Abstract
Orthodox seed serves as easily accessible model to study desiccation-sensitivity in plant tissues because once they undergo germination, they become sensitive to desiccation imposed injuries. In the proposed study, effects of rate of drying on the viability, electrolyte leakage, superoxide accumulation, lipid-protein oxidation and antioxidant enzymes were explored in excised radicles of Cicer arietinum L. under dehydration and wet storage. For both the drying conditions, desiccation could be explained by exponential and inverse functions. Under rapid drying tissue viability as scored by germination efficiency and tetrazolium staining remained 100 % all through the analysis (24 h) but declined remarkably after 0.30 g g−1 fresh mass water content (4 days) under slow drying. Moreover, precipitous fall in tissue viability was observed after 2 weeks of wet storage. Rapid drying was also accompanied with limited amounts of electrolyte leakage, superoxide radical, malondialdehyde and protein hydroperoxide, together with enhanced level of protein. Additionally, activities of both superoxide dismutase and ascorbate peroxidase were increased in rapidly dried radicles, but guaiacol peroxidase was declined. In contrary, above referred biomarkers were observed to perform either inversely or poorly during slow drying and wet storage suggesting that above documented alterations might be the resultant of ageing and not desiccation. Gathered data demonstrated that increased drying lowers the critical water content for tissue survival and also reduces the risk of damage resulting from aqueous-based deleterious reactions. Additionally, it also showed that growing radicles are a popular model to explore desiccation-sensitivity in plant tissues and/or seeds.
Keywords: Antioxidants, Cicer arietinum L, Desiccation sensitivity, Lipid peroxidation, Reactive oxygen species, Water content
Introduction
Due to high persistence of water, recalcitrant seeds are quite sensitive towards desiccation, hence their germplasms can not be stored for longer time applying conventional approaches fixed for orthodox seeds, and also may not be available after a particular season for study or propagation purposes (Song et al. 2003). Radicles of orthodox seeds become desiccation sensitive once they undergo the process of germination (Ntuli et al. 2013). Therefore, germinated radicles can be exploited as a popular model to study the underlying mechanisms of desiccation sensitivity in seeds (Ntuli et al. 2013).
Desiccation sensitivity is the degenerative changes taking place during dehydration of desiccation-sensitive seeds (Chaitanya et al. 2000). Dehydration conditions viz. drying rate and surrounding temperature, are two key determinants widely affecting the responses of seeds towards desiccation (Rego et al. 2013). It has been noted that under slow drying (SD), seed tissues spend long periods at intermediate water contents (WC), thus allowing the accumulation of injury linked with altered metabolism. In contrast, during rapid drying (RD) embryonic axes and cotyledonary tissues pass through the intermediate WC stage quite rapidly, minimizing the risk of damage accumulation (Ntuli and Pammenter 2009). Thus, excised axes or embryos of recalcitrant seeds can possibly survive up to longer durations at low WCs when desiccated rapidly over silica gel or in a flow of air current in a flash drier (Pammenter and Berjak 1999).
In addition to physical stresses the desiccation-intolerant (recalcitrant) seed tissues incur metabolic imbalance as an outcome of dehydration. It has already been suggested that during desiccation, electron transport chain of mitochondrial and microsomal membranes becomes disrupted, which results in reactive oxygen species (ROS)-induced oxidative situation that culminates with loss of seed viability (Parkhey et al. 2012; 2014a). Moreover, Song and co-workers (2009) recently reported a fall in the activity of cytochrome c oxidase in desiccating radicles and embryos of Antaris toxicaria and Zea mays respectively, allowing excessive accumulation of ROS in these tissues. However, it remains to be explored fully whether oxidative injury is the cause or consequence of the tissue damage or viability loss (Hendry et al. 1992). Desiccation promoted ROS accumulation linked loss of viability has already been documented in several recalcitrant species like Shorea robusta (Chaitanya and Naithani 1994; Parkhey et al. 2012), Acer saccharinum (Pukacka and Ratajczak 2006) and Antiaris toxicaria (Cheng and Song 2008) as well as in orthodox species; Helianthus annuus (Bailly 2004) and Glycine max (Liu et al. 2008). A wide variety of ROS are known, out of which three [hydroxyl radical, hydrogen peroxide and superoxide (.O2−) radical] are designated to be potentially cytotoxic (Keshavkant and Naithani 2010).
The ROS so produced, if accumulates in excess, results in membrane perturbation via peroxidation of lipid moieties (Keshavkant and Naithani 2001). Lipid peroxidation was shown to be one of the most common deleterious events, resulting oxidation of polyunsaturated fatty acids, thereby releasing several cytotoxic by-products that includes malondialdehyde (MDA), alkoxyl radical, epoxides and alkanes (Parkhey et al. 2012). Keshavkant et al. (2013) reported that lipid peroxidation is one of the well-established and foremost mechanisms of cellular injury in both plant and animal cells. Desiccation prompted ROS regulated alterations in the membrane integrity has also been shown to confer enhanced leakage of cellular electrolytes during loss of viability of Artocarpus heterophyllus (Wesley-Smith et al. 2001) and Shorea robusta (Parkhey 2013) seeds.
Additionally, ROS are also known to attack over cellular proteins (Chaitanya et al. 2000). It was shown to alter both structure and functions of proteins through oxidative modifications (Parkhey et al. 2014a). Reactive oxygen species oxidizes to proteins by directly attacking on it following various strategies like carbonylation, nitrosylation, glutathionylation and disulphide bond formation with specific amino acids, and indirectly by making adducts of it with lipid peroxidized products such as MDA (Parkhey et al. 2014a). Accumulation of large amount of protein hydroperoxide (PrOOH), a well recognized derivative of oxidized protein, has also been indicated during accelerated ageing (Keshavkant et al. 2013) and desiccation (Parkhey et al. 2014a) of seeds. It was shown to be the most reactive and prime product generated during ROS attack on proteins.
It has been suggested that a range of processes or mechanisms are operating to contribute desiccation tolerance (DT) in seeds (Berjak and Pammenter 2008). Among which, existence and efficient functioning of antioxidant machinery, which includes both enzymatic {superoxide dismutase (SOD), catalase (CAT), guaiacol peroxidase (POX), ascorbate peroxidase (APX), etc.} and non-enzymatic {ascorbic acid, α-tocopherol, carotenoid, etc.} components, was considered as one of the potent requirements conferring DT to seeds (Berjak and Pammenter 2008). However, above protective system might become impaired during desiccation or hydrated storage and certainly are inefficient in removal of ROS, resulting oxidative injury in seed tissues (Chaitanya and Naithani 1994; Ntuli et al. 2013).
Previously it was already indicated that RD of desiccation-intolerant seeds allows survival to lower water contents than SD (Huang et al. 2009; Ntuli et al. 2011; 2013). Researchers have suggested that such behaviour depends upon removal of water molecules sufficiently fast in view to inhibit the accumulation of injury resulting from aqueous-based detrimental metabolic reactions (Pammenter and Berjak 1999). Further, wet storage (WS) has been suggested to be one of the long-term, low intensity desiccation protocols, applicable for seeds/ seedlings (Pammenter et al. 1994). It is very much obvious that detrimental metabolic reactions (metabolic imbalance, lipid peroxidation, antioxidant impairment) participating during hydrated storage are very much similar (Ntuli et al. 2013).
Major objectives fixed for the present study were to monitor the effects of rate of drying on viability, ROS level, lipid-protein oxidation and free radical processing enzymes during desiccation (both RD and SD) and WS of Cicer arietinum L. radicles.
Material and methods
Experimental material
Seeds of Cicer arietinum L. were purchased from the market; healthy uninfected seeds were sorted out. Selected seeds were sterilized initially with 1 % (v/v) sodium hypochlorite solution for 5 mins followed by washing with MilliQ water (MW) (Millipore, Gradient A-10, USA). Sterilized seeds were then placed in germination boxes (30x15x5 cm), over two layers of MW moistened filter paper towels (Parkhey et al. 2012). These boxes were then placed in darkness at 26 ± 2 °C. The MW was supplied to the germinating seeds, whenever needed. Germinated seeds exhibiting 10 mm long radicles were harvested, their radicles were excised carefully using a sharp razor and stored over a moist filter paper in a closed Petri dish.
Desiccation trials
The excised radicles were initially divided into three separate groups. Each group was placed in sterile containers and then subjected to three distinct types of desiccation trials. 1. Rapid drying: Executed with self indicating activated silica gels (close to 0 % RH). Radicles were initially placed in net bags, which were then kept over a layer of silica gel, half-filled in an air tight glass desiccator, and above this a layer of silica gel was spread again, to extract tissue water from all the sides. Radicles were desiccated for 6, 12, 18 and 24 h, and hydrated silica was replaced after each two hour with fresh lots. 2. Slow drying: Radicles were placed on a plastic mesh bridge made over saturated solution of NaCl, yielding desired relative humidity (75 ± 1 % RH) inside the air tight container. Radicles were dried for 1, 2, 4 and 7 days. 3. Wet storage: Radicles were placed over a MW saturated filter paper towel in order to maintain 100 % RH inside the sterile Petri dish and were stored for 1, 2, 3 and 4 weeks. All the experimental containers were stored at 20 ± 1 °C until no further desiccation occurred.
Determination of water content
Water content of the radicles was assessed gravimetrically following Parkhey et al. (2014b). Five independent sets of 10 radicles each were weighed electronically (Sartorius, Sweden) before and after oven drying at 103 °C for 17 h. Water content was calculated as g g−1 fresh mass (FM).
Determination of viability, germination and leakage loss
In order to reduce the imbibitional injury, desiccated radicles were initially incubated over moist filter paper towel in Petri plates for 12 h. Afterwards radicles were subjected to the tetrazolium (TZ), germination and electrolyte leakage tests.
To evaluate germinability, moistened radicles were grown in Petri dishes on half strength MS medium (Murashige and Skoog 1962) with 30 g l−1 sucrose and 1 mg l−1 benzylaminopurine for a period of 25 days (16 h of photoperiod and 25 ± 2 °C temperature). Only those radicles were considered as germinated that showed significant change in elongation/ expansion or greening.
Viability of the radicles was assessed following TZ staining (Parkhey 2013). 0.2 g of radicles were soaked for overnight in 0.1 % (w/v) 2,3,5- triphenyl tetrazolium chloride solution, in darkness. Soaked radicles were extracted with 2 ml of absolute ethanol and centrifuged at 5000 g for 10 mins at room temperature (26 ± 2 °C). Supernatant thus collected were read at 520 nm using a UV-vis spectrophotometer (Lambda 25, Perkin Elmer, USA) and values were expressed as A520 g−1 FM.
Conductivity of the electrolytes leaked from radicles was measured following Parkhey (2013). 0.2 g of radicles were soaked in 15 ml of MW and then incubated for 24 h at room temperature. Electrical conductance of the soaking solution was recorded using Multi-cell Conductivity Meter (CM 183, Elico, India) and data were expressed in terms of mS g−1 FM.
Assessment of superoxide
0.2 g of radicle was extracted in chilled sodium phosphate buffer (0.2 M, pH 7.2) containing 10−3 M diethyldithiocarbamate to inhibit SOD activity (Sangeetha et al. 1990). The homogenate was centrifuged for 5 mins at 10,000 g. In the extract, .O2− content was determined by its capacity to reduce nitro blue tetrazolium (2.5 × 10−4 M). The absorbance of the end product was measured at 540 nm and .O2− content was expressed as μM min−1 g−1 FM.
Measurement of lipid peroxidation
Peroxidation of lipid was evaluated by measuring amount of MDA (Hodges et al. 1999). 0.2 g of radicle tissue was homogenized with 0.5 % (w/v) 2-thiobarbituric acid prepared in 20 % (w/v) trichloroacetic acid (TCA). The homogenate was boiled for 30 mins, cooled and centrifuged at 10,000 g for 10 mins. The absorbance of the supernatant was measured at 532 nm and extinction coefficient of 157 mM−1 cm−1 was used to calculate the amount of MDA. Content of MDA was written in terms of mM g−1 FM.
Protein and enzyme: extraction
Zero point two grams of radicle was homogenized in chilled potassium phosphate buffer (10 mM, pH 7.2) consisting 1 mM ethylenediaminetetraacetic acid, 2 mM dithiothreitol and 0.2 % (v/v) Triton X-100. Homogenate was then centrifuged at 14,000 g for 20 mins at 4 °C so as to get clear supernatant, which can be used for measuring protein, PrOOH and antioxidant enzymes.
Protein quantification
Content of protein was determined following the method of Bradford (1976) and expressed as mg g−1 FM.
Determination of protein hydroperoxide
Protein hydroperoxide content was estimated following Gay et al. (1999). Initially, isolated protein was precipitated by mixing with 2 ml of cold 10 % (w/v) TCA. The protein pellets were washed repeatedly with cold 10 % (w/v) TCA and then mixed properly in 1.8 ml of 25 mM H2SO4. To it, 100 μl each of ferrous ammonium sulphate (5 mM) and xylenol orange (5 mM) were added. Above mixture was allowed to stand at room temperature for 30 mins in the darkness, and then centrifuged (10,000 g, 20 mins). Supernatant thus obtained was measured at 560 nm. The PrOOH contents were calculated using a molar extinction coefficient of 20,100 M−1 cm−1 and expressed as nM g−1 FM.
Assessment of antioxidants
Activity of SOD (EC 1.15.1.1) was assayed following the method of Marklund and Marklund (1974) by estimating percent inhibition of pyrogallol auto-oxidation at 420 nm. One unit of SOD activity was defined as the quantity of enzyme that inhibited the nitro blue tetrazolium photoreduction by 50 %. Activity of SOD was expressed as Unit of SOD min−1 g−1 FM.
The POX (EC 1.11.1.17) activity was assayed by following the method of Chance and Maehly (1955). The tetraguaiacol formed in the assay mixture has a maximum absorption at 470 nm. Activity of POX was expressed as A470 min−1 g−1 FM.
Activity of APX (EC 1.11.1.11) was estimated following the method of Nakano and Asada (1981) by evaluating the rate of ascorbate oxidation at 290 nm. Its activity was given as A290 min−1 g−1 FM.
Statistical analyses
The influence of desiccation treatments on different parameters were assessed by using One-way ANOVA. The associations between studied parameters were evaluated by Pearson’s correlation coefficient test. Values presented are mean ± SD of five replicates. All statistical analyses were performed using SPSS software version 16.0.
Results
Water content
The C. arietinum L. radicles containing 0.94 g g−1 FM of WC showed significant change under distinct desiccation trials. During RD, enormous fall (6.7 fold, P < 0.05) in WC was observed that reaches to 0.14 g g−1 FM within 24 h only (Fig. 1a), exhibiting a negative (r = −0.85, P < 0.05) correlation in between. But, under SD, loss in WC was around 3.7 fold only, even after 7 days of storage, which was measured to be 0.25 g g−1 FM (Fig. 1a). In contrast to other two treatments, the WS radicle maintained constant level of WC throughout (Fig. 1b).
Fig. 1.
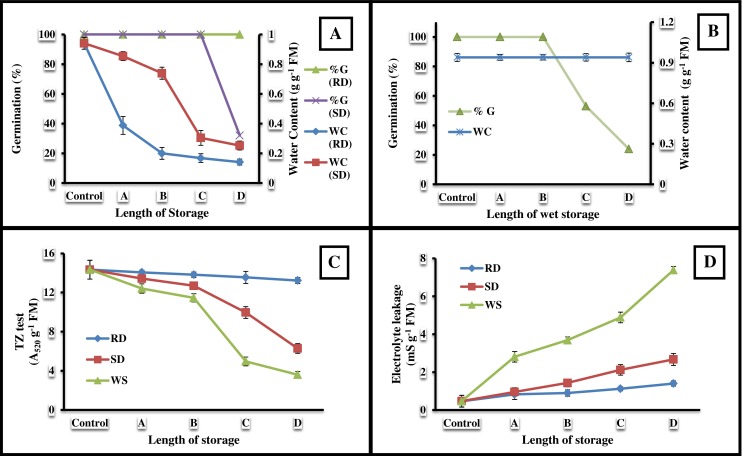
Variations in water content (a, b), viability (c) and electrical conductance (d) of Cicer arietinum L. radicles under three different sets of desiccation; rapid drying, slow drying and wet storage. In the current experimentation, length of incubation varies with type of treatment, viz; in case of RD: A = 6 h, B = 12 h, C = 18 h and D = 24 h; for SD: A = 1 day, B = 2 days, C = 4 days and D = 7 days, and for WS: A = 1 week, B = 2 weeks, C = 3 weeks and D = 4 weeks. Each observation plotted in the Figures is mean ± SD of five separate observations
Viability
Germination of non-treated radicle was 100 %, and was remained at that level throughout the RD (Fig. 1a). However, remarkable (P < 0.05) decline in germinability was recorded after 0.30 g g−1 FM of radicle WC, following SD (Fig. 1a). In line, gradual fall in germinability was recorded immediately after 2 weeks of WS (Fig. 1b).
During RD, constant value of TZ staining was recorded throughout (Fig. 1c). Other hand, it was declined slightly between initial days of both SD and WS, and then sharply (Fig. 1c).
Electrolyte leakage measured for fresh radicle was only 0.46 mS g−1 FM (Fig. 1d). During RD, the rise in it was found to be 3 fold higher, than the control, after 24 h of desiccation. Whereas, under two other treatments; SD and WS, remarkable (P < 0.05) shoot-ups (5.6 and 16 fold respectively) in the leakage rate was calculated, compared to control (Fig. 1d). Data exhibited positive (r = 0.99, P < 0.05) association between treatments and electrolyte leakage.
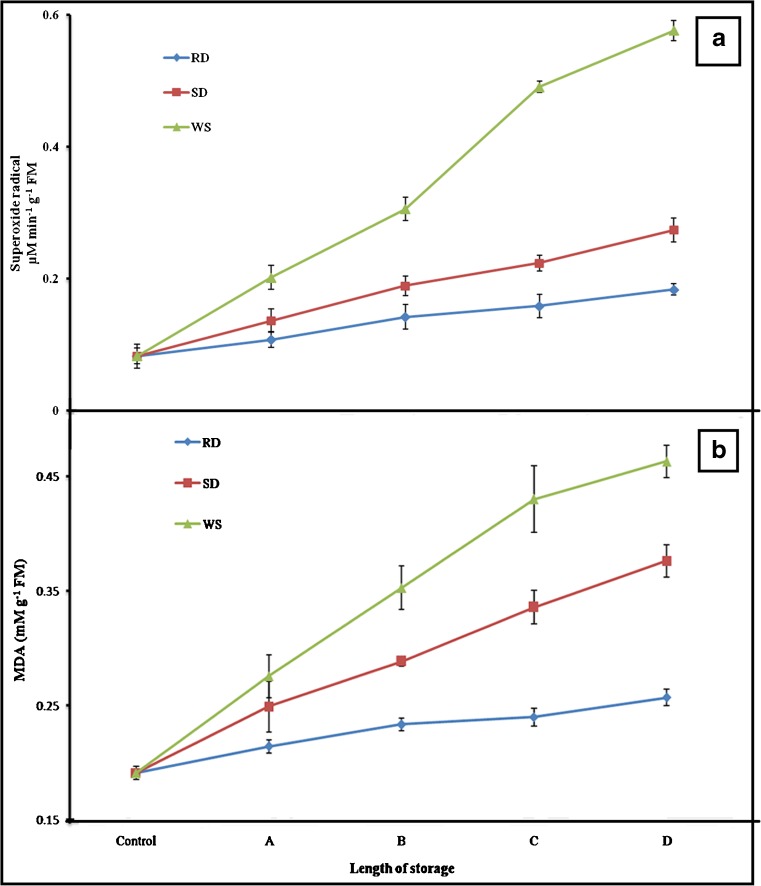
Superoxide radical
In the fresh radicles, superoxide content was very limited (0.08 μM min−1 g−1 FM) (Fig. 2a). The pace of its accumulation was 2.2, 3.3 and 7 folds under RD, SD and WS respectively (Fig. 2a). Its least was measured in RD radicles, whereas maximum (P < 0.05) in WS. A positive (r = 0.99, P < 0.05) link between WS and superoxide accumulation was observed.
Fig. 2.
Superoxide production (a) and MDA accumulation (b) in Cicer arietinum L. radicles under rapid drying, slow drying and wet storage conditions. In the current investigation, radicles were desiccated for various time lengths viz; in case of RD: A = 6 h, B = 12 h, C = 18 h and D = 24 h; for SD: A = 1 day, B = 2 days, C = 4 days and D = 7 days, and for WS: A = 1 week, B = 2 weeks, C = 3 weeks and D = 4 weeks. Each observation is mean ± SD of five replications
Lipid peroxidation
In respect to control (0.19 mM g−1 FM), maximum (2.4 fold, P < 0.05) level of MDA was measured in WS radicles and least (1.3 fold) was in RD (Fig. 2b). Amount of MDA (1.9 fold) estimated in SD radicle was in between the above two (Fig. 2b). MDA accumulation was found to be linked closely (r = 0.99, P < 0.05) with tested treatments.
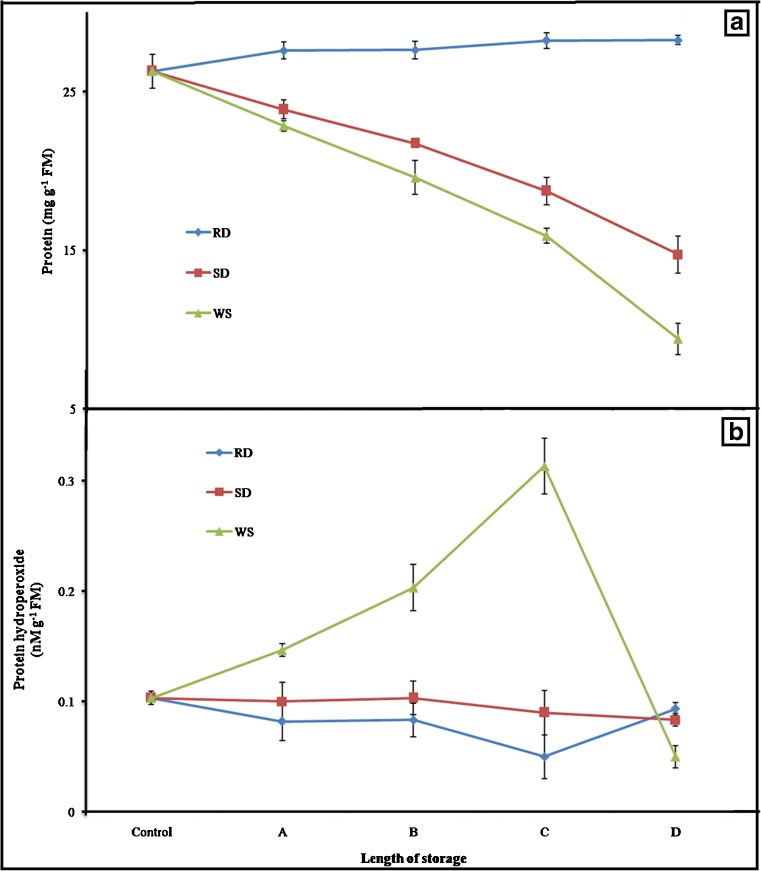
Protein content
Amount of protein measured in control radicle was 26.28 mg g−1 FM (Fig. 3a). A small rise (2 mg g−1 FM only) in the protein was observed after 24 h of RD (Fig. 3a). In contrast, remarkable (1.7 and 2.8 folds, P < 0.05) drop in it was discernible under SD and WS (Fig. 3a), exhibiting its negative association (r = −0.99, P < 0.05) with treatments.
Fig. 3.
Influence of rapid drying, slow drying and wet storage on protein (a) and protein hydroperoxide (b) contents of Cicer arietinum L. radicles. In this approach, radicles were dried for distinct timings such as; during RD: A = 6 h, B = 12 h, C = 18 h and D = 24 h; for SD: A = 1 day, B = 2 days, C = 4 days and D = 7 days, and for WS: A = 1 week, B = 2 weeks, C = 3 weeks and D = 4 weeks. Plotted values are mean ± SD of five replicates
Protein hydroperoxide
Regular fall in PrOOH was observed under both RD and SD, although its level was little higher in the SD radicles (Fig. 3b). In contrast, notable rise (3.1 fold, P < 0.05) in it was observed up to 3 weeks of WS, then declined suddenly and reached to its initial (Fig. 3b).
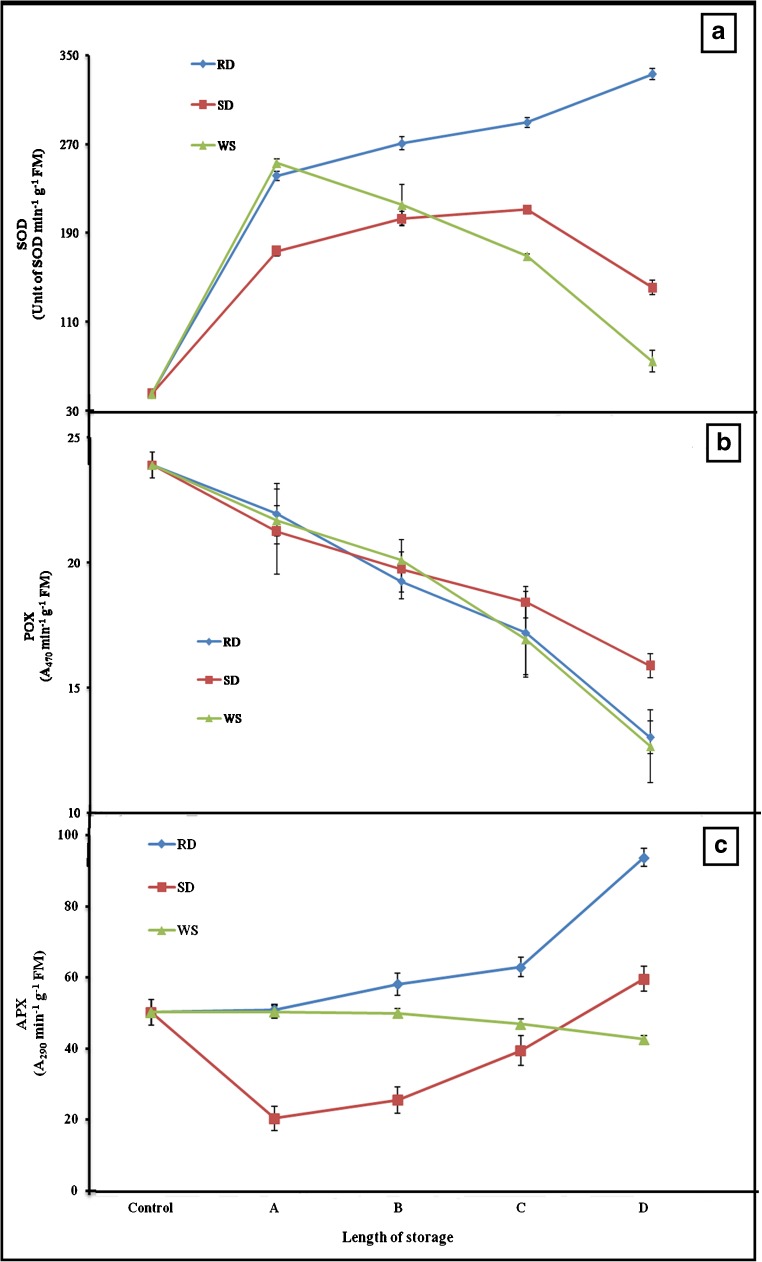
Antioxidants
Continuous upsurge in the SOD was discernible in RD radicles and its highest (7.3 fold) was measured after 24 h (Fig. 4a), exhibiting its closeness (r = 0.87, P < 0.05) with drying. Similar to RD, rise in SOD was noted initially up to 4th day (4.6 fold) and 1 week (5.6 fold) of SD and WS respectively. Later on, declining trend (P < 0.05) of it was noticed under both SD and WS, and reached to their initials finally (Fig. 4a).
Fig. 4.
Activities of SOD (a), POX (b) and APX (c) in Cicer arietinum L. radicles desiccated for various timings under varied conditions. In this research, radicles were stored differently for various time lengths like; in case of RD: A = 6 h, B = 12 h, C = 18 h and D = 24 h; for SD: A = 1 day, B = 2 days, C = 4 days and D = 7 days, and for WS: A = 1 week, B = 2 weeks, C = 3 weeks and D = 4 weeks. Data presented are mean ± SD of five independent recordings
In general, declining pattern of POX was observed along with treatments. Under both RD and WS, rate of activity reduction was quite similar i.e., 1.8 fold (Fig. 4b). Slight differently, SD discernible little less reduction (1.5 fold) in its activity (Fig. 4b). A negative association (r = −0.99, P < 0.05) between POX and tested treatment was investigated.
Parallel to desiccation, gradual rise in APX was discernible up to 18 h of RD, and then rose abruptly (1.4 fold, P < 0.05) up to 24 h (Fig. 4c). Likewise, SD also showed increases in APX although its level was less than the RD (Fig. 4c). Other hand, a small reduction in APX was registered in WS samples (Fig. 4c). APX exhibited positive association (r = 0.86, P < 0.05) with SD, but was negative (r = −0.88, P < 0.05) with WS.
Discussion
Current investigation compared the physiological and biochemical responses of C. arietinum L. radicles in affiliation to different regimes of desiccation. In connection, it must be remembered that the difference in water potentials between the cell and the surrounding air instigates release of cellular water (Wexler 1997). That is why WC of the WS radicles was measured to be constant (0.94 g g−1 FM) throughout as the surrounding air is full of humidity (Fig. 1b). Experiments revealed that RD significantly reduced the radicle WC (r = −0.85, P < 0.05), but not the germinability (Fig. 1a). Further, remarkable drop (3.1 fold, P < 0.05) in tissue viability was recorded when were stored under both SD and WS (Fig. 1a and c), and for this dehydration injury and ageing respectively, were held responsible. In contrast, highest (P < 0.05) value of electrolyte leakage was recorded for WS radicles and least for RD (Fig. 1d), because under RD, membranes are damaged a little only as they were exposed towards stress condition for a while compared to the SD and WS. In agreement, similar results were also published for tissues of Quercus robur and Pisum sativum when were kept under defined conditions by Ntuli et al. (2011; 2013).
Desiccation implies over production of ROS in seeds kept under storage (Pukacka and Ratajczak 2006; Parkhey et al. 2014a). Like ours (Fig. 2a), Li and Sun (1999) and Parkhey et al. (2012) also perceived increased accumulation of ⋅O2− during storage/ desiccation of seeds. During RD, radicles were exposed for a while towards stress condition, hence resulted in low levels of ⋅O2− (Ntuli et al. 2013). In contrast, during both SD and WS, it was overproduced chiefly due to age related imbalance in cellular metabolism and not desiccation (Wesley-Smith et al. 2001; Berjak and Pammenter 2008).
Desiccation prompted loss of viability during storage of Trichilia dregeana, Clausena lansium and Shorea robusta seed was accompanied by increased peroxidation of lipids (Song et al. 2004; Huang et al. 2009; Parkhey et al. 2012). Similarly, at excessive WCs also, oxidative reactions that are dependent upon altered cellular metabolism can occur, leading deteriorative changes (Wesley-Smith et al. 2001; Ntuli et al. 2013). In SD and WS radicles, MDA content was significantly (P < 0.05) higher (r = 0.99, P < 0.05) than in the RD (Fig. 2b), suggesting that RD radicle spends a limited time at intermediate WCs which was insufficient for damage consequent upon the detrimental water-based reactions to accumulate (Pammenter et al. 1991). Our data depicted an inverse relationship (r = −94, P < 0.05) between DT capacity and MDA accumulation.
In agreement to Ntuli et al. (2013) and Parkhey et al. (2014a), C. arietinum L. exhibited considerable (P < 0.05) fall in protein content under both SD and WS, but was maintained high enough throughout the RD (Fig. 3a). Reduced protein during SD and WS might be the outcome of increased activities of proteases or other catabolic enzymes or due to the fragmentation of proteins or excessive availability of ROS (Parkhey et al. 2014a). Inverse to protein, increased PrOOH was invariably measured in seeds kept under storage (Parkhey et al. 2014a), and was observed here also, in particular during both SD and WS (Fig. 3b). This high PrOOH may be the resultant of deteriorative processes going on in stressed tissues (Parkhey et al. 2014a). Additionally, sudden fall in PrOOH in dead tissues of WS may possibly be due to the breakdown of unstable cytotoxic molecules (Ntuli et al. 2011).
The ROS processing enzymes performs differently species to species, and also among tissues of a seed (Chaitanya and Naithani 1994; Li and Sun 1999). In this study, a rise in SOD was observed under RD (r = 0.87, P < 0.05), whereas it was increased initially and then declined under both SD and WS (Fig. 4a). Other hand, gradual fall in POX was discernible under all three treatments (r = −0.99, P < 0.05) (Fig. 4b). Additionally, the APX increased upon desiccation (RD and SD, r = 0.86, P < 0.05) but declined in WS radicles (r = −0.88, P < 0.05) (Fig. 4c). It is argued that the rise in both SOD and APX during RD contributed to lower down the injury. In contrast, both SD and WS allowed insufficient antioxidants resulting in over production of ROS, thereby enhanced MDA and PrOOH, and leakage of electrolytes, as observed previously by Ntuli et al. (2011; 2013).
Enlisted findings suggested that RD significantly lowers the critical WC for survival of desiccation-intolerant seeds. Data also indicated that such a phenomenon arises as an outcome of limited incidence of metabolic inequity together with efficient operation of ROS processing enzymes. In addition, concept floated in the proposed study can routinely be applied in order to unravel the underlying mechanisms of desiccation sensitivity in recalcitrant seeds as due to their typical inherent nature they may not be available after a particular season for studies.
Acknowledgments
The authors gratefully acknowledge financial assistance awarded to Jipsi Chandra by Pt. Ravishankar Shukla University, Raipur (Research Fellowship No. 79/8/Fin.Sch/2014, dated 16.04.14). Authors are also grateful to Department of Science and Technology, New Delhi for providing financial support under DST-FIST scheme (Sanction No. 2384/IFD/2014-15, dated 31.07.2014).
References
- Bailly C. Active oxygen species and antioxidants in seed biology. Seed Sci Res. 2004;14:93–107. doi: 10.1079/SSR2004159. [DOI] [Google Scholar]
- Berjak P, Pammenter NW. From Avicennia to Zizania: seed recalcitrance in perspective. Ann Bot. 2008;101:213–228. doi: 10.1093/aob/mcm168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for quantitation of microgram quantities of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chaitanya KSK, Keshavkant S, Naithani SC. Changes in total protein and protease activity in dehydrating recalcitrant sal (Shorea robusta) seeds. Silva Fenn. 2000;34:71–77. doi: 10.14214/sf.646. [DOI] [Google Scholar]
- Chaitanya KSK, Naithani SC. Role of superoxide, lipid peroxidation and superoxide dismutase in membrane perturbations during loss of viability in sal (Shorea robusta) seeds. New Phytol. 1994;126:623–627. doi: 10.1111/j.1469-8137.1994.tb02957.x. [DOI] [Google Scholar]
- Chance B, Maehly AC. Assays of catalase and peroxidase. In: Colowick SP, Kaplan NO, editors. Methods in enzymology. New York: Academic; 1955. pp. 443–450. [Google Scholar]
- Cheng HY, Song SQ. Possible involvement of reactive oxygen species scavenging enzymes in desiccation sensitivity of Antiaris toxicaria seeds and axes. J Integr Plant Biol. 2008;50:1549–1556. doi: 10.1111/j.1744-7909.2008.00723.x. [DOI] [PubMed] [Google Scholar]
- Gay C, Collins J, Gebicki JM. Hydroperoxide assay with the ferric xylenol orange complex. Anal Biochem. 1999;273:149–155. doi: 10.1006/abio.1999.4208. [DOI] [PubMed] [Google Scholar]
- Hendry GAF, Finch-Savage WE, Thorpe PC, Atherton NM, Buckland SM, Nilsson KA, Seel WE. Free radical processes and loss of viability during desiccation in the recalcitrant species Quercus robur L. New Phytol. 1992;122:273–279. doi: 10.1111/j.1469-8137.1992.tb04231.x. [DOI] [PubMed] [Google Scholar]
- Hodges DM, Delong JM, Forney CF, Prang RK. Improving the thiobarbituric acid reactive substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta. 1999;207:604–611. doi: 10.1007/s004250050524. [DOI] [PubMed] [Google Scholar]
- Huang H, Song SQ, Wu XJ. Responses of Chinese wampee radicles and maize embryos to dehydration at different rates. J Integr Plant Biol. 2009;51:67–74. doi: 10.1111/j.1744-7909.2008.00772.x. [DOI] [PubMed] [Google Scholar]
- Keshavkant S, Naithani SC. Chilling-induced oxidative stress in young sal (Shorea robusta) seedlings. Acta Physiol Plant. 2001;23:457–466. doi: 10.1007/s11738-001-0056-3. [DOI] [Google Scholar]
- Keshavkant S, Naithani SC. Chilling induced superoxide production, lipid peroxidation and leakage loss in Shorea robusta seedlings. Ind J Plant Physi. 2010;15:191–196. [Google Scholar]
- Keshavkant S, Sahu B, Parkhey S. Artificial ageing induced metabolic changes in Cicer arietinum seeds: ROS catabolism, lipid peroxidation, protein carbonylation, nucleic acid integrity and antioxidants. Germany: Lambert Academic Publishing; 2013. pp. 1–102. [Google Scholar]
- Li CR, Sun WQ. Desiccation sensitivity and activities of free radical-scavanging enzymes in recalcitrant Theobroma cocoa seeds. Seed Sci Res. 1999;9:209–217. doi: 10.1017/S0960258599000252. [DOI] [Google Scholar]
- Liu SJ, Wei ZM, Huang JQ. The effect of co-cultivation and selection parameters on Agrobacterium-mediated transformation of Chinese soybean varieties. Plant Cell Rep. 2008;27:489–498. doi: 10.1007/s00299-007-0475-8. [DOI] [PubMed] [Google Scholar]
- Marklund S, Marklund G. Involvement of the superoxide anion radical in the auto-oxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays of tobacco tissue cultures. Physiol Plantarum. 1962;15:473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplast. Plant Cell Physiol. 1981;22:867–880. [Google Scholar]
- Ntuli TM, Finch-Savage WE, Berjak P, Pammenter NW. Increased drying rate lowers the critical water content for survival in embryonic axes of English Oak (Quercus robur L.) seeds. J Integr Plant Biol. 2011;53:270–280. doi: 10.1111/j.1744-7909.2010.01016.x. [DOI] [PubMed] [Google Scholar]
- Ntuli TM, Pammenter NW. Dehydration kinetics of embryonic axes from desiccation-sensitive seeds: an assessment of descriptive models. J Integr Plant Biol. 2009;51:1002–1007. doi: 10.1111/j.1744-7909.2009.00875.x. [DOI] [PubMed] [Google Scholar]
- Ntuli TM, Pammenter NW, Berjak P. Increasing the rate of drying reduces metabolic imbalance, lipid peroxidation and critical water content in radicles of garden pea (Pisum sativum L.) Biol Res. 2013;46:121–130. doi: 10.4067/S0716-97602013000200002. [DOI] [PubMed] [Google Scholar]
- Pammenter NW, Berjak P. A review of recalcitrant seed physiology in relation to desiccation- tolerance mechanisms. Seed Sci Res. 1999;9:13–37. doi: 10.1017/S0960258599000033. [DOI] [Google Scholar]
- Pammenter NW, Berjak P, Farrant JM, Smith MT, Ross G. Why do stored, hydrated recalcitrant seeds die? Seed Sci Res. 1994;4:187–191. doi: 10.1017/S0960258500002178. [DOI] [Google Scholar]
- Pammenter NW, Vertucci CW, Berjak P. Homeohydrous (recalcitrant) seeds: dehydration, the state of water and viability characteristics in Landolphia kirkii. Plant Physiol. 1991;96:1093–1098. doi: 10.1104/pp.96.4.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhey S (2013) Reactive oxygen species mediated lipid peroxidation, protein carbonylation and DNA fragmentation in Shorea robusta seeds during natural ageing [Ph. D. Thesis]. Pt. Ravishankar Shukla University, Raipur, India, 1–183
- Parkhey S, Naithani SC, Keshavkant S. ROS production and lipid catabolism in desiccating Shorea robusta seeds during aging. Plant Physiol Biochem. 2012;57:261–267. doi: 10.1016/j.plaphy.2012.06.008. [DOI] [PubMed] [Google Scholar]
- Parkhey S, Naithani SC, Keshavkant S. Protein metabolism during natural ageing in desiccating recalcitrant seeds of Shorea robusta. Acta Physiol Plant. 2014;36:1649–1659. doi: 10.1007/s11738-014-1540-x. [DOI] [Google Scholar]
- Parkhey S, Tandan M, Keshavkant S. Salicylic acid and acquisition of desiccation tolerance in Pisum sativum seeds. Biotechnology. 2014 [Google Scholar]
- Pukacka S, Ratajczak E. Antioxidative response of ascorbate glutathaione-pathway enzymes and metabolites to desiccation of recalcitrant Acer saccharinum seeds. Plant Physiol. 2006;163:1259–1266. doi: 10.1016/j.jplph.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Rego SS, Nogueira AC, Medeiros ACDS, Petkowicz CADO, Santos AFD. Physiological behavior of Blepharocalyx salicifolius and Casearia decandra seeds on the tolerance to dehydration. J Seed Sci. 2013;35:1–3. doi: 10.1590/S2317-15372013000300008. [DOI] [Google Scholar]
- Sangeetha P, Das VN, Koratkar R, Suryaprabha P. Increase in free radical generation and lipid peroxidation following chemotherapy in patients with cancer. Free Rad Biol Med. 1990;8:15–19. doi: 10.1016/0891-5849(90)90139-A. [DOI] [PubMed] [Google Scholar]
- Song SQ, Berjak P, Pammenter N. Desiccation sensitivity of Trichilia dregeana axes and antioxidant role of ascorbic acid. Acta Bot Sin. 2004;46:803–810. [Google Scholar]
- Song SQ, Berjak P, Pammenter N, Ntuli TM, Fu JR. Seed recalcitrance: a current assessment. Acta Bot Sin. 2003;45:638–643. [Google Scholar]
- Song SQ, Tian MH, Kan J, Cheng HY. The response difference of mitochondria in recalcitrant Antiaris toxicaria axes and orthodox Zea mays embryos to dehydration injury. J Integr Plant Biol. 2009;51:646–653. doi: 10.1111/j.1744-7909.2009.00836.x. [DOI] [PubMed] [Google Scholar]
- Wesley-Smith J, Walters C, Pammenter NW, Berjak P. Interactions of water content, rapid (non-equilibrium) cooling to −196 °C and survival of embryonic axes of Aesulus hippocastanum L seeds. Ann Bot. 2001;88:653–664. doi: 10.1006/anbo.2001.1519. [DOI] [PubMed] [Google Scholar]
- Wexler A. Constant humidity solutions. In: Lide DR, editor. Handbook of chemistry and physics. New York: CRC Press; 1997. pp. 24–25. [Google Scholar]