Abstract
Objectives
To generate synthesized information on the epidemiology of VZV infection, as well as an estimation of prevalence of age-specific antibody in Iranian less than 40.
Material and Methods
After exclusion of irrelevant and overlapping reports, 15 papers were included (from nine major cities). Studies were pooled according to the heterogeneity test results. Random effect model methods were used for meta-analysis where significant heterogeneity was observed (age 1-16years).For other age groups, fixed model were used.
Results
Significant heterogeneity was observed in prevalence rates of all childhood age-groups. The seropositivity prevalence increased steeply from the age of 1-5 to 6-10 [from 21.9% (95% CI; 10.8-33.1) to 42.1 %(95% CI; 33.6-50.6)]. At the age of 11 15, 59.4% (95% CI; 46.1-72.8) of children showed to be infected. The rate of seropositivity was more than 87% in individuals of 40 and older.
Conclusion
The varicella seroeprevalence in Iran is in accordance with average tropical and temperate areas. Comparison of conducted studies during 2003 to 2011 didn’t show any alteration in VZV seroprovalence in Iran.
Keywords: Varicella, meta-analysis, seroepidemiologic study, Iran
INTRODUCTION
Varicella-zoster virus (VZV) is a human –herpes virus that causes varicella (chicken pox) and zoster (zona). Primary VZV infection is a common and generally benign disease of childhood and occurs mostly during the first decade of life (1-2). Although mortality is uncommon, varicella causes considerable hospitalization (3). Symptomatic disease is more common in neonates, older ages, immunocompromised individuals, and pregnant women (1, 4). Following a primary infection, VZV may become latent in the dorsal root ganglia and reactivate later to cause zoster (shingles). Life time risk of zoster in infected individuals is estimated about 15%(1). Varicella during pregnancy is a serious condition. Congenital varicella syndrome which causes significant morbidity and mortality is occasionally associated with this condition (4). In the pre-antiviral era, mortality of pregnant women was as high as 20–45% and up to 10% were able to develop pneumonia (5). Also, a primary maternal infection may be responsible for severe neonatal varicella during perinatal period(1).
Iran (located in the Middle East, Asia) is the sixteenth in size among all countries of the world (Fig.1), and its climate ranges from sub polar to subtropical (7). Iran has a population of approximately 78 million of different ethnic groups and about 25% of population is 15 years old or younger (8). Although mass vaccination in the childhood is expected to result in a substantially lower incidence, hospitalization, and mortality of the disease(6), but vaccination against VZV is not a component of any immunization program in Iran (9). Despite a shortage of comprehensive data on epidemiologic patterns of varicella seroprevalence in Iran, there are some well-designed cross sectional studies to report local rates of varicella seroprevalence. Epidemiological studies of varicella seroprevalence in the country are constantly bound to numerous limitations: inadequate nationwide data sets, lack of standard population-based studies, flawed disease registries, and finally discontinuity of data maintenance between public and private health sectors as well as family physicians. Furthermore, papers are not able to easily find when they are published in the local or national Persian language journals. This systematic review aimed to: 1) generate synthesized information on the epidemiology of VZV infections (2), estimate the prevalence of age-specific antibody in Iranian population at different age groups (up to 40); and (3) compare the seroprevalence between different time periods. To our knowledge, there is no systematic review on varicella seroprevalence in Iran so far.
Fig. 1. Map of Iran and Coordinates.
S, South; W, West; http://www.daftlogic.com, http://www.infoplease.com/atlas/country/iran.html
METHODS
We compared the standardized VZV antibody levels reported in the sub national serological surveys undertaken in 9 major cities of Iran in the different geographical region. The study was designed as an age-stratified systematic review of VZV seroprevalence in Iranian population between 0 to 40 years of age.
Data collection
The data was collected in four stages: Stage 1) Search on international database (Pub Med, Science Direct, and Scopus) Publications on varicella immunity prevalence in Iran were identified by searching on Pub Med, Science Direct and Scopus using these keywords: “varicella or chicken pox” in combination with “Iran, Iranian, Persian” and “seroprevalence or seroepidemiology”.
Stage 2) search through national database (“IranMedex”, the Scientific Information Database (SID), and Iran Doc) IranMedex (http://health.barakatkns.com/irmedex/query.asp) and SID (www.SID.ir) are databases for indexing Iranian medical scientific papers. The databases are using to index published papers in Persian or English, including articles in Iranian or International journals, scientific reports or medical thesis (only IranMedex). Iran Doc (http://thesis.irandoc.ac.ir) includes more than 650,000 records of which 220,000 are devoted to Iranian students’ thesis and dissertations and other gray literature such as national, regional and international medical science congress and seminars’ proceedings. Titles of all related articles and medical theses were reviewed.
We also reviewed related articles, hand-searched reference lists, and performed author contact.
Stage 3) Selection of relevant articles
1. Titles and abstracts were screened by authors separately to identify eligible studies according to agreed inclusion and exclusion criteria. Full papers of potentially eligible studies were retrieved for more detailed assessment. We selected papers for this systematic review if they could fulfill the following criteria:
Were conducted in Iran,
Contained data on frequency (prevalence) of VZV immunity,
Were published before 17thMarch 2014, and written in English or Persian, Studies on clinical pattern (types, risk factors and outcome) or mortality rate of varicella were excluded. We included studies on voluntary blood donors, pregnant women, and community studies. Analyses were done separately on studies from the following special groups (who were assumed to be at high risk for varicella): hospitalized patients, patients on haemodialysis, and hospital staff. Discrepancies were resolved by consensus.
Stage 4) Searching the references of the relevant papers. Each reference in relevant papers was checked for forward and backward citation of searched citations (to find more articles).
Data extraction
The following data were extracted from identified papers: authors’ name, place of the study, year of the study, varicella seropositivity prevalence, gender, age, and job status.
Measurement of heterogeneity
Statistical heterogeneity of results was checked by using Cochrane Q-test with significance level at <0.1. Heterogeneity was calculated as the weighted sum of squared differences between prevalence in individual studies and the pooled prevalence across all studies, with the weights which were used in the pooling method. We assumed the same prevalence in all studies as the null hypothesis. To test the heterogeneity, we calculated the amount of Q and compared it with a table of standard critical values. If our calculated Q was lower than the standard, then we failed to reject the null hypothesis (the studies are similar). If the Cochran Q was statistically significant or Q/degree of freedom (df) was greater than 1, heterogeneity was explored or leastwise was clearly stated. If the Cochran Q was not statistically significant and Q/df was less than 1, important heterogeneity considered very unlikely. Also, we used I2 statistic for quantifying inconsistency (I2 = [(Q-df)/Q] x 100%, where Q is the chi-squared statistic and df is its degrees of freedom). This describes the percentage of the variability in effect estimates that is due to heterogeneity rather than sampling error (chance). Negative values of I2 are put equal to zero so that I2 lies between 0 and 100%. A value of 0% indicates no observed heterogeneity, and larger values show increasing heterogeneity. A value greater than 50% was considered substantial heterogeneity. (10-11)
Pooling data and analysis
First Fix/random model meta-analysis was done using a Microsoft excel spreadsheet (10) and then studies were pooled according to the heterogeneity test results. In presence of significant heterogeneity, we used random model effect methods for meta-analysis. An overall prevalence with its 95% confidence intervals was calculated as a weighted average of individual summary statistics for eight age groups (1-5, 6-10, 11-15, 16-20, 21-25, 26-30, 31-40 and >40y). The results and forest plots were shown using Forest plot Viewer software (12). Since a large number of studies are included in the forest plots, the sample size and confidence intervals of pooled studies are listed in a separate table to reduce the clutter and to improve readability.
RESULTS
Search result
We found 72 relevant papers (after deletion of overlapping studies) out of all 112 searched citations in electronic search but failed to find more evidence during searching literature. Three new results were retrieved by backward and forward search of citations and Google scholar search. After exclusion of overlapping reports, we finally selected 15 studies. The detailed search process is demonstrated in Fig.2.
Fig. 2. Selection of studies for inclusion in review: varicella seroprevalence in I.R. Iran.
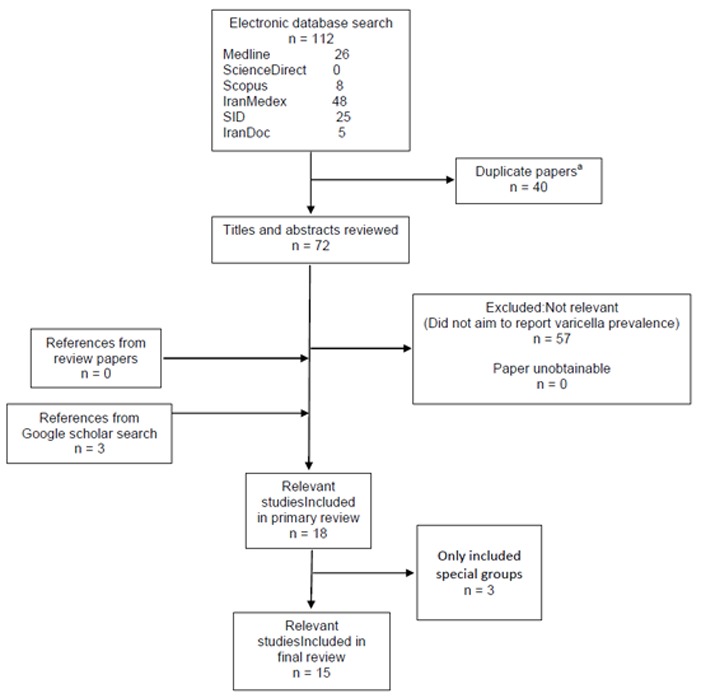
a. Duplicated papers due to searching multiple databases.
b. Special groups: hospitalized patients, patients on haemodialysis, and hospital staff
Studies
There were 18 relevant studies with satisfactory quality from 9 (out of 30) provinces (approximately 40% of the country’s population). Five studies were from Tehran (the Capital) covering from 2003 to 2010 (13-17), two from Fars covering 2002-2003 and 2008 (18-19), and two from Kerman in 2008 and 2006 to 2008 (20-21). Other studies were from Bushehr 2009 (22), Hamadan (2009-2010) (23), Mazandaran (2010-2011) (24), Isfahan (2011) (25), Kermanshah (2012)(26), and Qazvin (2012) (27). All included papers were cross sectional studies conducted in Iran from 2002 to 2012 with sample sizes between 62 and 843. (Table 1) Age of the subjects was between 1-70. Included studies have a female proportion between 100% (6 of 15) to 35%. Result of studies on the special groups (26, 28-30) who were assumed to be at groups with a higher risk for varicella (patients on hemodialysis, and health care workers) has been shown in Table 2. All studies had used ELISA methods, mainly Germany ELISAVZV IgG detection kits.
Table 1.
Characteristics of included studies (varicella seropositivity prevalence in Iranian cities between 2002 and 2012 by regions)
| Study’s First Author (Year of collection*) | City | Target population | Prevalence of seropositivity in age groups [positive seroprevalence/ sample size (%)] | Reference | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1-5 | 6-10 | 11-15 | 16-20 | 21-25 | 26-30 | 31-35 | 36-40 | 41-45 | 46-50 | 51≥ | Total | ||||
| Motamedifar (2002- 2003) | Shiraz | Primary school children | 95/270 (35.2) | 95/270 (35.2) | 18 | ||||||||||
| Sharifi (2003-2005) | Tehran | NA | 46/77 (59.7) | 31/51 (60.8) | 91/104 (87.5) | 133/151 (88) | 42/47 (89.4) | 51/58 (87.9) | 43/49 (87.7) | 64/74 (86.5) | 511/611 (83.6) | 13 | |||
| Ehsanipour (2005) | Tehran | referred to hospital clinics | 25/82 (30.6) | 16/26 (61.5) | 10/12 (83.3) | 51/120 (42.5) | 14 | ||||||||
| Pourahmad (2006-2008) | Jahrom | Premarital women | 21/38 (55.3) | 104/145 (71.7) | 81/109 (74.3) | 25/28 (89.3) | 9/9 (100) | 2/2 (100) | 2/2 (100) | 244/333 (73.3) | 20 | ||||
| Ziyaeyan (2008) | Shiraz | referred to hospital clinics | 39/154 (25.3) | 60/139 (43.1) | 78/106 (73.5) | 84/101 (83.2) | 41/49 (83.7) | 41/48 (85.4) | 37/42 (88.1) | 31/35 (88.6) | 81/92 (88) | 67/77 (87) | 559/843 (66.3) | 19 | |
| Hosseininasab (2008) | Kerman | Premarital women | 315/370 (85.1) | 331/353 (93.8) | 646/723 (89.3) | 21 | |||||||||
| Pourakbari (2008) | Tehran | Children, adolescents and medical students | 138/216 (63.9) | 75/101 (74.2) | 57/95 (60.0) | 269/412 (65.3) | 15 | ||||||||
| Talebi-Taher (2008) | Tehran | referred to hospital clinics | 56/75 (74.7) | 75/98 (76.5) | 89/105 (84.8) | 93/122 (76.2) | 313/400 (78.2) | 17 | |||||||
| Barazesh (2009) | Bushehr | Premarital women | 111/150 (74) | 23/30 (76/67) | 134/180 (74.5) | 22 | |||||||||
| Mamani (2009-2010) | Hamedan | Pregnant women | 27/36 (75.0) | 75/94 (79.8) | 63/76 (82.9) | 27/38 (71.1) | 16/20 (80.0) | 4/6 (66.7) | 212/270 (78.4) | 23 | |||||
| Talebi-Taher (2010) | Tehran | Pregnant women | 35/45 (77.8) | 101/117 (86.3) | 116/123 (94.3) | 108/114 (94.7) | 360/400 (90.3) | 16 | |||||||
| Bayani (2010-2011) | Babol | Pregnant women | 40/47 (85.1) | 90/101 (89.1) | 137/150 (91.3) | 109/117 (93.2) | 9/12 (75) | 385/427 (90.2) | 24 | ||||||
| Taghavi (2011) | Kashan | Referral pediatric hospital and public health centers | 27/212 (12.7) | 66/192 (34.4) | 61/154 (39.6) | 154/558 (27.6) | 25 | ||||||||
| Farshchi (2012) | Kermanshah | Medical student | 12/19 (63.1) | 40/43 (93.0) | 52/62 (83.9) | 26 | |||||||||
| Allami (2012) | Qazvin | Medical science student | 112/160 (70) | 50/64 (78.1) | 14/17 (82.4) | 11/12 (91.7) | 187/253 (74.0) | 27 | |||||||
year of data collection / study
Table 2.
Study characteristics of specific group, (varicella seropositivity prevalence in Iranian areas between 2002 and 2012 by regions)
| Study’s First Author (year of collection*) | City | Target population | Prevalence of seropositivity in age groups [positive seroprevalence/ sample size (%)] | Reference | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 16- 20 | 21- 25 | 26- 30 | 31- 35 | 36- 40 | 41-45 | 46-50 | 51-55 | 56-70 | ≥ 70 | Total | ||||
| Talebi-Taher (2009) | Tehran | Health care workers | 95/136 (69.8) | 74/103 (71.8) | 40/58 (68.9) | 39/55 (71.0) | 41/53 (77.4) | 289/405 (71.4) | 9 | |||||
| Talebi-Taher (2010) | Tehran | Patients on Hemodialysis | 24/27 (88.8) | 57/58 (98.2) | 53/53 (100) | 48/48 (100) | 183/187 (97.9) | 12 | ||||||
| Bayani (2011-2012) | Babol | Healthcare workers | 146/160 (91.2) | 240/248 (96.8) | 48/51 (94.1) | 434/459 (94.6) | 15 | |||||||
| Farshchi (2012) | Kermanshah | Health care workers | 10/16 (62.5) | 30/39 (76.9) | 80/88 (90.9) | 39/45 (86.7) | 159/188 (84.5) | 26 | ||||||
VZV immunity prevalence
Range of the reported VZV prevalence in childhood was wide and the studies showed heterogeneity (Table 3). The meta-analysis of point estimations and 95% confidence interval for VZV prevalence in different age groups were shown as a forest plot in Fig 3. The seropositivity prevalence steeply increased from the age of 1-5 to 6-10 [from 21.9% (95% CI; 10.8-33.1) to 42.1% (95% CI; 33.6-50.6)]. At the age of 11 15, 59.4% (95% CI; 46.1-72.8) of children showed to be infected. The rate of seropositivity was more than 87% in individuals of 40 and older. A gender difference in the prevalence of anti-VZV antibodies was reported in only one study(17). Trend of age-specific prevalence of VZV antibody in Iranian population during 2002 to 2012 is shown in Fig 4.
Table 3.
Heterogeneity for meta-analyses of prevalence
| Fixedeffects model | Heterogeneity | Randomeffects model | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Age group | Q | I2(%) | Q/df | P value | Qv | I2v | Q/df | P value | |
| 1-5 | 12.03 | 83.38 | 6.02 | 0.0024* | substantial | 1.75 | 0 | 0.88 | 0.4160 |
| 6-10 | 10.54 | 62.06 | 2.64 | 0.032* | substantial | 5.31 | 24.66 | 1.33 | 0.257 |
| 11-15 | 18.26 | 72.61 | 3.65 | 0.002* | substantial | 3.57 | 0 | 0.71 | 0.613 |
| 16-20 | 3.99 | 0 | 0.50 | 0.8579 | non-significant | 10.97 | 27.06 | 1.37 | 0.2035 |
| 21-25 | 11.84 | 7.08 | 1.08 | 0.376 | non-significant | 10.77 | 0 | 0.98 | 0.463 |
| 26-30 | 3.85 | 0 | 0.43 | 0.921 | non-significant | 10.78 | 16.52 | 1.20 | 0.290 |
| 31-40 | 3.97 | 0 | 0.57 | 0.783 | non-significant | -18.85 | 100 | -2.69 | 1 |
| >40 | 0.74 | 0 | 0.19 | 0.995 | non-significant | 1.31 | 0 | 0.33 | 0.859 |
df=degrees of freedom.
significant P-value ≤ 0.10
Fig. 3.
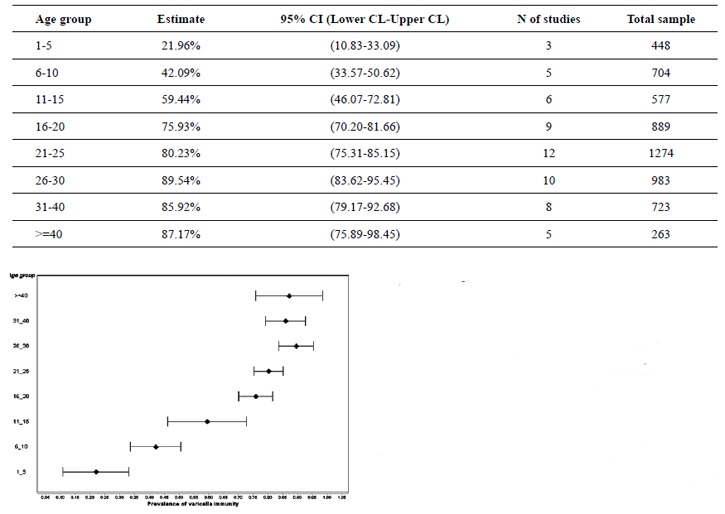
Forest Plot of varicella immunity (prevalence estimation) by age group
Fig.4.
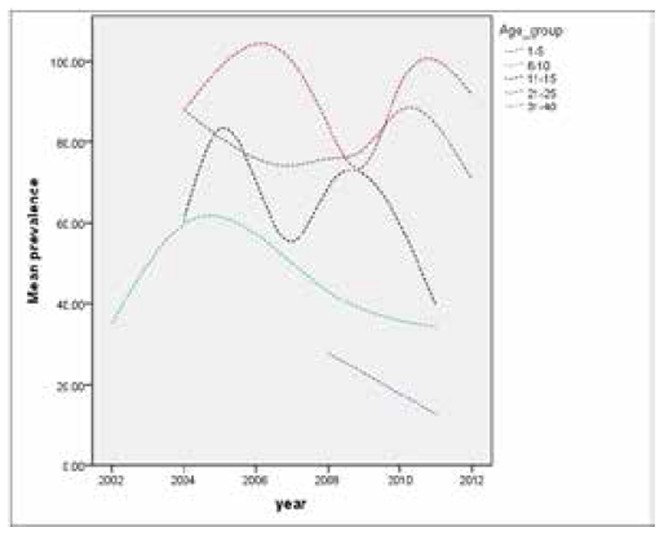
Age-specific prevalence of varicella-zoster virus (VZV) antibody in Iranian population (2002-2012)
DISCUSSION
This study is an age-stratified systematic review and meta-analysis on VZV seroprevalence rates in Iran. Result of our study provides secondary (synthesized) epidemiological information on VZV infection based on results of seroprevalence studies in various regions of a climatically heterogeneous country. Also, it prepares the baseline information to design a more effective strategy for the national VZV control programs. We focused on published papers during 2002-2014 to estimate a more accurate estimation of VZV immunity prevalence rates. This meta-analysis indicates that varicella immunity tends to get higher in the older ages and VZV IgG antibodies remain detectable over a lifetime; while the rate of sero positivity is not decreased even among the individuals of 40s and more. This may be described by numerous re-exposures or endogenous reactivation of VZV which preserve immunity (31). Seropositivity rates were low in the early childhood as the frequency of positive samples was 21.96% (95% CI; 10.83-33.09) during the first 1-5 years. By the age 6–10 years, 42.1% of the population had already been infected by VZV and at the age of 10–15 years, 59.4% of children were positive for anti-VZV antibodies. Finally, only a few individuals [12.83% (95% CI; 1.55-24.11)] were still susceptible to VZV infection by the age of 40 and more.
This study revealed that less than 60% of the populations have experienced infection before the age of 15. Also there is a relatively rapid rise in the seropositivity until the age of 25. This is important because varicella is considered as a benign and self-limiting disease of children, but it can be a potentially serious and life threatening condition in adults. (32)
Epidemiology of varicella shows different patterns in various climates. In tropical areas such as south Asian countries (e.g. Pakistan, Sri Lanka and India), the majority of varicella infections occur in young adults, while a few children (under 10 years old) are infected (33-35). A delayed onset of natural immunity (i.e. lower herd immunity and higher susceptibility to VZVin younger adults) happens in tropical regions while no apparent seasonal trend is observed. About one-fifth of the population remains at risk and VZV outbreaks can result complications such as pneumonia, hospitalization, and a greater burden of care especially in the middle-aged individuals (36-37). In temperate climates and in the absence of vaccination, varicella is relatively common in childhood with a high burden but low mortality rates. Many cases are presented before the age of 4, with a seasonal pattern (annual peak of late winter and early spring)(38-39).The majority of cases in temperate countries such as Germany, and Netherlands (before varicella vaccination), were found among young children and seroprevalence was increased steeply (40-41). Recent data from Switzerland showed that anti-VZV antibodies were detectable in 96.5% of 13 15-year old adolescents (42). Reasons for the different age distribution have remained unclear, although it could be a result of:1) different climates in these countries,2) a high degree of humidity and temperatures that inactivate the virus, thereby interrupting its transmission (43), and 3) the degree of childhood social interaction or population density in developed countries (44). As it is expected, the varicella seroepidemiology in Iran is compatible with average of both tropical and temperate regions. The results of the meta-analysis demonstrated a lower rate of varicella immunity in Iran than temperate countries and higher than tropical countries.
In our study, the comparison of prevalence rates in all childhood age-groups showed significant heterogeneity. Most of these studies were in Iranian temperate region. When there are only few studies, investigations of heterogeneity has a limited value (45). So, we performed a random effects model for meta-analysis instead of determining the causes of heterogeneity among results.
Considering overlap of VZV prevalence in two periods of time (2002-2005 and after 2005), we may conclude that prevalence rate of VZV in Iran has not changed significantly during those years. In Iran, absence of a mass vaccination program against VZV causes lack of significant changes in VZV immunity pattern. For example, in two studies from Tehran on the age group of11-15 (2003-2005 and 2008), positive seroprevalence rates were 60.78(95% C: 47.09-72.97) and 63.89 (95% CI: 57.29-70.00), respectively (13, 15). Also, studies conducted in adjacent countries to Iran have a similar finding. Comparison of conducted studies during 2002 to 2013 didn’t show any alteration in VZV seroprevalence in Turkey (western adjacent) (46-49).
Result of this study could identify future potential research areas and help in medical service planning in Iran. The study provides baseline information to assess appropriateness of a mass vaccination program, design the most effective strategy, and evaluate national programs once in place. However, the main limitation of the study is limited generalizability of the findings to whole Iranian population. The initial studies were conducted in nine major cities and did not cover rural population as well as people in other provinces and cities, so the results may not be easily generalized to whole Iranian population. This also could be a logical reason for more nationwide studies in this field.
CONCLUSION
The varicella seroepidemiology in Iran is in accordance with average tropical areas (south Asia) and temperate regions in the absence of vaccine (European countries). The seropositivity prevalence increased steeply from the age of 1-5 to 6-10 [from 21.9% (95% CI; 10.8-33.1) to 42.1 %(95% CI; 33.6-50.6)]. At the age of 11 15, 59.4% (95% CI; 46.1-72.8) of children showed to be infected. The rate of seropositivity was more than 87% in individuals of 40 and older. Comparison of conducted studies in Iran during 2003 to 2011 didn’t show any alteration in VZV seroprevalence. In conclusion, our findings are consistent with prior information gathered in Iranian adjacent countries.
Acknowledgments
We would like to thank all corresponding authors of primary draft of manuscript.
References
- 1.Whitley RJ. Mandell, Douglas, and Bennett’s principles and practice of infectious diseases. 7. Philadelphia, PA: Elsevier Churchill Livingstone; 2010. Varicella-Zoster Virus; pp. 1963–1969. [Google Scholar]
- 2.Miller E, Marshall R, Vurdien J. Epidemiology, outcome and control of varicella-zoster infection. Rev Med Microbiol. 1993;4:222–230. [Google Scholar]
- 3.Gil A, Oyaguez I, Carrasco P, Gonzalez A. Epidemiology of primary varicella hospitalisations in Spain. Vaccine. 2002;20:295–298. doi: 10.1016/s0264-410x(01)00370-x. [DOI] [PubMed] [Google Scholar]
- 4.Sauerbrei A, Wutzler P. Herpes simplex and varicella zoster virus infections during pregnancy: current concepts of prevention, diagnosis and therapy. Part 2: varicella-zoster virus infections. Med Microbiol Immunol. 2007;196:95–102. doi: 10.1007/s00430-006-0032-z. [DOI] [PubMed] [Google Scholar]
- 5.Harger JH, Ernest JM, Thurnau GR, Moawad A, Momirova V, Landon MB, et al. Risk factors and outcome of varicella-zoster virus pneumonia in pregnant women. J Infect Disease. 2002;185:422–427. doi: 10.1086/338832. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen HQ, Jumaan AO, Seward JF. Decline in mortality due to varicella and implementation of varicella vaccination in the United States. N Engl J Med. 2005;352:450–458. doi: 10.1056/NEJMoa042271. [DOI] [PubMed] [Google Scholar]
- 7.Geography of Iran: facts and figures. Iran Chamber Society; 2001. [ http://www.iranchamber.com] webcite. [Google Scholar]
- 8.The Results of Census 2006 (1385 AD) from Statistical Centre of Iran. Statistical Centre of Iran; 2009. [ http://www.sci.org.ir] webcite. [Google Scholar]
- 9.Moradi-Lakeh M, Esteghamati A. National Immunization Program in Iran: Whys and why nots. Hum Vaccin Immunother. 2013;9:112. doi: 10.4161/hv.22521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neyeloff JL, Fuchs SC, Moreira LB. Meta-analyses and Forest plots using a microsoft excel spreadsheet: step-by-step guide focusing on descriptive data analysis. BMC Res Notes. 2012;5:52. doi: 10.1186/1756-0500-5-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Br Med J. 2003;327(7414):557. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.U.S. Department of health and human services. National toxicology program. The Forest Plot Viewer software. Available from: http://ntp.niehs.nih.gov/go/tools_forestplotviewer [cited March 28, 2014]
- 13.Sharifi Z, Emadi Ghanjin S. The seroepidemiology of varicella zoster (VZV) in different age groups in Tehran, Iran. Iran J Allergy Asthma Immunol. 2005;4:95–98. [PubMed] [Google Scholar]
- 14.Ehsani pour F, Shayanfar N, Salariyan K. Surveying of protective antibody against varicella zoster virus (VZV) infection in children referring to Hazrat-e-RasoolAkram Hospital (2005) Iran Uni Med Sci J. 2009;16(64):38–44. [Google Scholar]
- 15.Pourakbari B, Shahbaznezhad L, Parvaneh N, Nikkhah S, Mahmoudi S, Teymuri M, et al. Seroepidemiology of Varicella zoster virus among children, adolescents and medical students in a referral children medical center, Tehran, Iran. Iran J Microbiol. 2012;4:136–138. [PMC free article] [PubMed] [Google Scholar]
- 16.Talebi-Taher M, Kashanian M, Khalili K. Seroprevalence of varicella-zoster virus among pregnant women in two teaching hospitals, Tehran, Iran. Iran J Microbiol. 2014;6:37–40. [PMC free article] [PubMed] [Google Scholar]
- 17.Talebi-Taher M, Rezaie O. Seroepidemiology of VZV in the young adults referring to Rasoul-Akram Hospital in Tehran. Sci J Kurdistan Univ Med Sci. 2012;17:36–42. [Google Scholar]
- 18.Motamedifar M, Handjani F, Hadi N, Shahkarami MK, Mehrabani D. Seroprevalence of varicella – zoster virus in children from Shiraz-Iran. Iran J Immunol. 2006;3:43–46. [Google Scholar]
- 19.Ziyaeyan M, Alborzi A, Jamalidoust M, Moieni M, Pourabbas B. Seroepidemiology of Varicella zoster virus infection among 1–70 year individuals in Iran. Iran Red Crescent Med J. 2012;12:176–180. [Google Scholar]
- 20.Pourahmad M, Davami MH, SotoodehJahromi AR. Evaluation of anti-varicella antibody in young women before their marriage: a sero-epidemiologic study in Iran. J Clin Virol. 2010;48:260–263. doi: 10.1016/j.jcv.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 21.Hosseininasab A, Arabzadeh AM, Haghdoost AA, Helmi Z. Immunity against varicella zoster virus based on history of previous chickenpox: a study in premarital Iranian women. Int J Infect Dis. 2013;17:e568–e569. doi: 10.1016/j.ijid.2013.01.033. [DOI] [PubMed] [Google Scholar]
- 22.Barazesh A, Zandi K, Hadavand F, Moatamed N, Hefzollah F, Hefzollah B, Vahdat K. Seroepidemiology of rubella, cytomegalovirus, herpes simplex & Varicella zoster virus in college women of Bushehr. Iran South Med J. 2014;16:459–466. [Google Scholar]
- 23.Mamani M, Zamani M, Hashemi SH, Akhtari M, Niayesh A. Seroepidemiology of varicella zoster virus among pregnant women in Hamedan, Iran. Afr J Microbiol Res. 2012;6:1829–1832. [Google Scholar]
- 24.Bayani M, Siadati S, Esmaeilzadeh S, Asgari S, Salmani S. Seroprevalence of Varicella zoster antibodies among pregnant women in Babol, Northern Iran. Iran J Pathol. 2013;8:171–177. [Google Scholar]
- 25.TaghaviArdakani A, Soltani B, Sehat M, Namjoo S. Seroprevalence and Risk Factors of Varicella-Zoster Among Children in Kashan - Center of Iran. Jundishapur J Microbiol. 2013;6:e83–88. [Google Scholar]
- 26.Farshchi A, Niayesh A. Seroprevalence of Varicella antibodies in healthcare workers in Imam Reza Hospital of Kermanshah-Iran. J Pharma& Health Sci. 2012;1:37–40. [Google Scholar]
- 27.Allami A, Mohammadi N, Najar A. Seroepidemiology of varicella and value of self-reported history of varicella infection in Iranian medical students. Int J Occup Med Environ Health. 2014;27:304–313. doi: 10.2478/s13382-014-0265-9. [DOI] [PubMed] [Google Scholar]
- 28.Talebi-Taher M, Noori M, Shamshiri AR, Barati M. Varicella Zoster antibodies among health care workers in a university hospital, Tehran, Iran. Int J Occup Med Environ Health. 2010;23:27–32. doi: 10.2478/v10001-010-0011-x. [DOI] [PubMed] [Google Scholar]
- 29.Talebi-Taher M, Hassanzadeh T, Ossareh Sh. Seroprevalence of antibodies against Varicella-Zoster virus among prevalent hemodialysis patients. Iran J Kidney Dis. 2013;7:475–478. [PubMed] [Google Scholar]
- 30.Bayani M, Hasanjani-Roushan MR, Siadati S, Javanian M, Sadeghi-Haddad-Zavareh M, Shokri M, et al. Seroepidemiology of varicella zoster virus in healthcare workers in Babol, Northern Iran. Caspian J Intern Med. 2013;4:686. [PMC free article] [PubMed] [Google Scholar]
- 31.Arvin AM. Immune responses to varicella-zoster virus. Infect Dis Clin North Am. 1996;10:529–570. doi: 10.1016/s0891-5520(05)70312-3. [DOI] [PubMed] [Google Scholar]
- 32.Mohsen AH, McKendrick M. Varicella pneumonia in adults. Eur Respir J. 2003;21:886–891. doi: 10.1183/09031936.03.00103202. [DOI] [PubMed] [Google Scholar]
- 33.Akram DS, Qureshi H, Mahmud A, Khan AA, Kundi Z, Shafi S, et al. Seroepidemiology of varicella-zoster in Pakistan. Southeast Asian J Trop Med Public Health. 2000;31:646–649. [PubMed] [Google Scholar]
- 34.Liyanage NP, Fernando F, Malavige GN, Mallikahewa R, Sivayogan S, Jiffry MT, et al. Sero-prevalence of varicella zoster virus infections in Colombo district, Sri Lanka. Indian J Med Sci. 2007;61:128–134. [PubMed] [Google Scholar]
- 35.Venkitaraman AR, Seigneurin JM, Baccard M, Lenoir GM, John TJ. Measurement of antibodies to varicella-zoster virus in a tropical population by enzyme-linked immunosorbentassay. J Clin Microbiol. 1984;20:582–583. doi: 10.1128/jcm.20.3.582-583.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ooi PL, Goh KT, Doraisingham S, Ling AE. Prevalence of varicella-zoster virus infection in Singapore. Southeast Asian J Trop Med Public Health. 1992;23:22–25. [PubMed] [Google Scholar]
- 37.Lolekha S, Tanthiphabha W, Sornchai P, Kosuwan P, Sutra S, Warachit B, et al. Effect of climatic factors and population density on varicella zoster virus epidemiology within a tropical country. Am J Trop Med Hyg. 2001;64:131–136. doi: 10.4269/ajtmh.2001.64.131. [DOI] [PubMed] [Google Scholar]
- 38.Schmid S, Jumaan AO. Impact of varicella vaccine on varicella-zoster virus dynamics. Clin Microbiol Rev. 2010;23:202–217. doi: 10.1128/CMR.00031-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Masuet-Aumatell C, Ramon-Torrell JM, Casanova-Rituerto A, Banqué-Navarro M, Dávalos-Gamboa MR, Montaño-Rodríguez SL. Seroprevalence of varicellazoster virus infection in children from Cochabamba: tropical or temperate pattern? Trop Med Int Health. 2013;18:296–302. doi: 10.1111/tmi.12040. [DOI] [PubMed] [Google Scholar]
- 40.Wutzler P, Farber I, Wagenpfeil S, Bisanz H, Tischer A. Seroprevalence of varicella-zoster virus in the German population. Vaccine. 2001;20:121–124. doi: 10.1016/s0264-410x(01)00276-6. [DOI] [PubMed] [Google Scholar]
- 41.de Melker H, Berbers G, Hahne S, Rumke H, van den Hof S, de Wit A, et al. The epidemiology of varicella and herpes zoster in The Netherlands: implications for varicella zoster virus vaccination. Vaccine. 2006;24:3946–3952. doi: 10.1016/j.vaccine.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 42.Aebi C, Fischer K, Gorgievski M, Matter L, Muhlemann K. Age-specific seroprevalence to varicella-zoster virus: study in Swiss children and analysis of European data. Vaccine. 2001;19:3097–3103. [Google Scholar]
- 43.Garnett GP, Cox MJ, Bundy DA, Didier JM, St Catharine J. The age of infection with varicella-zoster virus in St Lucia, West Indies. Epidemiol Infect. 1993;110:361–372. doi: 10.1017/s0950268800068308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arvin AM. Varicella-zoster virus. Clin Microbiol Rev. 1996;9:361–381. doi: 10.1128/cmr.9.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Higgins JPT, Green S. Formulating the problem. [accessed 6th April 2014];Cochrane Handbook for Systematic Reviews of Interventions 4.2.6 [updated September 2006]; Section 8.7.3. http://www.cochrane.org/resources/handbook/hbook.htm.
- 46.Kanra G, Tezcan S, Badur S. Varicella seroprevalence in a random sample of the Turkish population. Vaccine. 2002;20:1425–1428. doi: 10.1016/s0264-410x(01)00459-5. [DOI] [PubMed] [Google Scholar]
- 47.Ozkan S, Maral I, Ilhan F, Aycan S, Cirak MY, Beyazova U, et al. Varicella zoster seroprevalence in children less than 5 years old. J Trop Pediatr. 2005;51:141–144. doi: 10.1093/tropej/fmh102. [DOI] [PubMed] [Google Scholar]
- 48.Koturoglu G, Kurugol Z, Turkoglu E. Seroepidemiology of varicella-zoster virus and reliability of varicella history in Turkish children, adolescents and adults. Paediatr Perinat Epidemiol. 2011;25:388–393. doi: 10.1111/j.1365-3016.2010.01180.x. [DOI] [PubMed] [Google Scholar]
- 49.Kose S, Mandiracioglu A, Senger SS, Ulu Y, Cavdar G, Gol B, et al. Seroprevalence of varicella-zoster virus in the prevaccine era: A population-based study in Izmir, Turkey. J Infect Public Health. 2013;6:115–119. doi: 10.1016/j.jiph.2012.10.003. [DOI] [PubMed] [Google Scholar]


