Abstract
The sunflower (Helianthus annuus L. cv. PAC 36) seedlings were inoculated with plant growth promoting rhizobacteria (PGPR), viz. Azotobacter chroococcum (A+), Bacillus polymyxa (B+), separately and in combination of the two (AB+). Relative water content and seedling growth were maximum in AB+ seedlings under control. Water stress significantly decreased the RWC, growth and dry mass of non-inoculated seedlings. However, inoculated seedlings maintained higher growth even under water stress. Pigments and protein contents decreased under water stress, but higher amount of the same was observed in stressed AB+ seedlings. Enhanced activity of nitrate reductase was recorded in AB+ seedlings with maximum in control. Water stress significantly decreased the nitrate reductase activity. A significant increase in the activity of superoxide dismutase (SOD) in leaves was recorded under water stress except in B+ with maximum increase in non-inoculated seedlings. Catalase (CAT) activity decreased in stressed non-inoculated seedlings while increased in the leaves of A+ and AB+ seedlings. Almost similar trends were recorded for both leaves and cotyledons. PGPR improved the water status in stressed seedlings and thereby physiological and biochemical parameters and thus ameliorated the severe effects of water stress.
Keywords: Azotobacter chroococcum, Bacillus polymyxa, Helianthus annuus, Plant growth promoting rhizobacteria, Water stress
Introduction
Water stress caused by irregular rains and insufficient ground water is a major constraint for the growth and development of plant. The negative effects at the hand of water stress on growth of plant is due to the loss in evolution of O2 and decline in CO2 fixation which further leads to photodamage of the PS II reaction (Biswal 1997a,b) and oxidative stress in cell components. Oxidative stress generates free radicals and toxic derivatives of O2 such as singlet oxygen and superoxide anions (Smirnoff 1993) and consequent lipid peroxidation during senescence (Ishida et al. 1997). An antioxidative defense system comprising of catalase (CAT), peroxidase (POX) and superoxide dismutase (SOD) has been shown to be activated in response to a variety of oxidative stress (Mǿller et al. 2007; Kohler et al. 2008). These enzymes play various complementary roles in the concerted cellular defense, such as direct scavenging of reactive oxygen species (ROS) for detoxification of the cell (Rubio et al. 2002).
Plant-growth promoting rhizobacteria (PGPR) are a group of bacteria which are associated with roots of many plants and increase the plant growth. PGPR such as Azotobacter and Bacillus species are being more widely used in agriculture as biofertilizers because of their ability to fix nitrogen and to solubilize phosphorus respectively (Mahfouz and Sharaf-Eldin 2007). They also promote the growth of plants by their efficient role by producing growth promoting substances such as indole acetic acid (IAA), gibberillic acid (GA), cytokinin and vitamins and several other hormones (Gomes et al. 2001). Protective role of PGPR against various stresses, viz. water stress (Arshad et al. 2007 and Timmusk et al. 1999b), salinity stress (Saravanakumar et al. 2007), water logging stress (Grichko and Glick 2001), temperature stress (Barka et al. 2006) and heavy metal stress (Arshad et al. 2007) are well documented.
Sunflower (Helianthus annuus L.) is an important economic crop. H. annuus cv. PAC 36 is one of the most commonly grown cultivars in North India. In the present study the sunflower seeds were inoculated with Azotobacter chroococcum and Bacillus polymyxa separately and also in combination. Different biochemical parameters were assayed to observe the protective nature of inoculants in enhancing the adaptability of plants during water deficit.
Materials and methods
Growth, inoculation and stress condition
The seeds of sunflower (Helianthus annuus L. cv. PAC 36) were surface sterilized with 30% ethanol and divided into four sets for different treatments. Nitrogen fixing Azotobacter chroccoccum (A) and phosphate solubilizing Bacillus polymyxa (B) were taken as biofertilizers. The seeds were inoculated separately (A+, B+) and also in combination (AB+) in heavy bacterial suspension (containing > 108 cells/mL) by soaking them for 20 min. The inoculated seeds were air dried in shade for 30 min. The inoculated and the non-inoculated seeds were sown in pots filled with well manured sandy loam soil (1:4) in the experimental plot in the Department of Botany, University of Allahabad, Allahabad (24°47’ and 50°47’ N latitude; 81°91’ and 82°21’E longitude; 78 m above sea level) in the month of April, 2007 (Temperature: 32 ± 5 °C, Relative humidity: 60 ± 5 %). Irrigation was done as and when required. Fifteen days old seedlings of uniform size with the first pair of fully expanded leaves were selected for further experimentation. Each set of treatments was subdivided into two sets. One set was subjected to water stress by withholding water supply for five days while other set was regularly irrigated and was treated as control. The stressed plants were rewatered and recovery was recorded after 24 h. Cotyledons provide a major proportion of assimilate, which is required for growth and establishment of seedling during the developing stage of the first pair of leaves and hence, the senescing cotyledons and first fully expanded leaves from different treatments were sampled for biochemical analyses.
Relative water content
For the measurement of relative water content (RWC) leaves and cotyledons samples were cut into discs of uniform size, weighed for a fresh weight (FW) and then they were immediately floated on distilled water at 25 °C in darkness. The cotyledons being thick and fleshy deteriorated when immersed in water for more than 4 h. The turgid weight (TW) of discs of cotyledons was taken after 4 h and that of leaf discs after 12 h. The discs were dried in oven at 80 °C for 48 h for the dry weight (DW). The RWC was calculated following Bars and Weatherley (1962): RWC (%) = (FW-DW)/ (TW-DW) × 100.
Measurement of pigments and protein contents
The pigments of leaves and cotyledons viz. chlorophyll a, chlorophyll b and carotenoids were extracted with 80 % acetone and quantified following Lichtenthaler (1987). Protein content was determined following Lowry et al. (1951). The amount of protein was calculated with reference to standard curve obtained from bovine serum albumin.
Extraction and assay of enzymes
Nitrate reductase (NR) (EC 1.7.99.4) activity was assayed by modified procedure of Jaworski (1971) based on incubation of fresh tissue (0.25 g) in 4.5 mL medium containing 100 mM phosphate buffer, 3 % KNO3 and 5 % propanol. 0.4 ml aliquot was treated with 0.3 ml 3 % sulphanilamide in 3 N HCL and 0.3 ml 0.02 % N-1 naphthyl ethylenediamine dihydrochloride (NEDD). The absorbance was measured at 540 nm. NR activity was calculated with a standard curve prepared from NaNO2.
Superoxide dismutase (EC 1.15.11) activity was determined by the nitroblue tetrazolium (NBT) phototochemical assay method following Beyer and Fridovich (1987). 0.2 g fresh leaf tissue was homogenized in 1 % polyvinyl pyrrolidone (PVP) prepared in 50 mM potassium phosphate buffer (pH 7.0) and centrifuged at 15,000 g for 30 min at 4 °C. The reaction mixture contained 0.5 ml clear supernatant, 2 ml 0.15 mM ethylene diaminetetra aceticacid (EDTA), 20 mM of methionine, 0.12 mM NBT and 0.5 ml 11.96 μM riboflavin, 0.5 mL PVP and the activity determined colorimetrically against blank at 560 nm. One unit of SOD activity was defined as the amount of enzyme that was required to cause 50 % inhibition of the reduction of NBT.
Catalase (EC 1.11.1.6) was assayed according to the method of Sinha (1972). 0.2 g fresh leaf tissue was homogenized in 100 mM potassium phosphate buffer (pH 7.0) and centrifuged at 10,000 g for 30 min at 4 °C. The reaction mixture contained enzyme extract, 1.25 ml 0.2 M H2O2, potassium phosphate buffer. The reaction mixture was mixed with potassium dichromate acetic acid reagent for absorbance at 570 nm.
Statistical analysis
Treatments were arranged in a randomized block design with three replications. Data were statistically analyzed using analysis of variance (ANOVA) by using SPSS (Ver.10; SPSS Inc., Chicago, IL, USA). Appropriate standard deviation of means (±SD) was calculated for presentation with tables and graphs. The treatment means were analyzed by Duncan’s multiple range test (DMRT) at P < 0.05.
Results
Relative water content (RWC) and plant growth
RWC decreased under water stress Table 1. Minimum decrease in RWC was recorded in cotyledons and in the leaves of AB+ plants. Maximum loss was exhibited by non-inoculated plants. Inoculated plants exhibited improved water status during stress. Recovery on watering was more pronounced in plants with inoculants (Tables 2 and 3). Maximum growth of seedlings was recorded in AB+ under control condition as evinced from highest dry mass of seedlings. All inoculated seedlings had higher DM as compared to non-inoculated ones under control as well as under water stress. Seedlings inoculated with bacteria exhibited higher root length even under water stress, except A+ under control. Non-inoculated seedlings exhibited a decrease of 10.61 % in root length under water stress. Maximum shoot length was recorded in AB+ seedlings under control. Interestingly, no significant difference in SL was observed between stressed B+, AB+ and control non inoculated seedlings. Difference in dry mass, root and shoot length was not significantly different between stressed and stress recovered seedlings (Table 1).
Table 1.
Effect of water stress on growth parameters of inoculated and non-inoculated sunflower seedlings
| Treatments | Inoculation | Seedling length (cm) | Dry mass of seedling (mg seed−1) | ||||
|---|---|---|---|---|---|---|---|
| Shoot | Root | Total length | Shoot | Root | Total DM | ||
| Control | − | 12.37 ± 0.12d | 8.20 ± 0.10c | 20.57 ± 0.15e | 408.33 ± 30.14e | 145.90 ± 11.00c | 554.23 ± 19.24e |
| A+ | 14.03 ± 0.15c | 8.73 ± 0.12bc | 22.77 ± 0.06c | 531.67 ± 16.07b | 155.78 ± 3.02c | 687.44 ± 13.49b | |
| B+ | 15.33 ± 0.15b | 9.47 ± 0.31b | 24.80 ± 0.26b | 689.73 ± 36.51a | 165.71 ± 4.21b | 855.44 ± 40.59a | |
| AB+ | 16.57 ± 0.31a | 10.39 ± 0.39a | 26.96 ± 0.29a | 697.13 ± 6.59a | 177.40 ± 6.47a | 874.53 ± 9.11a | |
| Stress | − | 10.27 ± 0.12f | 7.33 ± 1.01d | 17.60 ± 0.92 h | 355.00 ± 15.00f | 110.48 ± 5.26d | 465.48 ± 19.52f |
| A+ | 11.50 ± 0.10e | 8.13 ± 0.32c | 19.53 ± 0.21f | 419.67 ± 15.50de | 145.13 ± 5.00c | 564.79 ± 13.58de | |
| B+ | 12.55 ± 0.51d | 9.17 ± 0.35b | 21.71 ± 0.69d | 460.67 ± 20.03 cd | 146.71 ± 5.81c | 607.37 ± 15.55 cd | |
| AB+ | 12.57 ± 0.12d | 9.43 ± 0.38b | 22.00 ± 0.46 cd | 485.00 ± 40.93c | 148.93 ± 3.68c | 633.93 ± 43.10c | |
| Recovery | − | 10.77 ± 0.60f | 7.86 ± 1.05de | 18.63 ± 0.89 g | 355.96 ± 15.06f | 111.33 ± 5.39d | 467.30 ± 19.68f |
| A+ | 11.40 ± 0.20e | 8.65 ± 0.40 cd | 20.05 ± 0.41ef | 421.10 ± 15.11de | 145.91 ± 4.88c | 567.01 ± 13.31de | |
| B+ | 12.55 ± 0.51d | 9.87 ± 0.12ab | 22.41 ± 0.40 cd | 462.14 ± 20.34 cd | 146.71 ± 5.81c | 608.84 ± 15.75 cd | |
| AB+ | 12.57 ± 0.12d | 9.93 ± 0.06ab | 22.50 ± 0.10 cd | 486.37 ± 40.47c | 149.69 ± 3.58c | 636.05 ± 42.73c | |
Mean ± (SD) values followed by the same letters within each column are not significantly different at 0.05 (ANOVA and Duncan’s multiple range test) n = 3
−non-inoculated, A+ inoculated with A. chroococcum, B+ inoculated with B. polymyxa, AB+ inoculated with both bacteria
Table 2.
Effect of water stress on relative water content (RWC), pigments and protein contents of cotyledons of inoculated and non-inoculated sunflower seedlings
| Treatments | Inoculation | Cotyledon | |||||
|---|---|---|---|---|---|---|---|
| RWC (%) | Pigments (μg/mgFW) | Protein (mg/g FW) | |||||
| Chl a | Chl b | Total chl | Carotenoids | ||||
| Control | − | 96.52 ± 3.61ab | 0.61 ± 0.08c | 0.16 ± 0.04c | 0.76 ± 0.07def | 0.03 ± 0.02f | 21.89 ± 1.81de |
| A+ | 97.02 ± 1.58ab | 0.77 ± 0.08ab | 0.22 ± 0.03abc | 0.99 ± 0.07b | 0.08 ± 0.03ef | 32.35 ± 2.48b | |
| B+ | 93.87 ± 2.94ab | 0.64 ± 0.06c | 0.21 ± 0.02bc | 0.85 ± 0.05 cd | 0.14 ± 0.03 cd | 23.77 ± 1.83 cd | |
| AB+ | 97.43 ± 1.46a | 0.88 ± 0.05a | 0.24 ± 0.05ab | 1.12 ± 0.04a | 0.24 ± 0.04b | 39.27 ± 4.46a | |
| Stress | − | 67.33 ± 1.53f | 0.41 ± 0.03e | 0.22 ± 0.03abc | 0.64 ± 0.03 g | 0.18 ± 0.04c | 17.19 ± 2.58 fg |
| A+ | 87.08 ± 1.87 cd | 0.57 ± 0.06 cd | 0.25 ± 0.04ab | 0.81 ± 0.04cde | 0.30 ± 0.03a | 14.73 ± 2.14gh | |
| B+ | 82.11 ± 2.00e | 0.45 ± 0.06de | 0.24 ± 0.04ab | 0.70 ± 0.05efg | 0.27 ± 0.03ab | 11.75 ± 0.87hi | |
| AB+ | 88.06 ± 2.01 cd | 0.64 ± 0.05c | 0.28 ± 0.04a | 0.92 ± 0.06bc | 0.31 ± 0.02a | 26.36 ± 3.46c | |
| Recovery | − | 84.54 ± 1.91de | 0.47 ± 0.06de | 0.17 ± 0.03c | 0.64 ± 0.05 fg | 0.12 ± 0.03de | 9.87 ± 0.65i |
| A+ | 88.65 ± 1.16c | 0.66 ± 0.06bc | 0.14 ± 0.03d | 0.80 ± 0.05cde | 0.18 ± 0.02c | 19.33 ± 1.75ef | |
| B+ | 93.33 ± 1.53b | 0.72 ± 0.46de | 0.19 ± 0.03bc | 0.91 ± 0.90bc | 0.17 ± 0.03 cd | 14.76 ± 1.26gh | |
| AB+ | 95.28 ± 2.58ab | 0.80 ± 0.16a | 0.03 ± 0.04d | 0.83 ± 0.14 cd | 0.15 ± 0.03 cd | 20.31 ± 2.25def | |
Mean ± (SD) values followed by the same letters within each column are not significantly different at 0.05 (ANOVA and Duncan’s multiple range test) n = 3
−non-inoculated, A+ inoculated with A. chroococcum, B+ inoculated with B. polymyxa, AB+ inoculated with both bacteria
Table 3.
Effect of water stress on relative water content (RWC), pigments and protein contents of leaves of inoculated and non-inoculated sunflower seedlings
| Treatments | Inoculation | Leaf | |||||
|---|---|---|---|---|---|---|---|
| RWC (%) | Pigments (μg/mgFW) | Protein (mg/g FW) | |||||
| Chl a | Chl b | Total chl | Carotenoids | ||||
| Control | − | 82.64 ± 2.25c | 2.55 ± 0.23d | 0.47 ± 0.06c | 3.03 ± 0.22d | 0.83 ± 0.02def | 47.53 ± 3.37f |
| A+ | 88.32 ± 1.85ab | 3.30 ± 0.17bc | 0.56 ± 0.03b | 3.86 ± 0.15b | 0.91 ± 0.01def | 86.54 ± 3.41b | |
| B+ | 89.13 ± 1.39a | 3.61 ± 0.28ab | 0.71 ± 0.03a | 4.32 ± 0.27a | 0.97 ± 0.03cde | 64.40 ± 1.92d | |
| AB+ | 89.43 ± 0.61a | 3.77 ± 0.29a | 0.65 ± 0.04a | 4.42 ± 0.26a | 1.24 ± 0.12bc | 104.50 ± 3.40a | |
| Stress | − | 49.26 ± 1.24f | 1.57 ± 0.25e | 0.35 ± 0.02de | 1.92 ± 0.23f | 0.84 ± 0.03def | 21.51 ± 1.71i |
| A+ | 52.42 ± 2.39f | 2.29 ± 0.19d | 0.38 ± 0.04d | 2.67 ± 0.17e | 0.77 ± 0.03ef | 41.98 ± 2.42 g | |
| B+ | 62.33 ± 2.45e | 2.50 ± 0.13d | 0.51 ± 0.03bc | 3.01 ± 0.10d | 1.01 ± 0.11cde | 57.21 ± 2.06e | |
| AB+ | 70.80 ± 2.07d | 3.18 ± 0.11c | 0.34 ± 0.03def | 3.52 ± 0.11c | 1.60 ± 0.43a | 74.50 ± 3.22c | |
| Recovery | − | 74.28 ± 2.46d | 1.14 ± 0.11f | 0.14 ± 0.04 g | 1.28 ± 0.09 g | 0.44 ± 0.03 g | 33.62 ± 2.59 h |
| A+ | 82.74 ± 3.83c | 1.88 ± 0.15e | 0.31 ± 0.02ef | 1.93 ± 0.22f | 1.10 ± 0.09 cd | 44.13 ± 2.00 fg | |
| B+ | 83.65 ± 2.91c | 1.58 ± 0.14e | 0.35 ± 0.03de | 1.92 ± 0.12f | 0.63 ± 0.04 fg | 45.93 ± 3.59 fg | |
| AB+ | 84.83 ± 2.68c | 1.64 ± 0.24e | 0.28 ± 0.03f | 2.19 ± 0.13f | 1.39 ± 0.31ab | 55.55 ± 3.97e | |
Mean ± (SD) values followed by the same letters within each column are not significantly different at 0.05 (ANOVA and Duncan’s multiple range test) n = 3
−non-inoculated, A+, inoculated with A. chroococcum, B+ inoculated with B. polymyxa, AB+ inoculated with both bacteria
Pigments and Protein
The plants inoculated with the bacteria, viz. A+, B+ and AB+ showed marked increase in chlorophyll and carotenoid contents. Lesser amount of chlorophyll and carotenoids was observed in senescing cotyledons as compared with their respective first fully expanded leaf irrespective of water regime. Under control condition maximum amount of pigment was observed in AB+ seedlings, while minimum was observed in non-inoculated ones. Water stress caused a decrease of pigments in all treatments as compared with their respective control. The cotyledons and leaves of inoculated seedlings under stress exhibited higher amount of total chlorophyll as compared with non- inoculated stressed seedlings with maximum in AB+ ones. Maximum amount of carotenoids was recorded in AB+ seedlings among the stressed plants. In comparison to the non-inoculated seedlings higher amount of total chlorophyll and carotenoids was maintained on re-watering of stressed inoculated ones. Leaves and senescing cotyledons exhibited almost similar pattern under stress and recovery treatments. Water deficit decreased the protein content in both. In control AB+ plants exhibited maximum respond index 79.39 % in cotyledons and 119.86 % in leaves. The leaves of inoculated seedlings maintained higher amount of protein content with maximum in AB+ ones as compared with that of non- inoculated one in their respective treatments. Cotyledons of AB+ seedlings exhibited higher amount of protein in all the treatments (Tables 1 and 2).
Enzymes
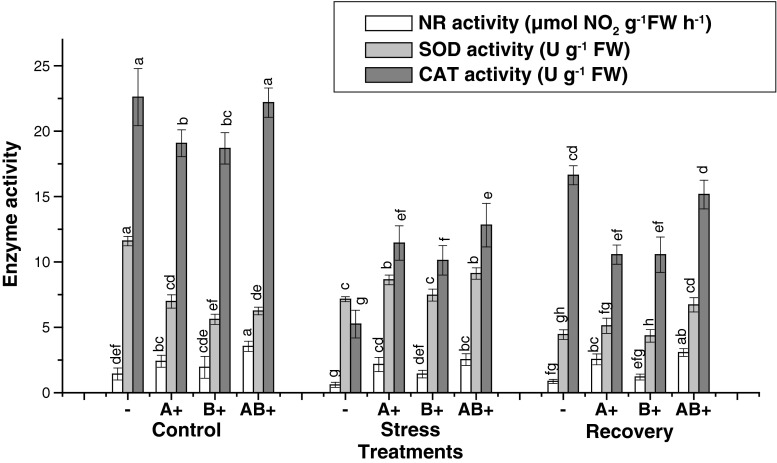
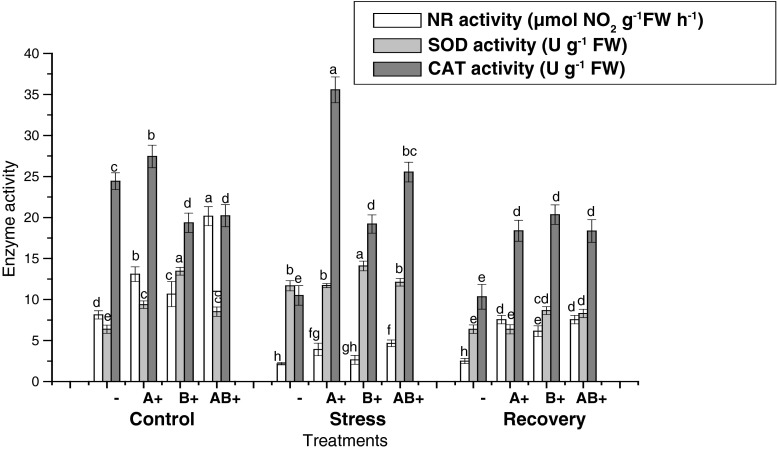
Nitrate reductase activity (NRA) in cotyledon and first fully expanded leaf was studied in plants with and without bacteria with different water regimes. Irrigated inoculated seedlings exhibited increase in NRA with maximum in AB+. Inoculated stressed seedlings exhibited higher NR activity as compared with stressed non inoculated seedlings. Similar trend was followed in recovery treatments. Though the water deficit reduced the NRA, but the decrease was not significantly different in leaves of A+ and B+. Lowest NRA was recorded in seedlings without bacteria and the same was maintained in stress and recovery treatments. Highest NRA was found in AB+ plants in all the treatments. Leaf NRA was always greater than cotyledon NRA in respective treatments.
Water stress enhanced the SOD activity except in cotyledons of non-inoculated seedlings. Under stress treatment leaves of non-inoculated plants showed 1.8 fold increase in SOD activity, which was greater than that of inoculated ones, i.e., 1.2, 1.3 and 1.04 folds in A+, AB+ and B+ respectively. A significant decrease in SOD activity was observed in plants relieved from stress. Senescing cotyledons exhibited successive decrease in SOD activity under stress and recovery treatments in non-inoculated seedlings.
Maximum activity of CAT was recorded in leaves of A+ plants followed by cotyledons of non-inoculated ones under control. In stress condition CAT activity decreased in non inoculated seedlings but increased in the leaves of A+ and AB+ plants. However no significant difference was recorded in B+ seedlings. Inoculated plants always maintained higher CAT activity as compared with the non-inoculated stressed plants (Figs. 1 and 2).
Fig. 1.
Effect of water stress on enzyme activities in the cotyledons of seedlings of sunflower. Mean ± (SD) values followed by the same letters within each column are not significantly different at 0.05 (ANOVA and Duncan’s multiple range test) n = 3
Fig. 2.
Effect of water stress on enzyme activities in the leaves of seedlings of sunflower. Mean ± (SD) values followed by the same letters within each column are not significantly different at 0.05 (ANOVA and Duncan’s multiple range test) n = 3
Discussion
Reduction of plant growth is one of the most common effects of water stress (Anjum et al. 2008 and Marulanda et al. 2009). The inoculation of plants with PGPR helped mitigating the effect of water stress. Timmusk et al. (1999b) also reported that inoculation with B. polymyxa enhanced drought tolerance. They suggested that PGPR helped in formation of a biofilm around the roots which enhanced the drought tolerance. According to Kohler et al. (2008) inoculation helped maintaining higher level of proline, which served as osmolyte. In our opinion proline might have helped maintaining RWC. Maintenance of RWC has been considered to be a drought resistance mechanism (Grashoff and Ververke 1991). Inoculation helped in maintaining RWC in stressed seedlings at the level of control. The bacteria enhanced the water use efficiency of plants by increasing water absorptive surface of the seedlings (Zhang and Zhang 2001). There are reports that root and shoot growth stimulatory activity of bacteria may be attributed to the production of growth regulators and/ or better availability of minerals or due to combination of their diverse activities (Glick et al. 1999). According to Smirnoff (1993) decrease in chlorophyll is an index of water stress. The decrease in chlorophyll contents might be due to inhibition of biosynthesis or degradation of chlorophyll contents in the plants (Iturbe-Oxmaetxe et al. 1998 and Rahman et al. 2002, 2004). Increase in carotenoids, accessory pigments which absorb photon and transfer excitation energy to reaction centers via chlorophyll, protect chlorophyll from photo-oxidation. Carotenoids also serve as antioxidants against free radicals and photochemical damage (Mishra et al. 2006). PGPR helped accumulation of pigments in stressed seedlings. Increased seedling growth and accumulation of dry matter is due to maintenance of photosynthetic efficiency due to PGPR. Photosynthetic efficiency of inoculated seedlings was improved due to increase in pigment contents in the leaves of inoculated stressed seedlings as compared with non inoculated stressed ones. Similar results of pigment accumulation were obtained by Arkhipova et al. (2005) in lettuce plants inoculated with Bacillus subtilis.
In germinating fatty seeds lipids are converted to sugars via ß-oxidation and central metabolic pool. In the seeds of sunflower, when stored reserve food (lipid) was exhausted during germination and seedling growth, the cotyledons became photosynthetic. Higher photosynthetic efficiency maintained by the cotyledons helped in the establishment and growth of seedlings at early stages (Ampofo et al. 1976). After the first pair of leaves was fully developed, the photosynthetic efficiency of cotyledons declined and they began to senesce. The PGPR delayed the senescing of cotyledons which increased the photosynthetic area of developing seedlings. This delay in senescence of cotyledons might be mediated by cytokinins (Wingler et al. 1998). Several workers have reported the production of cytokinins by PGPR (Salamone et al. 2001; Timmusk et al. 1999a and Gomes et al. 2001). The decrease in protein in plants under water stress might be due to inhibition of biosynthesis of protein (Rahman et al. 2002, 2004) and/or degradation of proteins (Møller et al. 2007) or due to oxidation of protein caused by reactive oxygen species (ROS) (Iturbe-Ormaetxe et al. 1998). The plants inoculated with bacteria maintained higher amount of protein in stress as well as in recovery treatments as compared with the non-bacterial stressed plants. Improved water status of inoculated seedlings helped in maintaining metabolic activities.
NRA was sensitive to water stress. NRA declined on induction of water stress. However, it regained as stress was released (Hsiao 1973). Several complementary factors and reactions are responsible for decline of NRA under water stress. According to Huber et al. (1996) the reduction of photosynthesis under water deficit was correlated with the reduction of NRA. The effect of water stress on NRA could be minimized in the presence of high nutrient supply. Smirnoff et al. (1985) reported that NRA was highest in the plants growing with ample nitrogen supply irrespective of water regime. However, our results clearly revealed sensitivity of NRA to WS. High nutrient and water availability due to PGPR might be the reason of higher NRA in inoculated seedlings as NR is a substrate inducible enzyme (Crawford 1995). Direct nitrogen supply enhanced the NRA which accumulated in plants in the presence of N-fixative agent, A. chroococcum. The increased supply of NO3− in inoculated plants helped in the acclimation of stressed plants by producing osmolytes like soluble sugars and amino acids which were synthesized by using photochemical energy (Smirnoff et al. 1985).
According to Radin (1984) leaves of P- deficient plants accumulated more ABA in stressed plants, which resulted in stomatal closure. Increased supply of phosphate due to phosphate solubilizing activity of PGPR have ameliorated the effects of water stress by influencing the increased stomatal conductance (Radin 1984) which enhanced the photosynthesis efficiency. High amount of photosynthates, i.e., sucrose and phosphorus level contributed in osmotic adjustment in stressed plants as reported by Ackerson (1981).
In order to limit oxidative damage under stress condition, plants have developed a series of detoxifying systems which caused the breakdown of highly toxic ROS. SOD activity increased in plants under water stress prevented the loss of cellular homeostasis as well as oxidative damage of membrane, lipids, protein and nucleic acids (Srivalli et al. 2003) which were caused by production of ROS. SOD converts one form of ROS (singlet oxygen) to another equally toxic H2O2, which is further detoxified by the coordinated action of H2O2 scavenging enzymes, viz. catalase (CAT), peroxidase (POX), glutathione reductase (GR) (Foyer et al. 1997). However in present study, CAT activity decreased in stress and recovery treatments in cotyledons and leaves except in the leaves of A+ and AB+ plants where increase was recorded. The CAT activity was always higher in stressed plants with inoculants as compared with that of non-inoculated stressed plants. Thus, it was evident that bacteria ameliorated the effect of water stress and enhanced the adaptive capability of inoculated stressed plants. According to Ünyayar et al. (2005) CAT activity was increased in tolerant Lycopersicon peruvianum and decreased in sensitive L. esculentum under drought stress. In oily seeds of H. annuus L. CAT played an important role in early growth and establishment of seedlings by detoxifying H2O2 which was produced in water deficit (Bailly et al. 2001; Jiang and Zhang 2002).
The present investigation showed that water stress severely affected the metabolism of plant and inhibited the growth of sunflower seedlings. The PGPR delayed the senescing of cotyledons resulting in increased photosynthetic area which maintained the health of seedlings and helped the plants to mitigate the effects of water stress since early developmental stages. Recovery on watering was more pronounced in inoculated plants. PGPR helped in maintaining higher RWC, strong anti-oxidative defence system and activity of nitrate reductase in stressed seedlings, which resulted in more accumulation of dry matter and increased seedling growth.
References
- Ackerson RC. Osmoregulation in cotton in response to water stress. II Leaf carbohydrate status in relation osmotic adjustment. Plant Physiol. 1981;67:489–493. doi: 10.1104/pp.67.3.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ampofo ST, Moore KG, Lovell PH. The influence of leaf on cotyledon photosynthesis and export during seedling development in Acer. New Phytol. 1976;76:247–255. doi: 10.1111/j.1469-8137.1976.tb01458.x. [DOI] [Google Scholar]
- Anjum NA, Umar S, Iqbal M, Khan NA. Growth characteristics and antioxidant metabolism of mung bean genotypes differeing in photosynthetic capacity subjected to water deficit stress. J Plant Interact. 2008;3(2):127–136. doi: 10.1080/17429140701810732. [DOI] [Google Scholar]
- Arkhipova TN, Veselov SU, Melentieu AI, Martynenko EV, Kudoyarova GR. Ability of bacterium Bacillus subtilis to produce cytokinin and to influence the growth and endogenous hormone content of lettuce plants. Plant Soil. 2005;272:201–209. doi: 10.1007/s11104-004-5047-x. [DOI] [Google Scholar]
- Arshad M, Saleem M, Hussain S. Perspectives of bacterial ACC diaminase in phytoremediation. Trends Biotechnol. 2007;25(8):356–362. doi: 10.1016/j.tibtech.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Bailly C, Audigier C, Ladonne F, Wagner MH, Coste F, Corbineau F, Côme D. Changes in oligosaccharide content and antioxidant enzyme activities in developing bean seeds as related to acquisition of drying tolerance and seed quality. J Exp Bot. 2001;357:701–708. doi: 10.1093/jexbot/52.357.701. [DOI] [PubMed] [Google Scholar]
- Barka EA, Nowak J, Clément C. Enhancement of chilling resistance of inoculated grapevine plantlets with a plant growth promoting rhizobactrium, Burkholderia phytofirmans Strain PsJN. Appl Environ Microbiol. 2006;72:7246–7252. doi: 10.1128/AEM.01047-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bars HD, Weatherley PE. A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust J Biol Sci. 1962;15:413–428. [Google Scholar]
- Beyer WF, Fridovich I. Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Anal Biochem. 1987;161:559–566. doi: 10.1016/0003-2697(87)90489-1. [DOI] [PubMed] [Google Scholar]
- Biswal B. Chloroplasts, pigments and molecular responses of photosynthesis. New York- Basel-Hong Kong: Marcel Dekker; 1997. pp. 877–885. [Google Scholar]
- Biswal B. Chloroplast metabolism during leaf greening and degreening. In: Pessarakli M, editor. Handbook of photosynthesis. New York- Basel-Hong Kong: Marcel Dekker; 1997. pp. 72–81. [Google Scholar]
- Crawford NM. Nitrate: nutrient and signal for plant growth. Plant Cell. 1995;7:859–868. doi: 10.1105/tpc.7.7.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer CH, Lopez-Delgado H, Dat JF, Scott IM. Hydrogen peroxide and glutathione- associated mechanisms of acclamatory stress tolerance and signaling. Physiol Plant. 1997;100:241–254. doi: 10.1111/j.1399-3054.1997.tb04780.x. [DOI] [Google Scholar]
- Glick BR, Patten CL, Holguin G, Penrose DM. Biochemical and genetic mechanisms used by plant growth promoting bacteria. London: Imperial College Press; 1999. [Google Scholar]
- Gomes NCM, Heuer H, Schonfeld J, Costa R, Mendonca- Hafler L, Smalla K. Bacterial diversity of the rhizosphere of maize (Zea mays) grown in tropical soil studied by temperature gradient gel electrophoresis. Plant Soil. 2001;232:167–180. doi: 10.1023/A:1010350406708. [DOI] [Google Scholar]
- Grashoff C, Ververke DR. Effect of pattern of water supply on Vicia faba L.3. Plant water relations, expansive growth and stomatal reactions. Neth J Agric Sci. 1991;39:247–262. [Google Scholar]
- Grichko VP, Glick BR. Amelioration of flooding stress by ACC containing plant growth promoting bacteria. Plant Physiol Biochem. 2001;39:11–17. doi: 10.1016/S0981-9428(00)01212-2. [DOI] [Google Scholar]
- Hsiao TC. Plant responses to water stress. Annu Rev Plant Physiol. 1973;24:519–570. doi: 10.1146/annurev.pp.24.060173.002511. [DOI] [Google Scholar]
- Huber SC, McMichael RW, Bachmann M, Huber JL, Shannon JC, Kang KK, Paul M. Regulation of leaf sucrose-phosphate synthase and nitrate reductase by reversible protein phosphorylation. In: Shewry PR, Halford NG, Hooley R, editors. Protein phosphorylation in plants. Oxford: Proceedings of the Phytochemical Society of Europe. Oxford Science Publications, Clarendon Press; 1996. pp. 19–34. [Google Scholar]
- Ishida H, Nishimori Y, Sugisawa M, Makino A, Mae T. The large subunit of ribulose—1,5 bisphosphate carboxylase / oxygenase is fragmented into 37 kDa and 16 kDa polypeptides by active oxygen in the lysates of chloroplasts from primary leaves of wheat. Plant Cell Physiol. 1997;38:471–479. doi: 10.1093/oxfordjournals.pcp.a029191. [DOI] [PubMed] [Google Scholar]
- Iturbe-Oxmaetxe I, Escuredo PR, Arrese-Igor C, Becana M. Oxidative damage in pea plants exposed to water deficit or paraquat. Plant Physiol. 1998;116:173–181. doi: 10.1104/pp.116.1.173. [DOI] [Google Scholar]
- Jaworski E. Nitrate reductase assay in intact plant tissue. Biochem Biophys Res Commun. 1971;43:1274–1279. doi: 10.1016/S0006-291X(71)80010-4. [DOI] [PubMed] [Google Scholar]
- Jiang M, Zhang J. Water stress induced abscisic acid accumulation triggers the increased generation of reactive oxygen species and up-regulates the activity of antioxidant enzymes in maize leaves. J Exp Bot. 2002;53:2401–2410. doi: 10.1093/jxb/erf090. [DOI] [PubMed] [Google Scholar]
- Kohler J, Hernández JA, Carabaca F, Roldán A. Plant growth promoting rhizobacteria and arbuscular mycorrhizal fungi modify alleviation biochemical mechanism in water stressed plants. Funct Plant Biol. 2008;35:141–151. doi: 10.1071/FP07218. [DOI] [PubMed] [Google Scholar]
- Lichtenthaler HK. Chlorophyll and carotenoids: Pigments of photosynthetic bio-membranes. In: Packer L, Douce R, editors. Methods in enzymology. Sandiego: Academic; 1987. pp. 350–382. [Google Scholar]
- Lowry OH, Rosenbrough RJ, Farr AL, Randall RJ. Protein measurement with Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Mahfouz SA, Sharaf-Eldin MA. Effect of mineral vs. biofertilizer on growth, yield and essential oil content of fennel (Foeniculum vulgare Mill.) Int Agrophys. 2007;21:361–366. [Google Scholar]
- Marulanda A, Barea JM, Azcón R. Stimulation of plant growth and drought tolerance by native microorganisms (AM fungi and Bacteria) from dry environments: mechanisms related to bacterium effectiveness. J Plant Grow Reg. 2009;28:115–124. doi: 10.1007/s00344-009-9079-6. [DOI] [Google Scholar]
- Mishra S, Srivastava S, Tripathi RD, Govindrajan R, Kuriakose SV, Prasad MNV. Phytochelatin synthesis and response of antioxidants during cadmiunm stress in Bacopa monnieri L. Plant Physiol Biochem. 2006;44:25–37. doi: 10.1016/j.plaphy.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Mǿller IM, Jensen PH, Hansson A. Oxidative modifications to cellular components in plants. Annu Rev Plant Physiol. 2007;58:459–481. doi: 10.1146/annurev.arplant.58.032806.103946. [DOI] [PubMed] [Google Scholar]
- Radin JW. Stomatal response to water stress and to abscisic acid in phosphorus- deficient cotton plants. Plant Physiol. 1984;76:392–394. doi: 10.1104/pp.76.2.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman SML, Mackay WA, Nawata E, Sakuratani T, Uddin ASMM, Quebedauex B. Superoxide dismutase and stress tolerance of four tomato cultivars. Hortsci. 2004;39:983–986. [Google Scholar]
- Rahman SML, Mackay WA, Quebedauex B, Nawata E, Sakuratani T. Superoxide dismutase, leaf water potential, relative water content, growth and yield of a drought-sensitive tomato cultivars. Subtrop Plant Sci. 2002;54:16–22. [Google Scholar]
- Rubio MC, Gonzalez EM, Minchin FR, Webb KJ, Arrese-Igor C, Ramos J, Becana M. Effect of water stress on antioxidant enzymes of leaves and nodules of transgenic alfalfa over expressing superoxide dismutases. Physiol Plant. 2002;115:531–540. doi: 10.1034/j.1399-3054.2002.1150407.x. [DOI] [PubMed] [Google Scholar]
- Salamone GIE, Hynes RK, Nelson LM. Cytokinin production by plant growth promoting rhizobacteria and selected mutants. Can J Microbiol. 2001;47:404–411. doi: 10.1139/w01-029. [DOI] [PubMed] [Google Scholar]
- Saravanakumar D, Samiyappan R. ACC deaminase from Pseudomonas fluorescens mediated saline resistance in groundnut (Arachis hypogea) plants. J Appl Microbiol. 2007;102:1283–1292. doi: 10.1111/j.1365-2672.2006.03179.x. [DOI] [PubMed] [Google Scholar]
- Sinha AK. Colorimetric assay of catalase. Anal Biochem. 1972;47:389–394. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- Smirnoff N. The role of active oxygen in the response of plants to water deficit and desiccation. New Phytol. 1993;125:27–58. doi: 10.1111/j.1469-8137.1993.tb03863.x. [DOI] [PubMed] [Google Scholar]
- Smirnoff N, Winslow MD, Stewart GR. Nitrate reductase activity in leaves of barley (Hordeum vulgare) and durum wheat (Triticum durum) during field and rapidly applied water deficits. J Exp Bot. 1985;36:1200–1208. doi: 10.1093/jxb/36.8.1200. [DOI] [Google Scholar]
- Srivalli B, Vishanathan C, Renu KC. Antioxidant defense in response to abiotic stresses in plants. J Plant Biol. 2003;30:121–139. [Google Scholar]
- Timmusk S, Nicander B, Granhall U, Tillberg E. Cytokinin production by Paenibacillus polymyxa. Soil Biol Biochem. 1999;31:1847–1852. doi: 10.1016/S0038-0717(99)00113-3. [DOI] [Google Scholar]
- Ünyayar S, Keleş Y, Çekiç FÖ. The antioxidative response of two tomato species with different drought tolerances as a result of drought and cadmium stress combinations. Plant Soil Environ. 2005;51(2):57–64. [Google Scholar]
- Wingler A, von Schaewen A, Leegood RC, Lea PJ, Quick WP. Regulation of leaf senescence by cytokinin, sugars and light: effects on NADH-dependent hydroxypyruate reductase. Plant Physiol. 1998;116:329–335. doi: 10.1104/pp.116.1.329. [DOI] [Google Scholar]
- Zhang GS, Zhang RZ. The influence of nitrogen, phosphorus nutrition on the root characteristic matters of spring wheat under water stress. J Gansu Agric Univ. 2001;36:163–167. [Google Scholar]