Abstract
RNA–protein interactions influence many biological processes. Identifying the binding sites of RNA-binding proteins (RBPs) remains one of the most fundamental and important challenges to the studies of such interactions. Capturing RNA and RBPs via chemical crosslinking allows stringent purification procedures that significantly remove the non-specific RNA and protein interactions. Two major types of chemical crosslinking strategies have been developed to date, i.e., UV-enabled crosslinking and enzymatic mechanism-based covalent capture. In this review, we compare such strategies and their current applications, with an emphasis on the technologies themselves rather than the biology that has been revealed. We hope such methods could benefit broader audience and also urge for the development of new methods to study RNA−RBP interactions.
Keywords: Protein−RNA interactions, High-throughput sequencing, Crosslinking, RNA-binding proteins, Aza-IP, miCLIP
Introduction
RNAs undergo multiple RBP-mediated processing and regulatory steps to exert their biological functions. To understand RNA processing and regulation, great efforts have been made to study protein–RNA interactions in the cellular context. Among many strategies that have been developed, mapping RNA–protein interactions on a genomic scale (thus often coupled with high-throughput analyses) represents one great example. In the initial attempts to identify RNAs that are bound by specific RBPs, RNA immunoprecipitation was combined with microarray analysis (RIP-ChIP) [1,2]. However, these methods are limited to stable ribonucleoprotein particles (RNPs), which are prone to contamination of indirect or non-physiological interactions [3]. In order to achieve high specificity (and also resolution), methods that allow covalent capture of RNA–protein interactions in vivo have been developed. In the following sections, we will describe these novel methods for mapping the binding sites of RBPs on a genome-wide scale.
Panoramic views of protein–RNA interactions enabled by UV crosslinking
CLIP and HITS-CLIP
UV cross-linking and immunoprecipitation (CLIP) is a milestone technology invented by the Darnell Laboratory in 2003 (Figure 1) [4]. Back in the 1980s, it was reported that UV light induces covalent crosslinks between proteins and RNAs, without causing crosslinks between proteins [5,6]. CLIP makes use of short wave UV irradiation at 254 nm to induce the formation of covalent crosslinks only at sites of direct contact between proteins and RNAs, in the context of whole tissues, organisms or individual cell types. After UV irradiation, cells are lysed and the cross-linked RNA–RBP is first treated with RNase to trim the RNA size to about 60–100 nucleotides in length. Since the protein–RNA complex is covalently linked, stringent conditions can be applied during immunoprecipitation to purify the protein–RNA complexes. Such immunoprecipitation procedure is generally enabled with antibodies against the proteins of interest or protein epitope tags. Subsequently, the protein–RNA complexes are separated on SDS–polyacrylamide gel electrophoresis (SDS–PAGE), and transferred to nitrocellulose membrane followed by proteinase K treatment. Such step also helps to remove contamination caused by the antibody or during immunoprecipitation step. The RNA molecules recovered are then 5′ and 3′ ligated, allowing PCR amplification of the target RNA from RNPs of interest.
Figure 1.
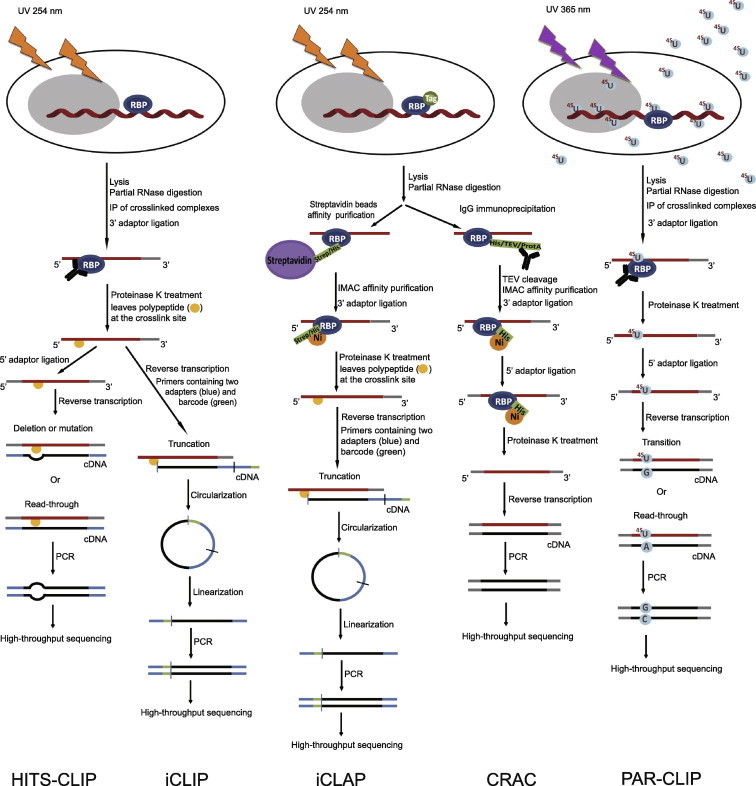
Outline of HITS-CLIP, PAR-CLIP and several variants, iCLIP, iCLAP and CRAC High-throughput sequencing CLIP (HITS-CLIP) and individual-nucleotide resolution CLIP (iCLIP) are in the left panels; individual-nucleotide resolution crosslinking affinity purification (iCLAP) and crosslinking and cDNA analysis (CRAC) are in the middle panels; photoactivatable ribonucleoside-enhanced crosslinking and immunoprecipitation (PAR-CLIP) is in the right panel. PAR-CLIP uses thioribonucleosides and UV at 365 nm to form the complex of RNA and RNA-binding protein (RBP), while the other four methods utilize UV at 254 nm. Isolation of RNA–RBP complexes is achieved either by immunoprecipitation (IP) (PAR-CLIP, HITS-CLIP and iCLIP) or by double affinity purification (iCLAP and CRAC). iCLAP and CRAC use immobilized metal ion affinity chromatography (IMAC) under denaturing conditions as a secondary purification. To achieve individual-nucleotide resolution, HITS-CLIP utilizes deletion or mutation during reverse transcription, iCLIP and iCLAP take advantage of truncated cDNAs, and PAR-CLIP makes use of thymidine (T) to cytidine (C) transition in cDNA. TEV, tobacco etch virus; ProtA, Staphylococcus aureus protein A.
This original CLIP protocol was first applied to identify Nova RNA targets in the brain by the Darnell group [4]. Nova is a neuron-specific RBP, which regulates neuronal RNA splicing. In 2005, Darnell and colleagues optimized the original CLIP protocol and further improved the specificity of the method. In the current CLIP protocol, the 3′ RNA linker ligation step is done on-bead [7]. There are three advantages conferred by this modification. First, free 3′-linkers can be removed during SDS–PAGE, so that it can prevent the ligation of-5′-linker and 3′-linker, self-ligation, circularization of target RNA and the ligation of bacterial rRNA that may come from the commercial RNA ligase or proteinase K. Therefore, signal-to-noise ratio can be greatly improved. Second, there is no need to separate free RNAs on urea–PAGE, which is a necessary step to separate linker−linker ligation from previous CLIP protocol. Third, the usage of high concentration of RNA linkers can be avoided, which alleviates the aforementioned self-ligation problem as well. In 2008, a new modification to CLIP, high-throughput sequencing of RNA isolated by crosslinking immunoprecipitation (HITS-CLIP), was adopted to harness the power of next-generation sequencing (NGS). Thus, HITS-CLIP has made it highly feasible to obtain genome-wide protein–RNA interactions. HITS-CLIP was first applied to study Nova–RNA interactions and uncovered the 3’ end RNA processing rules in the brain [8].
Control experiments are key components in every technique, and CLIP or HITS-CLIP is no exception. There are two essential controls in CLIP or HITS-CLIP. First, to verify that UV light has indeed caused covalent crosslinks between proteins and RNA, a non-UV treated sample should be used for immunoprecipitation, in which no RNA should be detected. Second, the antibody that recognizes the protein of interest must be specific. Therefore, it is very necessary to run a control without antibody, or a control with knockout cell or tissue, and do a mock IP experiment.
Apart from Nova, the RNA targets of several RBPs have been successfully identified by CLIP or HITS-CLIP in various biological systems. For example, with the help of the CLIP method, hnRNP A1, a nucleo-cytoplasmic shuttling protein, is shown to be required for processing of miR-18a [9] and SF2/ASF, a prototype member of the SR protein family, was reported to regulate processing of specific mRNAs with the help of CLIP [10]. CLIP was also used to study the function of the RNA-binding protein Rrm4, discovering that Rrm4 may transport RNAs from the nucleus to cell poles [11]. Additionally, HITS-CLIP was also applied to study other RBPs, such as Argonaute in mouse brain [12], polypyrimidine tract-binding protein (PTB, also known as hnRNP I) in HeLa cells [13], FOX2 in human embryonic stem cells [14] and so on.
Nonetheless, HITS-CLIP and CLIP methods discussed above cannot achieve individual-nucleotide resolution of the binding site. In 2011, it was reported that the cross-linked nucleotide could be deleted or mutated during reverse transcription [15,16]. Therefore, through the analysis of deletion or mutation in sequencing reads, HITS-CLIP can identify exact crosslink sites and achieve individual-nucleotide resolution of the binding site (Figure 1) [15,16].
In summary, CLIP and HITS-CLIP are very useful techniques to study protein–RNA interactions, which can be applied to different biological samples such as bacteria, fungi, yeast, Caenorhabditis elegans, mammalian tissue including brain, and culture cells including human embryonic stem cells and HeLa cells.
PAR-CLIP
It was estimated that in CLIP or HITS-CLIP, the maximal crosslinking efficiency ranges from 1% to 5% with purified protein and radiolabeled RNA [17]. To improve the crosslinking efficiency, photoactive nucleoside analogues, 4-thiouridine (4-SU) and 6-thioguanosine (6-SG), and hence photoactivatable ribonucleoside-enhanced CLIP (PAR-CLIP) were used by Tuschl and colleagues [18]. In PAR-CLIP, 4-SU and 6-SG are added to the growth medium, which are then taken up by cells and eventually incorporated into newly-synthesized RNA molecules without obvious toxicity. The formation of covalent crosslinks between proteins and RNAs is performed under UV irradiation at 365 nm, instead of UV 254 nm used in CLIP. The following steps are similar to CLIP protocol, including RNase treatment, immunoprecipitation, recovery of RNA fragment, reverse transcription and sequencing.
It is noteworthy that the incorporated 4-SU can lead to T to C transition in the sequenced cDNA. Therefore, it is possible to identify crosslink sites at individual-nucleotide resolution by analyzing the mutations in cDNA sequences (Figure 1). The accuracy of PAR-CLIP has been verified by identifying the RNA targets of several RBPs in recent years. At the very beginning, Thomas Tuschl and colleagues selected several intensely-studied RBPs and microRNA-containing RNP complexes (miRNPs), including pumilio homologue 2 (PUM2), quaking (QKI), insulin-like growth factor 2 mRNA-binding proteins 1–3 (IGF2BP1–3), the Argonaute proteins (AGO) and trinucleotide repeat-containing proteins 6 (TNRC6A−C) in HEK293 cells, to identify the binding sites [18]. One year later, transcriptome-wide binding sites of the RBP defective germline development protein 1 (GLD-1) in C. elegans [19], RNA targets of the RNA-binding protein HuR [16,20,21], the binding targets of the sequence-specific RBP complex Nrd1 and Nab3 in yeast [22] and the binding motif of PAP-associated domain-containing 5 (PAPD5) [23] were also identified using PAR-CLIP.
In addition, PAR-CLIP is particularly suitable for studies using cultured cells, because of its high uptake efficiency of nucleoside analogues (incorporation efficiency is up to 4% for 4-SU in relative to uridine [18]). In short, the PAR-CLIP method has higher cross-linking efficiency and can also achieve individual-nucleotide resolution of the binding sites.
CLIP-derived methods
In the case of the protein Nova, 85% of cDNAs are truncated, because reverse transcriptase stops at the UV-induced crosslink site, in which peptides may not be removed by proteinase K [24]. However, every coin has two sides. Individual-nucleotide resolution CLIP (iCLIP), a variation of CLIP, was developed by the Ule laboratory, which made use of this experimental observation to identify crosslinked sites [25]. In iCLIP protocol, co-immunoprecipitated RNA is ligated to a 3′ adaptor, followed by proteinase K treatment, resulting in a covalently-bound peptide on the RNA. Using primers containing two cleavable adaptor regions and one random barcode, cDNA will be truncated at crosslink site during reverse transcription. Subsequently, cDNA is circularized, linearized, amplified and sequenced. Thus, it is easy to find out the residue next to the crosslink site, which ideally should be the first nucleotide after the barcoded sequence (Figure 1). iCLIP was first applied to study the function of hnRNP in splicing [25]. Subsequently, this technique was used to identify the RNA targets of the T-cell-restricted intracellular antigen-1 (TIA1), TIA-like 1 (TIAL1) [26] and TAR DNA-binding protein 43 (TDP-43) [27].
Crosslinking and affinity purification (iCLAP), a method to purify streptavidin/histidine (Strep/His) double-tagged RBP using stringent affinity purification instead of immunoprecipitation, is a variation of iCLIP (Figure 1). In the case of TIA1 and TIAL1, the TIA1 antibody has a slight cross-reactivity during affinity purification to TIAL1 [26]. Therefore, it is necessary to use RBP with a tag at the N- or C-terminus to co-purify the RNP complex, under the conditions of poor antibodies. In the iCLAP technique, Jernej Ule and colleagues, who invented the iCLIP method, used magnetic streptavidin beads in the first purification step followed by cobalt beads under denaturing conditions as a secondary purification step [26]. Subsequently, cDNAs are amplified and sequenced in the similar way as using iCLIP.
Another variation of CLIP is the crosslinking and cDNA analysis (CRAC), where RBPs are tagged with C-terminal 6 × His, tobacco etch virus (TEV) protease site and protein A tags (Figure 1). Similar to iCLIP, immunoglobulin G (IgG) beads are used in the first purification step. The RNA–protein complexes were subsequently treated with TEV protease, followed by immobilized metal-ion affinity chromatography (IMAC) as a secondary purification. Using the CRAC method, the Tollervey laboratory identified the binding sites of the probable pre-mRNA-splicing factor ATP-dependent RNA helicase Prp43 [28] and the small nucleolar RNPs (snoRNPs) Nop1, Nop56, Nop58 and rRNA processing 9 (Rrp9) [29] in yeast.
In summary, as CLIP variants, iCLIP, iCLAP and CRAC are similar to HITS-CLIP and PAR-CLIP in essence. All methods can achieve single nucleotide resolution and produce data with high precision.
Crosslinking to identify RNA-bound proteome
RBPs play diverse roles in many biological processes and influence the RNA metabolism [30]. Many RBPs have canonical RNA binding domains, including the RNA recognition motif (RRM), heterogeneous nuclear RNP K-homology domain (KH), zinc finger (Znf), etc. [31]. Based on such observation, the mammalian genome has been predicted to encode about 600 RBPs [32]. However, researchers also have reported that there are quite a few RBPs that do not contain such canonical RNA-binding domains [23,33]. Therefore, mere computational predictions will likely miss these non-canonical RBPs.
Two types of experiments can be performed in order to identify an RBP. The first one takes advantage of protein arrays, which were spotted with tagged recombinant proteins. In fact, two groups have identified about 200 RBPs in yeast via protein arrays [34,35]. The other one utilizes RNA pull-down methods, in which RNA of interest is immobilized and then used to pull out potential RBPs from (very often) cell lysates via affinity. Combining the use of an RNA tag and high-resolution quantitative mass spectrometry (MS), interacting partners for RNA motifs of general interest can be detected [36]. For instance, by hybridizing with affinity-tagged oligodT, mRNA-binding proteome can be studied [37]. Nonetheless, these methods cannot discriminate direct RNA–protein interactions from indirect ones.
In 2012, two groups independently reported a new approach to study the mRNA interactome. In the first step of their methods, they use UV light to covalently crosslink RNA and RBPs. The UV light can only crosslink protein bound to RNA directly but does not affect protein–protein interaction. The next step involves affinity purification of mRNAs using oligodT, instead of trimming down the RNAs as performed in CLIP-type experiments. In this way, mRNA-binding proteins that are covalently attached to mRNA molecules will also be pulled down at the same time. By high-resolution quantitative MS, mRNA interactome can be identified in a high throughput manner. These studies have identified about 800 RBPs, including about 300 RBPs that are not annotated previously [38,39].
Cellular protein–RNA interactions enabled by mechanistic crosslinking
More than 100 post-transcriptional RNA modifications have been identified in cellular RNAs until now [40]. Yet, identifying the modifying enzymes along with their physiological sites of modification remains a challenging task [41].
Among these RNA modifications, RNA cytosine methylation, occurring at the C5 position (m5C), has been detected in tRNAs, rRNAs and mRNAs [42,43]. There are six families of m5C RNA methyltransferase (RMT), but only two of them, NOP2/Sun RMT family member 2 (NSUN2) and DNA (cytosine-5-)-methyltransferase 2 (DNMT2), have been identified in higher eukaryotes [42]. Both were known previously to work on specific tRNA molecules [44–46]; yet the full spectrum of targets remains an open question for both enzymes. To identity the target sites of these RMT, two groups separately developed two new crosslinking methods to capture RNA-modifying enzymes that are linked directly to their targets. Both methods involve the formation of covalent RNP complexes, yet they are based on enzymatic mechanism instead of using UV-crosslinking [47,48].
It has been found that m5C-RMT could form a covalent enzyme−substrate intermediate during the methylation reactions [42]. The covalent bond is formed, at the early stage of methylation process, between a cysteine residue of the m5C-RMT and the C6 atom of cytosine of the RNA target. Then the RNA methyltransferase transfers a methyl group from cofactor S-adenosyl methionine (SAM) to the C5 of the target cytosine (Figure 2). The enzyme is finally released by β-elimination. Interestingly, studies have shown that if the cytosine of the RNA targets is replaced by a cytosine analogue, 5-azacytidine (5-aza-C), the methyltransferase cannot be released from the RNA target sites and thus remains covalently attached to its targets (Figure 2). Based on this mechanism, Khoddami and Cairns developed a mechanistic crosslinking method called Aza-IP. They incorporated 5-aza-C into nascent RNA by feeding cells with 5-aza-C; they then immunoprecipitated the m5C-RMT, together with its target RNAs. Finally, the target sites can be identified by deep sequencing and computational analysis [48].
Figure 2.
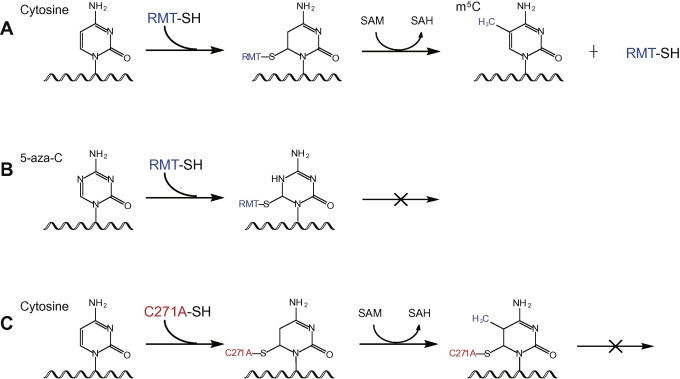
Mechanistic crosslinking of RNA-modifying enzymes and target RNA molecules A. Reaction mechanism of a typical m5C-RNA methyltransferase (RMT, in blue). B. 5-aza-cytidine (5-aza-C) traps RMT (in blue) with its target RNA molecules. C. Cysteine mutation (C271A, in red) in the active site of NSUN2 prevents release of the methyltransferase after methylation reaction, thereby enabling crosslinking of protein–RNA complex. SAM, S-adenosyl methionine; SAH, S-adenosyl homocysteine.
Besides the use of cytosine analogues to trap the m5C-RMT, mutation to the methyltransferase can also stabilize the covalently-linked protein–RNA during the catalytic process. For instance, Cys321 of NSUN2 can form the covalent bond with the cytosine of the target RNA sites. Another cysteine at position 271 (Cys271) of NSUN2 is required for the release of the methylated RNA. If Cys271 is mutated to alanine, the methyltransferase cannot be released from the RNA target sites (Figure 2). By utilizing such enzyme properties, Hussain and his colleagues successfully crosslinked the RNA targets of NSUN2; the remaining strategy for sequencing the RNA resembles those of iCLIP, therefore they termed the method methylation iCLIP (miCLIP) [47].
Towards more RNA-modifying enzymes
RNA molecules are intensively modified; yet the interactions between RNA targets and RNA-modifying enzymes are often transient. We discussed mechanistic crosslinking in the section above to study interactions between RNA targets and m5C-RMT; both “Aza-IP” and “miCLIP” rely on the detailed knowledge of the enzymatic mechanism and the usage of either a mechanism-based inhibitor, 5-aza-C, or mutant protein with altered enzymatic action. However, successful examples towards other RNA modifications and their related enzymes are still scarce at the moment.
We will use N6-methyladenosine (m6A) RNA modification as an example to formulate the challenges and opportunities in identifying, on the genomic level, protein–RNA interactions for RNA-modifying enzymes. m6A is the most abundant endogenous modification for mRNA in eukaryotes [49]; and the recent discoveries of two novel m6A demethylases have gained renewed interest for this long-known modification [50,51]. The fat mass and obesity-associated protein (FTO), which was first identified through genome-wide association studies to be linked to fat mass and obesity [52], shows efficient demethylation activity towards m6A [50]. Alpha-ketoglutarate-dependent dioxygenase alkB homologue 5 (ALKBH5), another homologue protein of FTO, was later shown to be another m6A demethylase, which impacts RNA metabolism and mouse fertility [51]. More recently, three labs independently reported the identification of m6A-methyltransferases [53–55]. In addition, reader proteins of m6A have been suggested [56]. For example, the YTH domain family member 2 (YTHDF2) protein, one of the reader proteins, selectively recognizes m6A and targets the bound mRNA to decay sites, thereby affecting the translation status and lifetime of mRNA [57].
Although the genome-wide characterization of m6A modification in mRNA has been reported [56,58], many open questions remain for the writer, reader and eraser proteins of the m6A modification. PAR-CLIP experiments have been used to investigate the protein–RNA interactions for the writer and reader proteins [53,57]; yet the detailed interaction profiles for the eraser proteins FTO and ALKBH5 are still lacking. Both FTO and ALKBH5 are Fe2+-dependent dioxygenases; they also use the putative “base-flipping” mechanism to gain access to the target m6A base for demethylation reactions [59]. While existing crosslinking methods might still not be able to capture the transient interactions for these eraser proteins, new strategies that take into account their unique enzymatic mechanism could be useful in stabilizing protein–RNA interactions.
Conclusion and outlook
Formation of covalent bond between protein and RNA offers an important tool to the identification of RNA–protein interaction, as covalently-crosslinked complex can bear stringent purification to remove nonspecifically-bound RNA and protein. While UV crosslink-based methods are preferentially used for RBPs, mechanism-based crosslinking methods will be particularly useful for RNA-modifying enzymes, since these proteins only stay transiently with their RNA targets. In this review, we summarize technical aspects of several UV-enabled crosslinking strategies, including HITS-CLIP, PAR-CLIP and several derived methods with individual nucleotide-resolution. While these methods have allowed many successful studies of RNA–protein interactions, challenges still exist for more robust assays. We hope our comparisons of the existing methods could really stimulate the development of new crosslinking methods with improved crosslinking efficiency. Ultimately, more high-resolution maps of RNA–protein interactions can be revealed to understand their biological roles in greater depth and detail.
Competing interests
The authors declare no competing financial interests.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 31270838) and the National Basic Research Foundation of China (Grant No. 2014CB964900).
Footnotes
Peer review under responsibility of Beijing Institute of Genomics, Chinese Academy of Sciences and Genetics Society of China.
References
- 1.Trifillis P., Day N., Kiledjian M. Finding the right RNA: identification of cellular mRNA substrates for RNA-binding proteins. RNA. 1999;5:1071–1082. doi: 10.1017/s1355838299981803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brooks S.A., Rigby W.F.C. Characterization of the mRNA ligands bound by the RNA binding protein hnRNP A2 utilizing a novel in vivo technique. Nucleic Acids Res. 2000;28:e49. doi: 10.1093/nar/28.10.e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mili S., Steitz J.A. Evidence for reassociation of RNA-binding proteins after cell lysis: implications for the interpretation of immunoprecipitation analyses. RNA. 2004;10:1692–1694. doi: 10.1261/rna.7151404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ule J., Jensen K.B., Ruggiu M., Mele A., Ule A., Darnell R.B. CLIP identifies Nova-regulated RNA networks in the brain. Science. 2003;302:1212–1215. doi: 10.1126/science.1090095. [DOI] [PubMed] [Google Scholar]
- 5.Wagenmakers A.J., Reinders R.J., van Venrooij W.J. Cross-linking of mRNA to proteins by irradiation of intact cells with ultraviolet light. Eur J Biochem. 1980;112:323–330. doi: 10.1111/j.1432-1033.1980.tb07207.x. [DOI] [PubMed] [Google Scholar]
- 6.Pinol-Roma S., Adam S.A., Choi Y.D., Dreyfuss G. Ultraviolet-induced cross-linking of RNA to proteins in vivo. Methods Enzymol. 1989;180:410–418. doi: 10.1016/0076-6879(89)80114-4. [DOI] [PubMed] [Google Scholar]
- 7.Ule J., Jensen K., Mele A., Darnell R.B. CLIP: a method for identifying protein–RNA interaction sites in living cells. Methods. 2005;37:376–386. doi: 10.1016/j.ymeth.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 8.Licatalosi D.D., Mele A., Fak J.J., Ule J., Kayikci M., Chi S.W. HITS-CLIP yields genome-wide insights into brain alternative RNA processing. Nature. 2008;456:464–469. doi: 10.1038/nature07488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guil S., Cáceres J.F. The multifunctional RNA-binding protein hnRNP A1 is required for processing of miR-18a. Nat Struct Mol Biol. 2007;14:591–596. doi: 10.1038/nsmb1250. [DOI] [PubMed] [Google Scholar]
- 10.Sanford J.R., Coutinho P., Hackett J.A., Wang X., Ranahan W., Caceres J.F. Identification of nuclear and cytoplasmic mRNA targets for the shuttling protein SF2/ASF. PLoS One. 2008;3:e3369. doi: 10.1371/journal.pone.0003369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Becht P., König J., Feldbrügge M. The RNA-binding protein Rrm4 is essential for polarity in Ustilago maydis and shuttles along microtubules. J Cell Sci. 2006;119:4964–4973. doi: 10.1242/jcs.03287. [DOI] [PubMed] [Google Scholar]
- 12.Chi S.W., Zang J.B., Mele A., Darnell R.B. Argonaute HITS-CLIP decodes microRNA-mRNA interaction maps. Nature. 2009;460:479–486. doi: 10.1038/nature08170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xue Y., Zhou Y., Wu T., Zhu T., Ji X., Kwon Y.S. Genome-wide analysis of PTB-RNA interactions reveals a strategy used by the general splicing repressor to modulate exon inclusion or skipping. Mol Cell. 2009;36:996–1006. doi: 10.1016/j.molcel.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yeo G.W., Coufal N.G., Liang T.Y., Peng G.E., Fu X.D., Gage F.H. An RNA code for the FOX2 splicing regulator revealed by mapping RNA–protein interactions in stem cells. Nat Struct Mol Biol. 2009;16:130–137. doi: 10.1038/nsmb.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang C., Darnell R.B. Mapping in vivo protein–RNA interactions at single-nucleotide resolution from HITS-CLIP data. Nat Biotechnol. 2011;29:607–614. doi: 10.1038/nbt.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kishore S., Jaskiewicz L., Burger L., Hausser J., Khorshid M., Zavolan M. A quantitative analysis of CLIP methods for identifying binding sites of RNA-binding proteins. Nat Methods. 2011;8:559–564. doi: 10.1038/nmeth.1608. [DOI] [PubMed] [Google Scholar]
- 17.Darnell R.B. HITS-CLIP: panoramic views of protein–RNA regulation in living cells. Wiley Interdiscip Rev RNA. 2010;1:266–286. doi: 10.1002/wrna.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hafner M., Landthaler M., Burger L., Khorshid M., Hausser J., Berninger P. Transcriptome-wide identification of RNA-binding protein and microRNA target sites by PAR-CLIP. Cell. 2010;141:129–141. doi: 10.1016/j.cell.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jungkamp A.C., Stoeckius M., Mecenas D., Grün D., Mastrobuoni G., Kempa S. In vivo and transcriptome-wide identification of RNA binding protein target sites. Mol Cell. 2011;44:828–840. doi: 10.1016/j.molcel.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mukherjee N., Corcoran D.L., Nusbaum J.D., Reid D.W., Georgiev S., Hafner M. Integrative regulatory mapping indicates that the RNA-binding protein HuR couples pre-mRNA processing and mRNA stability. Mol Cell. 2011;43:327–339. doi: 10.1016/j.molcel.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lebedeva S., Jens M., Theil K., Schwanhäusser B., Selbach M., Landthaler M. Transcriptome-wide analysis of regulatory interactions of the RNA-binding protein HuR. Mol Cell. 2011;43:340–352. doi: 10.1016/j.molcel.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 22.Jamonnak N., Creamer T.J., Darby M.M., Schaughency P., Wheelan S.J., Corden J.L. Yeast Nrd1, Nab3, and Sen1 transcriptome-wide binding maps suggest multiple roles in post-transcriptional RNA processing. RNA. 2011;17:2011–2025. doi: 10.1261/rna.2840711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rammelt C., Bilen B., Zavolan M., Keller W. PAPD5, a noncanonical poly(A) polymerase with an unusual RNA-binding motif. RNA. 2011;17:1737–1746. doi: 10.1261/rna.2787011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sugimoto Y., König J., Hussain S., Zupan B., Curk T., Frye M. Analysis of CLIP and iCLIP methods for nucleotide-resolution studies of protein–RNA interactions. Genome Biol. 2012;13:1–13. doi: 10.1186/gb-2012-13-8-r67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Konig J., Zarnack K., Rot G., Curk T., Kayikci M., Zupan B. ICLIP reveals the function of hnRNP particles in splicing at individual nucleotide resolution. Nat Struct Mol Biol. 2010;17:909–915. doi: 10.1038/nsmb.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Z., Kayikci M., Briese M., Zarnack K., Luscombe N.M., Rot G. ICLIP predicts the dual splicing effects of TIA-RNA interactions. PLoS Biol. 2010;8:e1000530. doi: 10.1371/journal.pbio.1000530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tollervey J.R., Curk T., Rogelj B., Briese M., Cereda M., Kayikci M. Characterizing the RNA targets and position-dependent splicing regulation by TDP-43. Nat Neurosci. 2011;14:452–458. doi: 10.1038/nn.2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bohnsack M.T., Martin R., Granneman S., Ruprecht M., Schleiff E., Tollervey D. Prp43 bound at different sites on the pre-rRNA performs distinct functions in ribosome synthesis. Mol Cell. 2009;36:583–592. doi: 10.1016/j.molcel.2009.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Granneman S., Kudla G., Petfalski E., Tollervey D. Identification of protein binding sites on U3 snoRNA and pre-rRNA by UV cross-linking and high-throughput analysis of cDNAs. Proc Natl Acad Sci U S A. 2009;106:9613–9618. doi: 10.1073/pnas.0901997106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glisovic T., Bachorik J.L., Yong J., Dreyfuss G. RNA-binding proteins and post-transcriptional gene regulation. FEBS Lett. 2008;582:1977–1986. doi: 10.1016/j.febslet.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lunde B.M., Moore C., Varani G. RNA-binding proteins: modular design for efficient function. Nat Rev Mol Cell Biol. 2007;8:479–490. doi: 10.1038/nrm2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Lima Morais D.A., Fang H., Rackham O.J., Wilson D., Pethica R., Chothia C. SUPERFAMILY 1.75 including a domain-centric gene ontology method. Nucleic Acids Res. 2011;39:D427–D434. doi: 10.1093/nar/gkq1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee I., Hong W. RAP – a putative RNA-binding domain. Trends Biochem Sci. 2004;29:567–570. doi: 10.1016/j.tibs.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 34.Tsvetanova N.G., Klass D.M., Salzman J., Brown P.O. Proteome-wide search reveals unexpected RNA-binding proteins in Saccharomyces cerevisiae. PloS One. 2010;5:e12671. doi: 10.1371/journal.pone.0012671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scherrer T., Mittal N., Janga S.C., Gerber A.P. A screen for RNA-binding proteins in yeast indicates dual functions for many enzymes. PloS One. 2010;5:e15499. doi: 10.1371/journal.pone.0015499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Butter F., Scheibe M., Mörl M., Mann M. Unbiased RNA-protein interaction screen by quantitative proteomics. Proc Natl Acad Sci U S A. 2009;106:10626–10631. doi: 10.1073/pnas.0812099106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lindberg U., Sundquist B. Isolation of messenger ribonucleoproteins from mammalian cells. J Mol Biol. 1974;86:451–468. doi: 10.1016/0022-2836(74)90030-8. [DOI] [PubMed] [Google Scholar]
- 38.Castello A., Fischer B., Eichelbaum K., Horos R., Beckmann B.M., Strein C. Insights into RNA biology from an atlas of mammalian mRNA-binding proteins. Cell. 2012;149:1393–1406. doi: 10.1016/j.cell.2012.04.031. [DOI] [PubMed] [Google Scholar]
- 39.Baltz A.G., Munschauer M., Schwanhausser B., Vasile A., Murakawa Y., Schueler M. The mRNA-bound proteome and its global occupancy profile on protein-coding transcripts. Mol Cell. 2012;46:674–690. doi: 10.1016/j.molcel.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 40.Cantara W.A., Crain P.F., Rozenski J., McCloskey J.A., Harris K.A., Zhang X. The RNA modification database, RNAMDB: 2011 update. Nucleic Acids Res. 2011;39:D195–D201. doi: 10.1093/nar/gkq1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He C. Grand challenge commentary: RNA epigenetics? Nat Chem Biol. 2010;6:863–865. doi: 10.1038/nchembio.482. [DOI] [PubMed] [Google Scholar]
- 42.Motorin Y., Lyko F., Helm M. 5-methylcytosine in RNA: detection, enzymatic formation and biological functions. Nucleic Acids Res. 2010;38:1415–1430. doi: 10.1093/nar/gkp1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Squires J.E., Patel H.R., Nousch M., Sibbritt T., Humphreys D.T., Parker B.J. Widespread occurrence of 5-methylcytosine in human coding and non-coding RNA. Nucleic Acids Res. 2012;40:5023–5033. doi: 10.1093/nar/gks144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jurkowski T.P., Meusburger M., Phalke S., Helm M., Nellen W., Reuter G. Human DNMT2 methylates tRNA(Asp) molecules using a DNA methyltransferase-like catalytic mechanism. RNA. 2008;14:1663–1670. doi: 10.1261/rna.970408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schaefer M., Pollex T., Hanna K., Tuorto F., Meusburger M., Helm M. RNA methylation by Dnmt2 protects transfer RNAs against stress-induced cleavage. Genes Dev. 2010;24:1590–1595. doi: 10.1101/gad.586710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tuorto F., Liebers R., Musch T., Schaefer M., Hofmann S., Kellner S. RNA cytosine methylation by Dnmt2 and NSun2 promotes tRNA stability and protein synthesis. Nat Struct Mol Biol. 2012;19:900–905. doi: 10.1038/nsmb.2357. [DOI] [PubMed] [Google Scholar]
- 47.Hussain S., Sajini A.A., Blanco S., Dietmann S., Lombard P., Sugimoto Y. NSun2-mediated cytosine-5 methylation of vault noncoding RNA determines its processing into regulatory small RNAs. Cell Rep. 2013;4:255–261. doi: 10.1016/j.celrep.2013.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khoddami V., Cairns B.R. Identification of direct targets and modified bases of RNA cytosine methyltransferases. Nat Biotechnol. 2013;31:458–464. doi: 10.1038/nbt.2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grosjean H. XXIV. Springer; New York: 2005. (Fine-tuning of RNA functions by modification and editing). p. 442. [Google Scholar]
- 50.Jia G., Fu Y., Zhao X., Dai Q., Zheng G., Yang Y. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat Chem Biol. 2011;7:885–887. doi: 10.1038/nchembio.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zheng G., Dahl J.A., Niu Y., Fedorcsak P., Huang C.M., Li C.J. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol Cell. 2013;49:18–29. doi: 10.1016/j.molcel.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Frayling T.M., Timpson N.J., Weedon M.N., Zeggini E., Freathy R.M., Lindgren C.M. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316:889–894. doi: 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu J., Yue Y., Han D., Wang X., Fu Y., Zhang L. A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat Chem Biol. 2014;10:93–95. doi: 10.1038/nchembio.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ping X.L., Sun B.F., Wang L., Xiao W., Yang X., Wang W.J. Mammalian WTAP is a regulatory subunit of the RNA N6-methyladenosine methyltransferase. Cell Res. 2014;24:177–189. doi: 10.1038/cr.2014.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang Y., Li Y., Toth J.I., Petroski M.D., Zhang Z., Zhao J.C. N6-methyladenosine modification destabilizes developmental regulators in embryonic stem cells. Nat Cell Biol. 2014;16:191–198. doi: 10.1038/ncb2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dominissini D., Moshitch-Moshkovitz S., Schwartz S., Salmon-Divon M., Ungar L., Osenberg S. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature. 2012;485:201–206. doi: 10.1038/nature11112. [DOI] [PubMed] [Google Scholar]
- 57.Wang X., Lu Z., Gomez A., Hon G.C., Yue Y., Han D. N6-methyladenosine-dependent regulation of messenger RNA stability. Nature. 2014;505:117–120. doi: 10.1038/nature12730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meyer K.D., Saletore Y., Zumbo P., Elemento O., Mason C.E., Jaffrey S.R. Comprehensive analysis of mRNA methylation reveals enrichment in 3′ UTRs and near stop codons. Cell. 2012;149:1635–1646. doi: 10.1016/j.cell.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yi C., He C. DNA repair by reversal of DNA damage. Cold Spring Harb Perspect Biol. 2013;5:a012575. doi: 10.1101/cshperspect.a012575. [DOI] [PMC free article] [PubMed] [Google Scholar]



