Abstract
Glycinebetaine is one of the most competitive compounds which play an important role in salt stress in plants. In this study, the enhanced salt tolerance in soybean (Glycine max L.) by exogenous application of glycinebetaine was evaluated. To improve salt tolerance at the seedling stage, GB was applied in four different concentrations (0, 5, 25 and 50 mM) as a pre-sowing seed treatment. Salinity stress in the form of a final concentration of 150 mM sodium chloride (NaCl) over a 15 day period drastically affected the plants as indicated by increased proline, MDA and Na+ content of soybean plants. In contrast, supplementation with 50 mM GB improved growth of soybean plants under NaCl as evidenced by a decrease in proline, MDA and Na+ content of soybean plants. Further analysis showed that treatments with GB, resulted in increasing of CAT and SOD activity of soybean seedlings in salt stress. We propose that the role of GB in increasing tolerance to salinity stress in soybean may result from either its antioxidant capacity by direct scavenging of H2O2 or its role in activating CAT activity which is mandatory in scavenging H2O2.
Keywords: Salt stress, Soybean, Glycinebetaine, SOD, CAT, K+/Na+ ratio
Introduction
Environmental stresses, such as salt stress, induce the production of methylglyoxal (Yadav et al. 2005a, b) and Reactive Oxygen Species (ROS) (Hasegawa et al. 2000; Apel and Hirt 2004) in plant cells. ROS are highly reactive and toxic to plants, and via damage to proteins, lipids and carbohydrates and glycine, can lead to cell death (Noctor and Foyer 1998; Apel and Hirt 2004; Miransari and Smith 2009). Methylglyoxal can react with and modify other molecules, such as DNA and proteins (Yadav et al. 2005b).
To reciprocate environmental stress, plants store multiple groups of compatible solutes such as sugars,free amino acids like glycinebetaine, proline and polyols (Hoque et al. 2007). Betaine and proline are the most popular compatible solutes that contribute to osmotic adjustment (Rhodes and Hanson 1993; Hasegawa et al. 2000; Ashraf and Foolad 2007), and stabilization and protection of proteins, enzymes and membranes from the damaging effects of salt stress (McNeil et al. 1999; Okuma et al. 2000; Ashraf and Foolad 2007).
Glycinebetaine as one of the compatible solutes, plays an important role in salinity by osmotic adjustment in plants (Subbarao et al. 2001; Munns 2002; Ashraf and Harris 2004), protecting the proteins (by maintaining the structure of enzymes such as Rubisco) (Bohnert and Jensen 1996), protecting the membrane structure (Crowe et al. 1992), protection of cytoplasm and chloroplasts from Na+ damage (Rahman et al. 2002), protection of photosynthetic mechanism (Sakamoto and Murata 2002) and by functioning as oxygen radical sweeper (Smirnoff and Cumbes 1989). As the synthesis of GB is energetically expensive, the use of external glycine as an alternative source through genetic engineering has been adopted to improve the salt tolerance in plants (Mäkelä et al. 1996). Exogenous use of GB with various environmental stresses, also improves growth, survival and abiotic stress tolerance of plants (Harinasut et al. 1996; Diaz-Zorita et al. 2001).
Exogenous betaine improves salt endurance by upregulating stress-preservative proteins (Khedr et al. 2003) and detracting oxidation of lipid membranes (Okuma et al. 2000; Demiral and Türkan 2004).
Most studies with GB have focused on its physiological role and biosynthetic pathway, with little interest in its effect on antioxidative defense system.
So, to our knowledge, to describe the effects of exogenous use of GB in relation to antioxidant content of soybean plants under salt stress are meagre.
With this object, we comparatively interrogated the function of the exogenous GB application on lipid peroxidation quantities, antioxidant enzyme activities and glycinebetaine agglomeration in leaves of soybean seedlings under salt stress. We report here that soybean plants take up GB and accumulate it in their leaves; leading to tolerance against oxidative damage caused by salt stress.
Materials and methods
Plant material
Seeds of ‘soybean’ (Glycine max L.), were disinfected in 1 % sodium hypochlorite solution (NaOCl) for 10 min to remove probable seed-borne microorganisms, washed for 1 min under running water then were dried for 30 min at room temperature.
One layer of soybean seeds was placed in covered crystal polystyrene boxes (10 cm × 10 cm × 4 cm) on double layers of filter paper wetted with 15 ml of 0 (control), 5, 25, and 50 mM GB (Sigma–Aldrich) solution. The boxes were kept at 20 °C in the dark for 24 h. After GB application, seeds were washed for 1 min under running water and left to dry on paper towels for 24 h under room condition (20–22 °C and 50–60 % RH).
The seeds were planted at a depth of 1.0 cm × 5.0 cm-deep flat cells (75 cm3) filled with growth medium containing of perlite and peat in the proportion of 1:3. The flats were watered regularly with tap water and kept in a growth chamber at 25 ± 1 °C (day/night) under cool fluorescent lamps (100 μmol m−2 s−1) for 16 h day−1 photoperiod.
When the seedlings were developed up to 4 leaved stage (42–45 days after planting), salt stress was imposed. Half of the seedlings were watered with distilled water containing 25 mM NaCl, and the NaCl concentration was increased up to a concentration of 150 mM with daily increments of 25 mM to avoid salinity shock, while the other half of the plants were continued to be watered with only distilled water. Salt stress lasted for 15 days, after which the plants were harvested for determining the effects of salt stress and GB treatments. The treatments were repeated three times with 12 plants in every replication and all treatments were classified in a randomized complete block design (RCBD).
Determination of proline and MDA contents
MDA content was measured by the thiobarbituric acid method described by Zhang et al. (2005). MDA content was estimated according to the following formula:
where A532, A600 and A450 represent the absorbance of the mixture at 532, 600, and 450 nm, respectively.
Proline content was measured using the acid ninhydrin method described by Shan et al. (2007). Proline content expressed as μg proline g−1 FW.
Determination of CAT, SOD,APX and LOX enzyme activity
The activity of SOD was estimated using the slightly modified method of Xu et al. (2008). Fresh leaf samples (0.5 g) were quickly extracted in a pre-chilled mortar on an ice bath with 5 ml of ice-cold 100 mM phosphate buffer (pH 7.8) containing 1 mM EDTA and 5 % (w/v) PVP. After centrifugation at 10,000 × g for 30 min at 4 °C, the supernatant was used for SOD (EC 1.15.1.1) analysis. One hundred μl of the enzyme extract was mixed with 2.465 ml of 100 mM phosphate buffer (pH 7.8), 75 μl of 55 mM methionine, 300 μl of 0.75 mM nitroblue tetrazolium (NBT) and 60 μl of 0.1 mM riboflavin in a test tube. The test tubes containing the reaction solution were irradiated under 2 fluorescent light tubes (40 μmol m−2 s−1) for 10 min. The absorbance measured at 560 nm with UV/visible spectrophotometer (HACH, USA). Blanks and controls were run in the same manner, but without illumination and enzyme, respectively. One unit of SOD activity was defined as the amount of enzyme that would inhibit 50 % of NBT photo reduction.
Catalase activity was determined according to Beers and Sizer (1952). Fresh samples (200 mg) were homogenized in 5 ml of 50 mM Tris-/NaOH buffer (pH 8.0) containing 0.5 mM EDTA, 2 % (w/v) PVP and 0.5 % (v/v) Triton X-100. The homogenate was centrifuged at 22,000×g for 10 min at 4 °C and after dialysis supernatant was used for enzyme assay. Assay mixture in a total volume of 1.5 ml contained 1000 μl of 100 mM KH2PO4 buffer (pH 7.0), 400 μl of 200 mM H2O2 and 100 μl enzyme. The decomposition of H2O2 was followed at 240 nm (extinction coefficient of 0.036 mM_1 cm_1) by a decrease in absorbance. Enzyme specific activity is expressed as mmol of H2O2 oxidized min_1 (mg protein) _1.
About 200 mg plant samples were homogenized in 5 ml of 50 mM K-phosphate buffer (pH 7.8) containing 1 % PVP, 1 mM ascorbic acid and 1 mM PMSF as described by Moran et al. (1994). After centrifugation at 22 000_g for 10 min at 4 8C, the supernatant was dialyzed against the same extraction buffer and it served as enzyme. Ascorbate peroxidase was assayed according to Nakano and Asada (1981) Reaction mixture in a total volume of 1 ml contained 50 mM K-phosphate buffer (pH 7.0), 0.2 mM ascorbic acid, 0.2 mM EDTA, 20 mM H2O2 and enzyme. H2O2 was the last component to be added and the decrease in absorbance was recorded at 290 nm (extinction coefficient of 2.8 mM_1 cm_1) using a UV–vis spectrophotometer (HACH, USA) at 30 s intervals up to 7 min. Correction was made for the low, non enzymic oxidation of ascorbic acid by H2O2. The specific activity of enzyme is expressed as mmol ascorbate oxidized (min_1(mg protein)-1).
LOX activity was assayed using the method of Todd et al. (1990). The standard assay mixture contained 200 μl of Tween 20 and 40 μl of linoleic acid (Aldrich) in 40 ml of 0.1 mol L−1 sodium phosphate buffer (pH 7). To 1 ml of standard assay mixture in a cuvette, 0.2 ml of LOX extract was added. One unit of LOX activity was defined as the amount of enzyme that caused an increase in absorption at 234 nm of 0.01 min−1 at 25 °C with linoleic acid as substrate.
H2O2 analyses
For H2O2 determination, 2 g of frozen tissue was homogenized with 5 ml of chilled pure acetone and centrifuged at 10 000 × g for 20 min at 4 °C. The supernatant was collected for H2O2 analysis by a method based on titanium oxidation (Patterson et al. 1984). The acetone extract (supernatant) was mixed with hydrochloric acid containing 200 ml L−1 TiCl4 and 17 mol L−1 ammonia solution. The precipitate was washed with acetone and dissolved in 2 mol L−1 sulfuric acid for spectrophotometric measurement at 410 nm. H2O2 concentration was determined from a standard graph (from 5 to 1 mmol L−1 H2O2, constructed by direct addition of H2O2 to the titanium solution) and expressed as nmol g−1 FW.
Determination of Na+ and K+ contents
Na+ and K+ in leaves and roots were determined by the methods described by Allen et al. (1986). Before determining Na + and K+ contents of soybean seedlings, dried shoots of all plants from each replicate were ground together. Na+ and K+ contents were determined by atomic absorption spectrophotometry (HACH, USA) after wet digestion of dried tissues in a 3:1 ratio of nitric: perchloric acid mixture.
Determination of glycinebetaine
The method described by Grieve and Grattan (1983) was employed to determine GB in the leaf tissues. The optical density of the organic layer was measured at 365 nm using a spectrophotometer (HACH, USA). Leaf glycinebetaine was determined following Grieve and Grattan (1983). Leaf glycinebetaine was extracted from the dry leaf material with warm distilled water (70 °C). The extract (0.25 ml) was mixed with 0.25 ml of 2 N HCl and 0.2 ml of potassium triiodide solution. The contents were shaken and cooled in an ice bath for 90 min. Then 2.0 ml of ice cooled distilled water and 20 ml of 1,2- dichloromethane (cooled at −10 °C) were added to the mixture. The two layers were formed in the mixture. The upper aqueous layer was discarded and optical density of the organic layer was measured at 365 nm. The concentrations of glycinebetaine were calculated on fresh weight basis.
Statistical analysis
The experiments were repeated three times, and 5 soybean seedling from each treatment were taken at each sampling. Experimental results are given as means ± standard error (SE) of three replicate measurements. Analysis of variance and the Duncan multiple range test were performed by a statistical product and service solutions (SPSS) program.
Results
Salt stress markedly suppressed the shoot and root lengths of soybean plants. Plants subjected to salt stress were half of the size than those are grown under control conditions. Pre-sowing seed treatment with increasing concentrations of GB positively affected plant growth.
Lipid peroxidation
Lipid peroxidation levels in the leaves of soybean plants determined as the content of MDA given in Table 1. Application of exogenous GB to seeds reduced lipid peroxidation (MDA content) in soybean plants exposed to salt stress and the highest reduction in MDA content was observed in 50 mM GB treated plants (Table 1). MDA contents of seedlings not to be exposed to salinity stress, was not affected by exogenous GB applications compared to the control plant. In leaves of soybean plants growing under normal conditions, a considerable increase in lipid peroxidation level occurred after 7 days.
Table 1.
The effect of pre-sowing seed treatment with GB on MDA and Proline content and Na+ and K+ contents, and Na+/K+ ratio of soybean seedlings
| Treatment | GB | MDA content | Proline content | Na+content | K+ content | Na+/K+ ratio |
|---|---|---|---|---|---|---|
| Salt (−) | 0 | 30.8 ± 3.6 e | 20.25 ± 2.5 c | 41 ± 2 c | 495 ± 9 ab | 0.08 d |
| 5 | 32.6 ± 1.9 e | 19.2 ± 1.2 c | 43 ± 2 c | 420 ± 8 c | 0.1 d | |
| 25 | 37.2 ± 2 e | 22.4 ± 1.6 c | 42 ± 2 c | 440 ± 11 c | 0.09 d | |
| 50 | 35.7 ± 2.4 e | 20.8 ± 2 c | 44 ± 4 c | 545 ± 15 a | 0.08 d | |
| Salt (+) | 0 | 78.4 ± 2.2 a | 94.2 ± 4 b | 312 ± 2a | 405 ± 15 c | 0.71 a |
| 5 | 69.2 ± 1.8 b | 106 ± 2.2 a | 275 ± 5 b | 415 ± 12 c | 0.64 c | |
| 25 | 62.6 ± 3.1 b | 98.4 ± 2 b | 240 ± 8 b | 430 ± 15 c | 0.58 c | |
| 50 | 48.4 ± 2.8 c | 104 ± 3.2 a | 220 ± 9 b | 443 ± 12 c | 0.52 b |
Vertical bars represent mean ± SE
Proline content
Proline concentration in the leaves of soybean seedlings increased significantly (20 to 94) in response to salt stress (Table 1). Although seed application of GB did not result in any significant increase in proline content of seedlings grown under control conditions, GB pre-treatment caused up to 4 fold increase in proline content of seedlings exposed to salt stress.
Antioxidant enzymes
Plant antioxidant defense system consists of antioxidant enzymes and non-enzymatic antioxidants. As shown in Fig. 1, NaCl stress caused a significant increase in CAT activity in soybean seedlings. In addition to, pre-sowing seed treatment with increasing concentrations of GB increased CAT enzyme activity under saline conditions (Fig. 1). Seedlings obtained from 25 mM and 50 mM GB treatments exhibited higher enzyme activity than those obtained from non-GB-treated seeds. Under control conditions (0 mM NaCl), however, pre-treatment with increasing concentrations of GB caused only a slight increase in the CAT enzyme activity.
Fig. 1.
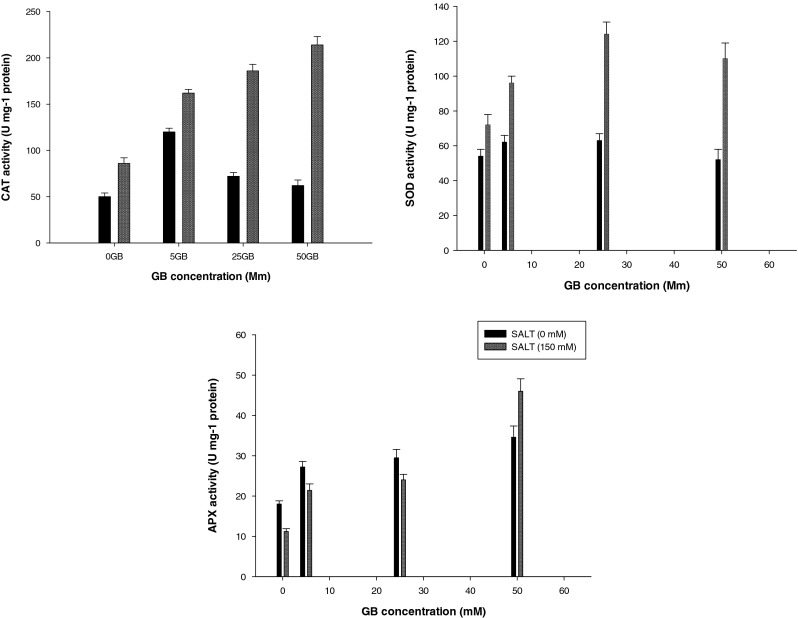
Effect of pre-sowing seed treatment with GB on CAT, SOD and APX activity of soybean plant under optimum (0 mM NaCl) and salt stress (150 mM NaCl) conditions. Vertical bars represent mean ± SE
NaCl stress caused a significant increase in SOD activity in soybean seedlings as shown in Fig. 2. Also, pre-sowing seed treatment with increasing concentrations of GB increased SOD enzyme activity under saline conditions (Fig. 2).
Fig. 2.
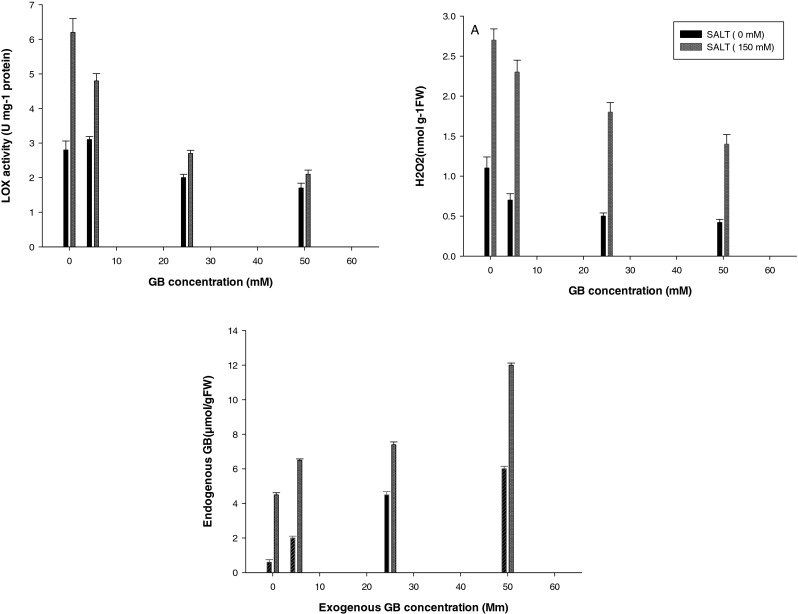
Effect of pre-sowing seed treatment with GB on LOX activity, GB and H2O2 content of soybean plant under optimum (0 mM NaCl) and salt stress (150 mM NaCl) conditions. Vertical bars represent mean ± SE
Under control conditions (0 mM NaCl), pre-treatment with increasing concentrations of GB caused only a slight increase in the SOD enzyme activity.
As shown in Fig. 1, the activities of APX increased under salt stress conditions in soybean. Exogenous application of GB increased APX activity significantly compared with control seedlings. In non-stressed seedling, exogenous GB, increased APX activity too.
The H2O2 content was measured and used as indicators of ROS level. The results (Fig. 2) showed that salt stress caused significant increases in H2O2 content. Salt stress further increased the H2O2 level reaching 245.45 % of the control plant. However, under salt stress, GB pretreatment significantly reduced ROS level.
H2O2 content in GB pretreated leaves exposed to salt stress (salt + GB) was increased by 209, 163.6 and 127.2 % in concentration of 5, 25 and 50 mM of GB respectively compared with the control group. The results suggested that exogenous GB application protected soybean leaves from oxidative damage under salt stress.
Na+ and K+ concentration
Na+ accumulation in the leaves of soybean increased by 7 fold when the plants were exposed to salt stress (Table 1). Pre-sowing seed treatment with exogenous concentration of GB, positively affected on reduction of Na+ contents in seedlings and seedlings pre-treated with 50 mM GB before sowing had the lowest Na+ contents. Exogenous GB at 25 mM reduced the Na+ content when compared with non-GB treatment under salinity conditions.
Salt stress reduced K+ content in soybean plants. K+ accumulation in the leaves decreased by 495 to 435 when the plants were exposed salt stress (Table 1). Pre-sowing seed treatment with exogenous GB has not effect K+ contents of soybean seedlings. However, leaf K+ content was not increased by GB applications (Table 1). Na+/K+ ratio was considerably reduced by exogenous GB treatments in salt-stressed plants (Table 1).
Effect of exogenous GB on endogenous GB contents
Endogenous glycinebetaine content was enhanced in both salt-treated and non-treated soybean plants with enhance the concentration of exogenously applied glycinebetaine (Fig. 2).
Discussion
In the present study, a marked decline in growth and yield of soybean plant due to salt stress was observed. These findings are parallel to what has been earlier reported for soybean plant (Xing et al. 2007; Miransari and Smith 2009). However, exogenous application of GB had a marked effect in improving soybean plant growth under salt stress condition.
These findings regarding the influence of glycinebetaine are parallel to some previous studies in which it was shown that exogenous glycinebetaine counteracts growth inhibition induced by salt stress in various crop plants, e.g., cotton (Naidu 1998), rice (Rahman et al. 2002), maize (Nawaz and Ashraf 2007), and wheat (Raza et al. 2007; Mahmood et al. 2009).
Many plants accumulate proline and betaine in response to salt stress, and increased levels of proline and betaine accumulated in plant cells correlate with enhanced stress tolerance (Munns 2002; Chen and Murata 2008; Malekzadeh et al. 2014). Known about the protective mechanisms of proline and betaine against salt stress is very little.
In addition to osmoprotectant role, GB may also have an antioxidant role in protecting plants from salt-induced oxidative damages. Under saline conditions, exogenous GB mitigates the inhibition of growth of plant cells. GB as an efficient antioxidant system often correlates with the alleviation of oxidative damage and improved tolerance to salt stress (Shalata and Tal 1998; Hasegawa et al. 2000; Shalata et al. 2001; Xing et al. 2007; Malekzadeh et al. 2012). Plants employ both enzymatic and non-enzymatic antioxidant defense systems against oxidative damage of ROS. Pre-sowing seed by GB treatment significantly (P ≤ 0. 05) declined the aggregation of leaf Na+ content, because of the application of 50 mM GB in low saline conditions, Na+ condensation slightly reduced.
Endogenous leaf glycinebetaine level increased in both salt-treated and non-treated soybean plants with increment in the concentration of exogenously applied GB (Fig. 2). Raza et al. (2007) have shown a similar endogenous GB accumulation in salt-treated wheat plants supplied with exogenous GB.
Previous studies have indicated that both LOX in plants play an important role in phospholipid catabolism by initiating a lipolytic cascade in membrane deterioration during stress (Bargmann et al. 2009). LOX plays a primary role in generating peroxidative damage in membrane lipids in plants. Lower LOX activity was shown to be related to chilling tolerance of plants as LOX could enhance the level of lipid unsaturation and enhance membrane fluidity (Lee et al. 2005).
If parallels are drawn between leaf osmotic potential and each of GB, proline, leaf Na+ and leaf K+, it is clear that K+ and GB contributed more to osmoregulatory process (Table 1).
It is well established that salt tolerance is commenly characterized to enhanced Na+ exclusion and increased absorption of K+ to maintain optimum K+/Na+ ratio in shoots (Gorham et al. 1990). As Ashraf and Harris (2004) assumed, K+/Na+ ratio might be valid selection criteria for assessing the salinity resistance of different species of plants.
Accordingly, keeping and acquisition of K+ content are major determinants of resistance to salinity. In the present study, concentration of Na+ content in the roots and shoots of soybean plants significantly increased because of the salt stress, while K+ content decreased. Moreover, application of exogenous GB declined the accumulation of Na+ content accompanied by an enhanced accumulation of K+ content, which would lead to an increased K+/Na+ ratio of soybean plants under the salt stress (Table 1). However, the mechanism by which GB accomplish this decline in Na+ and enhanced K+ accumulation needs further research. A few of the possible explanation could be (1) Biological membranes of plants use transporters for absorption of K+ from the growth medium (Chaum and Kirdmanee 2010), which play major role in maintaining cellular K+/Na+ ratios (Farooq et al. 2008). Glycinebetaine retains totality of cell membranes for appropriate action of enzymes and proteins under diverse environmental stress (Raza et al. 2007). (2) Analogously, Mahmood (2009) proposed that GB protects diverse transporters for natural functioning under drought stress. From this, it can be offered that glycinebetaine has actually a protective consequence in discriminating Na+ versus K+ under saline conditions. (3) The other possible duty of GB is that, it could increase the vacuolar output in the roots of salt stressed plants for accumulation of more Na+, as has earlier been noticed in rice (Rahman et al. 2002). Thus, these vacuoles act as reservoirs of Na+ in roots resulting in decreased Na+ transition to shoots (Table 1).
In conclusion, glycinebetaine at physiological concentrations has a direct role in supporting enzymes involved in protecting systems which participate in reducing salt stress. Exogenous glycinebetaine may improve salt tolerance in soybean plants via enhancing enzyme activity. According to the conclusions, it is suggested that the antioxidant protection activity of glycinebetaine against salt stress is powerful.
References
- Allen SE, Grimshaw HM, Rowland AP. Chemical analysis. In: Moore PD, Chapman SB, editors. Methods in Plant Ecology. 2. Oxford: Blackwell Scientific Publications; 1986. pp. 285–344. [Google Scholar]
- Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol. 2004;55:373–99. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- Ashraf M, Foolad MR. Roles of glycinebetaine and proline in improving plant abiotic stress tolerance. Environ Exp Bot. 2007;59:206–216. doi: 10.1016/j.envexpbot.2005.12.006. [DOI] [Google Scholar]
- Ashraf M, Harris PJC. Potential biochemical indicators of salinity tolerance in plants. Plant Sci. 2004;166:3–16. doi: 10.1016/j.plantsci.2003.10.024. [DOI] [Google Scholar]
- Bargmann BOR, Laxalt AM, ter Riet B, Testerink C, Merquiol E, Mosblech A, Leon-Reyes A, Pieterse CMJ, Haring MA, Heilmann I, Bartels D, Munnik T. Reassessing the role of phospholipase D in the Arabidopsis wounding response. Plant Cell Environ. 2009;32(7):837–850. doi: 10.1111/j.1365-3040.2009.01962.x. [DOI] [PubMed] [Google Scholar]
- Beers RF, Sizer IW. Colorimetric method for estimation of catalase. J Biol Chem. 1952;195:133–139. [PubMed] [Google Scholar]
- Bohnert HJ, Jensen RG. Metabolic engineering for increased salt tolerance-the next step. Aust J Plant Physiol. 1996;23:661–666. doi: 10.1071/PP9960661. [DOI] [Google Scholar]
- Chaum S, Kirdmanee C. Effect of glycinebetaine on proline, water use, and photosynthetic efficiencies, and growth of rice seedlings under salt stress. Turk J Agric Forest. 2010;34:517–527. [Google Scholar]
- Chen THH, Murata N. Glycinebetaine: an effective protectant against abiotic stress in plants. Trends Plant Sci. 2008;13:499–505. doi: 10.1016/j.tplants.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Crowe JH, Hoekstra FA, Crowe LM. Anhydrobiosis. Annu Rev Physiol. 1992;54:579–599. doi: 10.1146/annurev.ph.54.030192.003051. [DOI] [PubMed] [Google Scholar]
- Demiral T, Türkan I. Does exogenous glycinebetaine affect antioxidative system of rice seedlings under NaCl treatment? Plant Physiol. 2004;161:1089–100. doi: 10.1016/j.jplph.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Diaz-Zorita M, Fern’andez-Canigia MV, Grosso GA. Applications of foliar fertilizers containing glycinebetaine improve wheat yields. J Agron Crop Sci. 2001;186:209–215. doi: 10.1046/j.1439-037X.2001.00469.x. [DOI] [Google Scholar]
- Farooq M, Aziz T, Hussain M, Rehman H, Jabran K, Khan MB. Glycinebetaine improves chilling tolerance in hybrid maize. J Agron Crop Sci. 2008;194:152–160. doi: 10.1111/j.1439-037X.2008.00295.x. [DOI] [Google Scholar]
- Gorham J, Wyn RG, Bristol A. Partial characterization of the trait for enhanced K+-Na+ discrimination in the D genome of wheat. Planta. 1990;180:590–597. doi: 10.1007/BF02411458. [DOI] [PubMed] [Google Scholar]
- Grieve CM, Grattan SR. Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil. 1983;70:303–307. doi: 10.1007/BF02374789. [DOI] [Google Scholar]
- Harinasut P, Tsutsui K, Takabe T, Nomura M, Takabe T, Kishitani S. Exogenous glycinebetaine accumulation and increased salt-tolerance in rice seedlings. Biosci Biotechnol Biochem. 1996;60:366–368. doi: 10.1271/bbb.60.366. [DOI] [PubMed] [Google Scholar]
- Hasegawa PM, Bressan RA, Zhu JK, Bohnert HJ. Plant cellular and molecular responses to high salinity. Annu Rev Plant Physiol Plant Mol Biol. 2000;5:1463–99. doi: 10.1146/annurev.arplant.51.1.463. [DOI] [PubMed] [Google Scholar]
- Hoque MA, Banu MN, Okuma E, Amako K, Nakamura Y, Shimoishi Y, Murata Y. Exogenous proline and glycinebetaine increase NaCl-induced ascorbate-glutathione cycle enzyme activities, and proline improves salt tolerance more than glycinebetaine in tobacco Bright Yellow-2 suspension-cultured cells. J Plant Physiol. 2007;164(11):1457–1468. doi: 10.1016/j.jplph.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Khedr AHA, Abbas MA, Wahid AAA, Quick WP, Abogadallah GM. Proline induces the expression of salt-stress responsive proteins and may improve the adaptation of Pancratium maritimum L. to salt-stress. J Exp Bot. 2003;54:2553–62. doi: 10.1093/jxb/erg277. [DOI] [PubMed] [Google Scholar]
- Lee SH, Ahn SJ, Im YJ, Cho K, Chung GC, Cho BH, Han O. Differential impact of low temperature on fatty acid unsaturation and lipoxygenase activity in fig leaf gourd and cucumber roots. Biochem Biophys Res Commun. 2005;330:1194–1198. doi: 10.1016/j.bbrc.2005.03.098. [DOI] [PubMed] [Google Scholar]
- Mahmood T, Ashraf M, Shahbaz M. Does exogenous application of glycinebetaine as a pre-sowing seed treatment improve growth and regulate some key physiological attributes in wheat plants grown under water deficit conditions? Pak J Bot. 2009;41:1291–1302. [Google Scholar]
- Mäkelä P, Mantila J, Hinkkanen R, Pehu E, Peltonen-Sainio P. Effect of foliar applications of glycinebetaine on stress tolerance, growth, and yield of spring cereals and summer turnip rape in Finland. J Agron Crop Sci. 1996;176:223–234. doi: 10.1111/j.1439-037X.1996.tb00467.x. [DOI] [Google Scholar]
- Malekzadeh P, Khara J, Heydari R. Effect of exogenous Gama-aminobutyric acid on physiological tolerance of wheat seedlings exposed to chilling stress. Iranian J Plant Physiol. 2012;3:611–617. [Google Scholar]
- Malekzadeh P, Khara J, Heydari R. Alleviating effects of exogenous Gamma-aminobutiric acid on tomato seedling under chilling stress. Physiol Mol Biol Plants. 2014;20:133–137. doi: 10.1007/s12298-013-0203-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil SD, Nuccio ML, Hanson AD. Betaines and related osmoprotectants: targets for metabolic engineering of stress resistance. Plant Physiol. 1999;120:945–950. doi: 10.1104/pp.120.4.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miransari M, Smith DL. Alleviating salt stress on soybean (Glycine max L. Merr.) – Bradyrhizobium japonicum symbiosis, using signal molecule genistein. Eur J Soil Biol. 2009;45:146–152. doi: 10.1016/j.ejsobi.2008.11.002. [DOI] [Google Scholar]
- Moran JF, Becana M, Iturbe-Ormaetxe I, Frechilla S, Klucas RV, Aparicio-Tejo P. Drought induced oxidative stress in pea plants. Planta. 1994;194:346–352. doi: 10.1007/BF00197534. [DOI] [Google Scholar]
- Munns R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002;25:239–250. doi: 10.1046/j.0016-8025.2001.00808.x. [DOI] [PubMed] [Google Scholar]
- Naidu BP. Simultaneous estimation of sugars, polyols, proline analogues and betaines accumulating in stressed plants by high performance liquid chromatography-ultra violet detection. Aust J Plant Physiol. 1998;25:793–800. doi: 10.1071/PP97165. [DOI] [Google Scholar]
- Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981;22:867–880. [Google Scholar]
- Nawaz K, Ashraf M. Improvement in salt tolerance of maize by exogenous application of glycinebetaine: growth and water relations. Pak J Bot. 2007;39:1647–1653. [Google Scholar]
- Noctor G, Foyer CH. Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:249–279. doi: 10.1146/annurev.arplant.49.1.249. [DOI] [PubMed] [Google Scholar]
- Okuma E, Soeda K, Tada M, Murata Y. Exogenous proline mitigates the inhibition of growth of Nicotiana tabacum cultured cells under saline conditions. Soil Sci Plant Nutr. 2000;46:257–63. doi: 10.1080/00380768.2000.10408781. [DOI] [Google Scholar]
- Patterson BD, Mackae EA, Ferguson IB. Estimation of hydrogen peroxide in plant extracts using titanium. Anal Biochem. 1984;139:487–492. doi: 10.1016/0003-2697(84)90039-3. [DOI] [PubMed] [Google Scholar]
- Rahman MS, Miyake H, Takeoka Y. Effects of exogenous glycinebetaine on growth and ultra-structure of salt-stressed rice seedlings (Oryza sativa L.) Plant Prod Sci. 2002;5:33–44. doi: 10.1626/pps.5.33. [DOI] [Google Scholar]
- Raza SH, Athar HR, Ashraf M, Hameed A. Glycinebetaine-induced modulation of antioxidant enzymes activities and ion accumulation in two wheat cultivars differing in salt tolerance. Environ Exp Bot. 2007;60:368–376. doi: 10.1016/j.envexpbot.2006.12.009. [DOI] [Google Scholar]
- Rhodes D, Hanson AD. Quaternary ammonium and terriary sulfonium compounds in higher plants. Annu Rev Plant Physiol Plant Mol Biol. 1993;44:357–384. doi: 10.1146/annurev.pp.44.060193.002041. [DOI] [Google Scholar]
- Sakamoto A, Murata N. The role of glycinebetaine in the protection of plants from stress: clues from transgenic plants. Plant Cell Environ. 2002;25:163–171. doi: 10.1046/j.0016-8025.2001.00790.x. [DOI] [PubMed] [Google Scholar]
- Shalata A, Tal M. The effect of salt stress on lipid peroxidation and antioxidants in the leaf of the cultivated tomato and its wild salt-tolerant relative Lycopersicon pennellii. Physiol Plant. 1998;104:169–74. doi: 10.1034/j.1399-3054.1998.1040204.x. [DOI] [PubMed] [Google Scholar]
- Shalata A, Mittova V, Volokita M, Guy M, Tal M. Response of the cultivated tomato and its wild salt-tolerant relative Lycopersicon pennellii to salt-dependent oxidative stress: the root antioxidative system. Physiol Plant. 2001;112:487–94. doi: 10.1034/j.1399-3054.2001.1120405.x. [DOI] [PubMed] [Google Scholar]
- Shan DP, Huang JG, Yang YT, Guo YH, Wu CA, Yang GD, Gao ZG, Zheng CC. Cotton GhDREB1 increases plant tolerance to low temperature and is negatively regulated by gibberellic acid. New Phytol. 2007;176:70–81. doi: 10.1111/j.1469-8137.2007.02160.x. [DOI] [PubMed] [Google Scholar]
- Smirnoff N, Cumbes OJ. Hydroxyl radical scavenging activity of compatible solutes. Phytochemistry. 1989;28:1057–1060. doi: 10.1016/0031-9422(89)80182-7. [DOI] [Google Scholar]
- Subbarao GVL, Levine HE, Stutte GW. Glycinebetaine accumulation: its role in stress resistance in crops plants. In: Pessarakli M, editor. Handbook of Plant and Crop Physiology. NY: Marcel Dekker Inc; 2001. pp. 881–907. [Google Scholar]
- Todd JF, Paliyath G, Thompson JE. Characteristics of a membrane associated lipoxygenase in tomato fruit. Plant Physiol. 1990;94:1225–1232. doi: 10.1104/pp.94.3.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing SG, Jun YB, Zhang W H, Liu YL (2007) Higher accumulation of γ-aminobutyric acid induced by salt stress through stimulating the activity of diamine oxidases in Glycine max (L.) Merr. Roots. Plant Physiol and Biochem 560–566 [DOI] [PubMed]
- Xu PL, Guo YK, Bai JG, Shang L, Wang XJ. Effects of long-term chilling on ultrastructure and antioxidant activity in leaves of two cucumber cultivars under low light. Physiol Plantarum. 2008;132:467–478. doi: 10.1111/j.1399-3054.2007.01036.x. [DOI] [PubMed] [Google Scholar]
- Yadav SK, Singla-Pareek SL, Ray M, Reddy MK, Sopory SK. Methylglyoxal levels in plants under salinity stress are dependent on glyoxalase I and glutathione. Biochem Biophys Res Commun. 2005;337:61–67. doi: 10.1016/j.bbrc.2005.08.263. [DOI] [PubMed] [Google Scholar]
- Yadav SK, Singla-Pareek SL, Ray M, Reddy MK, Sopory SK. Transgenic tobacco plants overexpressing glyoxalase enzymes resist an increase in methylglyoxal and maintain higher reduced glutathione levels under salinity stress. FEBS Lett. 2005;579:6265–6271. doi: 10.1016/j.febslet.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Zhang JH, Huang WD, Liu YP, Pan QH. Effects of temperature acclimation pretreatment on the ultrastructure of mesophyll cells in young grape plants (Vitis vinifera L. cv, Jingxiu) under cross-temperature stresses. J Int Plant Biol. 2005;47:959–970. doi: 10.1111/j.1744-7909.2005.00109.x. [DOI] [Google Scholar]


