Abstract
Among the group of small round cell tumors of kidney Ewing’s sarcoma/PNET is a very rare entity which has aggressive clinical course. We report a case of renal mass in 24 years old male which was histologically diagnosed as small round cell tumor of kidney. Arrangement of the malignant cell along with vascular network in a filigree pattern was suspicious for a diagnosis of Ewing’s sarcoma/PNET which was confirmed by positivity of CD 99 immunohistochemically. Thus careful histological observation and immunohistochemical stains can give the proper diagnosis of primary Ewing’s sarcoma/PNET thus removing the diagnostic dilemma in the group of small round cell tumors.
Keywords: ewing's sarcoma, renal, diagnostic dilemma
Introduction
The small round cell tumors of kidney include wide range of entities with overlapping histomorphology and they have different prognostic values. These are monophasic wilm’s tumor, neuroblastoma, non-Hodgkin’s lymphoma, embryonal rhabdomyosarcoma, small cell neuroendocrine carcinoma, synovial sarcoma and Ewing’s sarcoma/peripheral primitive neuro ectodermal tumor (PNET). Among these entities Ewing’s sarcoma/PNET is an extraordinally rare tumor [1]. Fewer than 100 cases are reported till date in literature globally [2]. This aggressive tumor is more prevalent in adolescent and young adults with slight male preponderance [1, 3]. Most of the patients presents with flank pain and hematuria. Herein we report the clinical, histopathological and immunohistochemical finding in a new case of primary Ewing’s sarcoma/primitive neuroectodermal tumor of kidney.
Case Report
A 24 year old young female has presented with pain in right lower abdomen for 2 months and a vaguely palpable lump in right lumber region. History of hematuria was absent.
CT scan of whole abdomen has shown a heterogenous enhancing mass of 7 cm × 4.1 cm, involving right mid pole and indenting the calyceal system (Figure 1). Renal vein or Inferior vena cava has not involved. No radiological evidence of hepatic or pulmonary metastasis, and perirenal and hilar lymphadenopathy has seen. The patient has undergone radical nephrectomy.
Figure 1.
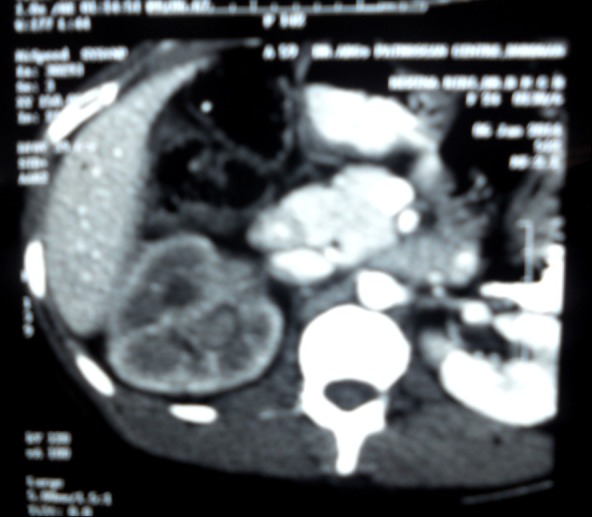
CT scan whole abdomen showing heterogenous enhancing mass in right kidney.
On gross examination after removal of renal capsule a 6 cm × 5 cm mass has seen in the medial part of the kidney. The cut section of the mass was grayish white with areas of focal hemorrhage, extending into renal pelvis. Both the upper and lower poles of the kidney have uninvolved (Figure 2).
Figure 2.
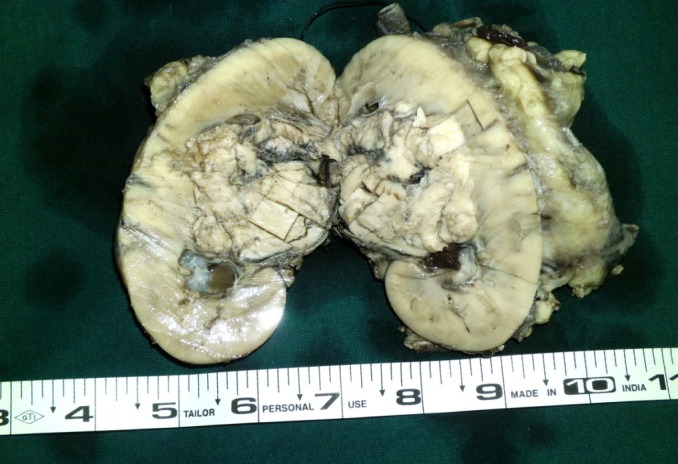
Grayish white mass of 6 cm × 5 cm extending into right renal pelvis.
Microscopic examination has shown proliferation of monomorphic small round cells arranged in vague lobulated pattern separated by thin firbrovascular septae. The individual cells were small having scanty cytoplasm, round nucleus and small nucleoli. In areas these neoplastic cells have invaded into normal renal parenchyma into broad sheets. In areas degenerated malignant round cells have seen which have associated by distinct vascular network, with thick wall and dilated lumina giving filigree pattern (Figure 3, 4). No lymphovascular invasion and rosette formation has seen. Areas of hemorrhage and necrosis have seen into the mass.
Figure 3.
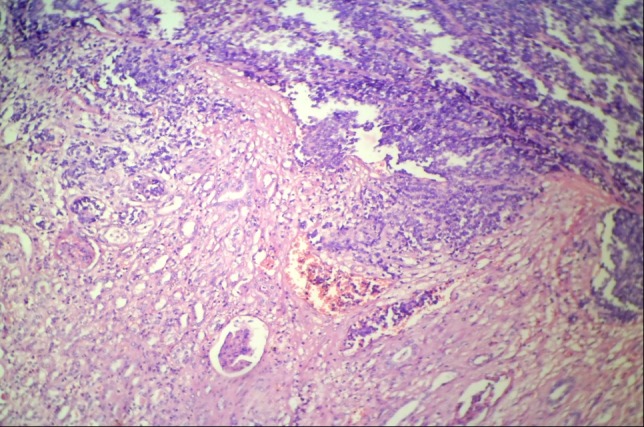
Photomicrograph showing small round cell tumor infiltrating into renal parenchyma (H & E stain, 100X).
Figure 4.
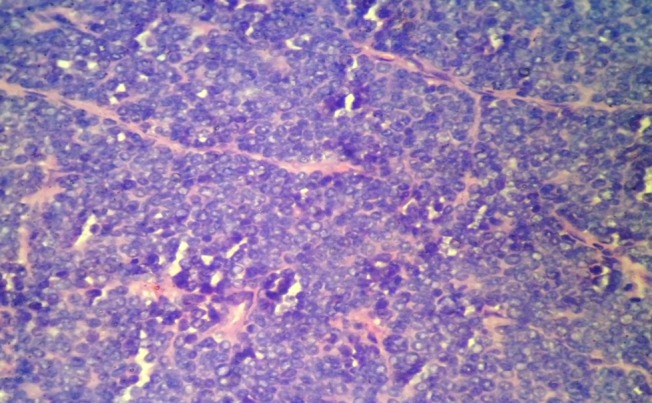
Photomicrograph showing the filigree pattern of small round cell tumor (H & E, 400X).
Immunohistochemically the tumor cells were diffusely and strongly positive for CD 99 (Figure 5) and negative for Cytokeratin AE1/AE3, CD 45 and WT-1. A diagnosis of extraskeletal Ewing’s sarcoma/PNET of kidney has made. The patient has undergone 6 cycles of chemotherapy with VAC -IE protocol using cyclophosphamide (1200 mg/m2 i.v), doxorubicin (250 mg/ m2 i.v bolus), vincristine (1.4 mg/m2 i.v) alternating with ifosfamide (1800mg/m2 ) and etoposide (100 mg/m2 i.v) within two weeks interval. The patient could not afford treatment after that and remained apparently disease free for 5 months. The disease has continued to progress and later on, she has developed bilateral pulmonary metastasis and died on the 15th months after initial diagnosis due to several terminal complications.
Figure 5.
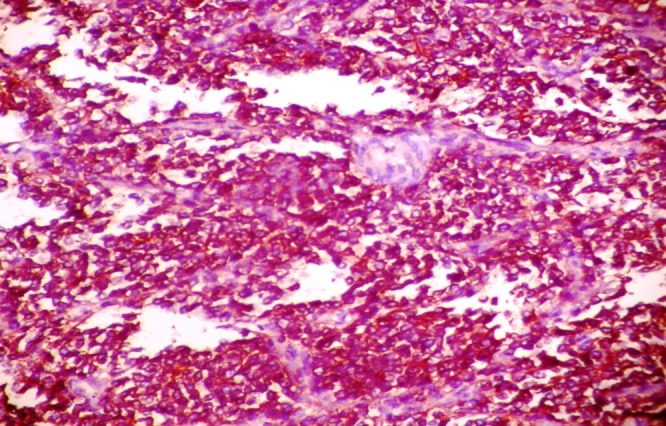
Photomicrograph showing diffuse CD 99 positivity of the neoplastic cells.
Discussion
Ewings’ sarcoma primarily has arisen from lower extremity (most commonly femur). Pelvis would be the second most common site. Apart from bony structures of pelvis, it might arise from non-osseous structures like gastrointestinal tract, kidney or adrenal glands. 25% of the patients have presented with metastasis at the time of diagnosis and the frequent sites of metastasis would be the lung, bone, and bone marrow. The most important prognostic factors were the presence of metastasis at the time of presentation, location of the tumour, tumour size and the serum LDH level. Pelvic primary tumours have been more aggressive and have shown a poor prognosis in comparison with tumors of extremity [4]. Primary renal Ewing’s sarcoma/PNET was an aggressive malignant tumor, and has male predominance with mean age of 28-34 years. Most commonly they have presented with heamturia and/or flank pain [5]. The previous reports have revealed the aggressive behaviour of this neoplasm with variability of its presentations. This highly aggressive tumour has been often diagnosed in advanced stage when it has already involved adjacent structures of kidney or liver, spleen, peritoneum, and lungs. The 5-years disease free survival rate would be around 45-55% in well-confined cases. Cases with advanced stage at presentation have shown a median relapse-free survival of only 2 years. Radiotherapy and chemotherapy were not useful for the treatment of this entity.
So the distinction from the other malignant neoplasms of kidney has been too much essential from the prognostic point of view [6].
Small blue round cell tumor would be a diagnostic challenge in the field of renal histopathology because great number of other tumors have presented in almost a similar histomorphology under microscope. This group includes Ewing’s sarcoma/PNET, non-Hodgkin Lymphoma, alveolar rhabdomyosarcoma, renal neuroblastoma, Wilm’s tumor, monophasic synovial sarcoma, desmoplastic small roumd cell tumor, carcinoid tumors, clear cell sarcoma of kidney. The blastemal cells of Wilm’s tumor might mimic renal PNET, but presence of epithelial component especially tubular differentiation has ruled out renal PNET. The Homer Wright type of rosettes commonly has scared of number or less defined in extra skeletal Ewing’s sarcoma were a typical histological feature for PNET and could address the diagnosis but they could also be found in neuroblastoma. Apart from rosettes neuroblastomas might show neurofibrillary background. Most of the non-Hodgkin’s lymphomas have shown polymorphic histology with small lymphocytes in the background. But filigree pattern in Ewing’s sarcoma/ PNET has not seen in the other small round cell tumors of kidney. For a better diagnostic approach, a detailed immunohistochemical analysis would be necessary. For this purpose the commonly used immunohistochemical markers were cytokeratin (for nephroblastoma, small cell carcinoma, synovial sarcoma), CD45 or Leucocyte Common Antigen (for lymphoma), Chromogranin or Neuron specific enolase (for neuroblastoma), CD 99 (for Ewing’s sarcoma/PNET) and WT-1 (for Wilm’s tumor). The bases of diagnosis of the present case as Ewing’s sarcoma/PNET were histomorphology of small blue round call tumor and CD 99 positivity immunohistochemically [7].
PNET of soft tissue and Ewing’s Sarcoma of bone were pathologically equivalent entities as they share common genetic alteration. Bony counterparts have been more undifferentiated than the PNET of soft tissue which shown more neuroectodermal differentiation.
Histogenesis of the renal Ewing’s sarcoma/PNET has been from the neural ramification investing the kidney. The innervations of kidney have come from the adrenergic fibres originating be from the celiac plexus and accompanying efferent arterioles and descending vasa recta. Another possibility of histogenesis was the migration of the embryonic neural crest cells into kidney and subsequent malignant transformation [8].
Apart from histopathology and immunohistochemistry, molecular analytic methods would be also important requirements for better confirmation of the diagnosis of ES/PNET group of tumors. More than 85% of Ewing’s sarcoma/PNET have characterized by presence of t(11;22) (q24;q12) translocation that results fusion of EWS gene located on chromosome 22 to FLI1 gene on chromosome 11 resulting formation of chimeric product of EWS-FLI1. These have detected by FISH or RT-PCR techniques [9]. But here we could not perform molecular genetic methods because of poor socioeconomic condition of the patient and patient party.
Management of renal Ewings’ Sarcoma/PNET has included surgery, chemotherapy, and radiation. Surgery would be an important method for local control of the disease. According to some school local radiotherapy to the surgical bed has shown yields good response and has prevented local recurrence [10]. The survival rate of the patients with local disease without metastasis at presentation, and who has received multimodality treatment was almost 70% where it has come down to 9 - 41% among the patients with metastasis at presentation [11,12]. In the study of Mukkunda et al. 7 patients with renal Ewing’s sarcoma have undergone median follow-up of 36 months (range from 5 to 149) and had a median disease-free survival (in patients with nonmetastatic disease) of 30.35 months (range from 5.1 to 149) with a 5-years overall survival rate of 42%. The EURO-E.W.I.N.G. 99 study has recommended a multidrug chemotherapy, delivered before and continued after local control. No randomized studies have been published in the setting of the rarity of renal Ewings; sarcoma/PNET. Most of the regimens have contained vincristine, doxorubicin, cyclophosphamide, ifosfamide and etoposide similar to GPOH-Ewing Sarcoma study protocols. But the combination of used multiple chemotherapeutic agents have differed extensively inter-individually [10]. Despite receiving VAC-IE chemotherapy protocol, our patient had extensive pulmonary metastasis with an apparent disease free period of 5 month proving the high fatality of the disease entity.
Conclusion
It has been much important to distinguish primary renal Ewing’s sarcoma/PNET from the other small blue round cell tumours by histopathological, immunohistochemical methods for its aggressive behaviour and therapeutic response. In absence of molecular genetic analysis in poor socioeconomic conditions, careful histopathological and immunohistochemical studies might be valuable in the differential diagnosis of small blue round cell tumours of kidney and to overcome the diagnostic dilemma in cases of Ewing’s sarcoma/PNET.
Acknowledgments
Prof. (Dr.) Uma Banerjee, Head of the Department, Department of Pathology, Burdwan Medical College, Burdwan, West Bengal, India.
Footnotes
Conflicts of Interest
The authors had no conflict of interest.
REFERENCES
- 1.Jimenez RE, Folpe AL, Lapham RL, Ro JY, O'Shea PA, Weiss SW, Amin MB. Primary Ewing's sarcoma/primitive neuroectodermal tumor of the kidney: a clinicopathologic and immunohistochemical analysis of 11 cases. Am J Surg Pathol. . 2002;26(3):320–7. doi: 10.1097/00000478-200203000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Almeida MF, Patnana M, Korivi BR, Kalhor N, Marcal L. Ewing sarcoma of the kidney: a rare entity. Case Rep Radiol. . 2014;2014:283902. doi: 10.1155/2014/283902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parham DM, Roloson GJ, Feely M, Green DM, Bridge JA, Beckwith JB. Primary malignant neuroepithelial tumors of the kidney: a clinicopathologic analysis of 146 adultand pediatric cases from National Wilms' Tumor Study Group Pathology Center. Am J Surg Pathol. . 2001;25(2):133–46. doi: 10.1097/00000478-200102000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Helman LJ, Malkin D. Cancer s of Childhood. In: DeVita VT, et al., editors. Hellman and Rosenberg's Cancer: Principles and Practice of Oncology. 8th. pp. 2061–2067. [Google Scholar]
- 5.Parada D, Godoy A, Liuzzi F, Peña KB, Romero A, Parada AM. Primary Ewing's sarcoma/primitive neuroectodermal tumor of the kidney. An infrequent finding. Arch Esp Urol. 2007;60(3):321. doi: 10.4321/s0004-06142007000300020. [DOI] [PubMed] [Google Scholar]
- 6.Pomara G, Cappello F, Cuttano MG, Rappa F, Morelli G, Mancini P, Selli C. Primitive Neuroectodermal Tumor (PNET) of the kidney: a case report. BMC Cancer. 2004;4(3) doi: 10.1186/1471-2407-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu Y, Zhu Y, Chen H, Huang Y, Wei Q, Chen H, Xie X, Li X, Zhou Q, Yang Y, Zeng H. Primitive neuroectodermal tumor of the kidney with inferior vena cava tumor thrombus during pregnancy response to sorafenib. Chin Med J (Engl). 2010;123(15):2155. [PubMed] [Google Scholar]
- 8.Clapp W, Crocker B. Adult Kidney. In: Sternberg S, editor. Histology for Pathologists. New York: Raven Press; 1997. p. 799. [Google Scholar]
- 9.May WA, Lessnick SL, Braun BS, Klemsz M, Lewis BC, Lunsford LB, Hromas R, Denny CT. The Ewing's sarcoma EWS/FLI-1 fusion gene encodes a more potent transcriptional activator and is a more powerful transforming gene than FLI-1. Mol Cell Biol. . 1993;13(12):7393. doi: 10.1128/mcb.13.12.7393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zöllner S, Dirksen U, Jürgens H, Ranft A. Renal Ewing tumors. Ann Oncol. 2013;24(9):2455–61. doi: 10.1093/annonc/mdt215. [DOI] [PubMed] [Google Scholar]
- 11.Ladenstein R, Potschger U, Le Deley MC, Whelan J, Paulussen M, Oberlin O, et al. Primary disseminated multifocal Ewing sarcoma: results of the Euro-EWING 99 trial. J Clin Oncol. 2010;28:3284–91. doi: 10.1200/JCO.2009.22.9864. [DOI] [PubMed] [Google Scholar]
- 12.Mukkunda R, Venkitaraman R, Thway K, Min T, Fisher C, Horwich A, et al. Primary adult renal Ewing’s Sarcoma: a rare entity. Sarcoma. 2009 doi: 10.1155/2009/504654. [DOI] [PMC free article] [PubMed] [Google Scholar]


