Abstract
Curcuma longa L., commonly known as turmeric, is one of the economically and medicinally important plant species. It is predominantly cultivated in the tropical and sub tropical countries. India is the largest producer, and exporter of turmeric in the world, followed by China, Indonesia, Bangladesh and Thailand. In the present study, Directed Amplification of Minisatellite DNA (DAMD) and Inter Simple Sequence Repeats (ISSR), methods were used to estimate the genetic variability in indigenous turmeric germplasm. Cumulative data analysis for DAMD (15) and ISSR (13) markers resulted into 478 fragments, out of which 392 fragments were polymorphic, revealing 82 % polymorphism across the turmeric genotypes. Wide range of pairwise genetic distances (0.03–0.59) across the genotypes revealed that these genotypes are genetically quite diverse. The UPGMA dendrogram generated using cumulative data showed significant relationships amongst the genotypes. All 29 genotypes studied grouped into two clusters irrespective of their geographical affiliations with 100 % bootstrap value except few genotypes, suggesting considerable diversity amongst the genotypes. These results suggested that the current collection of turmeric genotypes preserve the vast majority of natural variations. The results further demonstrate the efficiency and reliability of DAMD and ISSR markers in determining the genetic diversity and relationships among the indigenous turmeric germplasm. DAMD and ISSR profiling have identified diverse turmeric genotypes, which could be further utilized in various genetic improvement programmes including conventional as well as marker assisted breeding towards development of new and desirable turmeric genotypes.
Keywords: DAMD, Genetic diversity, ISSR, Turmeric germplasm
Introduction
The genus Curcuma (Zingiberaceae) contains about 80 species all over Asia, South East Asia and Africa (Velayudhan et al. 1999). Turmeric (Curcuma longa L.), also known as “Golden Spice” is one of the economically important species under the genus Curcuma, which is predominantly cultivated in the tropical and sub tropical countries. India is the largest producer and exporter of turmeric in the world, followed by China, Indonesia, Bangladesh and Thailand (Selvan and Thomas 2002). There are innumerable medicinal uses of the turmeric, varying from cosmetic face cream to the prevention of Cox-2 inhibitors (Duke 2003). Turmeric spice is obtained from the underground rhizomes of the plant which, after drying and processing, results in a bright yellow powder used by the food industry as a natural dye. Curcumin, the yellow colour pigment of turmeric, has different pharmacological activities (Sasikumar 2005; Ravindran et al. 2007). Curcumin has proved to be a powerful antioxidant, anti-parasitic, antispasmodic and anti-inflammatory compounds that can also inhibit carcinogenesis and cancer growth (Araujo and Leon 2001).
The International Trade Centre, Geneva, has estimated an annual growth rate of 10 % in the world demand for turmeric. Because of its increasing demand by the food industry, turmeric crops have been the focus of a variety of studies aiming at increasing its productivity (Meenakshi and Sulikeri 2003; May et al. 2005). Turmeric plants are exclusively propagated by vegetative means using its underground rhizomes as there is essentially no seed formation. As hybridization programmes are ineffective in most cases, genetic improvement of the turmeric crop is limited to germplasm selection and to eventual sports arising during vegetative propagation. As selection of superior genotypes is usually made in field trials, the identification of divergent genotypes could be enhanced using molecular markers. Moreover, molecular markers are a useful tool for the characterization of genetic variability available in natural populations and germplasm collections. Knowledge of genetic variability is essential for breeding programmes and plant genetic resource conservation. Molecular marker techniques overcome many of the limitations of morphological and biochemical techniques, and can detect variation at the DNA level (Tingey and Tufo 1993). Characterization of turmeric germplasm using molecular markers is very limited, except few stray information on isozymes (Shamina et al. 1998), RAPD (Salvi et al. 2001; Panda et al. 2007; Tyagi et al. 2007; Thaikert and Paisooksantivatana 2009; Jan et al. 2011), RAPD, ISSR and AFLP (Nayak et al. 2006; Hussain et al. 2008; Syamkumar 2008; Vijayalatha and Chezhiyan 2008; Das et al. 2011) and SSR markers (Sigrist et al. 2010; Joshi et al. 2010; Siju et al. 2010; Hayakawa et al. 2011a, b). In the present paper Directed Amplification of Minisatellite DNA (DAMD-Heath et al. 1993) and Inter Simple Sequence Repeats (ISSR- Prevost and Wilkinson 1999), methods were used to estimate the genetic variability in indigenous turmeric germplasm. Identification of elite genotype(s) with these markers would be helpful in breeding programmes for further improvement of turmeric germplasm in terms of its qualitative traits.
Materials and methods
Leaf samples of twenty nine genotypes of C. longa were used in the present study (Table 1). These genotypes are grown and maintained at Distant Banthra Research Centre of CSIR-National Botanical Research Institute (CSIR-NBRI), Lucknow, India. Closely related taxon Costus speciosus (Koenig) Sm. was considered as the out-group for comparison with C. longa genotypes. Total genomic DNA was extracted from the fresh leaf tissues following CTAB method (Doyle and Doyle 1990) and its quality and quantity was checked by gel electrophoresis on 0.8 % agarose gel, stained with ethidium bromide, and compared with a set of known DNA concentration standards (100 bp ladder), and by UV spectroscopy using a Nanodrop ND-1000 Spectrophotometer (NanoDrop Technologies Inc. USA).
Table 1.
Details of Curcuma longa genotypes used in the present study and their total curcuminoid contents
| Sl. No. | Genotype identity | Locality | Geographical co-ordinates | Total curcuminoids | Reference |
|---|---|---|---|---|---|
| 1 | NBH 1 | Banthra UP | 26° 43′ 00″ N, 80° 51′ 00″ E | 0.804 ± 0.025 | Singh et al. 2011 |
| 2 | NBH 2 | Banthra UP | 26° 43′ 00″ N, 80° 51′ 00″ E | 0.772 ± 0.026 | -do- |
| 3 | NBH 3 | Banthra UP | 26° 43′ 00″ N, 80° 51′ 00″ E | 0.916 ± 0.013 | -do- |
| 4 | NBH 4 | East UP | 26° 47′ 00″ N, 82° 08′ 00″ E | 1.105 ± 0.016 | -do- |
| 5 | NBH 5 | East UP | 26° 47′ 00″ N, 82° 08′ 00″ E | 0.541 ± 0.028 | -do- |
| 6 | NBH 6 | East UP | 26° 47′ 00″ N, 82° 08′ 00″ E | 1.810 ± 0.040 | -do- |
| 7 | NBH 7 | East UP | 26° 47′ 00″ N, 82° 08′ 00″ E | 1.895 ± 0.024 | -do- |
| 8 | NBH 8 | East UP | 26° 47′ 00″ N, 82° 08′ 00″ E | 2.277 ± 0.233 | -do- |
| 9 | NBH 9 | East UP | 26° 47′ 00″ N, 82° 08′ 00″ E | 2.413 ± 0.075 | -do- |
| 10 | NBH 10 | East UP | 26° 47′ 00″ N, 82° 08′ 00″ E | 2.845 ± 0.015 | -do- |
| 11 | NBH 11 | East UP | 26° 47′ 00″ N, 82° 08′ 00″ E | 1.471 ± 0.026 | -do- |
| 12 | NBH 12 | East UP | 26° 47′ 00″ N, 82° 08′ 00″ E | 2.380 ± 0.075 | -do- |
| 13 | NBH 13 | Deoria UP | 26° 30′ 17″ N, 83° 47′ 14″ E | 1.962 ± 0.015 | -do- |
| 14 | NBH 14 | Jhansi UP | 25° 26′ 00″ N, 78° 35′ 00″ E | 2.157 ± 0.050 | -do- |
| 15 | NBH 15 | Jhansi UP | 25° 26′ 00″ N, 78° 35′ 00″ E | 1.914 ± 0.074 | -do- |
| 16 | NBH 16 | Jhansi UP | 25° 26′ 00″ N, 78° 35′ 00″ E | 2.235 ± 0.019 | -do- |
| 17 | NBH 17 | Haryana | 28° 26′ 00″ N, 77° 19′ 00″ E | 0.432 ± 0.019 | -do- |
| 18 | NBH 18 | Hyderabad (AP) | 17° 22′ 31″ N, 78° 28′ 28″ E | 1.162 ± 0.032 | -do- |
| 19 | NBH 19 | Jeevani North East | 26° 11′ 00″ N, 91° 41′ 00″ E | 1.689 ± 0.017 | Present work |
| 20 | NBH 20 | Jeevani North East | 23° 50′ 11″ N, 91° 16′ 30″ E | 1.651 ± 0.016 | Present work |
| 21 | NBH 21 | Pant selection Pant Nagar | 29° 03′ 00″ N, 79° 31′ 00″ E | 1.254 ± 0.015 | Present work |
| 22 | NBH 22 | Lakadong Shillong | 25° 11′ 00″ N, 92° 17′ 00″ E | 6.498 ± 0.709 | Present work |
| 23 | Prabha | Faizabad UP | 26° 47′ 00″ N, 82° 08′ 00″ E | 1.664 ± 0.058 | Singh et al. 2011 |
| 24 | RH-5 | Basti UP | 26° 48′ 00″ N, 82° 43′ 00″ E | 1.174 ± 0.023 | Present work |
| 25 | ROMA | Basti UP | 26° 48′ 00″ N, 82° 43′ 00″ E | 1.381 ± 0.043 | Present work |
| 26 | AZAAD 1 | Basti UP | 26° 48′ 00″ N, 82° 43′ 00″ E | 1.192 ± 0.062 | Present work |
| 27 | KTS-2 | Basti UP | 26° 48′ 00″ N, 82° 43′ 00″ E | 2.044 ± 0.015 | Present work |
| 28 | Rajendra Sonia | Basti UP | 26° 48′ 00″ N, 82° 43′ 00″ E | 3.441 ± 0.583 | Present work |
| 29 | Pant Peetabh | Pant Nagar | 29° 03′ 00″ N, 79° 31′ 00″ E | 1.133 ± 0.013 | Present work |
| 30 | Out-group | Costus speciosus | 26° 51′ 19″ N, 80° 57′ 05″ E | – | – |
Twenty DAMD primers already available in the public domain (Jeffereys et al. 1985; Huey and Hall 1989; Vergnaud 1989; Winberg et al. 1993; Zhou et al. 1997; Murray et al. 1988; Nakamura et al. 1988; Kang et al. 2002) were custom synthesized from Sigma Aldrich Chemicals Pvt. Ltd. India. These primers were tested with two template DNAs, out of which 15 primers resulting in distinct and clear banding patterns were selected for further DAMD profiling. PCR amplification was carried out according to Zhou et al. (1997). The reaction mixture (20 μl) contained 1.6 μl of 10 mM dNTP mix (2.5 mM each dNTP), 0.4 μl of 25 mM MgCl2, 2 μl suitable 10X assay buffer with 15 mM MgCl2 (Buffer A) supplied along with the enzyme, 0.8 μl of 20 μM primer, 1.2 unit Taq DNA polymerase (Merck Specialities Pvt. Ltd. India) and 40 ng/2ul genomic DNA. Optimal DNA amplification was obtained through 35 cycles (92 °C for 1 min, 55 °C for 2 min and 72 °C for 2 min)) in a thermal cycler (Proflex PCR System; Applied Biosystems, Life Technologies, USA).
A set of 100 anchored microsatellite primers was procured from University of British Columbia, Canada. PCR amplification of 20 ng DNA was performed in 1.6 μl of 10 mM dNTP mix (2.5 mM each dNTP), 1.6 μl of 25 mM MgCl2, 2 μl suitable 10X assay buffer (Buffer F) supplied along with the enzyme, 0.3 μl of 15 μM primer and 0.9 U Taq DNA polymerase (Merck Specialities Pvt. Ltd. India) in a 20 μl reaction using Proflex PCR System (Applied Biosystems, Life Technologies, USA). After initial denaturation at 94 °C for 4 min, each cycle consisted of 1 min denaturation at 94 °C, 1 min of annealing at 54 °C, 2 min extension at 72 °C along with 7 min extension at 72 °C at the end of 35 cycles.
The amplified PCR products were electrophoresed on 1.5 % agarose gel using 0.5X TBE buffer (1 X TBE buffer is Tris-borate 89 mM; 2 mM EDTA, pH 8.3) at constant voltage of 5 V/cm. After electrophoresis the gel was stained in ethidium bromide and then visualized and archived using UV Tech Gel Documentation System (UK). The patterns were photographed and stored as digital images in gel documentation system. Representative gel profiles of DAMD and ISSR methods used in the present investigation have been provided in Fig. 1.
Fig. 1.
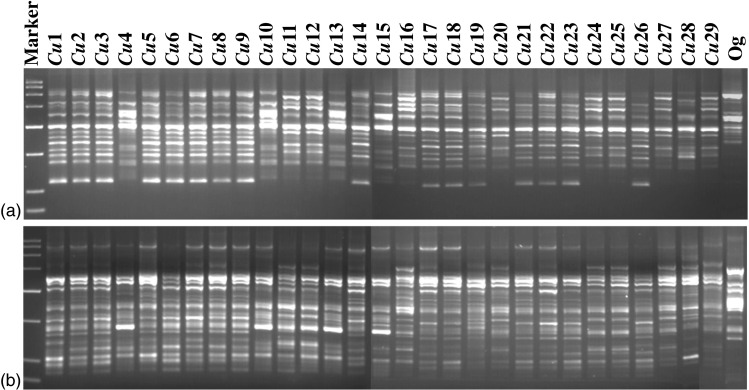
Representative gel images showing PCR profiles of Curcuma longa genotypes using (a) DAMD primer 14C2 (b) ISSR primer UBC 835. Lanes indicated by ‘Marker’ contains low range molecular weight marker as the size marker
Data analysis was carried out only for those genotypes that resulted in consistent and reproducible profiles. For each primer, the molecular sizes of each fragment were estimated on the basis of the corresponding marker lane. Data were scored as presence (1) or absence (0) of a band. Only distinct and well-separated bands were included in the further analyses. A pair wise matrix of similarity between genotypes was determined for the band data using Jaccard’s similarity coefficient for UPGMA method in the FreeTree program (ver. 0.9.1.5) (Pavlicek et al. 1999). From this data, the UPGMA tree was computed after allowing a 1000 replicate bootstrap test using the same program. The trees were viewed, annotated and printed using Tree View (ver. 1.6.5) (Page 2001).
The data were further used to calculate different diversity parameters such as total number of bands, polymorphic bands, percent polymorphism and genetic distances. Furthermore, to measure the usefulness of marker systems used in the present study, Diversity Index (DI), Multiplex Ratio (MR), Effective Multiplex Ratio (EMR), Marker Index (MI) and Resolving power (Rp) were calculated according to Powell et al. (1996). Polymorphic Information Content (PIC) value has been primarily and most extensively used as a measure of discriminatory power or informativeness of markers in most of the diversity studies. PIC value was calculated according to Botstein et al. 1980. Mantel test (Mantel 1967) was carried out in NTSYS pc software ver. 2.02e (Rohlf 1998) using MXCOMP module to compute the matrix correlation (r) between the pairwise similarity matrices generated from different markers to ascertain the goodness of fit, amongst the data sets.
The curcuminoid contents for 17 genotypes out of 29 considered in the present study were already available (Singh et al. 2011) therefore, curcuminoid contents for rest of the genotypes (12) were estimated during the course of present study (Table 1), following the methodology adopted by Singh et al. (2011).
Results
Fifteen minisatellite core sequence primers (DAMD) resulted into a total of 257 scorable fragments in the size ranging from 160 to 3000 bp. The total number of fragments scored ranged from ten (URP 30F) to 21 (14C2), with an average of 17.1 per primer. All fifteen primers were found to be efficient to generate 217 polymorphic fragments varying from 08 (URP 30 F) to 18 (HVR), with a mean of 14.5 fragments. The rate of polymorphism revealed for different primers ranged from 69.2 % (HVA) to 94.1 % (HBV3, URP4R). The overall percent polymorphism was found to be 84.4 %. Rp value varied from 11.2 (URP 30 F) to 25.6 (M13) with a mean of 18.4. Lowest PIC value recorded was 0.24 (URP 2R), whereas primer OGRB 01 showed the highest (0.34) PIC value. Average PIC value for all the 15 DAMD primers was 0.29 (Table 2).
Table 2.
Sequences of DAMD and ISSR primers and the extent of polymorphism determined with these primers
| Primer name | Sequence 5′–3′ | Loci amplified | Polymorphic loci | Percentage polymorphism | Mean PIC | Rp value | Approx. band size range |
|---|---|---|---|---|---|---|---|
| DAMD | |||||||
| HVR | CCTCCTCCCTCCT | 20 | 18 | 90.0 | 0.31 | 22.1 | 250–3000 |
| HBV | GGTGTGTGTGTGCAT | 15 | 14 | 93.3 | 0.33 | 14.5 | 300–3000 |
| HBV3 | GGTGAAGCACAGGTG | 17 | 16 | 94.1 | 0.33 | 16.5 | 280–3000 |
| HVA | AGGATGGAAAGGAGGC | 13 | 09 | 69.2 | 0.26 | 20.0 | 500–2500 |
| 33.6 | AGGGCTGGAGG | 20 | 17 | 85.0 | 0.28 | 25.3 | 200–2500 |
| M13 | GAGGGTGGCGGTTCT | 20 | 15 | 75.0 | 0.26 | 25.6 | 200–2500 |
| URP 4R | AGGACTCGATAACAGGCTCC | 17 | 16 | 94.1 | 0.32 | 18.6 | 350–3000 |
| URP 9F | ATGTGTGCGATCAGTTGCTG | 19 | 16 | 84.2 | 0.29 | 19.6 | 200–3000 |
| URP 30F | GGACAAGAAGAGGATGTGGA | 10 | 08 | 80.0 | 0.25 | 11.2 | 400–1800 |
| URP 2R | CCCAGCAACTGATCGCACAC | 17 | 13 | 76.4 | 0.24 | 14.6 | 230–1200 |
| FV11ex8 | ATGCACACACACAGG | 15 | 12 | 80.0 | 0.26 | 13.7 | 300–3000 |
| FV11ex8C | CCTGTGTGTGTGCAT | 14 | 13 | 93.0 | 0.34 | 11.9 | 250–1600 |
| 14C2 | GGCAGGATTGAAGC | 21 | 17 | 81.0 | 0.31 | 22.3 | 250–2500 |
| OGRB01 | AGGGCTGGAGGAGGGC | 20 | 17 | 85.0 | 0.34 | 20.0 | 160–2000 |
| 6.2H(+) | AGGAGGAGGGGAAGG | 19 | 16 | 84.2 | 0.28 | 20.7 | 160–2500 |
| Total | 257 | 217 | 84.4 | 0.29 | 18.4 (Mean) | 160–3000 | |
| ISSR | |||||||
| UBC 808 | (AG) 8C | 20 | 15 | 75.0 | 0.28 | 21.8 | 350–2000 |
| UBC 809 | (AG) 8G | 16 | 12 | 75.0 | 0.29 | 19.9 | 200–1500 |
| UBC 811 | (GA) 8C | 10 | 08 | 80.0 | 0.26 | 11.2 | 500–2000 |
| UBC 835 | (AG) 8YC | 19 | 14 | 74.0 | 0.29 | 24.6 | 300–2000 |
| UBC 836 | (AG) 8YA | 16 | 12 | 75.0 | 0.27 | 18.8 | 300–1500 |
| UBC 841 | (GA) 8YC | 17 | 13 | 77.0 | 0.22 | 18.9 | 250–1200 |
| UBC 842 | (GA) 8YG | 21 | 18 | 86.0 | 0.29 | 19.8 | 250–1800 |
| UBC 866 | (CTC) 6 | 16 | 14 | 88.0 | 0.35 | 16.0 | 600–2500 |
| UBC 883 | (TA)7BVB | 21 | 16 | 76.1 | 0.30 | 26.0 | 250–2000 |
| UBC 888 | (CA)7BDB | 18 | 15 | 83.3 | 0.27 | 21.8 | 400–2000 |
| UBC 889 | (AC) 8DBD | 12 | 10 | 83.3 | 0.28 | 13.9 | 250–2000 |
| UBC 890 | (CT)7VHV | 19 | 16 | 84.2 | 0.29 | 21.9 | 300–1400 |
| UBC 891 | (TG)7HVH | 16 | 12 | 75.0 | 0.29 | 19.9 | 250–1600 |
| Total | 221 | 175 | 79.1 | 0.28 | 19.6 (Mean) | 200–2500 | |
| Cumulative | 478 | 392 | 82.0 | 0.29 | 160–3000 | ||
Among the 100 ISSR primers tested for their ability to detect polymorphism, 13 primers generated interpretable polymorphic amplifications. A total of 221 fragments, varying from 10 (UBC 811) to 21 (UBC 842, UBC 883) were generated with an average of 17 fragments per primer, of which 175 (79.2 %) were polymorphic. Average fragment size for 13 ISSR primers ranged from 200 to 2500 bp. The number of polymorphic fragments varied from 08 (UBC 811) to 18 (UBC 842), with an average of 13.5 polymorphic fragments per primer across all 29 genotypes. Resolving power (Rp) was used to determine the ability of the primers to distinguish genotypes. Rp value for ISSR ranged from 11.2 (UBC 811) to 26.0 (UBC 886) with a mean of 19.6. The lowest and highest PIC values were 0.22 (UBC 841) and 0.35 (UBC 866), respectively. The average PIC value for the 13 ISSR primers was 0.28. Over all polymorphism detected by both markers (DAMD and ISSR) was 82.0 % (Table 2).
Cumulative data of both the markers (DAMD and ISSR) were considered to calculate the genetic distances among pairs of genotypes. Minimum genetic distance (0.03) was observed between NBH 07 and NBH 08, whereas the maximum genetic distance (0.59) was recorded in NBH 04 and NBH 19; NBH 10 and NBH 18; NBH 10 and NBH 19; NBH 10 and Rajendra Sonia; NBH 11 and NBH 18; NBH 13 and NBH 18; NBH 13 and NBH 19; NBH 16 and Rajendra Sonia. Average genetic distance was 0.43 across the turmeric genotypes analyzed in the present investigation (Data not shown).
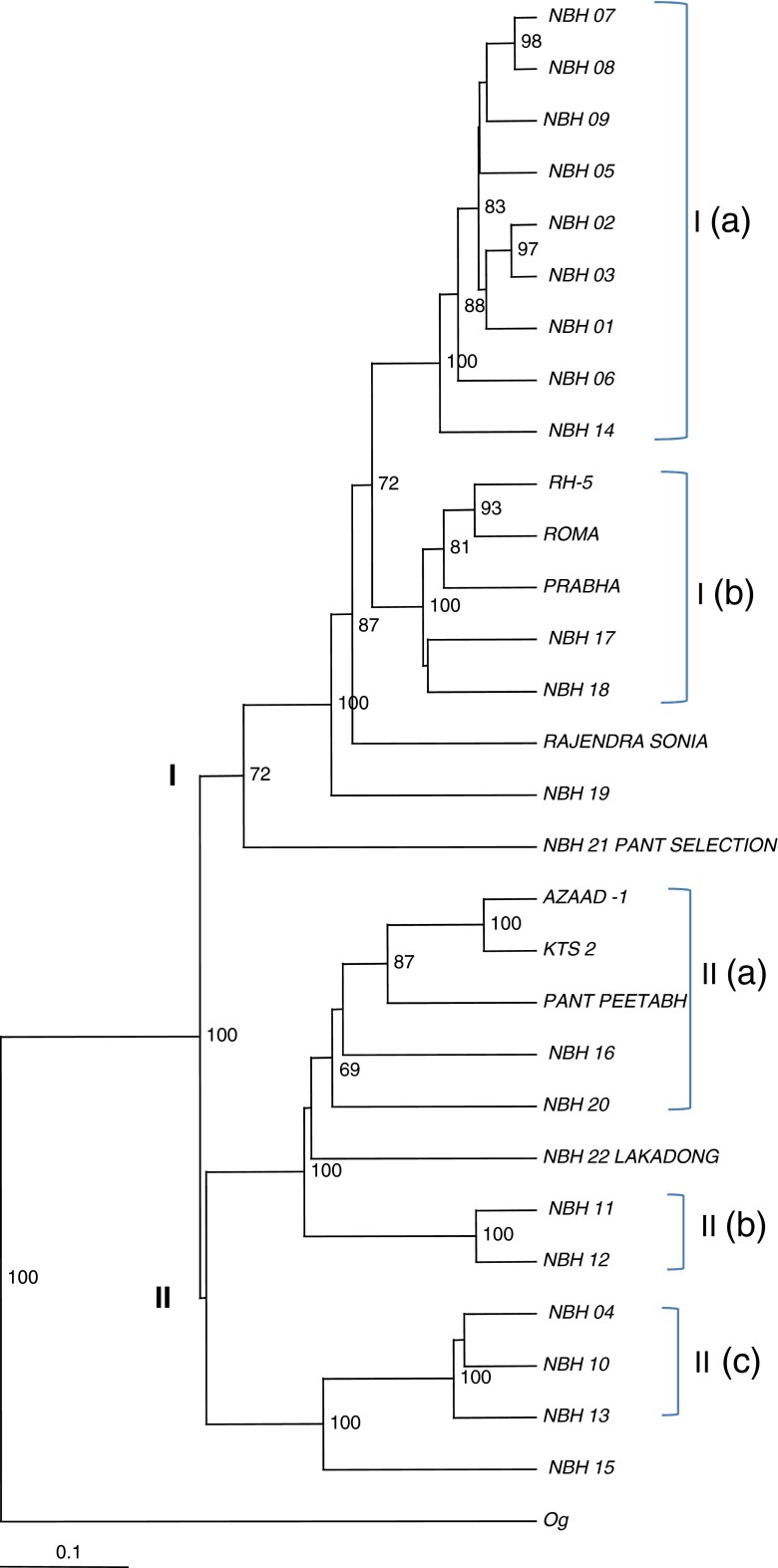
UPGMA dendrograms generated using Jaccard’s similarity matrix based on DAMD as well as ISSR data revealed almost consistent patterns of groupings of all 29 genotypes studied. However, a more comprehensive dendrogram constructed based on the combined data of DAMD and ISSR identified two main clusters: cluster 1 and cluster II, separated clearly with 100 % bootstrap values (Fig. 2). These two main clusters were further divided into five sub-clusters. The outgroup taxon Costus speciosus rooted out from all turmeric genotypes. Variable numbers of the genotypes were grouped in different sub-clusters. Sub-cluster I (a) grouped nine genotypes viz., NBH 01, NBH 02, NBH 03, NBH 05, NBH 06, NBH 07, NBH 08, NBH 09 and NBH 14 (Uttar Pradesh), whereas sub-cluster I (b) consisted of five genotypes (RH- 5, ROMA, PRABHA (Uttar Pradesh), NBH 17 (Haryana) and NBH 18 (Andhra Pradesh). Similarly, sub-cluster II (a) consisted of five genotypes AZAAD1 and KTS2 (Uttar Pradesh ), Pant Peetabh (Uttarakhand), NBH 16 (Uttar Pradesh ) and NBH 20 (Tripura). Sub-cluster II (b) consisted of two genotypes NBH 11 and NBH 12 (Uttar Pradesh ) and sub-cluster II (c) grouped NBH04, NBH10 and NBH13 (Uttar Pradesh ). Two pairs of genotypes AZAAD1 and KTS2 (Uttar Pradesh ); NBH 11 and NBH 12 (Uttar Pradesh ) revealed the highest bootstrap (100 %) values between them. High bootstrap values observed amongst different clusters are indicated (69–100 %) on the nodes of the dendrogram (Fig. 2). Genotypes in the clusters did not exclusively group according to their geographical provenances. However, region specific grouping was limited to sub-cluster I (a), as all the genotypes in this cluster were originally from Uttar Pradesh. Similarly, sub-cluster II (b) and sub-cluster II (c) also grouped genotypes from same locality of Uttar Pradesh. Two genotypes from Uttarakhand grouped in different clusters I and cluster II. Sub-cluster I (b) and II (a) were found to be most diverse sub-clusters, which represented from different states. Rajendra Sonia (Uttar Pradesh ), NBH 19 (Assam), Pant Peetabh (Uttarakhand), Lakadong (Meghalaya) and NBH 15 (Uttar Pradesh ) did not show significant grouping within their respective clusters (Table 1 and Fig. 2).
Fig. 2.
UPGMA dendrogram based on cumulative (DAMD and ISSR) data showing relationships among Curcuma longa genotypes
To determine the overall utility of a given marker, MI was calculated for both markers used in the present investigation. DAMD showed higher MI value (5.1) as compared to ISSR (4.9). Effective Marker Index (EMR) considers all possible attributes such as polymorphic information content, fraction of polymorphic fragments, multiplex ratio as well as the qualitative issues for a given marker system. Calculated values of EMI were higher in DAMD (14.5) as compared to ISSR (13.5) in the present study (Table 3).
Table 3.
Comparison of DAMD, ISSR and cumulative band data analyses in indigenous turmeric genotypes
| Markers | DAMD | ISSR | *Cumulative |
|---|---|---|---|
| No. of accessions | 29 | 29 | 29 |
| Total no of assays /primer | 15 | 13 | 28 |
| Total no. of bands amplified (n) | 257 | 221 | 478 |
| Polymorphic bands (p) | 217 | 175 | 392 |
| Polymorphism (%) | 84.4 | 79.2 | 82.0 |
| Band size range (bp) | 160–3000 | 200–2500 | 160–3000 |
| Genetic distance range | 0.06–0.61 | 0.00–0.60 | 0.03–0.59 |
| Average PIC | 0.29 | 0.28 | 0.29 |
| Average diversity index (DIav) | 0.35 | 0.36 | 0.01 |
| Multiplex ratio (MR) | 17.1 | 17.0 | 17.1 |
| Effective multiplex ratio (EMR) | 14.5 | 13.5 | 14.0 |
| Marker index (MI) | 5.1 | 4.9 | 0.2 |
*Cumulative: combined data of DAMD and ISSR
DAMD, ISSR and cumulative (DAMD+ISSR) data were correlated using the Mantel test at a significance level of 1000 permutations. DAMD versus cumulative (0.982) and ISSR versus cumulative (0.982) revealed similar correlation coefficient, whereas individual markers (DAMD versus ISSR) showed lower correlation coefficient (Table 4). This indicates that the individual marker data are the best fit to cumulative data than amongst the individual markers.
Table 4.
Mantel correlations between the genetic distances obtained from DAMD, ISSR and cumulative data analyses among the turmeric genotypes
| Marker pairs | Correlation coefficient (r) | (p) value |
|---|---|---|
| ISSR vs DAMD | 0.93084 | 0.0020 |
| ISSR vs *cumulative | 0.98297 | 0.0020 |
| DAMD vs *cumulative | 0.98212 | 0.0020 |
*Combined DAMD and ISSR band data
On the basis of the plant height, the genotypes were grouped into three groups: short (<60 cm), medium (60-90 cm) and tall (>90 cm). Majority of the genotypes (NBH 01, NBH 02, NBH 03, NBH 04, NBH 05, NBH 06, NBH 07, NBH 10, NBH 12, NBH 14, NBH 16, NBH 17, NBH 18, NBH 22, ROMA) were of medium size, while rest of the genotypes were short (NBH 08, NBH 09, NBH 11, NBH 13, NBH 15, KTS-2, Pant Peetabh, Rjendra Sonia, RH-5, AZAD-1, Prabha,) and tall (NBH 19, NBH 20, NBH 21), respectively. The amount of curcuminoid contents in 29 genotypes varied from 0.432 to 6.498 %. Lakadong (NBH 22) showed maximum (6.498 %) amount of curcuminoid contents, followed by Rajendra Sonia (3.441 %), NBH 10 (2.845 %), NBH 09 (2.413 %) and NBH 12 (2.380 %), respectively. The lowest curcuminoids were recorded in NBH 17 (0.432 %) followed NBH 05 (0.541 %), and NBH 02, respectively (Table 1).
Discussion
The diversity analysis efficiency and discriminatory power of the DNA based markers largely depends upon the rate of polymorphism detected amongst the genotypes. In the present investigation DAMD and ISSR markers were used to characterize the indigenous collection of turmeric germplasm. These markers are highly informative, cost effective, and require less time and labour for diversity analysis. Despite being vegetatively propagated, turmeric genotypes revealed significant level of polymorphism (82.0 %), in the present study, which is indicative of prevalence of wide genetic diversity in these genotypes. Wide range of pairwise genetic distances (0.03–0.59) across the genotypes revealed that these genotypes are genetically quite diverse. These results suggested that the current collection of genotypes preserve the vast majority of natural variations in turmeric germplasm. Earlier studies on genetic variability in Curcuma species including C. longa using RAPD (Angle et al. 2008; Thaikert and Paisooksantivatana 2009; Jan et al. 2011), AFLP (Keeratinijakal et al. 2010) and RAPD, ISSR and AFLP (Das et al. 2011) also revealed high degree of polymorphism within and between the species, therefore, corroborating the present study.
DAMD and ISSR markers used in the present study differed, not only in its underlying principle, but also in generating information with regard to the extent of polymorphisms detected. Relatively higher level of polymorphism was detected using DAMD (84.4 %) than ISSR markers (79.2 %), indicating the presence of numerous highly variable minisatellite loci across the genotypes. The difference may also be attributed to different approaches which may provide different results (Nesbitt et al. 1995), suggesting that the character or scope of variation examined by each marker may differ (Hodgkin et al. 2001; Verma et al. 2009).
PIC as well as the Rp values of the DAMD and ISSR markers used in the present investigation indicated the potential use of some of these primers (UBC 866 UBC 883 and FVIIex8C, OGRB01, 33.6, M13) for further characterization of the turmeric germplasm. Marker index (MI) which is a convenient estimate for marker efficiency may further compliment to the markers (Milbourne et al. 1997). The higher MI of the DAMD assay calculated in the present study possibly derives from its high effective multiplex ratio (EMR) estimated as compared to the ISSR. Nevertheless, the total number of scorable as well as polymorphic fragments for DAMD was higher in comparison to ISSR that could be an additional advantage of DAMD, thus revealing its power to detect more polymorphism across the turmeric genotypes. Strong Mantel correlation observed between DAMD and ISSR markers revealed high degree of correlation coefficient between these markers. Similar significant correlation in Curcuma species was reported in earlier studies using RAPD, ISSR and AFLP markers (Das et al. 2011).
The UPGMA dendrogram generated using cumulative (DAMD+ISSR) data showed significant relationships amongst the genotypes. All 29 genotypes studied grouped in two clusters with the 100 % bootstrap value, irrespective of their geographical affiliations except few genotypes, suggesting considerable diversity amongst the genotypes. Lack of region specific affinities in turmeric genotypes was also reported earlier (Thaikert and Paisooksantivatana 2009), which is in congruence with the present investigation. The lowest genetic distance revealed between NBH 07 and NBH 08 (Uttar Pradesh) shows that these genotypes are closely related to each other. Similarly, the highest genetic distances were found in NBH 04 (Uttar Pradesh) – NBH 19 (Assam); NBH 10 (Uttar Pradesh) - NBH18 and NBH19 (Andhra Pradesh and Assam, respectively); NBH 16 (Jhansi, Uttar Pradesh) - Rajendra Sonia (Basti, Uttar Pradesh) is evident as these genotypes are separated in different clusters with 100 % bootstrap values in the dendrogram, suggesting that they are genetically diverse. The genotypes like Lakadong (Meghalaya) and Rajendra Sonia (Basti, Uttar Pradesh) did not show region specific groupings in the dendrogram and therefore, they are geographically and genetically independent. Chandra et al. (1997, 1999), also in their study reported that Lakadong formed solitary group and were genetically most distant. Biochemical data also revealed that Lakadong (NBH 22) and Rajendra Sonia harbor maximum curcuminoid contents (6.498 and 3.441 %, respectively) among all the 29 genotypes of turmeric germplasm investigated in the present study. Therefore, these genotypes/chemotypes are very significant for further prospection of indigenous turmeric germplasm.
The present investigation seems to be a maiden attempt at molecular characterization of indigenous turmeric germplasm using combination of DAMD and ISSR markers. The results also demonstrate the efficiency and reliability of DAMD and ISSR markers in determining the genetic diversity and relationships among the indigenous turmeric germplasm, and therefore, they are suitable markers for the large scale sorting of germplasm. DAMD and ISSR profiling have identified diverse turmeric genotypes which could be further utilized in various genetic improvement programmes including conventional as well as marker assisted breeding towards development of new and desirable turmeric genotypes.
Acknowledgments
This work was financially supported by the Council of Scientific and Industrial Research (CSIR), New Delhi under the grant BSC0110 (AGTEC). The authors would like to thank Director, CSIR-National Botanical Research Institute, Lucknow (India) for facilities and encouragements.
References
- Angle GR, Makeshkumar T, Mohan C, Vimala B, Nambisan B. RAPD genetic diversity analysis of starchy Curcuma species using markers. Plant Biochem Biotechnol. 2008;17:0971–781. [Google Scholar]
- Araujo CAC, Leon LL. Biological activities of Curcuma longa L. Mem. Inst. Mem Inst Oswaldo Cruz. 2001;96:723–728. doi: 10.1590/S0074-02762001000500026. [DOI] [PubMed] [Google Scholar]
- Botstein D, White RL, Skolnick M, Davis RW. Construction of a genetic linkage map in man using restriction fragment length polymorphism. Am J Hum Genet. 1980;32:314–331. [PMC free article] [PubMed] [Google Scholar]
- Chandra R, Desai AR, Govind S, Gupta PN. Metroglyph analysis in turmeric (Curcuma longa L.) germplasm in India. Sci Hortic. 1997;70:211–222. doi: 10.1016/S0304-4238(97)00036-8. [DOI] [Google Scholar]
- Chandra R, Govind S, Desai AR. Growth, yield and quality performance of turmeric (Curcuma longa L.) genotypes in mid-altitudes of Meghalaya. J Appl Hortic (Lucknow) 1999;1:142–144. [Google Scholar]
- Das A, Kesari V, Satyanarayana VM, Parida A, Rangan L. Genetic relationship of Curcuma species from Northeast India using PCR-based markers. Mol Biotechnol. 2011;49(1):65–76. doi: 10.1007/s12033-011-9379-5. [DOI] [PubMed] [Google Scholar]
- Doyle JJ, Doyle JL. Isolation of plant DNA from fresh tissue. Focus. 1990;12:13–15. [Google Scholar]
- Duke JA. CRC handbook of medicinal spices. Boca Raton: CRC Press; 2003. [Google Scholar]
- Hayakawa HYM, Ito K, Yamamoto Y, Fukuda T. Difference of curcumin content in Curcuma longa L (Zingiberaceae) caused by Hybridization with other Curcuma species. Am J Plant Sci. 2011;2(2):111–119. doi: 10.4236/ajps.2011.22013. [DOI] [Google Scholar]
- Hayakawa TK, Minamiya Y, Ito K, Miyazaki A, Fukuda T, Yamamoto Y. Development of a molecular marker to identify a candidate line of turmeric (Curcuma longa L.) with a high curcumin content. Am J Plant Sci. 2011;2(1):15–26. doi: 10.4236/ajps.2011.21002. [DOI] [Google Scholar]
- Heath DD, Iwana GK, Delvin RH. PCR primed with VNTR core sequences yield species specific patterns and hypervariable probes. Nucleic Acids Res. 1993;21:5782–5785. doi: 10.1093/nar/21.24.5782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin T, Roviglioni R, de Vicente MC, Dudnik N. Molecular methods in the conservation and use of plant genetic resources. Acta Hortic. 2001;546:107–118. [Google Scholar]
- Huey B, Hall J. Hypervariable DNA fingerprinting in Escherichia coli: minisatellite probe from bacteriophage M13. J Bacteriol. 1989;171:2528–2532. doi: 10.1128/jb.171.5.2528-2532.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain Z, Tyagi RK, Sharma RA, Agarwal A. Genetic diversity in in vitro conserved germplasm of Curcuma longa as revealed by RAPD markers. Biol Plant. 2008;52:627–633. doi: 10.1007/s10535-008-0123-3. [DOI] [Google Scholar]
- Jan UH, Rabbani MA, Shinwari ZK. Assessment of genetic diversity of indigenous turmeric (Curcuma longa L.) germplasm from Pakistan using RAPD markers. Med Plant Res. 2011;5(5):823–830. [Google Scholar]
- Jeffereys AJ, Wilson V, Thein SL. Hypervariable ‘minisatellite’ regions in human DNA. Nature. 1985;314:67–73. doi: 10.1038/314067a0. [DOI] [PubMed] [Google Scholar]
- Joshi RK, Kuanar A, Mohanty S, Subudhi E, Nayak S. Mining and characterization of EST derived microsatellites in Curcuma longa L. Bioinformatics. 2010;5(3):128–132. doi: 10.6026/97320630005128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HW, Park DS, Go SJ, Eun MY. Fingerprinting of diverse genomes using PCR with universal rice primers generated from repetitive sequences of Korean weedy rice. Mol Cells. 2002;13:281–287. [PubMed] [Google Scholar]
- Keeratinijakal V, Kladmook M, Laosatit K. Identification and characterization of Curcuma comosa Roxb, phytoestrogens-producing plant, using AFLP markers and morphological characteristics. J Med Plant Res. 2010;4(24):2651–2657. [Google Scholar]
- Mantel N. The detection of disease clustering and a generalized regression approach. Cancer Res. 1967;27:209–220. [PubMed] [Google Scholar]
- May A, Cecilio FAB, Cavarianni RL, Barbosa JC. Turmeric (Curcuma longa L.) development and productivity in function at nitrogen and potassium doses Rev. Bras. Planta Med. 2005;7:73–78. [Google Scholar]
- Meenakshi N, Sulikeri GS. Effect of different planting materials on growth, yield and productivity of turmeric (Curcuma longa L.) Int J Trop Agric. 2003;21:37–44. [Google Scholar]
- Milbourne D, Meyer RC, Bradshaw JE, Baird E, Bonar N, Provan J, Powell W, Waugh R. Comparison of PCR-based marker systems for the analysis of genetic relationships in cultivated potato. Mol Breed. 1997;3:127–136. doi: 10.1023/A:1009633005390. [DOI] [Google Scholar]
- Murray MJ, Haldeman BA, Grant FJ, O’Hara PJ. Probing the human genome with minisatellite-like sequences from the human coagulation factor VII gene. Nucleic Acids Res. 1988;16:4166. doi: 10.1093/nar/16.9.4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Carlson M, Krapcho K, Kanamori M, White R. New approach for the isolation of VNTR markers. Am J Hum Genet. 1988;43:854–859. [PMC free article] [PubMed] [Google Scholar]
- Nayak S, Naik P, Acharya L, Pattnaik A. Detection and evaluation of genetic variation in 17 promising cultivars of turmeric (Curcuma longa L.) using 4C nuclear DNA content and RAPD markers. Cytologia. 2006;71:49–55. doi: 10.1508/cytologia.71.49. [DOI] [Google Scholar]
- Nesbitt KA, Potts BM, Vaillancourt RE, West AK, Reid JB. Partitioning and distribution of RAPD variation in a forest tree species, Eucalyptus globulus (Myrtaceae) Heredity. 1995;74:628–637. doi: 10.1038/hdy.1995.86. [DOI] [Google Scholar]
- Page RDM, TreeView (Win32), ver. 165, 2001; http://taxonomy.zoology.gla.ac.uk/rod/treeview.html
- Panda MK, Mohanty S, Subudhi E, Acharya L, Nayak S. Assessment of genetic stability of micropropagated plants of Curcuma longa L. by cytophotometry and RAPD analysis. Int J Integr Biol. 2007;1:189–195. [Google Scholar]
- Pavlicek A, Hrda S, Flegr J. FreeTree – freeware program for construction of phylogenetic trees on the basis of distance data and bootstrapping/jackknife analysis of the tree robustness application in the RAPD analysis of the genus Frenkelia. Folia Biol (Praha) 1999;45:97–99. [PubMed] [Google Scholar]
- Powell W, Morgante M, Andre C, Hanafey M, Vogel J, Tingey S, Rafalski A. The comparison of RFLP, RAPD, AFLP and SSR (microsatellite) markers for germplasm analysis. Mol Breed. 1996;2:225–238. doi: 10.1007/BF00564200. [DOI] [Google Scholar]
- Prevost A, Wilkinson MJ. A new system comparing PCR primers applied to ISSR fingerprinting of potato cultivars. Theor Appl Genet. 1999;98:107–12. doi: 10.1007/s001220051046. [DOI] [Google Scholar]
- Ravindran PN, Babu KN, Sivaranan K. Turmeric: the genus Curcuma. Medicinal and aromatic plants - industrial profiles. Boca Raton: CRC Press; 2007. [Google Scholar]
- Rohlf FJ. NTSYS-pc: numerical taxonomy and multivariate analysis system, version 202e Applied Biostatistics Inc. Setauket: Exeter Software; 1998. [Google Scholar]
- Salvi ND, George L, Eapen S. Plant regeneration from leaf base callus of turmeric and random amplified polymorphic DNA analysis of regenerated plants. Plant Cell Tissue Organ Cult. 2001;6:113–119. doi: 10.1023/A:1010638209377. [DOI] [Google Scholar]
- Sasikumar B. Genetic resources of Curcuma: diversity, characterization and utilization. Plant Genet Res. 2005;3:230–251. doi: 10.1079/PGR200574. [DOI] [Google Scholar]
- Selvan TM, Thomas KG (2002) Turmeric In: Singh HP, Sivaraman KT, Selvan M, (Eds) Indian spices - production and utilization proceedings, national consultative meeting for accelerated production and export of spices 97-109 coconut development board, Cochin
- Shamina A, Zachariah TJ, Sasikumar B, Johnson KG. Biochemical variation in turmeric (C. longa) accessions based on isozyme polymorphism. J Hortic Sci Biotechnol. 1998;73:479–483. [Google Scholar]
- Sigrist MS, Pinheiro JB, Azevedo-Filho JA. Development and characterization of microsatellite markers for turmeric (Curcuma longa) Plant Breed. 2010;129(5):570–573. [Google Scholar]
- Siju S, Dhanya K, Syamkumar S, Sheeja TE, Sasikumar B, Bhat AI, Parthasarathy VA. Development, characterization and utilization of genomic microsatellite markers in turmeric (Curcuma longa L) Biochem Syst Ecol. 2010;38:641–646. doi: 10.1016/j.bse.2010.08.006. [DOI] [PubMed] [Google Scholar]
- Singh S, Niranjan A, Sharma SK, Tewari SK. Curcuminoids, phenolic contents and yield investigations of Curcuma longa L. accessions, grown on partially reclaimed sodic soil. Med Plants Int J PhytomedicineRelat Ind. 2011;3(3):203–207. doi: 10.5958/j.0975-4261.3.3.033. [DOI] [Google Scholar]
- Syamkumar S (2008) Molecular biochemical and morphological characterization of selected Curcuma accessions. Ph D Thesis Calicut University, Calicut, India
- Thaikert R, Paisooksantivatana Y. Variation of total curcuminoids content, antioxidant activity and genetic diversity in turmeric (Curcuma longa L.) collections. Kasetsart J (Nat Sci) 2009;43:507–518. [Google Scholar]
- Tingey SV, Tufo JP. Genetic analysis with random amplified polymorphic DNA markers. Plant Physio. 1993;101:349–352. doi: 10.1104/pp.101.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagi RK, Agarwal A, Mahalakshmi C, Hussain Z, Tyagi H. Low cost media for in vitro conservation of turmeric (Curcuma longa L.) and genetic stability assessment using RAPD markers. In Vitro Cell Dev Biol Plant. 2007;43:51–58. doi: 10.1007/s11627-006-9000-y. [DOI] [Google Scholar]
- Velayudhan KC, Muralidharan VK, Amalraj VA, Gautam PL, Mandal S, Kumar D. Curcuma genetic resources scientific monograph No 4 (1-5) New Delhi: National Bureau of Plant Genetic Resources; 1999. [Google Scholar]
- Vergnaud G. Polymers of random short oligonucleotides detect polymorphic loci in the human genome. Nucleic Acids Res. 1989;17:7623–7630. doi: 10.1093/nar/17.19.7623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma S, Rana TS, Ranade SA. Genetic variations and clustering in Murraya paniculata complex, revealed by single primer amplification reaction (SPAR) methods. Curr Sci. 2009;96(9):1210–1216. [Google Scholar]
- Vijayalatha KR, Chezhiyan N. Multivariate based marker analysis in turmeric (Curcuma longa L.) J Hortic Sci. 2008;3(2):107–111. [Google Scholar]
- Winberg BC, Zhou Z, Dallas JF, McIntyre CL, Gustafson JP. Characterization of minisatellite sequences from Oryza sativa. Genome. 1993;36:978–983. doi: 10.1139/g93-128. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Bebeli PJ, Somers DJ, Gustafson JP. Direct amplification of minisatellite-region DNA with VNTR core sequences in the genus Oryza. Theor Appl Genet. 1997;95:42–949. doi: 10.1007/s001220050645. [DOI] [Google Scholar]