Abstract
Background and Objectives
Women reproductive system is a suitable environment for growth of various pathogen and nonpathogenic microorganisms. Mycoplasmataceae is a family of bacteria which cause oligosymptomatic genital infections. The complications caused by these bacteria may lead to infertility in women. The aim of this study was detection of genital Mycoplasma hominis, Ureaplasmaurealyticum and Mycoplasma genitalium among infertile females who referred to the infertility clinics.
Materials and Methods
A total of 104 infertile women (in reproductive age) who referred to infertility clinics in the city of Sanandaj, Kurdistan, Iran, from February to May 2013 were selected for this study. Cervical swabs were collected from all patients. The presence of genital Mycoplasmas was detected by multiplex-PCR. All data were analyzed statistically.
Results
Out of 104 patients, 39 cases (37.5%) were infected with U. urealyticum. Mycuplasma. genitalium and M. homimis were detected in 3 (2.9%) of the infertile women. Co-infection was seen in 3.8% of the patients. There was no statistically the infections and patient age, educational levels, literacy, situation of employment, age of first significant sexual intercourse, history of abortion, type of infertility and infertility duration (p value > 0.05).
Conclusions
The data showed a low percentage of infection for M. genitalium and M. hominis in the studied women while the prevalence of U. urealyticum was high. Despite having no symptoms of an ongoing acute inflammation of the reproductive tract, many women may have genital mycoplasmas in the cervix. We concluded that multiplex PCR using a pair of primers is a useful and cost-effective method for diagnosis of female genital infections.
Keywords: PCR, Mycoplasma hominis, Mycoplasma genitalium, Ureaplasmaurealyticum
INTRODUCTION
Infertility can be defined as the lack of conception after at least one year of constant, sexual intercourse without using a contraceptive device (1,2). More than 70 million couples in the world suffer from infertility. The majority of them are residents of developing countries (3). In female, the main causes of infertility include endocrine disorders, ovulatory dysfunction, tubal and peritoneal diseases. Uterine infections are relatively uncommon. Furthermore, a great number of infertility cases still remain unexplained (4). Women reproductive system is a suitable environment for growth of various pathogen and nonpathogen microorganisms. Mycoplasmataceae is a family of bacteria which cause oligosymptomatic genital infections and the complications caused by these bacteria may lead to infertility in women (1).
Mycoplasma genitalium is associated with urethritis, cervicitis and endometritis, salpingitis and pelvic inflammatory disease (PID), and may be considered as a cause of infertility in women (5). Mycoplasma hominis also as a common commensal of the female genital tract has been associated with pyelonephritis, bacterial vaginosis, cervicitis, endometritis, PID and postpartum septicemia (6). Ureaplasma urealyticum is considered as the main cause of nonchlamydial, nongonococcal urethritis. Furthermore, this bacterium can cause chorioamnionitis, preterm delivery, abortion, preterm birth, bacterial vaginosis and cervicitis (7). The role of these microorganisms in the etiology of infertility has been very controversial (2, 8). Studies indicate that inappropriate diagnosis, prevention and treatment of mycoplasma infections can led to chronic disease such as PID and infertility (9). The aim of the present study was to determine the prevalence of M. hominis, M. genitalium and U. urealyticum in infertile women by multiplex PCR.
MATERIALS AND METHODS
Patients
In this cross sectional study, 104 married infertile women aged 14 to 40 years who referred to an infertility clinic (Besat Hospital, Kurdistan University of Medical Sciences, Sanandaj, Iran) from February to May 2013 were included. The patients included 62 women with primary infertility and 42 women with secondary infertility. Primary infertility refers to women who have not become pregnant after at least 1 year of having sex without using control methods. Secondary infertility refers to women’s who have been able to get pregnant at least once, but now are unable (10). The patient had no symptoms related to the genitourinary tract infections. Excluding criteria included the male factor infertility, reproductive organ infection, anatomical abnormalities, benign ovarian tumors and uterine myeloma.
All the subjects also have not been taking antibiotics from two weeks before sampling and written consent was obtained from all participants. Cervical swabs were collected from all the patients using a sterile Dacron swab (Eurotubo, Deltalab). Samples were transported to the laboratory in 5 ml Phosphate Buffered Saline (PBS).
DNA extraction and PCR amplification
DNA extraction of the specimens was carried with High pure PCR template Preparation Kit (Roche Co, Germany Cat.No.11796828001) in accordance with the instructions of the suppliers. Multiplex-PCR was used for detection of genital Mycoplasmas. The utilized primers which were capable of detecting M. hominis, M. genitalium and U. urealyticum simultaneously were as follows: 16 S rRNA forward primer sequence: MyUu F5-TGGAGTTAAGTCGTAACAAG-3, and reverse primer sequence: MyUu R5-CTGAGATGTTTCACTTCACC-3 (7). PCR reaction was performed using the PCR master mix (Ampliqon Co, Skovlunde, Denmark, Cat. No. 180301) in 25μl final volume for each sample including: 12.5 μl master mix 2X (1.5 mM magnesium chloride,15mM deoxy nucleotide triphosphate,1.25 U Taq DNA polymerase), 5 μl DNA template, 1μl of each primer pair in a total volume of 25μl. The PCR reaction was performed using a GenAmp PCR system (Corbiet, Sydney, Australia) according to the following program: pre denaturation for 5 minutes at 95°C followed by 30 cycles each containing denaturation at 94°C for 30 seconds, annealing at 56°C for 30 seconds and extension at 72°C for 60 seconds, followed by final extension at 72°C for 5 minutes. The M. genitalium (ATCC: 33530), M. hominis(ATCC: 15056) and U. urealyticum (ATCC: 29557) extracted DNA, as well as sterile double-distilled water were used as positive and negative controls for PCR reaction, respectively. PCR products were electrophoresed in a 1.5% agarose gel, followed by staining by SYBER green (CinaGen Co, Tehran, Iran) and visualizing under an UV transilluminator.
Statistical analysis
Statistical analysis was conducted to determine how many samples were positive for each bacterium, as well as for those positive for 2 or 3 bacterial isolates. In order to observe relation between these variables and the presence of an infection in our patients, we used the chi-square test. A p value <0.05 was considered statistically significant. The tests were performed using SPSS for windows version 16.0 (SPSS Inc, Chicago, IL).
RESULTS
The length of PCR products were 630, 559 and 465 bp for16 SrRNA gene of M. hominis and U. urealyticum and M. genitalium, respectively (Fig. 1). The studied group age was 14-40 (The mean age was 29.2 years old). In 40 patients (38.4%), there were at least one infection. Thirty nine cases (37.5%) were co-infected with U. urealyticum. M. genitalium and M. hominis were detected from 3(2.9%) of the patients (Table 1). U. urealyticum was detected in a decisive majority of patients.
Fig. 1. Agarose gel electrophoresis of PCR amplified products.
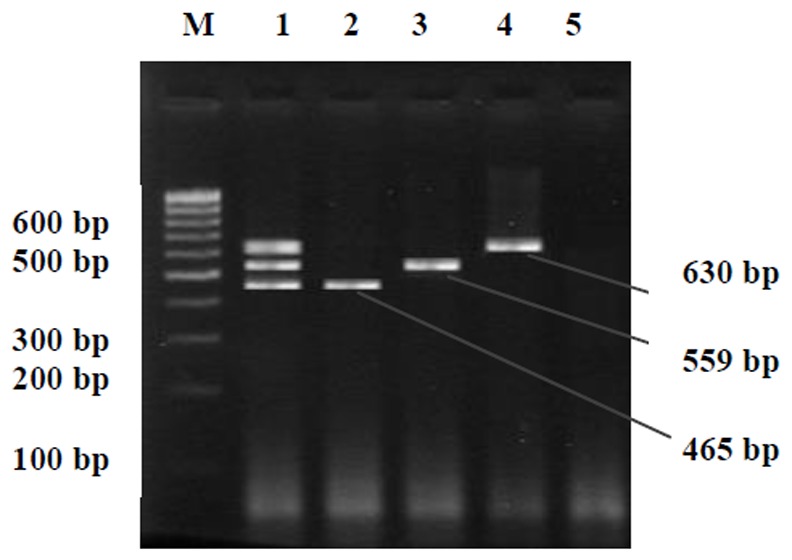
Lane 1 shows positive control for M. genitalium(465 bp), U. urealyticum(559 bp) and M. hominis (630 bp). Lane 2 shows 465 bp M. genitalium. Lane 3 shows 559 bp U. urealyticum. Lane 4 shows 630 bp M. hominis amplification product. Lanes 5 shows the negative control showing no Mycoplasma infection and Lane M is the DNA size marker (100 bp DNA ladder, SM#333)
Table 1.
Detection of genital Mycoplasmas by using multiplex PCR
| PCR Results | Positive | Percent |
|---|---|---|
| M. genitalium | 3 | 2.88 |
| U. urealyticum | 39 | 37.5 |
| M. hominis | 3 | 2.88 |
| M. genitalium+U. urealyticum | 1 | ./96 |
| M. genitalium + M. hominis | 1 | ./96 |
| U. urealyticum + M. hominis | 1 | ./96 |
| M. genitalium + U. urealyticum + M. hominis | 1 | ./96 |
| Total positive patients | 40 | 38.46 |
| Total negative patients | 64 | 61.54 |
| Total | 104 | 100 |
The patients characteristics have been summarized in Table 2. In terms of socio-economic indicators (age, literacy, situation of employment, age of the first sexual intercourse), their history of abortion, type of infertility(primary and secondary), duration of infertility and Mycoplasma infections. No significant difference was observed between infected and uninfected individuals (P value >0.05).
Table 2.
The characteristics of M. genitalium, M. hominis and U.uralyticum- infected patients
| Total | Prevalence of at least one infection n subjects/n total | P | Mycoplasma hominis n subjects/n total | Mycoplasma genitalium | Ureaplasma urealyticum |
|---|---|---|---|---|---|
| 40/104 | 3/104 | 3/104 | 39/104 | ||
| Age distrubition | |||||
| <23 years old(34) | 12 | 3 | 2 | 12 | |
| 23-31 years old (41) | 16 | 0.736 | 0 | 1 | 15 |
| >31 years old(26) | 12 | 0 | 0 | 12 | |
| Literacy | |||||
| Illiterate(13) | 6 | 0 | 0 | 5 | |
| Low – literate(52) | 19 | 2 | 2 | 19 | |
| Diploma(32) | 12 | 0.975 | 1 | 1 | 12 |
| Higher edacatione(7) | 3 | 0 | 0 | 3 | |
| Situation of employment | |||||
| Unemployed (4) | 3 | 0.126 | 0 | 0 | 3 |
| Employee (100) | 37 | 3 | 3 | 36 | |
| Age on first intercourse | |||||
| (14-27)years old (88) | 34 | 0.932 | 3 | 3 | 33 |
| >27 years old (16) | 6 | 0 | 0 | 6 | |
| History of Abortion | |||||
| Abortion (20) | 6 | 0.387 | 1 | 0 | 5 |
| Not Abortion (84) | 34 | 2 | 3 | 34 | |
| Duration of infertility | |||||
| (1-3)years old (58) | 22 | 2 | 2 | 22 | |
| (4-6)years old (24) | 9 | 0.693 | 1 | 1 | 8 |
| (7-9)years old (9) | 5 | 0 | 0 | 5 | |
| >9 years old (13) | 4 | 0 | 0 | 4 | |
| Type of infertility | |||||
| Primary(62) | 24 | 0.950 | 1 | 1 | 23 |
| Secondary (42) | 16 | 2 | 2 | 16 | |
DISCUSSION
Mycoplasma and Ureaplasma are agents of sexual transmitted diseases. They are considered to be a threat to community health (11). Most of these infections are not diagnosable due to lack of symptoms, the antibacterial effect of sperm, the high possibility of contamination with other urethral organisms and the difficulty of culturing (11, 12). Numerous researchers have attempted to study the association between genital Mycoplasmal infections and infertility. Several epidemiological reports have documented the presence of M. hominis, M. genitalium and U. urealyticum in infertile women (13, 14). Also in vitro studies have shown that sperm samples infected with these Mycoplasmas undergo detrimental changes in sperm count, sperm velocity and motility parameters (15). The pregnancy success rate of in vitro fertilization (IVF) might be reduced as a result of prior mycoplasma colonization of the female and male genital tract (11).
The prevalence of U. urealyticum and M. hominis was shown to be in an equal range as reported by Miron et al. (Romania- 2013), Günyeli (2011- Turkey), Verteramoet al. (2013 - Italy) and Zdrodowska et al.(2006 - Poland)(11, 16-18). There were no prominent differences in infection rates beside discrepancy in study population being geographically and socially different in each report. The infertile women in this study had no symptoms of acute infection of the genital tract, so the low prevalence of infection with M. hominis and M. genitalium was normal (20). High percentage of U. urealyticum infection may be related to the age and sexual activity the selected group of young women. Low prevalence of M. hominis infections may probably be due to the lack of any patients suffering from bacterial vaginosis (19, 20).
Mycolplasma genitalium was shown to be associated with infertility due to fallopian tube abnormalities (21). The frequency of this bacterium in the infertile women was almost similar to those reported by Tomusiak et al.(2013- Poland) and Gowin et al. (2011-Turkey) (22,23); but lower than those observed by Grześko et al. (24). In a few studies performed on women with tubal factor infertility, M. genitalium has not been isolated (25, 26).
Since the prevalence of M. genitalium is higher in women with PID, it’s presence is always indicative of pathologic conditions (27). Different rates of infection indicate discrepancy in sampling and other condition. However, more studies with large study group are needed to evaluate the rate of M. genitalium infection.
Detection of Mycoplasmas is very difficult and time consuming in many countries. Meanwhile numerous reports documented the role of these bacteria in women infertility and introduced them as true pathogens to the medical community. Obviously, adequate detection of these bacteria is urgently needed. An obstacle to diagnosis is high cost of Mycoplasmas culture. This study used multiplex PCR with a pair of primers in order to detect, in less than 8 hours, without interference from other microorganisms present in the sample.
In conclusion, our data showed a low rate of M. genitalium and M. hominis infections and high prevalence of U. urealyticum. These results could be a reflection of the regional and social conditions (muslim women with limited partners) which reduce bacterial infection in this population. In overall, this study results were in the same range of the reviewed articles. Despite having no symptoms of an ongoing acute inflammation of the reproductive tract, many women may have genital mycoplasmas in the cervix. We concluded that multiplex PCR method using a pair of primers is a useful and low cost method for diagnosis of genital infections in women.
Acknowledgments
This study was part of Atefeh Mousavi (Medical Microbiology Msc. student) thesis and was supported by a grant (91/134) from Research Deputy of Kurdistan University of Medical Sciences. We thanks Dr NourAmirmozafari for English revising of our manuscript. The authors wish to extend their gratitude to Avicenna Research Institute (ARI) for providing the standard bacterial strains.
References
- 1.Lee JS, Kim KT, Lee HS, Yang KM, Seo JT, Choe JH. Concordance of Ureaplasma urealyticum and Mycoplasma hominis in infertile couples: impact on semen parameters. Urology. 2013;81:1219–1224. doi: 10.1016/j.urology.2013.02.044. [DOI] [PubMed] [Google Scholar]
- 2.Pellati D, Mylonakis I, Bertoloni G, Fiore C, Andrisani A, Ambrosini G, et al. Genital tract infections and infertility. Eur JObstetGynecol Reprod Biol. 2008;140:3–11. doi: 10.1016/j.ejogrb.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 3.Ombelet W, Cooke I, Dyer S, Serour G, Devroey P. Infertility and the provision of infertility medical services in developing countries. Hum Reprod Update. 2008;14:605–621. doi: 10.1093/humupd/dmn042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hull M, Glazener C, Kelly N, Conway D, Foster P, Hinton R, et al. Population study of causes, treatment, and outcome of infertility. Brit Med. 1985;291(6510):1693. doi: 10.1136/bmj.291.6510.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Falk L, Fredlund H, Jensen J. Signs and symptoms of urethritis and cervicitis among women with or without Mycoplasma genitalium or Chlamydia trachomatis infection. Sex Transm Infect. 2005;81:73–78. doi: 10.1136/sti.2004.010439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor BD, Darville T, Haggerty CL. Does bacterial vaginosis cause pelvic inflammatory disease? Sex Transm Dis. 2013;40:117–122. doi: 10.1097/OLQ.0b013e31827c5a5b. [DOI] [PubMed] [Google Scholar]
- 7.Amirmozafari N, Mirnejad R, Kazemi B, Sariri E, Bojari MR, Darkahi FD. Comparison of polymerase chain reaction and culture for detection of genital mycoplasma in clinical samples from patients with genital infections. Saudi Med J. 2009;30:1401–1405. [PubMed] [Google Scholar]
- 8.Gdoura R, Kchaou W, Ammar-Keskes L, Chakroun N, Sellemi A, Znazen A, et al. Assessment of Chlamydia trachomatis, Ureaplasma urealyticum, Ureaplasma parvum, Mycoplasma hominis, and Mycoplasma genitalium in semen and first void urine specimens of asymptomatic male partners of infertile couples. J Androl. 2008;29:198–206. doi: 10.2164/jandrol.107.003566. [DOI] [PubMed] [Google Scholar]
- 9.Dhawan B, Gupta V, Khanna N, Singh M, Chaudhry R. Evaluation of the diagnostic efficacy of PCR for Ureaplasma urealyticum infection in Indian adults with symptoms of genital discharge. Jpn J Infect Dis. 2006;59:57. [PubMed] [Google Scholar]
- 10.Balen AH. Infertility in Practice. Fourth Edition. Taylor & Francis; 2014. pp. 5–8. [Google Scholar]
- 11.Günyeli İ, Abike F, Dünder İ, Aslan C, Tapısız ÖL, Temizkan O, et al. Chlamydia, Mycoplasma and Ureaplasma infections in infertile couples and effects of these infections on fertility. Arch Gynecol Obstet. 2011;283:379–385. doi: 10.1007/s00404-010-1726-4. [DOI] [PubMed] [Google Scholar]
- 12.Dabaja AA, Schlegel PN. Medical treatment of male infertility. Transl Androl Urol. 2014;3:9–16. doi: 10.3978/j.issn.2223-4683.2014.01.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fenkci V, Yilmazer M, Aktepe O. Have Ureaplasma urealyticum and Mycoplasma hominis infections any significant effect on women fertility? Infez Med. 2002;10:220–223. [PubMed] [Google Scholar]
- 14.Rosemond A, Lanotte P, Watt S, Sauget A, Guerif F, Royere D, et al. Systematic screening tests for Chlamydia trachomatis, Mycoplasma hominis and Ureaplasma urealyticum in urogenital specimens of infertile couples. Patholol Biol. 2006;54:125–129. doi: 10.1016/j.patbio.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 15.Gdoura R, Kchaou W, Chaari C, Znazen A, Keskes L, Rebai T, et al. Ureaplasmaurealyticum, Ureaplasma parvum, Mycoplasma hominis and Mycoplasma genitalium infections and semen quality of infertile men. BMC Infect. 2007;7:129. doi: 10.1186/1471-2334-7-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miron ND, SoColov D, Mareş M, Anton G, Nastasa V, Moraru RF, et al. Bacteriological agents which play a role in the development of infertility. Acta Microbiol Immunol Hung. 2013;60:41–53. doi: 10.1556/AMicr.60.2013.1.5. [DOI] [PubMed] [Google Scholar]
- 17.Verteramo R, Patella A, Calzolari E, Recine N, Marcone V, Osborn J, et al. An epidemiological survey of Mycoplasma hominis and Ureaplasma urealyticum in gynaecological outpatients, Rome, Italy. Epidemiol Infect. 2013;141:2650–2657. doi: 10.1017/S0950268813000277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zdrodowska-Stefanow B, Kłosowska W, Ostaszewska-Puchalska I, Bułhak-Kozioł V, Kotowicz B. Ureaplasma urealyticum and Mycoplasma hominis infection in women with urogenital diseases. Adv Med Sci. 2006;51:250–253. [PubMed] [Google Scholar]
- 19.Paavonen J, Miettinen A, Stevens C, Chen K, Holmes K. Mycoplasma hominis in nonspecific vaginitis. Sex Transm Dis. 1982;10(4 Suppl):271–275. [PubMed] [Google Scholar]
- 20.Shafer M, Sweet R, Ohm-Smith M, Shalwitz J, Beck A, Schachter J. Microbiology of the lower genital tract in postmenarchal adolescent girls: differences by sexual activity, contraception, and presence of nonspecific vaginitis. J Pediatr. 1985;107:974–981. doi: 10.1016/s0022-3476(85)80208-0. [DOI] [PubMed] [Google Scholar]
- 21.Clausen HF, Fedder J, Drasbek M, Nielsen PK, Toft B, Ingerslev HJ, et al. Serological investigation of Mycoplasma genitalium in infertile women. Hum Reprod. 2001;16:1866–1874. doi: 10.1093/humrep/16.9.1866. [DOI] [PubMed] [Google Scholar]
- 22.Tomusiak A, Heczko PB, Janeczko J, Adamski P, Pilarczyk-Zurek M, Strus M. Bacterial infections of the lower genital tract in fertile and infertile women from the southeastern Poland. Ginekol Pol. 2013;84:352–358. doi: 10.17772/gp/1588. [DOI] [PubMed] [Google Scholar]
- 23.McGowin CL, Anderson-Smits C. Mycoplasma genitalium: an emerging cause of sexually transmitted disease in women. PLoS pathogens. 2011;7:e1001324. doi: 10.1371/journal.ppat.1001324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grześko J, Elias M, Mączyńska B, Kasprzykowska U, Tłaczała M, Goluda M. Occurrence of Mycoplasma genitalium in fertile and infertile women. Fertil Steril. 2009;91:2376–2380. doi: 10.1016/j.fertnstert.2008.03.060. [DOI] [PubMed] [Google Scholar]
- 25.Costoya A, Morales F, Borda P, Vargas R, Fuhrer J, Salgado N, et al. Mycoplasmateceae species are not found in Fallopian tubes of women with tubo-peritoneal infertility. Braz J Infect. 2012;16:273–278. [PubMed] [Google Scholar]
- 26.Svenstrup HF, Fedder J, Kristoffersen SE, Trolle B, Birkelund S, Christiansen G. Mycoplasma genitalium, Chlamydia trachomatis, and tubal factor infertility―a prospective study. Fertil Steril. 2008;90:513–520. doi: 10.1016/j.fertnstert.2006.12.056. [DOI] [PubMed] [Google Scholar]
- 27.Anagrius C, Lore B, Jensen J. Mycoplasma genitalium: prevalence, clinical significance, and transmission. Sex Transm Infect. 2005;81:458–462. doi: 10.1136/sti.2004.012062. [DOI] [PMC free article] [PubMed] [Google Scholar]


