Abstract
Two non-polar fractions viz. hexane (Hex-LI) and chloroform fraction (CHCl3-LI) of Lawsonia inermis were studied for their antiproliferative potential in various cancer cell lines viz. HeLa, MCF-7, A549 and C6 glioma cells. Both the fractions showed more than 60 % of growth inhibition in all the tested cell lines at highest tested concentration. In clonogenic assay, different concentrations of Hex-LI and CHCl3-LI decreased the number and size of colonies as compared to control in HeLa cells. The apoptotic effects as nuclear condensation, fragmentation were visualized with Hoechst-33342 staining of HeLa cells using confocal microscope. Both fractions induced apoptotic cell death in human cervical carcinoma (HeLa) cells as evident from flow cytometric analysis carried out using Annexin V-FITC and propidium iodide dyes. CHCl3-LI treated cells significantly induced apoptosis (25.43 %) in comparison to control. Results from Neutral Comet assay demonstrated that both fractions induced double stranded breaks (DSB’s) in HeLa cells. Our data indicated that Hex-LI and CHCl3-LI treated cells showed significant increase of 32.2 and 18.56 % reactive oxygen species (ROS) levels in DCFH-DA assay respectively. Further, experimental studies to decipher exact pathway via which these fractions induce cell death are in progress.
Keywords: Lawsonia inermis, HeLa cells, Cytotoxicity, Apoptosis, ROS
Introduction
Cancer is characterized by several features such as uncontrolled cell division and growth, cell invasion and finally leading to metastasis. Cancer is major cause of mortality among human population globally after cardiovascular diseases (Kutluk and Kars 1998). Apoptosis is a kind of cell death characterized by various morphological alterations such as chromatin condensation, nuclear fragmentation, formation of apoptotic bodies etc. (Kerr et al. 1972). During apoptosis, caspases get activated and induce several molecular cascades which results in cell death (Allen et al. 1997; Hu and Kavanagh 2003; Wang et al. 2005; Elmore 2007; Millan and Huerta 2009). Apoptosis is the main mechanism which maintains tissue homeostasis. Alterations in the regulation of apoptosis may result in many pathological conditions and/or diseases such as cancer, autoimmune disorders, neurodegenerative diseases etc. (Kroemer and Reed 2000; Wang et al. 2012, 2013; Ramasamy et al. 2013). Once the cell looses control over apoptotic mechanism, cell evades death and divides continuously leading to neoplastic transformation (Igney and Krammer 2002; Hu and Kavanagh 2003; Reed 2003; Qi et al. 2010). Since ages, natural products are utilized in the traditional health care medicinal practices from centuries (Akerele 1988; Gurib-Fakim 2006). Plants are only sources which account for providing medicine or medicinal formulations for treating 80 % world population (Owoabi et al. 2007). Plants are considered as safe, better substitutes for various synthetic drugs and pose no side effects (Dai and Mumper 2010; Vasanthi et al. 2014). In last few years, studies on medicinal plants have shown that crude extracts as well as pure phytochemicals possess potential to induce apoptosis (Rao et al. 2009; Wu et al. 2009; Lee et al. 2009; Xu et al. 2009). Targeting cancer cells via inducing apoptosis is one of the strategies for the development of anticancer drugs (Kroemer et al. 1995; Wyllie et al. 1980; Kerr et al. 1972; Kelloff et al. 2000).
Lawsonia is a monotypic genus represented by only one species Lawsonia inermis Linn (Lythraceae). This plant is a native of North Africa and South-West Asia. Lawsonia inermis Linn. is a traditional medicinal and multipurpose plant commonly known as Henna in India and commonly used for dyeing of hands and hairs. Leaves of the plant are used in traditional medicine system as expectorant, anti-inflammatory, liver tonic, haematinic, styptic, febrifuge, diuretic etc. (Reddy 1988; Ahmed et al. 2000; Bich et al. 2004; Warrier 2004; Jiny Varghese et al. 2010; Dhiman et al. 2012). Leaves of the plant were reported as modulators of antioxidant and xenobiotic metabolizing enzymes viz. phase I and II enzymes (Dasgupta et al. 2003). Extracts as well as various pure constituents from this plant have been reported with antioxidant (Hsouna et al. 2011; Guha et al. 2011; Kumar et al. 2014), antimutagenic, antigenotoxic, anticlastogenic (Raja et al. 2008; Basirian et al. 2013; Kumar et al. 2014) and anitumor activity (Dasgupta et al. 2003; Priya et al. 2011). From the last 25 years, our laboratory have been studying medicinal plants for their bioactive potential. In the present investigation, two fractions from L. inermis were studied for anti-proliferative and apoptosis inducing activities in cervical carcinoma HeLa cells.
Materials and methods
Chemicals
Annexin V-FITC apoptosis detection kit, 2′, 7′-dichlorofluorescin diacetate and Hoechst-33342 were purchased from Sigma Chemical Co. (St Louis, MO, USA). 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) and trypan blue dye were purchased from Hi-Media, Mumbai, India. All other chemicals used in the present studies were of AR grade.
Collection of plant material
The leaves of the Lawsonia inermis L. were obtained from local market (Majeeth Mandi) at Amritsar, Punjab, India and plant has been identified with voucher specimen (No. 6773) and has been kept in the Herbarium of the Department of Botanical and Environmental Sciences, Guru Nanak Dev University, Amritsar, Punjab.
Preparation of extracts
Leaves were crushed to fine powder and dipped into 80 % methanol for 2 days at room temperature with constant shaking so that phytoconstituents get dissolved into solvent. The miscella (solvent containing phytoconstituents) so obtained was concentrated using rotary vacuum evaporator and finally lyophilized. The mother extract (MeOH-LI) i.e., 120 g was dissolved in doubled distilled water and fractionated with hexane and chloroform in series. The respective fractions so obtained were named as Hex-LI and CHCl3-LI fractions.
Antiproliferative studies
Procurement and mantainence of cancer cell lines
HeLa (Human cervical cancer), MCF-7 (human breast adenocarcinoma), A549 (Human alveolar adenocarcinoma) and C6 glioma (Rat glioblastoma) cell lines were purchased from the National Centre for Cell Science (NCCS, Pune, India). Different cell lines were cultured in DMEM medium containing 10 % FBS and antibiotic-antimycotic solution. Cells were grown at 37 °C and 5 % CO2 using CO2 incubator.
Measurement of cell viability
Before carrying out all the experiments, cell viability was checked using method of Militao et al. (2006) with slight modifications. Cells were washed with PBS (pH 7.4), trypsinised and then finally centrifuge at 2000 rpm. Cell pellet was suspended in media and stained with trypan blue dye (0.4 % in PBS) to determine number of viable cells (not stained). After determining viability, cells were used to carry out different experiments.
Cytotoxicity
MTT assay was carried out to assess cytotoxic potential of both the fractions using method of Mickisch et al. (1990) with slight modifications. HeLa cells were seeded at density of 8000 cells per well of the 96 well plate. After time period of 24 h, cells were treated with extract concentrations for next 24 h. On the completion of treatment time, MTT was added and incubated for 2 h. Finally media was removed from the wells and DMSO (100 μl) was added per well to dissolve the purple coloured formazan crystals (MTT metabolic product) and finally reading was taken at 570 nm.
Colonogenic assay
Colonogenic potential of both the fractions was assessed using protocol of Gundala et al. (2013) with slight modifications. The HeLa cells at a density of 4000 cells/well were seeded into 6‑well plates containing 3 ml complete growth medium. Cells were treated with different concentrations of test samples for 24 h and incubated in CO2 incubator for 8 days. Finally, the media was removed and colonies were stained with crystal violet stain. The numbers of colonies were counted for control as well as for each concentration.
Nuclear morphology assessment using confocal microscopy
The morphology of nucleus was examined using Hoechst 33342 dye. Nuclear condensation, fragmentation etc. are the distinct attributes of apoptosis (Lizard et al. 1995; Zhang and Xu 2002). HeLa cells at a density of 2 × 105 cells/well were cultured into 6-well plates. After the treatment of different extract/fractions to HeLa cells (24 h), cells were observed under inverted microscope. Finally stained with Hoechst 33342 for 10 min at 37 °C and scanned under a Nikon A1R Laser Scanning Confocal Microscope system (Nikon Corporation, Japan) using NIS Elements AR analysis software version 4.11.00 for observing nuclear morphology.
Determination of apoptosis using flow cytometry
Apoptosis inducing potential of Hex-LI and CHCl3-LI fractions was evaluated by using Annexin V-FITC Apoptosis Detection Kit (Sigma) as described in Haneef et al. (2012). About 1 × 106 HeLa cells were seeded and allowed to attach for 24 h. After 24 h, cells were treated with Hex-LI (250 μg/ml) and CHCl3-LI (450 μg/ml). After treating the cells for 24 h, all adhering as well as floating cells were collected in tubes and centrifuged to obtain cell pellet. Cell were washed twice with PBS and centrifuged again to obtain cell pellet. After that cell pellet was suspended in binding buffer followed by addition of 5 μl of Annexin V-FITC conjugate and 10 μl of Propidium iodide to 500 μl of cell suspension. After incubation of 10 min, cells were analyzed by flow cytometry (BD Accuri C6 Flow Cytometer, BD Biosciences) using BD Accuri software. [Viable cells (annexin V and PI negative), early apoptotic cells (annexin V positive and PI negative), necrotic cells (annexin V negative, PI-positive) and late apoptotic cells (annexin V positive and PI positive)].
Single cell gel electrophoresis assay (neutral comet assay)
Neutral comet assay was carried out to determine double strand breaks in DNA using the method of Wojewodzka et al. (2002) and Matkar et al. (2006) with slight modifications. HeLa cells were seeded at the density of 4 × 105 cells in 6 well plates. After the complete adherence of cells, cells were treated with Hex-LI (250 μg/ml) and CHCl3-LI (450 μg/ml) for 24 h. After the treatment, cells were washed with PBS, trypsinised, centrifuged to get pellet and finally suspended in 500 μl of PBS. Frosted slides were coated with normal melting point agarose (NMPA). After solidification of agarose, next layer of low melting point agarose (LMPA) containing treated and untreated HeLa cells was laid and allowed for solidifying followed by the third layer of LMPA and covered with coverslip. After agarose layering, coverslip was removed and slides were lowered down in lysing jars containing lysis buffer (2.5 M NaCl, 100 mM EDTA, 10 mM Tris–HCl, pH 8.3, 10 % DMSO, 1 % Triton X-100) for 45 min. After that slides were removed from lysing jar and placed in electrophoretic buffer (300 mM sodium acetate, 100 mM Tris–HCl, pH 8.3) followed by electrophoresis for 45 min (20 V). The slides were stained with 20 μg/ml ethidium bromide and placed in moist chamber before scoring. To measure the DNA damage induced in HeLa cells on treatment with fractions, 50 comets from each slide were examined under an Epifluorescent Nikon microscope. Analysis of results was done with the visual scoring method; a total of 50 comets per slide were categorized into one to five categories according to level of DNA damage in the comets. Each comet was given grade between 0 (undamaged) and 4 (completely damaged) (Collins et al. 1997).
Reactive oxygen species measurement
ROS measurement was carried out by method of Deeb et al. (2010) with slight modifications. HeLa cells were seeded in 6-well plates and allowed to adhere completely. The cells were treated with test fractions Hex-LI (250 μg/ml) and CHCl3-LI (450 μg/ml) for 24 h. At the end of treatment period, cells were incubated in 2′, 7′-dichlorofluorescin diacetate (DCFH-DA) dye for 30 min at 37 °C. Cells were then washed twice with PBS and finally analyzed by BD Accuri C6 Flow Cytometer (BD Biosciences) (ExCitation 488 nm; Emission 530 nm) for 2,7-dichlorofluorescein (DCF) positive cells.
Statistical analysis
The results were expressed as the average and standard error. GI50 values were calculated using regression equation. The data were also analyzed for statistical significance using Student’s t test and analysis of variance (One-way ANOVA) and the difference among means was compared by high-range statistical domain (HSD) using Tukey’s test. The significance of results was checked at *p ≤ 0.05.
Results
Measurement of cell viability
The cells showing viability above 90–95 % were used to carry out experiments. Viable cells are colourless while dead cells are stained blue in color after treatment with trypan blue dye.
Cytotoxicity among different cancer cell lines
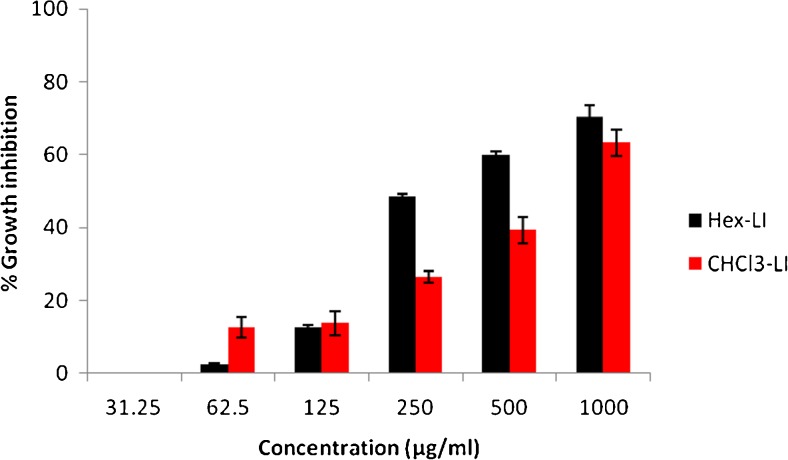
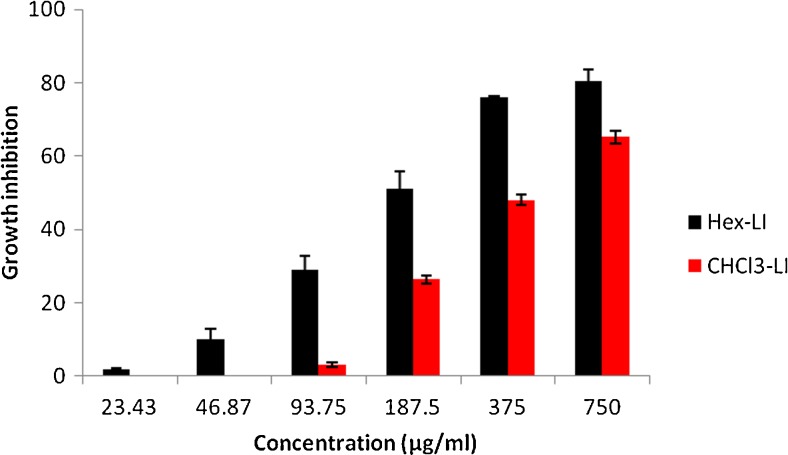
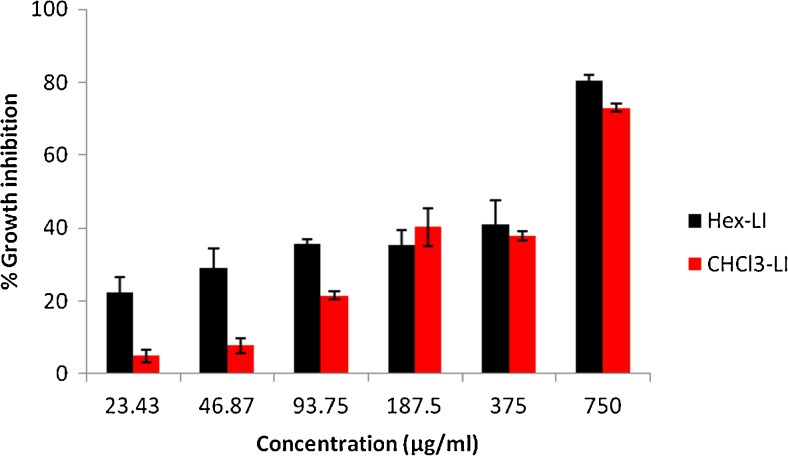
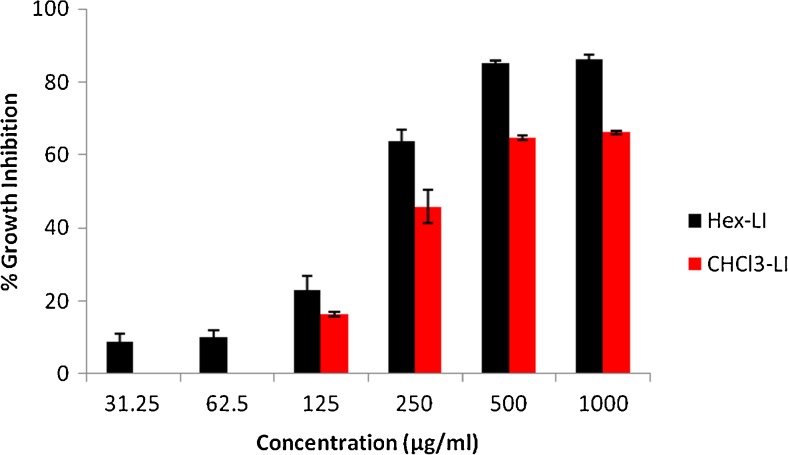
The antiproliferative effect of both fractions viz. Hex-LI and CHCl3-LI was studied against Hela, MCF-7, A549 and C6 glioma cell lines. The antiproliferative potency of fractions was seen in dose dependent manner (Figs. 1, 2, 3 and 4). Hex-LI showed GI50 (concentration at which 50 % inhibition of growth has been observed) of 382.44, 186.47, 378.73, 204.27 μg/ml in HeLa, MCF-7, A549, C6 cancer cell lines respectively whereas CHCL3-LI showed GI50 of 741.44, 524.46, 469.80 and 387.14 μg/ml in HeLa, MCF-7, A549, C6 cancer cell lines rerspectively.
Fig. 1.
Growth inhibitory effects on HeLa cells treated with various concentrations of Hex-LI and CHCl3-LI. [Hex-LI (Regression equation: y = 23.03ln (x)-86.95; *r = 0.9664) and CHCl3-LI (Regression equation: y = 0.059x + 6.255; *r = 0.9767)] *p ≤ 0.05
Fig. 2.
Growth inhibitory effects on MCF-7 cells treated with various concentrations of Hex-LI and CHCl3-LI. [Hex-LI (Regression equation: y = 25.23ln(x)-81.91; *r = 0.9859) and CHCl3-LI (Regression equation: y = 0.095x + 0.176; *r = 0.9576)] *p ≤ 0.05
Fig. 3.
Growth inhibitory effects on A549 cells treated with various concentrations of Hex-LI and CHCl3-LI. [Hex-LI (Regression equation: y = 0.071x + 23.11; *r = 0.9648) and CHCl3-LI (Regression equation: y = 0.086x + 9.597; *r = 0.9481)] *p ≤ 0.05
Fig. 4.
Growth inhibitory effects on C6 glioma cells treated with various concentrations of Hex-LI and CHCl3-LI. [Hex-LI (Regression equation: y = 26.92ln(x)-93.20; *r = 0.9555) and CHCl3-LI (Regression equation: y = 22.82ln(x)-85.98; *r = 0.9643)] *p ≤ 0.05
Colonogenic assay
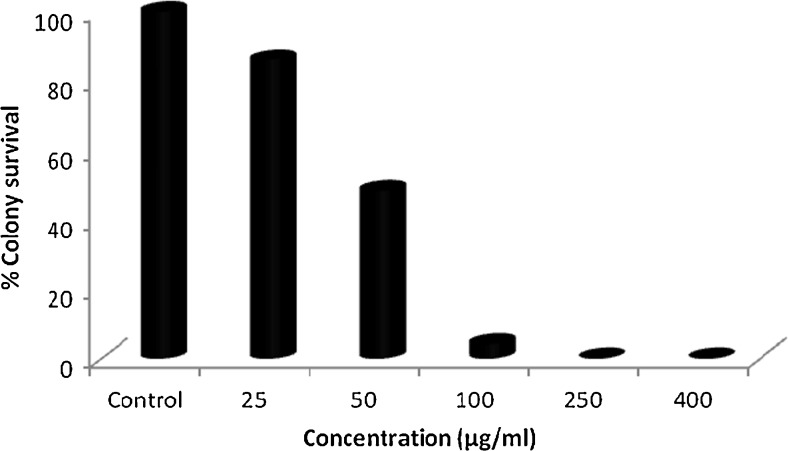
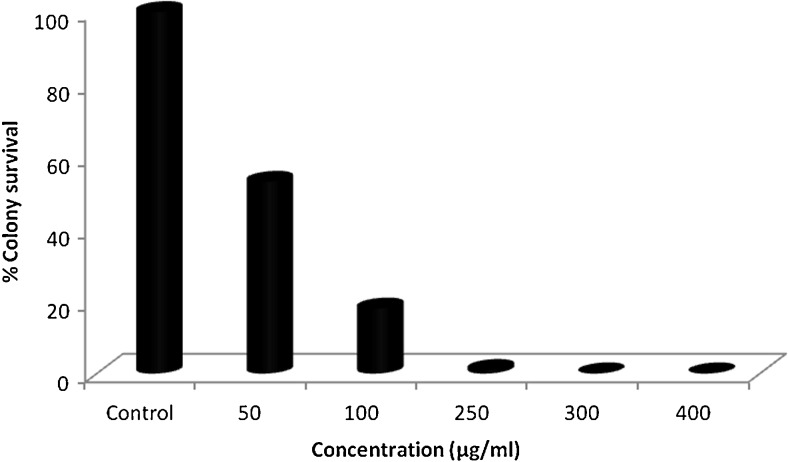
Colonogenic assay is an in vitro experiment used to check ability of cell to grow indefinitely and form colony. Such type of cell is known as colonogenic as it retains the capacity of proliferation after treatment with chemotherapeutic agents (Munshi et al. 2005; Franken et al. 2006). In the colony formation assay, both the fractions viz. Hex-LI and CHCl3-LI effectively inhibited the growth of colonies in dose dependent manner. It was observed that there was complete inhibition of colony formation at highest concentration of 400 μg/ml. Along with decrease in the number of colonies, it was also observed that there was decrease in the size of colonies as compared to size of control colonies (Figs. 5 and 6). Both fractions were taken forward to study apoptotic cell death in HeLa cells.
Fig. 5.
Colony formation inhibition in HeLa cells on treatment with Hex-LI. Cell survival was determined by considering the control cells survival as 100 %
Fig. 6.
Colony formation inhibition in HeLa cells on treatment with CHCl3-LI. Cell survival was determined by considering the control cells survival as 100 %
Nuclear morphology assessment using confocal microscopy
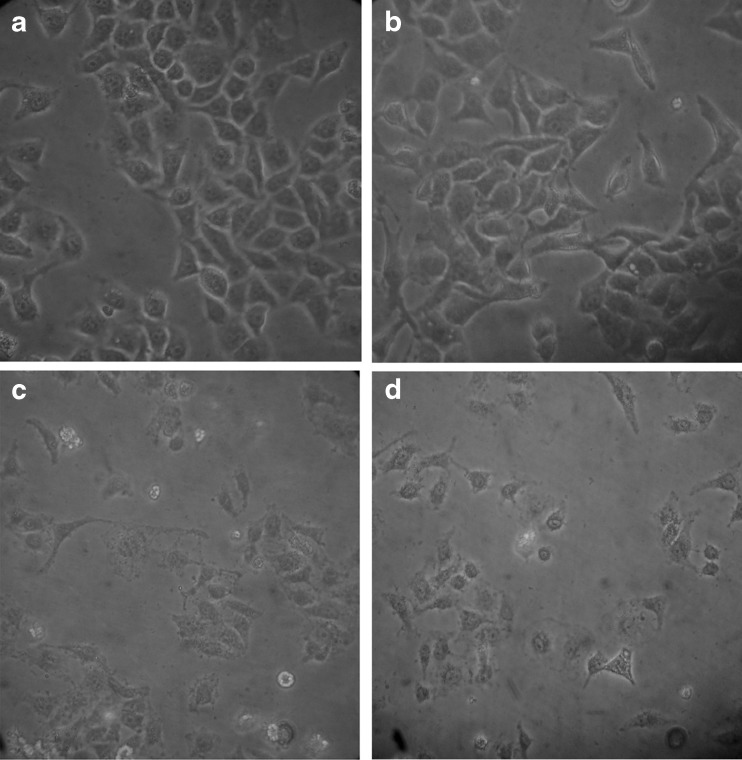
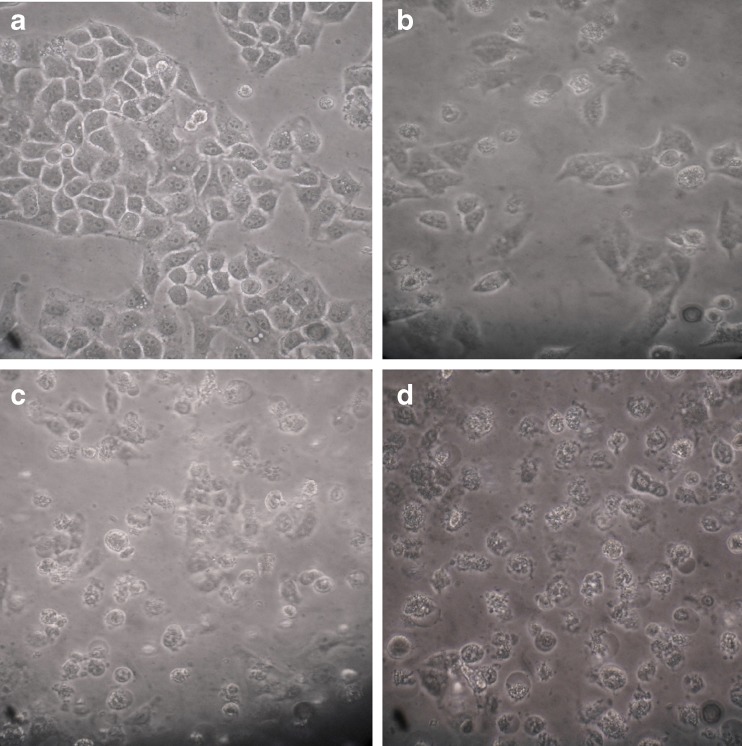
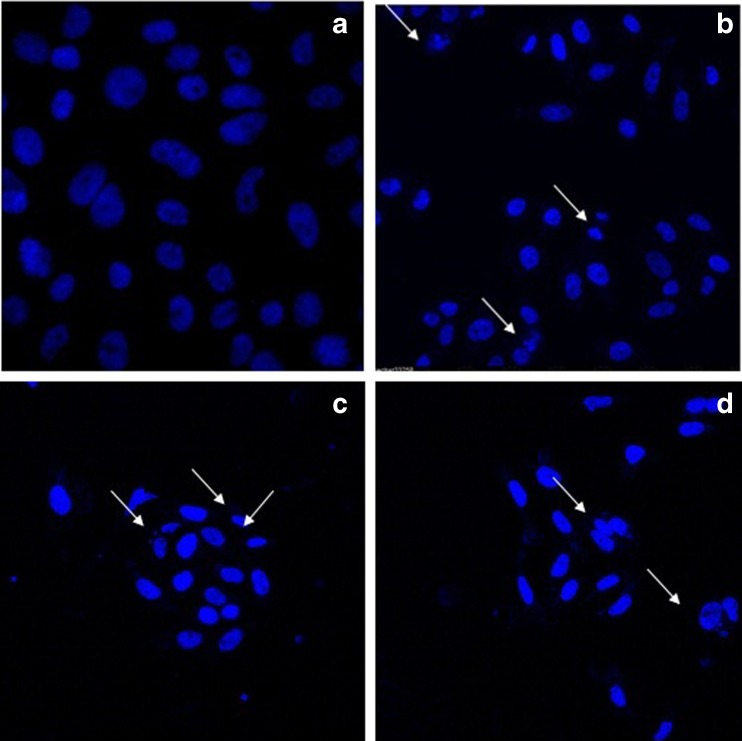
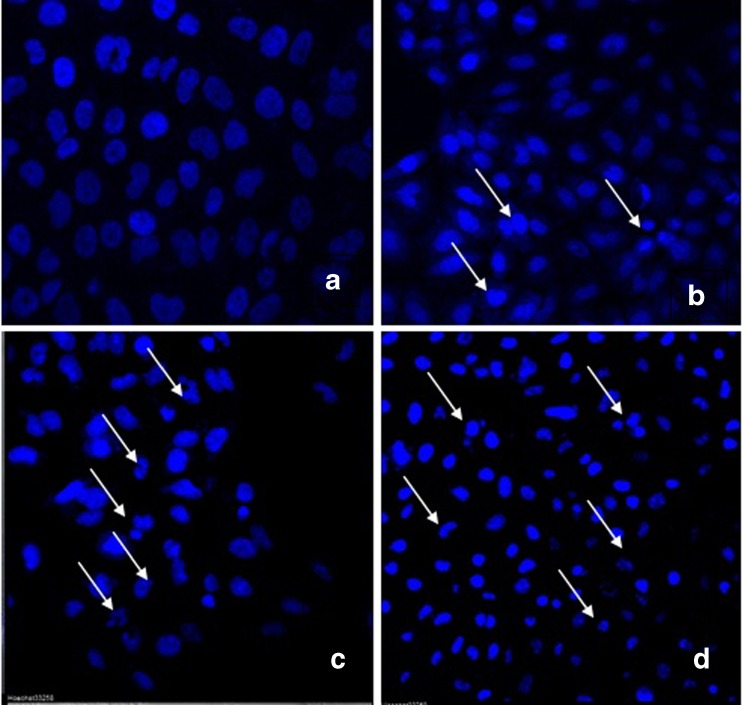
Hoechst 33342 is a DNA specific fluorescent dye which enters easily into the cell and intercalates in A-T regions of DNA. After treatment of 24 h, HeLa cells were observed for morphology changes under inverted microscope (Figs. 7 and 8). For nuclear morphological assessment, cells were washed with PBS followed by fixation with paraformaldehyde. After fixation, cells were stained with Hoechst 33342 dye to observe nuclear morphological alterations such as DNA condensation, fragmentation etc. It was observed that on treatment with Hex-LI and CHCl3-LI, nuclei showed condensation, fragmentation (Figs. 9 and 10). However, the control cells showed prominent nucleus without any type of condensation and fragmentation.
Fig. 7.
Morphological features visualized under phase contrast inverted microscope. The cells were incubated with various Hex-LI concentrations for 24 h. a Control; b 100 μg/ml; c 250 μg/ml; d 350 μg/ml
Fig. 8.
Morphological features visualized under phase contrast inverted microscope. The cells were incubated with various CHCl3-LI concentrations for 24 h. a Control; b 350 μg/ml; c 400 μg/ml; d 450 μg/ml
Fig. 9.
Nuclear alterations observed in HeLa cells after treatment with Hex-LI (24). Cells were stained with Hoechst 33342. Arrows designates cells exhibiting nuclear condensation, fragmentation and formation apoptotic bodies. The cells were incubated with various concentrations for 24 h. a control; b 100 μg/ml of Hex-LI; c 250 μg/ml of Hex-LI; d 350 μg/ml of Hex-LI
Fig. 10.
Nuclear alterations observed in HeLa cells after treatment with CHCl3-LI (24 h). Cells were stained with Hoechst 33342. Arrows designates cells exhibiting nuclear condensation, fragmentation and formation apoptotic bodies. The cells were incubated with various concentrations for 24 h. a control; b 350 μg/ml of CHCl3-LI; c 400 μg/ml of CHCl3-LI; d 450 μg/ml of CHCl3-LI
Determination of apoptosis using flow cytometry
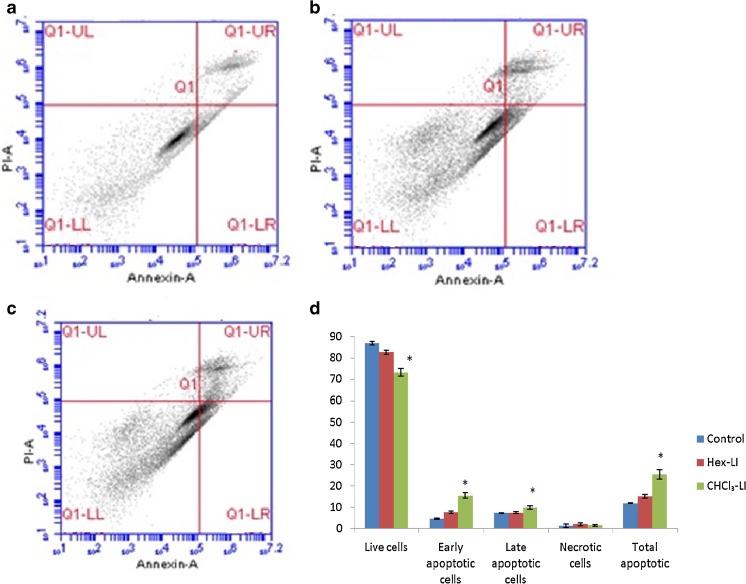
To evaluate, whether the fractions induce cell death through apoptotic or necrotic mode, flow cytometric analysis was carried out by using Annexin V-FITC and PI dye. During apoptosis, there is translocation of phosphatidylserine to outer surface of plasma membrane. Annexin V is Ca2+− dependent phospholipid-binding protein possesses high affinity for phosphatidylserine (Vermes et al. 1995). Translocation of phosphatidylserine to outer surface of plasma membrane also takes place during necrosis. So the use of both dyes viz. Annexin V-FITC and PI helps in estimation of early apoptotic, late apoptotic and necrotic cell population. From the Fig. 11, it is clear that Hex-LI and CHCl3-LI treatment caused 7.63 and 15.53 % of early apoptosis as compared to control value of 4.66 %. However, percentage of late apoptotic cells were 7.46 and 9.9 % in Hex-LI and CHCl3-LI treated cells. The total number of apoptotic cells was 15.1 and 25.43 % as compared to control value of 11.93 %.
Fig. 11.
Induction of apoptosis in HeLa cells after 24 h of treatment with Hex-LI and CHCl3 analyzed by flow cytometry. a Control; b Hex-LI (250 μg/ml); c CHCl3-LI (450 μg/ml) and d Graph showing number of live cells (LL quadrant), early apoptotic (LR quadrant), late apoptotic (UR quadrant), necrotic (UL quadrant) and total apoptotic cells. *p ≤ 0.05
Single cell gel electrophoresis assay (neutral comet assay)
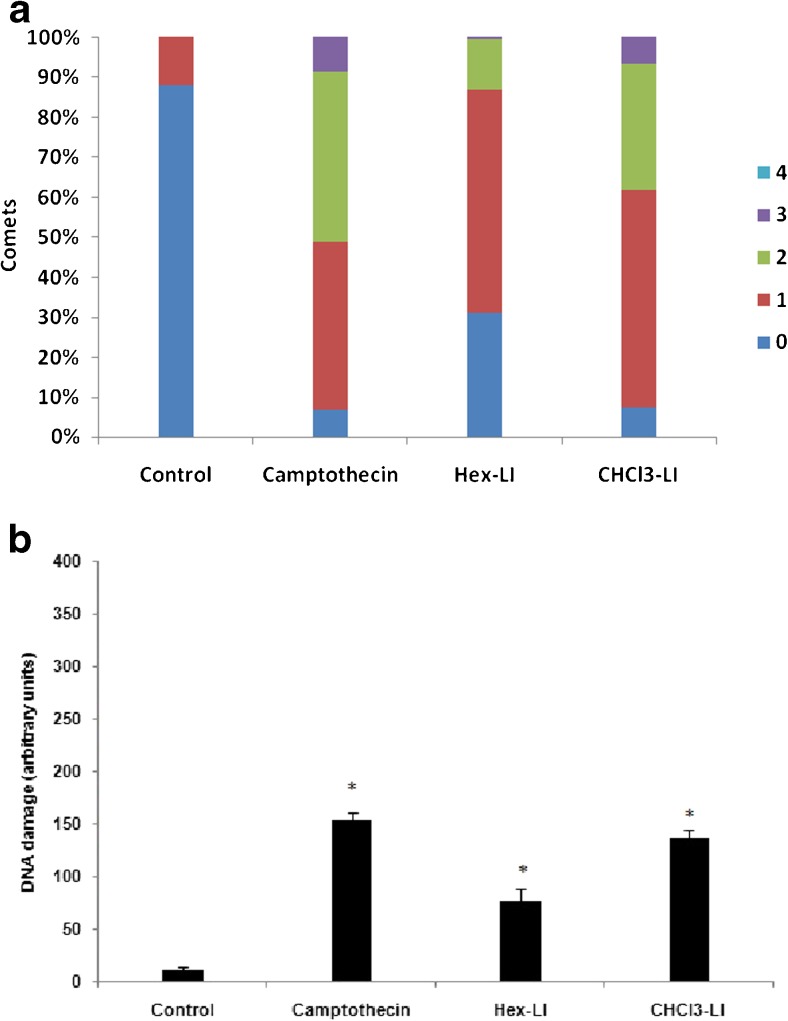
After evaluating apoptosis in Hex-LI and CHCl3-LI treated HeLa cancer cells, same concentrations of the both fractions were used to determine double stranded breaks in HeLa cells. From the results it was seen that both fractions showed DSB’s breaks with DNA damage (arbitrary units) of 137.3 and 75 in Hex-LI and CHCl3-LI treated HeLa cancer cells respectively (Fig. 12). Positive control i.e., camptothecin showed more DNA damage (153.3) as compared to both fractions.
Fig. 12.
a Various kinds of comets in different grades (1–4) by visual scoring. b DNA damage induced in Hex-LI (250 μg/ml) and CHCl3-LI (450 μg/ml) treated HeLa cells evaluated by visual scoring. *p ≤ 0.05
Reactive oxygen species measurement
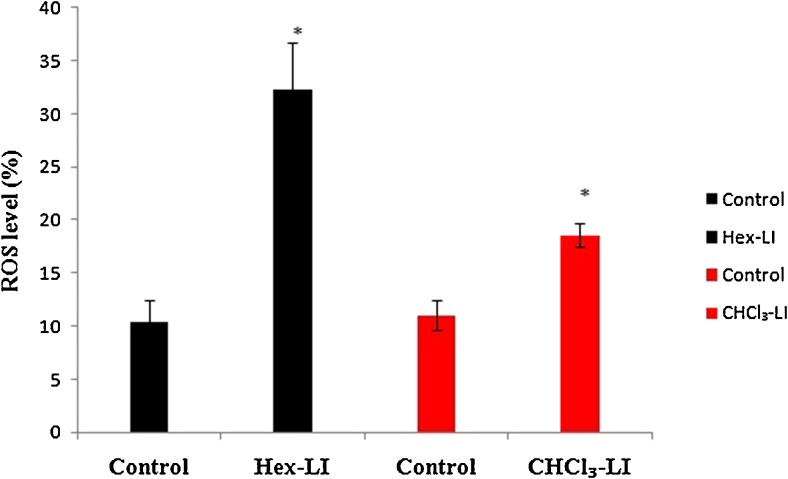
2′, 7′-dichlorofluorescin diacetate (DCFH-DA), was used to measure ROS in treated cells. DCFH-DA, a non polar compound when it enters into the cells, it gets cleaved by cellular estrases into DCFH. Later on DCFH get oxidized by hydrogen peroxide/low molecular weight peroxides into 2,7-dichlorofluorescein (DCF) (highly fluorescent) which is analyzed by flow cytometry. Both the fraction viz. Hex-LI and CHCl3-LI significantly induced generation of reactive oxygen species. It has been observed that ROS levels were found to be 32.2 % as compared to its control (10.4 %) in Hex-LI and 18.56 % as compared to its control (11.03 %) in CHCl3-LI treated cells (Fig. 13).
Fig. 13.
ROS level analyzed by flow cytometry in HeLa cells treated with Hex-LI (250 μg/ml) and CHCl3 (450 μg/ml) in comparison to untreated cells (Control). *p ≤ 0.05
Discussion
Apoptosis is a well regulated process, characterized by cell shrinkage, chromatin condensation, fragmentation, formation of apoptotic bodies. It plays a vital role in cellular homeostasis by controlling cell proliferation (Millan and Huerta 2009; Ramasamy et al. 2013). Numerous plants and their constituents are known to possess apoptosis inducing potential (Hsieh et al. 2006; Hsu et al. 2011; Kim et al. 2008). Any compound could possibly be developed into an anti-cancer drug, if it shows less inhibitory effects on normal cells than the cancer cells (Taraphdar et al. 2001). In recent years, it has been seen that scientific community is more engaged in isolating and screening natural molecules for drug discovery (Moure et al. 2001; Krishnaiah et al. 2011). Apoptosis can be exploited as an important target to develop anticancer drugs (Hu and Kavanagh 2003; Camero 2002). In the present work, we studied the growth inhibitory and apoptosis inducing effects of popular medicinal plant L. inermis on cancer cell lines viz. HeLa, MCF-7, A549 and C6. We found that two fractions viz. Hex-LI, CHCL3-LI were found to exhibit considerable dose dependent growth inhibitory activity i.e., more than 60 % in all the tested cell lines (Figs. 1–4). Antiproliferative activity of the extract depends upon the extraction procedure and the choice of solvent used for extraction (Chirinos et al. 2007; Lafka et al. 2007, 2013). Sadeghi-aliabadi et al. (2008) reported that chloroform extract of Convolvulus arvensis is quite effective in inducing anticancer effects in HeLa cells. Machana et al. (2012) reported that combination of hexane and water fraction of Polyalthia evecta showed high anticancer activity in HepG2 cells. Pacifico et al. (2013) reported CHCl3 extract from Laurus nobilis leaves induced strong antiproliferative activity in nervous system cell lines. Kim et al. (2012) studied hexane extract of garlic cloves and reported that the extract has the ability to induce apoptosis in Hep3B cells. Colonogenic assay was used to check the potential of cell to divide and form colony after the removal of test compound (Karna et al. 2011). Our results depicted that both the fractions inhibited the colony formation potential of cervical carcinoma cells (Figs. 5 and 6). According to Drees et al. (1997) test sample was regarded as effective if it decreased the colony formation potential to ≤30 % in comparison to control. Our data showed that both the fractions completely inhibited colony formation potential of HeLa cells as compare to control indicating high effectivity of test fractions. Pratumvinit et al. (2009) investigated Ficus hispida L.extract for colony formation inhibition in T47D breast cancer cell line and found that extract treatment showed reduction in number of colonies. Leaves of Moringa oleifera significantly inhibited colonogenecity in A549 cells in dose dependent manner (Jung 2014).
Plant extracts are centre of attraction for the search of anticancer agents because of minimal side effects and their potential to inhibit growth and proliferation of cancerous cells (Rao et al. 2009). Herbs or combinations of extracts of these herbs can act synergistically resulting in increased effectivity and can also have potential to reduce toxic effects. (Vickers 2002; Li et al. 2000). In the present study, after evaluating cytotoxicity, Hex-LI and CHCl3-LI were analyzed for cell morphological changes, nuclear changes, DNA fragmentation and formation of apoptotic bodies which are the characteristic features of apoptosis. It was observed that treated cells showed morphological alterations such as cell shrinkage, rounding off along with decline in cell number (Figs. 7 and 8). Further, to observe nuclear changes, cells were stained with Hoechst 33342 dye and observed under confocal microscope (Figs. 9 and 10). With increase in the concentration of both the extracts, nuclei of the cells showed shrinkage, condensation, nuclear fragmentation and formation of apoptotic bodies. Further, flow cytometric studies confirmed that both fractions induced apoptosis in HeLa cells. Hex-LI induced 15.1 % and 25.43 % of apoptosis in HeLa cells (Fig. 11). Earlier we reported that both the fractions were rich in polyphenolic constituents. HPLC revealed that Hex-LI contains ellagic acid in good proportion while CHCl3-LI contains ellagic acid along with kaempferol in high amounts (Kumar et al. 2014). Apoptosis inducing effects of these fractions might be a contribution of these phytochemicals as these have been reported as potential anticancer agents in many studies. Edderkaoui et al. (2008) reported that ellagic acid induced apoptosis in pancreatic cancer cell by suppressing NF-kappa B activity. Han et al. (2006) showed that ellagic acid induced apoptosis in human osteogenic sarcoma (HOS) through modulating expression of caspase 3 and bax proteins. Kaempferol stimulated apoptosis in human osteosarcoma U-2 OS cells (Huang et al. 2010). Detailed study revealed the involvement mitochondrial dependent and endoplasmic reticulum stress signaling pathway in kaempferol induced apoptosis. In an another study, kaempferol induced apoptosis in human bladder cancer cells by upregulating tumor suppressor PTEN i.e., Phosphatase and tensin homolog deleted on chromosome 10 and downregulating Akt phosphorylation (Xie et al. 2013). HeLa cells were evaluated using neutral comet assay for double stranded breaks (DSB’s) on treatment with Hex-LI and CHCl3-LI for 24 h. It was observed that cells treated with both the fractions showed DSB’s in DNA which may results from endonucleolytic activity of enzymes involved in apoptosis. These DSB’s were resulted from the apoptotic process occurring in the treated cells. Lim et al. (2011) reported that Acalypha wilkesiana extracts caused apoptotic cell death via DNA single and double stranded breaks in U87MG and A549 cells. Lam et al. (2012) studied that Fagonia cretica extract treatment induces DNA damage in human breast cancer (MCF-7) cells. As compared to normal cells, cancer cells show high metabolic activities which results in elevated levels of reactive oxygen species (Gundala et al. 2014; Schumacker 2006; Szatrowski and Nathan 1991; Trachootham et al. 2009). Various polyphenolic compounds are known to exert anticancer effects via generating oxidative stress in cancer cells (Azam et al. 2004). ROS in excess can cause damage to DNA, proteins, lipids and cell membranes. In response to ROS, mitochondrial membrane become permeable leading to the release of cytochrome c which induces caspases cascades ultimately resulting in apoptosis (Conklin 2004; Circu and Aw 2010; Simon et al. 2000; Thayyullathil et al. 2008). Sharma et al. (2007) reported that Kaempferol showed apoptogenic activity in human glioblastoma cells by elevating the levels of ROS. Ellagic acid caused apoptosis of tsgh8301 human bladder cancer cells (Ho et al. 2014). Increased ROS and Ca2+ production along with other factors played important role in inducing apoptosis in tsgh8301 human bladder cancer cells. Several natural products targets cancer cells by elevating ROS levels (Ding et al. 2009; Jeong et al. 2010; Widodo et al. 2010). Hex-LI and CHCl3-LI significantly increased production of ROS in HeLa cells as compared to their respective controls. Increased ROS production can be a one of the mechanism along with other signaling cascades responsible for the apoptosis inducing effects of Hex-LI and CHCl3-LI fractions of L. inermis.
Conclusion
In conclusion, fractions isolated from L. inermis possess good anticancer activity which can be exploited for the development of chemotherapeutic agents for the treatment of cancer after further toxicity studies. Studies are also in progress to explore the mechanism responsible for these activities.
Acknowledgments
This work was supported by the Council of Scientific and Industrial Research (CSIR) [38(1265)/10/EMR-II], New Delhi (India) and the UPE programme of University Grants Commission (UGC), New Delhi.
Conflict of interest
None.
References
- Ahmed S, Rahman A, Alam A, Saleem M, Athar M, Sultana S. Evaluation of the efficacy of Lawsonia alba in the alleviation of carbon tetrachloride induced oxidative stress. J Ethnopharmacol. 2000;69:157–164. doi: 10.1016/S0378-8741(99)00091-4. [DOI] [PubMed] [Google Scholar]
- Akerele O. Medicinal plants and primary health care: an agenda for action. Fitoterapia. 1988;59:355–363. [Google Scholar]
- Allen RT, Hunter WJ, 3rd, Agarwal DK. Morphological and biochemical characterization and analysis of apoptosis. J Pharmacol Toxicol Methods. 1997;37:215–228. doi: 10.1016/S1056-8719(97)00033-6. [DOI] [PubMed] [Google Scholar]
- Azam S, Hadi N, Khan NU, Hadi SM. Prooxidant property of green tea polyphenols epicatechin and epigallocatechin-3-gallate: implications for anticancer properties. Toxicol in Vitro. 2004;18:555–561. doi: 10.1016/j.tiv.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Basirian M, Manjula SN, Mruthunjaya K. In vitro anti-clastogenic activity of different fractions of roots of Lawsonia inermis on chromosomal aberration of human lymphocytes against doxocirubicin and cyclophosphamide as clastogens. Int J Med Med Sci. 2013;46:1239–1244. [Google Scholar]
- Bich DH, Chung DQ, Chuong BX, et al. The medicinal plants and animals in Vietnam. Hanoi: Science and Technology Publishing House; 2004. [Google Scholar]
- Camero A. Targeting the cell cycle for cancer therapy. Br J Cancer. 2002;87:129–133. doi: 10.1038/sj.bjc.6600458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirinos R, Rogez H, Campos D, Pedreschi R, Larondelle Y. Optimization of extraction conditions of antioxidant phenolic compounds from mashua (Tropaeolum tuberosum Ruíz & Pavón) tubers. Sep Purif Technol. 2007;55:217–225. doi: 10.1016/j.seppur.2006.12.005. [DOI] [Google Scholar]
- Circu ML, Aw TY. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic Biol Med. 2010;48:749–762. doi: 10.1016/j.freeradbiomed.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins A, Dusinska M, Franklin M, Somorovská M, Petrovská H, Duthie S, Fillion L, Panayiotidis M, Raslová K, Vaughan N. Comet assay in human biomonitoring studies: reliability, validation, and applications. Environ Mol Mutagen. 1997;30:139–146. doi: 10.1002/(SICI)1098-2280(1997)30:2<139::AID-EM6>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Conklin KA. Free radicals: the pros and cons of antioxidants. Cancer chemotherapy and antioxidants. J Nutr. 2004;134:3201–3204. doi: 10.1093/jn/134.11.3143S. [DOI] [PubMed] [Google Scholar]
- Dai J, Mumper RJ. Plant phenolics: extraction, analysis and their antioxidant and anticancer properties. Molecules. 2010;15:7313–7352. doi: 10.3390/molecules15107313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta T, Rao AR, Yadava PK. Modulatory effect of Henna leaf (Lawsonia inermis) on drug metabolising phase I and phase II enzymes, antioxidant enzymes, lipid peroxidation and chemically induced skin and forestomach papillomagenesis in mice. Mol Cell Biochem. 2003;245:11–22. doi: 10.1023/A:1022853007710. [DOI] [PubMed] [Google Scholar]
- Deeb D, Gao X, Jiang H, Janic B, Arbab AS, Rojanasakul Y, Dulchavsky SA, Gautam SC. Oleanane triterpenoid CDDO-Me inhibits growth and induces apoptosis in prostate cancer cells through a ROS-dependent mechanism. Biochem Pharmacol. 2010;79:350–360. doi: 10.1016/j.bcp.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhiman A, Sharma K, Goyal J, Garg M, Sharma A. Determination of Lawsone content in leaves of Lawsonia inermis Linn. Int J Pharm Pharm Sci. 2012;1:17–20. [Google Scholar]
- Ding H, Han C, Guo D, Chin YW, Ding Y, Kinghorn AD, D’Ambrosio SM. Selective induction of apoptosis of human oral cancer cell lines by avocado extracts via a ROS-mediated mechanism. Nutr Cancer. 2009;61:348–356. doi: 10.1080/01635580802567158. [DOI] [PubMed] [Google Scholar]
- Drees M, Dengler WA, Roth T, Labonte H, Mayo J, Malspeis L, Grever M, Sausville EA, Fiebig HH. Flavopiridol (L86-8275): selective antitumor activity in vitro and activity in vivo for prostate carcinoma cells. Clin Cancer Res. 1997;3:273–279. [PubMed] [Google Scholar]
- Edderkaoui M, Odinokova I, Ohno I, Gukovsky I, Go VL, Pandol SJ, Gukovskaya AS. Ellagic acid induces apoptosis through inhibition of nuclear factor kappa B in pancreatic cancer cells. World J Gastroenterol. 2008;14(23):3672–3680. doi: 10.3748/wjg.14.3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franken NAP, Rodermond HM, Stap J, Haveman J, Bree CV. Clonogenic assay of cells in vitro. Nat Protoc. 2006;5:2315–2319. doi: 10.1038/nprot.2006.339. [DOI] [PubMed] [Google Scholar]
- Guha G, Rajkumar V, Ashok Kumar R, Mathew L. Antioxidant activity of Lawsonia inermis extracts inhibits chromium(vi)-induced cellular and DNA toxicity. Evid Based Complement Alternat Med. 2011 doi: 10.1093/ecam/nep205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundala SR, Yang C, Lakshminarayana N, Asif G, Gupta MV, Shamsi S, Aneja R. Polar biophenolics in sweet potato greens extract synergize to inhibit prostate cancer cell proliferation and in vivo tumor growth. Carcinogenesis. 2013;34(9):2039–2049. doi: 10.1093/carcin/bgt141. [DOI] [PubMed] [Google Scholar]
- Gundala SR, Yang C, Mukkavilli R, Paranjpe R, Brahmbhatt M, Pannu V, Cheng A, Reid MD, Aneja R. Hydroxychavicol, a betel leaf component, inhibits prostate cancer through ROS-driven DNA damage and apoptosis. Toxicol Appl Pharmacol. 2014;280:86–96. doi: 10.1016/j.taap.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurib-Fakim A. Medicinal plants: traditions of yesterday and drugs of tomorrow. Mol Aspects Med. 2006;27:1–93. doi: 10.1016/j.mam.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Han DH, Lee MJ, Kim JH. Antioxidant and apoptosis-inducing activities of ellagic acid. Anticancer Res. 2006;26(5A):3601–3606. [PubMed] [Google Scholar]
- Haneef J, Parvathy M, Thankayyan RSK, Sithul H, Sreeharshan S. Bax translocation mediated mitochondrial apoptosis and caspase dependent photosensitizing effect of Ficus religiosa on cancer cells. PLoS ONE. 2012;7(7):e40055. doi: 10.1371/journal.pone.0040055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho CC, Huang AC, Yu CS, Lien JC, Wu SH, Huang YP, Huang HY, Kuo JH, Liao WY, Yang JS, Chen PY, Chung JG. Ellagic acid induces apoptosis in tsgh8301 human bladder cancer cells through the endoplasmic reticulum stress- and mitochondria-dependent signaling pathways. Environ Toxicol. 2014;29:1262–1274. doi: 10.1002/tox.21857. [DOI] [PubMed] [Google Scholar]
- Hsieh TJ, Liu TZ, Lu FJ, Hsieh PY, Chen CH. Actinodaphnine induces apoptosis through increased nitric oxide, reactive oxygen species and down regulation of NF-kappa B signaling in human hepatoma Mahlavu cells. Food Chem Toxicol. 2006;44:344–354. doi: 10.1016/j.fct.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Hsouna AB, Trigui M, Culioli G, Blache Y, Jaoua S. Antioxidant constituents from Lawsonia inermis leaves: isolation, structure elucidation and antioxidative capacity. Food Chem. 2011;125:193–200. doi: 10.1016/j.foodchem.2010.08.060. [DOI] [Google Scholar]
- Hsu H-F, Huang K-H, Lu K-J, Chiou S-J, Yen J-H, Chang C-C, Houng J-Y. Typhonium blumei extract inhibits proliferation of human lung adenocarcinoma A549 cells via induction of cell cycle arrest and apoptosis. J Ethnopharmacol. 2011;135(2):492–500. doi: 10.1016/j.jep.2011.03.048. [DOI] [PubMed] [Google Scholar]
- Hu W, Kavanagh JJ. Anticancer therapy targeting the apoptotic pathway. Lancet Oncol. 2003;4:721–729. doi: 10.1016/S1470-2045(03)01277-4. [DOI] [PubMed] [Google Scholar]
- Huang WW, Chiu YJ, Fan MJ, Lu HF, Yeh HF, Li KH, Chen PY, Chung JG, Yang JS. Kaempferol induced apoptosis via endoplasmic reticulum stress and mitochondria-dependent pathway in human osteosarcoma U-2 OS cells. Mol Nutr Food Res. 2010;54(11):1585–1595. doi: 10.1002/mnfr.201000005. [DOI] [PubMed] [Google Scholar]
- Igney F, Krammer P. Death and anti-death: tumour resistance to apoptosis. Nat Rev Cancer. 2002;2:277–288. doi: 10.1038/nrc776. [DOI] [PubMed] [Google Scholar]
- Jeong JC, Jang SW, Kim TH, Kwon CH, Kim YK. Mulberry fruit (Moris fruc-tus) extracts induce human glioma cell death in vitro through ROS-dependent mitochondrial pathway and inhibits glioma tumor growth in vivo. Nutr Cancer. 2010;62:402–412. doi: 10.1080/01635580903441287. [DOI] [PubMed] [Google Scholar]
- Jiny Varghese K, Silvipriya KS, Resmi S, Jolly CI. Lawsonia Inermis (Henna): a natural dye of various therapeutic uses - a review. Inventi Rapid: Cosmeceuticals. 2010;1:1–5. [Google Scholar]
- Jung IL. Soluble extract from Moringa oleifera leaves with a new anticancer activity. PLoS ONE. 2014;9(4):e95492. doi: 10.1371/journal.pone.0095492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karna P, Gundala SR, Gupta MV, Shamsi SA, Pace RD, Yates C, Narayan S, Aneja R. Polyphenol-rich sweet potato greens extract inhibits proliferation and induces apoptosis in prostate cancer cells in vitro and in vivo. Carcinogenesis. 2011;32(12):1872–1880. doi: 10.1093/carcin/bgr215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelloff GJ, Crowell JA, Steele VE, Lubet RA, Malone WA, Boone CW, Kopelovich L, Hawk ET, Lieberman R, Lawrence JA, Ali I, Viner JL, Sigman CC. Progress in cancer chemoprevention: development of diet-derived chemopreventive agents. J Nutr. 2000;130(2S):467S–471S. doi: 10.1093/jn/130.2.467S. [DOI] [PubMed] [Google Scholar]
- Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EJ, Lim SS, Park SY, Shin HK, Kim JS, Park JHY. Apoptosis of DU145 human prostate cancer cells induced by dehydrocostus lactone isolated from the root of Saussurea lappa. Food Chem Toxicol. 2008;46(12):3651–3658. doi: 10.1016/j.fct.2008.08.038. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Han MH, Kim GY, Choi YW, Choi YH. Hexane extracts of garlic cloves induce apoptosis through the generation of reactive oxygen species in Hep3B human hepatocarcinoma cells. Oncol Rep. 2012;28:1757–1763. doi: 10.3892/or.2012.1985. [DOI] [PubMed] [Google Scholar]
- Krishnaiah D, Sarbathy R, Nithyanandam R. A review of the antioxidant potential of medicinal plant species. Food Bioprod Process. 2011;89:217–233. doi: 10.1016/j.fbp.2010.04.008. [DOI] [Google Scholar]
- Kroemer G, Reed JC. Mitochondrial control of cell death. Nat Med. 2000;6(5):513–519. doi: 10.1038/74994. [DOI] [PubMed] [Google Scholar]
- Kroemer G, Petit PX, Zamzami N, Vayssiere JL, Mignotte B. The biochemistry of programmed cell death. FASEB J. 1995;9:1277–1287. doi: 10.1096/fasebj.9.13.7557017. [DOI] [PubMed] [Google Scholar]
- Kumar M, Kumar S, Kaur SJ. Identification of polyphenols in leaf extracts of Lawsonia inermis L. with antioxidant, antigenotoxic and antiproliferative potential. Int J Green Pharm. 2014;8:23–36. doi: 10.4103/0973-8258.126816. [DOI] [Google Scholar]
- Kutluk T, Kars A (1998) General knowledge about cancer. Ankara; Turkey cancer investigation and fight society publications, pp: 7–15
- Lafka TI, Sinanoglou VJ, Lazos ES. On the extraction and antioxidant activity of phenolic compounds from winery wastes. Food Chem. 2007;104:1206–1214. doi: 10.1016/j.foodchem.2007.01.068. [DOI] [Google Scholar]
- Lafka TI, Lazou AE, Sinanoglou VJ, Lazos ES. Phenolic extracts from wild olive leaves and their potential as edible oils antioxidants. Foods. 2013;2:18–31. doi: 10.3390/foods2010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam M, Carmichael AR, Griffiths HR. An aqueous extract of Fagonia cretica induces DNA damage, cell cycle arrest and apoptosis in breast cancer cells via FOXO3a and p53 expression. PLoS ONE. 2012;7(6):e40152. doi: 10.1371/journal.pone.0040152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HZ, Liu WZ, Hsieh WT, Tang FY, Chung JG, Leung HWC. Oxidative stress involvement in Physalis angulata -induced apoptosis in human oral cancer cells. Food Chem Toxicol. 2009;47:561–570. doi: 10.1016/j.fct.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Li XK, Motwani M, Tong W, Bornmann W, Schwartz GK, Huanglian A. Chinese herbal extract, inhibits cell growth by suppressing the expression of cyclin B1 and inhibiting CDC2 kinase activity in human cancer cells. Mol Pharm. 2000;58:1287–1293. doi: 10.1124/mol.58.6.1287. [DOI] [PubMed] [Google Scholar]
- Lim SW, Ting KN, Bradshaw TD, Zeenathul NA, Wiart C, Khoo TJ, Lim KH, Loh HS. Acalypha wilkesiana extracts induce apoptosis by causing single strand and double strand DNA breaks. J Ethnopharmacol. 2011;138(2):616–623. doi: 10.1016/j.jep.2011.10.005. [DOI] [PubMed] [Google Scholar]
- Lizard G, Fournel S, Genestier L. Kinetics of plasma membrane and mitochondrial alterations in cells undergoing apoptosis. Cytometry. 1995;21:275–283. doi: 10.1002/cyto.990210308. [DOI] [PubMed] [Google Scholar]
- Machana S, Weerapreeyakul N, Barusrux S, Thumanu K, Tanthanuch W. Synergistic anticancer effect of the extracts from Polyalthia evecta caused apoptosis in human hepatoma (HepG2) cells. Asian Pac J Trop Biomed. 2012;2:589–596. doi: 10.1016/S2221-1691(12)60103-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matkar SS, Wrischnik LA, Jones PR, Hellmann-Blumberg U. Two closely related nickel complexes have different effects on DNA damage and cell viability. Biochem Biophys Res Commun. 2006;343:754–761. doi: 10.1016/j.bbrc.2006.03.019. [DOI] [PubMed] [Google Scholar]
- Mickisch G, Fajta S, Keilhauer G, Schlike E, Tschada P, Alken P. Chemosensitivity testing of primary human renal cell carcinoma by tetrazolium based microculture assay (MTT) Urol Res. 1990;18:131–136. doi: 10.1007/BF00302474. [DOI] [PubMed] [Google Scholar]
- Militao GCG, Dantas INF, Pessoa C, Falcão MJC, Silveira ER, Lima MAS, Curi R, Lima T, Moraes MO, Costa-Lotufo LV. Induction of apoptosis by pterocarpans from Platymiscium floribundum in HL-60 human leukemia cells. Life Sci. 2006;78:2409–2417. doi: 10.1016/j.lfs.2005.09.044. [DOI] [PubMed] [Google Scholar]
- Millan A, Huerta MD. Apoptosis-inducing factor and colon cancer. J Surg Res. 2009;151:163–170. doi: 10.1016/j.jss.2007.05.020. [DOI] [PubMed] [Google Scholar]
- Moure A, Cruz JM, Franco D, Dominguez JM, Sineiro J, Dominguez H, Núñez MJ, Parajó JC. Natural antioxidants from residual sources. Food Chem. 2001;2:145–171. doi: 10.1016/S0308-8146(00)00223-5. [DOI] [Google Scholar]
- Munshi A, Hobbs M, Meyn RE (2005) Clonogenic cell survival assay . From: Methods in Molecular Medicine, vol 110:Chemosensitivity vol. 1. In: Blumenthal RD (ed) In vitro Assays. Humana Press Inc, Totowa, NJ [DOI] [PubMed]
- Owoabi J, Omogbai EKI, Obasuyi O. Antifungal and antibacterial activities of the ethanolic and aqueous extract of Kigella africana (Bignoniaceae) stem bark. Afr J Biotechnol. 2007;6:882–885. [Google Scholar]
- Pacifico S, Gallicchio M, Lorenz P, Potenza N, Galasso S, Marciano S, Fiorentino A, Stintzing FC, Monaco P. Apolar Laurus nobilis leaf extracts induce cytotoxicity and apoptosis towards three nervous system cell lines. Food Chem Toxicol. 2013;62:628–637. doi: 10.1016/j.fct.2013.09.029. [DOI] [PubMed] [Google Scholar]
- Pratumvinit B, Srisapoomi T, Worawattananon P, Opartkiattikul N, Jiratchariyakul W, Kummalue T. In vitro antineoplastic effect of Ficus hispida L. plant against breast cancer cell lines. J Med Plant Res. 2009;3(4):255–261. [Google Scholar]
- Priya R, Ilavenil S, Kaleeswaran B, Srigopalram S, Ravikumar S. Effect of Lawsonia inermis on tumor expression induced by Dalton’s lymphoma ascites in Swiss albino mice. Saudi J Biol Sci. 2011;18:353–359. doi: 10.1016/j.sjbs.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi F, Li A, Zhao L, Xu H, Inagaki Y, Wang D, Cui X, Gao B, Kokudo N, Nakata M, Tang W. Cinobufacini, an aqueous extract from Bufo bufo gargarizans Cantor, induces apoptosis through a mitochondria mediated pathway in human hepatocellular carcinoma cells. J Ethnopharmacol. 2010;128:654–661. doi: 10.1016/j.jep.2010.02.022. [DOI] [PubMed] [Google Scholar]
- Raja W, Agrawal RC, Ovais M. Evaluation of antimutagenocity effect of Lawsonia inermis (henna) leaf extract in Swiss albino mice. Res J Pharm Technol. 2008;1(3):278–279. [Google Scholar]
- Ramasamy S, Wahabb NA, Abidina NZ, Manickam S. Effect of extracts from Phyllanthus watsonii Airy Shaw on cell apoptosis in cultured human breast cancer MCF-7 cells. Exp Toxicol Pathol. 2013;65:341–349. doi: 10.1016/j.etp.2011.11.005. [DOI] [PubMed] [Google Scholar]
- Rao PS, Ramanadham M, Prasad MNV. Anti-proliferative and cytotoxic effects of Strychnos nux-vomica root extract on human multiple myeloma cell line – RPMI 8226. Food Chem Toxicol. 2009;47:283–288. doi: 10.1016/j.fct.2008.10.027. [DOI] [PubMed] [Google Scholar]
- Reddy KR. Folk medicine from Chittoor district Andhra Pradesh, India used in the treatment of jaundice. Int J Crude Drug Res. 1988;26:137–140. [Google Scholar]
- Reed JC. Apoptosis-targeted therapies for cancer. Cancer Cell. 2003;3:17. doi: 10.1016/S1535-6108(02)00241-6. [DOI] [PubMed] [Google Scholar]
- Sadeghi-aliabadi H, Ghasemi N, Kohi M. Cytotoxic effect of Convolvulus arvensis extracts on human cancerous cell line. Res Pharm Sci. 2008;3:31–34. [Google Scholar]
- Schumacker PT. Reactive oxygen species in cancer cells: live by the sword, die by the sword. Cancer Cell. 2006;10:175–176. doi: 10.1016/j.ccr.2006.08.015. [DOI] [PubMed] [Google Scholar]
- Sharma V, Joseph C, Ghosh S, Agarwal A, Mishra MK, Sen E. Kaempferol induces apoptosis in glioblastoma cells through oxidative stress. Mol Cancer Ther. 2007;6(9):2544–2553. doi: 10.1158/1535-7163.MCT-06-0788. [DOI] [PubMed] [Google Scholar]
- Simon HU, Haj-Yehia A, Levi-Schaffer F. Role of reactive oxygen species in apoptosis induction. Apoptosis. 2000;5:415–418. doi: 10.1023/A:1009616228304. [DOI] [PubMed] [Google Scholar]
- Szatrowski TP, Nathan CF. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991;51:794–798. [PubMed] [Google Scholar]
- Taraphdar AK, Roy M, Bhattacharya RK. Natural products as inducers of apoptosis: implication for cancer therapy and prevention. Curr Sci. 2001;80:1387–1396. [Google Scholar]
- Thayyullathil F, Chathoth S, Hago A, Patel M, Galadari S. Rapid reactive oxygen species (ROS) generation induced by curcumin leads to caspase-dependent and -independent apoptosis in L929 cells. Free Radic Biol Med. 2008;45:1403–1412. doi: 10.1016/j.freeradbiomed.2008.08.014. [DOI] [PubMed] [Google Scholar]
- Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov. 2009;8:579–591. doi: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- Vasanthi P, Ganapathy M, Evanjelene VK, Ayyavuv N, Angamuthu J. Phytochemical screening and antioxidant activity of extracts of the leaf and bark of Albizzia lebbeck (Benth) Acad J Med Plant. 2014;2:026–031. [Google Scholar]
- Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis, flow cytometric detection of phosphatidylserin expression on early apoptotic cells using fluorescein labeled annexin-V. J Immunol Methods. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-I. [DOI] [PubMed] [Google Scholar]
- Vickers A. Botanical medicines for the treatment of cancer: rationale, overview of current data, and methodological considerations for phase I and II trials. Cancer Investig. 2002;20:1069–1079. doi: 10.1081/CNV-120005926. [DOI] [PubMed] [Google Scholar]
- Wang ZB, Liu YQ, Cui YF. Pathways to caspase activation. Cell Biol Int. 2005;29:489–496. doi: 10.1016/j.cellbi.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Wang CZ, Calway T, Yuan CS. Herbal medicines as adjuvants for cancer therapeutics. Am J Chin Med. 2012;40:657–669. doi: 10.1142/S0192415X12500498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CZ, Zhang Z, Huang WH, Du GJ, Wen XD, Calway T, Yu C, Nass R, Zhao J, Du W, Li SP, Yuan CS. Identification of potential anticancer compounds from Oplopanax horridus. Phytomedicine. 2013;20(11):999–1006. doi: 10.1016/j.phymed.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrier PK (2004) Indian medicinal plants-a compendium of 500 species. Orient longman private limited, Chennai, pp 303–304
- Widodo N, Priyandoko D, Shah N, Wadhwa R, Kaul SC. Selective killing of cancer cells by Ashwagandha leaf extract and its component Withanone involves ROS signaling. PLoS ONE. 2010;5:e13536. doi: 10.1371/journal.pone.0013536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojewodzka M, Buraczewska I, Kruszewski M. A modified neutral comet assay: elimination of lysis at high temperature and validation of the assay with anti-single-stranded DNA antibody. Mutat Res. 2002;518:9–20. doi: 10.1016/S1383-5718(02)00070-0. [DOI] [PubMed] [Google Scholar]
- Wu SJ, Chang SP, Lin DL, Wang SS, Hou FF, Ng LT. Supercritical carbon dioxide extract of Physalis peruviana induced cell cycle arrest and apoptosis in human lung cancer H661 cells. Food Chem Toxicol. 2009;47:1132–1138. doi: 10.1016/j.fct.2009.01.044. [DOI] [PubMed] [Google Scholar]
- Wyllie AH, Kerr JF, Currie AR. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/S0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- Xie F, Su M, Qiu W, Zhang M, Guo Z, Su B, Liu J, Li X, Zhou L. Kaempferol promotes apoptosis in human bladder cancer cells by inducing the tumor suppressor, PTEN. Int J Mol Sci. 2013;14(11):21215–21226. doi: 10.3390/ijms141121215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Ge R, Du J, Xin H, Yi T, Sheng J, Wang Y, Ling C. Corosolic acid induces apoptosis through mitochondrial pathway and caspases activation in human cervix adenocarcinoma HeLa cells. Cancer Lett. 2009;284:229–237. doi: 10.1016/j.canlet.2009.04.028. [DOI] [PubMed] [Google Scholar]
- Zhang J, Xu M. Apoptotic DNA fragmentation and tissue homeostasis. Trends Cell Biol. 2002;12(2):84–89. doi: 10.1016/S0962-8924(01)02206-1. [DOI] [PubMed] [Google Scholar]