Scholar]
- Falleti MG, Maruff P, Collie A, Darby DG. Practice effects associated with the repeated assessment of cognitive function using the CogState battery at 10-minute, one week and one month test-retest intervals. J Clin Exp Neuropsychol. 2006;28(7):1095–112. doi: 10.1080/13803390500205718. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHuch PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gaoua N. Cognitive function in hot environments: a question of methodology. Scand J Med Sci Sports. 2010;20(Suppl 3):60–70. doi: 10.1111/j.1600-0838.2010.01210.x. [DOI] [PubMed] [Google Scholar]
- Gaoua N, Racinais S, Grantham J. Alterations in cognitive performance during passive hyperthermia are task dependent. Int J Hyperth. 2011;27(1):1–9. doi: 10.3109/02656736.2010.516305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaoua N, Grantham J, Racinais S, Massioui FE. Sensory displeasure reduces complex cognitive performance in the heat. J Environ Psychol. 2012;32:158–163. doi: 10.1016/j.jenvp.2012.01.002. [DOI] [Google Scholar]
- Guergova S, Dufour A. Thermal sensitivity in the elderly: a review. Ageing Res Rev. 2011;10:80–92. doi: 10.1016/j.arr.2010.04.009. [DOI] [PubMed] [Google Scholar]
- Hancock PA, Ross JM, Szalma JL. A meta-analysis of performance response under thermal stressors. Hum Factors. 2007;49(5):851–877. doi: 10.1518/001872007X230226. [DOI] [PubMed] [Google Scholar]
- Hocking C, Silberstein RB, Lau WM, Stough C, Roberts W. Evaluation of cognitive performance in the heat by functional brain imaging and psychometric testing. Comp Biochem Physiol A Mol Integr Physiol. 2001;128:719–734. doi: 10.1016/S1095-6433(01)00278-1. [DOI] [PubMed] [Google Scholar]
- Holowatz LA, Kenney WL. Peripheral mechanisms of thermoregulatory control of skin blood flow in aged humans. J Appl Physiol. 2010;109:1538–1544. doi: 10.1152/japplphysiol.00338.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue Y, Kuwahara T, Araki T. Maturation and aging-related changes in heat loss effector function. J Physiol Anthropol Appl Hum Sci. 2004;23:289–294. doi: 10.2114/jpa.23.289. [DOI] [PubMed] [Google Scholar]
- International Organization for Standardization. ISO 9241 Ergonomic requirements for office work with visual display terminals (VDTs). Part 6: guidance on the work environment.
- IPCC . Summary for policymakers. In: Stocker TF, Qin D, Plattner G-K, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM, editors. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge: University Press; 2013. [Google Scholar]
- Jager CA, Milwain E, Budge M. Early detection of isolated memory deficits in the elderly: the need for more sensitive neuropsychological tests. Psychol Med. 2002;32(3):483–491. doi: 10.1017/S003329170200524X. [DOI] [PubMed] [Google Scholar]
- Jones RN, Fong TG, Metzger E, TulebaevS YFM, Alsop DC, Marcantonio ER, Cupples LA, Gottlieb G, Inouye SK. Aging, brain disease and reserve: implications for delirium. Am J Geriatr Psychiatr. 2010;18(2):117–127. doi: 10.1097/JGP.0b013e3181b972e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney WL, Munce TA. Invited review: aging and human temperature regulation. J Appl Physiol. 2003;95:2598–2603. doi: 10.1152/japplphysiol.00202.2003. [DOI] [PubMed] [Google Scholar]
- Lowe C, Rabbitt P. Test/re-test reliability of the CANTAB and ISPOCD neuropsychological batteries: theoretical and practical issues. Neuropsychologia. 1998;36(9):915–923. doi: 10.1016/S0028-3932(98)00036-0. [DOI] [PubMed] [Google Scholar]
- Mavrogianni A, Wilkinson P, Davies M, Biddulph P, Oikonomou E. Building characteristics as determinants of propensity to high indoor summer temperatures in London dwellings. Build Environ. 2012;55:117–130. doi: 10.1016/j.buildenv.2011.12.003. [DOI] [Google Scholar]
- Minson CT, Wladkowski SL, Cardell AF, Pawelczyk JA, Kenney WL. Age alters the cardiovascular response to direct passive heating. J Appl Physiol. 1998;84:1323–1332. doi: 10.1152/jappl.1998.84.4.1323. [DOI] [PubMed] [Google Scholar]
- Missenard FA. Etude physiologique et technique de la ventilation. Encyclopédie industrielle et commerciale. Paris: Eyrolles; 1933. [Google Scholar]
- Moyen NE, Ellis CL, Ciccone AB, Thurston TS, Cochrane KC, Brown LE, Coburn JW, Judelson DA. Increasing relative humidity impacts low-intensity exercise in the heat. Aviat Space Environ Med. 2014;85(2):112–119. doi: 10.3357/ASEM.3787.2014. [DOI] [PubMed] [Google Scholar]
- Nedel AS, Gonçalves FLT, Cardoso MRA. Evaluation of thermal simulation of households in the metropolitan region of São Paulo, Brazil. Ecotoxicology. 2009;18(8):1143–9. doi: 10.1007/s10646-009-0379-1. [DOI] [PubMed] [Google Scholar]
- Pilcher JJ, Nadler E, Busch C. Effects of hot and cold temperature exposure on performance: a meta-analytic review. Ergonomics. 2002;45:682–698. doi: 10.1080/00140130210158419. [DOI] [PubMed] [Google Scholar]
- Racinais S, Gaoua N, Grantham J. Hyperthermia impairs short-term memory and peripheral motor drive transmission. J Physiol. 2008;586(19):4751–4762. doi: 10.1113/jphysiol.2008.157420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radakovic SS, Maric J, Surbatovic M, Radjen S, Stefanova E, Stankovic N, Filipovic N. Effects of acclimation on cognitive performance in soldiers during exertional heat stress. Mil Med. 2007;172:133–136. doi: 10.7205/MILMED.172.2.133. [DOI] [PubMed] [Google Scholar]
- Simmons SE, Saxby BK, McGlone FP, Jones DA. The effect of passive heating and head cooling on perception, cardiovascular function and cognitive performance in the heat. Eur J Appl Physiol. 2008;104(2):271–280. doi: 10.1007/s00421-008-0677-y. [DOI] [PubMed] [Google Scholar]
- Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc. 2002;8:448–460. doi: 10.1017/S1355617702813248. [DOI] [PubMed] [Google Scholar]
- Sun G, Qian S, Jiang Q, Liu K, Li B, Li M, Zhao L, Zhou Z, von Deneen KM, Liu Y. Hyperthermia-induced disruption of functional connectivity in the human brain network. PLoS One. 2013;8(4):e61157. doi: 10.1371/journal.pone.0061157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong S, Mackenzie J, Pitman AJ, FitzGerald G, Nicholls N, Selvey L. Global climate change: time to mainstream health risks and their prevention on the medical research and policy agenda. Intern Med J. 2008;38(6):445–447. doi: 10.1111/j.1445-5994.2008.01688.x. [DOI] [PubMed] [Google Scholar]
- United Nations Population Fund (UNFPA) and HelpAge International (2012) Ageing in the twenty-first century: a celebration and a challenge - executive summary
- Wunderlich CA. The course of the temperature in disease: a guide to clinical thermometry. Am J Med Sci. 1869;57:425–447. doi: 10.1097/00000441-186904000-00021. [DOI] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1983;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]