Abstract
Background and Objectives
Ureaplasma urealyticum can colonize women genital tract and be isolated from the amniotic fluid of women with adverse pregnancy outcomes. The association of U. urealyticum with spontaneous abortion remains controversial. The aim of this study was to evaluate the frequency of U. urealyticum infection among pregnant women and its association with spontaneous abortion.
Materials and Methods
In this case-control study we included 109 women with spontaneous abortion with gestation age between 10-20 weeks (Cases), and 109 women with normal pregnancy with gestation age between 20-30 weeks (Controls) in Sanandaj, Iran. Using specific primers and extracted DNA from endocervical swabs, a PCR test was conducted for detection of U. urealyticum in both women groups.
Results
Total prevalence of U. urealyticum infection in women was 26 out of 218 cases (11.92%). The prevalence of U. urealyticum infection was 18 out of 109 (16.5%) and 8 out of 109 (7.3%) in case (spontaneous abortion) and control groups, respectively. Using chi-square test, association between U. urealyticum infection and spontaneous abortion was statistically significant (P<0.05).
Conclusions
Colonization of U. urealyticum in genital tract of women, and its asymptomatic feature in combination with other factors such as other microorganisms or cervical incompetence may induce spontaneous abortion. Further studies are needed to confirm this possibility.
Keywords: Ureaplasma urealyticum, endocervical infection, spontaneous abortion, pregnancy outcomes
INTRODUCTION
Embryo-fetal infections have been associated with adverse pregnancy outcomes such as, spontaneous preterm birth, preterm premature rupture of membranes, chorioamnionitis and recurrent spontaneous abortions. They are significant contributors to perinatal morbidity and mortality (1–3). The possible mechanisms include toxic microbial byproducts, embryo-placental infection, endometrial infection, and inflammatory cascade during pregnancy. Chlamydia trachomatis, Ureaplasma urealyticum and Mycoplasma hominis may be associated with spontaneous abortion based on the previous reports (4–6).
U. urealyticum colonizes the women genital tract and can be found in vagina among 29-42% of all pregnant women. This organism is so prevalent that its role as a member of normal vaginal bacterial flora is not clear (7). It is frequently isolated from the amniotic fluid of women with adverse pregnancy outcomes. The presence of U. urealyticum and U. parvum on the samples of amniotic fluid from healthy asymptomatic pregnant women, and its association with preterm premature rupture of membranes or preterm birth have been reported (8). Women carrying both U. urealyticum and M. hominis simultaneously had more severe adverse pregnancy outcomes compared to patients who were only positive for U. urealyticum (9). Studies suggest that U. urealyticum is independently associated with fetal growth, low birth weight (7), ectopic pregnancy (10) and enhances the risk of these outcomes.
In addition to the well recognized bacterial invasion, uterus may also be infected by viruses and protozoa. Microorganisms may migrate the amniotic cavity and fetus by ascending from the vagina and cervix, hematogenous route through the placenta, or from the abdominal cavity through the fallopian tubes (11). However, the most common pathway of intrauterine infection is the vaginal and cervical infection and ascending route to amniotic cavity (2).
The association of U. urealyticum with pregnancy outcomes has been suggested by many observational studies and proof of causality in spontaneous abortion remains to be confirmed. As this organism can reside in the normal flora of vagina, there are controversies regarding its real role in pregnancy outcomes and the necessity to treat this bacterium (1, 12). Conflicting results seem primarily due to differences in study method, population and ethnic groups. The PCR assay has been used to detect U. urealyticum infections in women, because PCR has proved to be more sensitive and conventional microbiological methods (1, 8, 13).
The aim of this study was to evaluate the frequency of endocervical U. urealyticum infection among two groups of pregnant women (normal pregnancy and spontaneous abortion) and its association with spontaneous abortion.
MATERIALS AND METHODS
Study population
This case-control and non-interventional study, conducted in 2013. Pregnant women attended in the obstetrics and gynecology section or prenatal clinic and expected to deliver were included for this study in Beasat Hospital, Sanandaj, Kurdistan province, Iran. We included 109 women with spontaneous abortion with gestational age between 10-20 weeks (Cases), and 109 women with normal pregnancy with gestational age between 20-30 weeks (Controls). All women signed the informed-consent form to participate in this study.
Demographic data such as age, educational degree, and place of residence, occupation and obstetrical information such as number of childbirth, miscarriage (abortion), premature delivery, genital infection, urinary infection, the gestational age, and using contraceptive methods before pregnancy were gathered by gynecologist using a questionnaire.
Inclusion criteria for selected women were including pregnancy, gestational age, having sexual activity, no use of antibiotics two weeks before taking sample. Exclusion criteria were having immunodeficiency, chronic diseases (diabetes, endocrine disorders, and hypertension), recurrent miscarriage due to anatomic injury and positive results of first and second screening tests.
In addition to asking first day of last menstruation, ultrasound scan test was done for confirmation of gestational age. To eliminate the role of chromosomal abnormalities and probability of genetically miscarriage, fetal health assessments were done between 11-13 weeks of gestation, including nuchal translucency (NT), double tests such as pregnancy associated plasma protein A (PAPPA) and free βHCG. In addition, at 15-17 weeks of gestation the confirmatory triple tests were performed for elimination of probable neural tube and chromosomal anomalies.
After matching of two groups (gestational age and place of residence) and completing the questionnaire, subjects were asked for endocervical sampling by cotton swab. Samples immediately were taken into sterile tubes containing 5 ml of PBS (phosphate buffered saline) in 15 ml falcon tubes. Specimens were transported to the laboratory in a cold box and placed at -20°C until DNA extraction.
DNA extraction
Tubes containing cervical swab were centrifuged at 6000 rpm for 30 min. Then, the supernatant was discarded and the sediment poured into the 1.5 ml microtube. DNA was extracted from sediments using DNA extraction kit (High pure PCR Template Preparation, Roche, Germany) according to manufacturer’s instruction. After DNA extraction, to avoid being broken DNA, samples were aliquoted into separate 0.2ml microtubes and were maintained at -20°C until conducting PCR assay.
PCR test for U. urealyticum detection
We designed two specific primers for Urease gene of U.realyticum genome sequence in GeneBank. The primers sequences were as: Forward: 5’-ACG ACG TCC ATA AGC AAC T-3’ and Reverse: 5’-CAA TCT GCT CGT GAA GTA TTA C-3’ and the amplicon was 425bp (Fig. 1). PCR reaction was performed in a total volume of 25 μl using PCR master mix.
Fig 1.
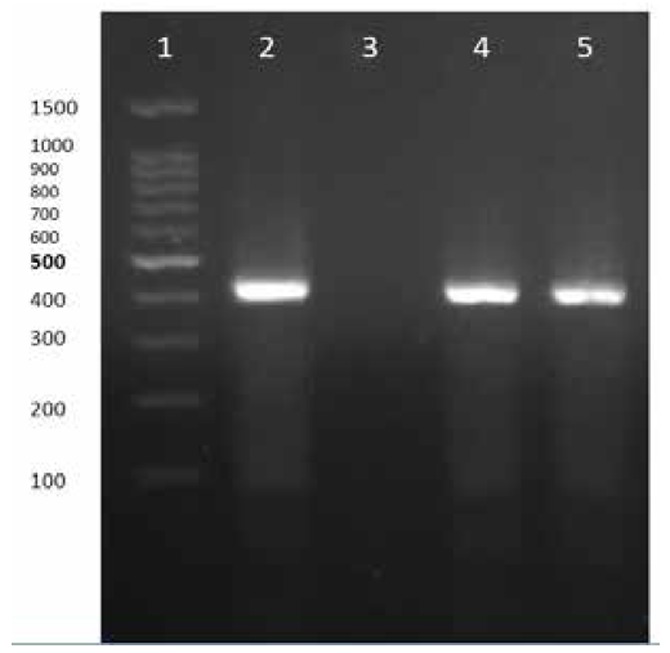
PCR test for Ureaplasma urealyticum detection.
Lane 1) DNA Ladder
Lane 2) PCR positive control
Lane 3) negative control
Lanes 4 and 5) positive PCR tests, (positive PCR products with 425bp length)
The amplification program consisted of initial denaturation at 94°C 5min, followed by 35 cycles of denaturation at 94°C 30s, annealing at 60°C 30s, extension at 72°C 60s; and final extension at 72°C 10min. PCR products were separated by electrophoresis in 1% gel agarose, stained by ethidum bromide, visualized by UV light and photographed. In addition to negative control U. urealyticum (ATCC 29557) was used as PCR positive control.
Statistical analysis
The data was analyzed using SPSS statistic software version 20. For comparison of qualitative variables, chi-square test was used in both groups.
RESULTS
The results of PCR for detection of Ureaplasma urealyticum DNA is demonstrated in Fig 1. Total prevalence of U. urealyticum infection in women (spontaneous abortion and normal pregnancy) was 26/218 (11.92%). The prevalence of U.realyticum infection was 18/109 (16.5%) in the case group (spontaneous abortion) and 8/109 (7.3%) in control group (normal pregnancy), respectively. The difference of prevalence rate among two groups was statistically significant (Chi-Square, p<0.032).
In our study, the gestational age of spontaneous abortion ranged between 10 and 20 weeks. Age range in case group was 19-43 years with the average of 29 year old; and in control group was 19-42 years, with the average of 27 year old.
The highest frequency of U. urealyticum infection in case group was for age groups 23 and 25 years (3 people), and in control group was for age group 21 years (2 people). The history of urinary tract infection in husbands of women was two in case group and zero in control group.
Numbers of pregnancies in control and patients group were similar. Average number of children in the family of both groups was two children. Summary of the results and history of vaginal and urinary infections in both groups are presented in Table 1.
Table 1.
Distribution of demographic data, history of risk factors, and prevalence of U. urealyticum infection in women with spontaneous abortion (Cases) and with normal delivery (controls).
| Variables | Cases (spontaneous abortion) n=109 | Controls (normal delivery) n=109 |
|---|---|---|
| Age range (years) | 19-43 | 19-42 |
| Illiterate | 5 | 3 |
| Primary education | 49 | 33 |
| High school education | 36 | 44 |
| Academic education | 19 | 29 |
| Housekeeper | 97 (89%) | 97 (89%) |
| Employee | 12 (11%) | 12 (11%) |
| Alcohol consumer | 0 | 0 |
| Smoking | 0 | 3 |
| History of Preterm delivery | 4 | 0 |
| History of preterm premature rupture of the membranes | 5 | 1 |
| History of Vaginal infection | 11 (10.1%) | 5 (4.6%) |
| History of Urinary infection | 9 (8.3%) | 8 (7.35%) |
| Prevalence of U. urealyticum infection | 18 (16.5%) | 8 (7.3%) |
| Coexistence of U. urealyticum and vaginal infection | 2 | 0 |
| Coexistence of U. urealyticum infection and preterm premature rupture of the membranes | 3 | 0 |
| Coexistence of U. urealyticum infection and Preterm delivery | 2 | 0 |
Using chi-square test, association between U. urealyticum infection and spontaneous abortion was statistically significant (P<0.05).
DISCUSSION
Ureaplasma urealyticum colonizes the women genital tract and is frequently isolated from amniotic fluid of healthy asymptomatic pregnant women. Its association with preterm premature rupture of membranes, preterm birth and spontaneous abortion has been reported. Also embryo-fetal infections have been suggested to cause recurrent spontaneous abortions at a rate about 4% (4). Using PCR, Aydin Yet al. found the prevalence of U. urealyticum infection in the cervices of 96 pregnant women and 124 non-pregnant controls as 26% and 15.3%, respectively, (14) which is higher than our results.
In a previous study the relationship between endocervical Mycoplasma infection and the spontaneous abortion was investigated using culture of endocervical swabs. In the spontaneous abortion group, the rate of U. urealyticum infection, was 74.1% (43/58), but in the normal group, the rate was 48.0% (24/50) and their difference was significant (P<0.01) (15). In a study conducted by Ye et al. (2004) 228 women who received routine prenatal care in Belgium during the first trimester (14 weeks’ gestation), U. urealyticum was associated with an increased risk of miscarriage (5). The effect of U. urealyticum infection on pregnancy and neonatal outcomes had also been evaluated in a prospective study in 170 infected women (cases) and 83 women with negative cultures (controls) by Abele-Horn et al. (1997). They concluded that U. urealyticum colonization is associated with amnionitis, chorioamnionitis and preterm delivery (16). In another study, women at mid-trimester of pregnancy with a positive cervical culture and high levels of antibodies, had a higher rate of pregnancy complications than those with a negative culture and absence of antibodies (P=0.0006) (17). In our study the prevalence of U. urealyticum was 18 out of 109 (16.5%) in the case group (spontaneous abortion) and 8 out of 109 (7.3%) in control group; and the association between U. urealyticum endocervical infection and spontaneous abortion at gestation age between 10-20 weeks was statistically significant (P<0.05). Considering the relatively similar sample size and study method, analysis the role of U. urealyticum in spontaneous abortion, our results are comparable to previous studies.
Our result contradicted those of some earlier studies with regard to pregnancy outcomes, i.e. no significant difference in the prevalence of M. hominis, and U. urealyticum in the spontaneous preterm birth and without preterm birth groups using vaginal swabs from 126 pregnant women was reported by Choi SJ et al. (2). Also, in another study mycoplasmas detected in four subjects were not associated with pregnancy outcomes (8). In the study conducted by Kataoka et al. (2006) U. urealyticum were detected in 8.7% by PCR method in a prospective cohort study of 877 women with singleton pregnancies at <11 weeks of gestation. However, U. urealyticum, was not associated with abortion and preterm birth (6). Moreover, endocervical infection with U. urealyticum was not found in relation with spontaneous abortion or preterm low birth weight in two large unselected cohorts of pregnant women as reported by Harrison (18). The conflicting results obtained in our study may be related to difference in populations examined by others.
Although the vaginal and endocervical secretion specimens are easily accessible but indirectly reflect the intrauterine environment infection (7). Also, it is not known why U. urealyticum invades the amniotic cavity only in some women despite heavy colonization of the vagina by this microorganism (6). Causality has been difficult to demonstrate due to the high prevalence of asymptomatic lower genital tract colonization and culture data from inaccessible or potentially contaminated sites.
PCR assay is superior to culture method to detect the presence of ureaplasma. However, culture method allows antibiotic susceptibility testing (1). Endocervical secretions in the present study were not cultured for mycoplasmas, which makes us unable to compare PCR and culture isolation rates in our samples. Further, we did not examine the bacterial susceptibility test.
Due to the difficulties in demonstrating the mechanism of microbial pathogenesis and detection, definite relationship between infections and spontaneous abortion is still unknown and treatment of related infections is controversial (4,12). As, there was urinary tract infection in two husbands of women in case group, screening program is important in high risk sexually active women and needed as part of case-finding strategies and treatment. Antibiotic treatment of Ureaplasma and its possible effect on prevention of pregnancy outcomes is another approach. Further randomized trials are necessary to confirm this possibility. Detection of this microorganism in aborted material would be another research suggestion.
In conclusion, U. urealyticum infection may be an important etiologic agent of spontaneous abortion in Sanandaj. Because of high colonization of U. urealyticum in genital tract of women and its asymptomatic feature of infection, its combination with other factors such as other microorganisms or cervical incompetence may induce spontaneous abortion. Further studies are needed to confirm this possibility.
Acknowledgments
This study was part of M.Sc thesis of Amjad Ahmadi. We would like to thank Kurdistan University of Medical Sciences for financial support. We also appreciate Department of Genecology, Beasat Hospital, Kurdistan University of Medical Sciences, for providing endocervical swab specimens and for data collection.
References
- 1.Capoccia R, Greub G, Baud D. Ureaplasma urealyticum Mycoplasma hominis and adverse pregnancy outcomes. Curr Opin Infect Dis. 2013;26:231–240. doi: 10.1097/QCO.0b013e328360db58. [DOI] [PubMed] [Google Scholar]
- 2.Choi SJ, Park SD, Jang IH, Uh Y, Lee A. The prevalence of vaginal microorganisms in pregnant women with preterm labor and preterm birth. Ann Lab Med. 2012;32:194–200. doi: 10.3343/alm.2012.32.3.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldenberg RL, Andrews WW, Faye-Petersen OM, Goepfert AR, Cliver SP, Hauth JC. The Alabama Preterm Birth Study: intrauterine infection and placental histologic findings in preterm births of males and females less than 32 weeks. Am J Obstet Gynecol. 2006;195:1533–1537. doi: 10.1016/j.ajog.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 4.Nigro G, Mazzocco M, Mattia E, Di Renzo GC, Carta G, Anceschi MM. Role of the infections in recurrent spontaneous abortion. J Matern Fetal Neonatal Med. 2011;24:983–989. doi: 10.3109/14767058.2010.547963. [DOI] [PubMed] [Google Scholar]
- 5.Donders GG, Van Bulck B, Caudron J, Londers L, Vereecken A, Spitz B. Relationship of bacterial vaginosis and mycoplasmas to the risk of spontaneous abortion. Am J Obstet Gynecol. 2000;183:431–437. doi: 10.1067/mob.2000.105738. [DOI] [PubMed] [Google Scholar]
- 6.Kataoka S, Yamada T, Chou K, Nishida R, Morikawa M, Minami M, et al. Association between preterm birth and vaginal colonization by mycoplasmas in early pregnancy. J Clin Microbiol. 2006;44:51–55. doi: 10.1128/JCM.44.1.51-55.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vogel I, Thorsen P, Hogan VK, Schieve LA, Jacobsson B, Ferre CD. The joint effect of vaginal Ureaplasma urealyticum and bacterial vaginosis on adverse pregnancy outcomes. Acta Obstet Gynecol Scand. 2006;85:778–785. doi: 10.1080/00016340500442423. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez N, Fernandez C, Zamora Y, Berdasquera D, Rivera JA. Detection of Ureaplasma urealyticum and Ureaplasma parvum in amniotic fluid: association with pregnancy outcomes. J Matern Fetal Neonatal Med. 2011;24:47–50. doi: 10.3109/14767058.2010.482609. [DOI] [PubMed] [Google Scholar]
- 9.Kwak DW, Hwang HS, Kwon JY, Park YW, Kim YH. Co-infection with vaginal Ureaplasma urealyticum and Mycoplasma hominis increases adverse pregnancy outcomes in patients with preterm labor or preterm premature rupture of membranes. J Matern Fetal Neonatal Med. 2014;27:333–7. doi: 10.3109/14767058.2013.818124. [DOI] [PubMed] [Google Scholar]
- 10.Liang XD, Gu TT, Wang JL, Cui H, Wei LH. Relationship between ureaplasma urealyticum infection and ectopic pregnancy. Zhonghua Fu Chan Ke Za Zhi. 2007;42:370–373. [PubMed] [Google Scholar]
- 11.Pararas MV, Skevaki CL, Kafetzis DA. Preterm birth due to maternal infection: Causative pathogens and modes of prevention. Eur J Clin Microbiol Infect Dis. 2006;25:562–569. doi: 10.1007/s10096-006-0190-3. [DOI] [PubMed] [Google Scholar]
- 12.Klein LL, Gibbs RS. Use of microbial cultures and antibiotics in the prevention of infection-associated preterm birth. Am J Obstet Gynecol. 2004;190:1493–1502. doi: 10.1016/j.ajog.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 13.Perni SC, Vardhana S, Korneeva I, Tuttle SL, Paraskevas LR, Chasen ST, et al. Mycoplasma hominis and Ureaplasma urealyticum in midtrimester amniotic fluid: association with amniotic fluid cytokine levels and pregnancy outcome. Am J Obstet Gynecol. 2004;191:1382–1386. doi: 10.1016/j.ajog.2004.05.070. [DOI] [PubMed] [Google Scholar]
- 14.Aydin Y, Atis A, Ocer F, Isenkul R. Association of cervical infection of Chlamydia trachomatis Ureaplasma urealyticum and Mycoplasma hominis with peritoneum colonisation in pregnancy. J Obstet Gynaecol. 2010;30(8):809–812. doi: 10.3109/01443615.2010.519063. [DOI] [PubMed] [Google Scholar]
- 15.Ye LL, Zhang BY, Cao WL. Relationship between the endocervical mycoplasma infection and spontaneous abortion due to early embryonic death. Zhonghua Fu Chan Ke Za Zhi. 2004;39:83–85. [PubMed] [Google Scholar]
- 16.Abele-Horn M, Peters J, Genzel-Boroviczeny O, Wolff C, Zimmermann A, Gottschling W. Vaginal Ureaplasma urealyticum colonization: influence on pregnancy outcome and neonatal morbidity. Infection. 1997;25:286–291. doi: 10.1007/BF01720398. [DOI] [PubMed] [Google Scholar]
- 17.Horowitz S, Horowitz J, Mazor M, Porath A, Glezerman M. Ureaplasma urealyticum cervical colonization as a marker for pregnancy complications. Int J Gynaecol Obstet. 1995;48:15–19. doi: 10.1016/0020-7292(94)02236-4. [DOI] [PubMed] [Google Scholar]
- 18.Harrison HR. Cervical colonization with Ureaplasma urealyticum and pregnancy outcome: prospective studies. Pediatr Infect Dis. 1986;5(6 Suppl):S266–299. doi: 10.1097/00006454-198611010-00013. [DOI] [PubMed] [Google Scholar]


