Abstract
Since the proposal for pangenomic study, there have been a dozen software tools actively in use for pangenomic analysis. By the end of 2014, Panseq and the pan-genomes analysis pipeline (PGAP) ranked as the top two most popular packages according to cumulative citations of peer-reviewed scientific publications. The functions of the software packages and tools, albeit variable among them, include categorizing orthologous genes, calculating pangenomic profiles, integrating gene annotations, and constructing phylogenies. As epigenomic elements are being gradually revealed in prokaryotes, it is expected that pangenomic databases and toolkits have to be extended to handle information of detailed functional annotations for genes and non-protein-coding sequences including non-coding RNAs, insertion elements, and conserved structural elements. To develop better bioinformatic tools, user feedback and integration of novel features are both of essence.
Keywords: Pangenomics, Pangenome, Comparative analysis, Genomic dynamics, Core genes
Introduction
In the past decade or so, the remarkable advancement of DNA sequencing technology and application has led to an astronomical accumulation of genomic data. This is especially true for the prokaryotic genomes as individual of them is only a few megabases in size. It is expected that in the next decade or two, there will be more data collected than what we can actually handle. Therefore, database construction, improvement, and consolidation, as well as new tool development, are especially welcome. In this way, the sibling fields of genomics, such as pangenomics and metagenomics, can all be ready for curating, sharing, and mining floods of the incoming genomic big data. Coming back to the reality and focusing on pangenomics, there were, as of December 2014, more than 40 bacterial species that have over 20 fully-assembled genomes from different strains and isolates, allowing for comprehensive pangenomic studies. The concept of pangenome was first proposed in 2005 by Tettelin et al. [1], [2], which is defined as the entire genomic repertoire of a given species or phylogenetic clade when multiple species are defined by systematics. According to the definition, gene profile (content) of a pangenome is divided into three groups: core (shared by all genomes), dispensable, and strain- (or isolate-) specific genes. A series of pangenomic studies have been performed in genomic dynamics [3], [4], [5], [6], pathogenesis and drug resistance [7], [8], [9], bacterial toxins [10], and species evolution [11]. The concept has also been extended to viral [12], plant [13], [14], [15], and fungal genome studies [16]. A review on ten-year history and field achievement of pangenomics has just been published at the beginning of 2014 [2], which detailed major projects as well as methodology and technology advancements.
Here, we provide a brief review on the pangenomic software packages and tools, including their basic function, general utility, and popularity based on their cumulative citation by peer-reviewed scientific publications. Although such a single-criterion evaluation may never be adequate and thorough, we hope that it provides a field guide for students and young scientists to make the right choice for their preferred applications.
Highlights of the software packages and tools
Since 2010, we have seen a dozen or so software packages and tools being put forward, which are capable of clustering orthologous genes, identifying single nucleotide polymorphisms (SNPs), constructing phylogenies, and profiling core/shared/isolate-specific genes. Although they may share similar functions, each has its own characteristics and limitations, leaving rooms for further improvement.
Among the early-developed packages, Panseq [17] and PanCGHweb [18] were published in 2010, followed by CAMBer [19] and the Prokaryotic-genome Analysis Tool (PGAT) [20] in 2011. PanCGHweb is a web tool for pangenomic microarray analysis based on PanCGH algorithm [21]. It enables users to group genes into orthologs and to construct gene-based phylogenies of related strains and isolates. However, this package is rather specific for handling microarray data but not RNA-seq data. Panseq, another online pangenomic tool, is able to determine core and accessory regions of genome assemblies based on MUMmer and BLASTn, as well as to identify SNPs among the core genomic regions. In addition, Panseq also has a locus selector module that selects the most discriminatory loci among the accessory loci or core gene SNPs [17]. Panseq, however, is not able to provide pangenomic profile and functional enrichment analysis that is important for the biologists to filter out functional relevance of the pangenomic elements. The later released CAMBer is designed to identify multi-gene families from multiple bacterial strains and isolates. These multi-gene families can be used for sequencing error detection, mutation identification, and pangenomic profile computation [19]. CAMBer is supreme in refining gene function prediction according to multi-gene family information, but it does not provide tools for comparative or evolutionary analysis among strains and isolates. As a web-based database, PGAT integrates several useful functions, such as plotting the presence and absence of genes among members of a pangenome, identifying SNPs among orthologs and syntenic regions, comparing gene orders among different strains and isolates, providing KEGG pathway analysis tools, and searching for genes through different annotations such as the Cluster of Orthologous Groups of proteins (COG), PSORT, SignalP, the Tied Mixture Hidden Markov Model (TMHMM), and Pfam. However, PGAT is just a database with a limited number of species curated and it cannot perform analysis for new sequencing data from users.
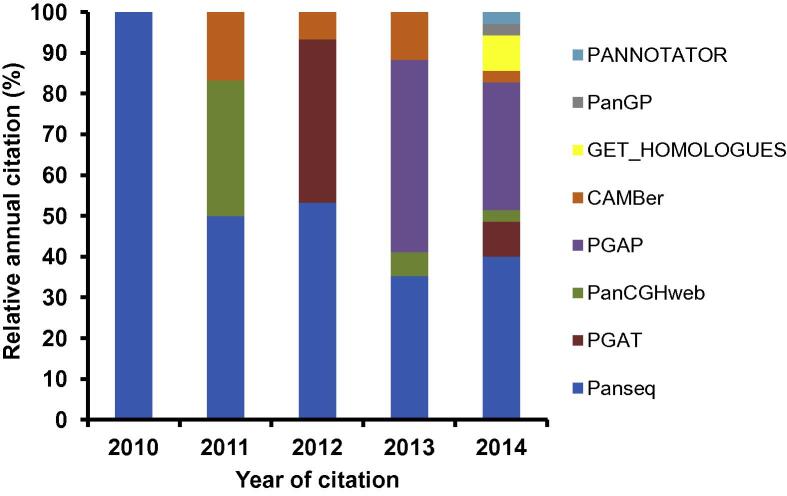
PGAP is a stand-alone program developed by Zhao et al. in 2012, which contains five functional models [22]. Based on functional gene clustering and analysis, PGAP presents pangenomic profile (partitions of pangenomic elements or gene categories), genetic variation, species evolution, and function enrichment of different strains and isolates of a given pangenome. In addition, all analyses are performed with a single command, and such integration is rather user-friendly and efficient. Nonetheless, PGAP has its limitation as well. For instance, all its output files of the five models are text files, which lacks of intuitiveness. Contreras-Moreira et al. subsequently proposed a program called GET_HOMOLOGUES in 2013, which is also a versatile software package for pangenomics [23]. This software package integrated data download, sequence feature extraction, homologous gene identification, pangenome profiling, graphical display, and phylogenetic tree construction into one powerful toolkit. Several other tools were also available in 2013, such as PanCake [24] and PANNOTATOR [25]. PanCake was developed for identifying singletons and core regions in arbitrary sequence sets, while PANNOTATOR, a web-based automated pipeline, was designed for the annotation of closely-related genomes for pangenomic analysis. However, these two tools only focus on simple functions, such as clustering homologous genes and gene curation. In 2014, a powerful and flexible toolkit, the Integrated Toolkit for Exploration of microbial Pan-genomes (ITEP), was published by Benedict and colleagues [26]. ITEP integrates plenty of existing bioinformatics tools for pangenomic analysis, including protein family prediction, ortholog detection, functional domain analysis, pangenomic profiling, and metabolic network integration. Moreover, ITEP also integrates some visualization scripts that assist biologists in phylogenetic tree construction, annotation curation, and specific query for conserved protein domain identification. In 2014, another rapid core-genome alignment and visualization pangenomic software package, Harvest, was proposed by Treangen et al. Harvest contains tools, such as Parsnp and Gingr, for core gene alignment, variant calling, recombination detection, and phylogenetic trees construction [27]. To analyze pangenomic profile in a larger scale, a software package PanGP was developed with a graphic interface by Zhao et al. in 2014 [28]. Spine and AGEnt were also developed in 2014, which are capable of profiling pangenomes based on both finished and draft genomic sequences [29]. We summarized all the software packages and tools in Table 1, highlighting their platforms and major features. We went one step further and ranked them according to their citations by peer-reviewed scientific publications (Figure 1), which were collected from ISI Web of Science-Science Citation Index Expanded. Our summary indicates that Panseq and PGAP have been the most popular packages up to the end of 2014.
Table 1.
Software tools for pangenomic studies
| Name | Link | Platform | Main features | Ref. |
|---|---|---|---|---|
| Panseq | https://lfz.corefacility.ca/panseq/ | Online Windows Linux | a, b | [17] |
| PGAT | http://nwrce.org/pgat | Online | a, b, e | [20] |
| PanCGHweb | http://bamics2.cmbi.ru.nl/websoftware/pancgh/pancgh_start.php | Online | a, d | [18] |
| PGAP | http://pgap.sourceforge.net/ | Linux | a, b, c, d, e | [22] |
| ITEP | https://price.systemsbiology.net/itep | Linux | a, b, d, e, f, g | [26] |
| CAMBer | http://bioputer.mimuw.edu.pl/camber/index.html | Windows Linux | a, c, f | [19] |
| Harvest | https://github.com/marbl/harvest | Mac OSX Linux | a, b, d, g | [27] |
| GET_HOMOLOGUES | http://www.eead.csic.es/compbio/soft/gethoms.php | Mac OSX Linux | a, c, d, f, g | [23] |
| PanCake | https://bitbucket.org/CorinnaErnst/pancake/wiki/Home | Windows Linux | a | [24] |
| PanGP | http://PanGP.big.ac.cn | Windows Linux | c, g | [28] |
| PANNOTATOR | http://bnet.egr.vcu.edu/pannotator/index.html | Online | a, f | [25] |
| Spine and AGEnt | http://vfsmspineagent.fsm.northwestern.edu/index_age.html | Online Mac OSX Linux | a | [29] |
Note: Only letters are used in main features column, their corresponding feature descriptions are listed as below: (a) Clustering homologous genes, assigning their presence/absence or analyzing core/accessory genomes; (b) Identifying SNPs; (c) Plotting pangenomic profiles; (d) Building phylogenetic relationships of orthologous genes/families of strains/isolates; (e) Function-based searching or analysis; (f) Annotation and/or curation; and (g) Visualization.
Figure 1.
Relative citation of the pangenomic software tools from peer-reviewed scientific publications
A wish list for improving the current software
Although single-tool solution could not usually satisfy the need for understanding the whole picture, a wish list from the users is always helpful for prioritizing goals for the package developers, hence providing directions for improving each package.
First, the performance of pangenomic analysis strongly depends on the accuracy of genome assembly and annotation. Therefore, an adequate number of complete sequence assemblies are a prerequisite. Currently, most of the existing bacterial genome sequences are actually incomplete (in most of the cases, contigs are not joined together into single chromosomes), and some only have high-quality and high-coverage raw data available. The inclusion of incomplete genome assemblies for pangenomic analysis may need scaffold building that requires reformatting of the contig data files. Despite the development of the third-generation sequencing technology, which would certainly help the assembly and finishing of prokaryotic genomes [30], incomplete prokaryotic genomes are expected to be deposited into public databases in mass. It would be a waste if such data are left unused.
Second, orthologous gene identification is a key step in pangenomic analysis. At present, the existing software for ortholog detection is mainly based on sequence similarity, phylogenetic relationship, or other annotation information such as functional information. The development of novel and more efficient ortholog identification method for multiple closely-related strains and isolates can greatly improve the accuracy of pangenomic analysis. One possibility is to integrate gene gain-and-loss information for phylogeny building among strains and isolates.
Third, sampling is also important for pangenomics in a couple of counts. One is how many strains or isolates to choose for a pangenomic analysis. The other is how to implement a filter that differentiates more diverse strains or isolates from the less diverse for pangenomic analysis. For instance, if we choose all genomes of a species for an analysis, which include one or a few divergent genomes, the core genome will be much shorter or reduced. Obviously, individual genomes should be selected and regrouped for better representation of average nucleotide identity (ANI). ANI is one of the most useful measurements for species delineation [31]. Therefore, for a better pangenomic analysis, detailed information for the available samples is of essence, which should include their genotypes, phenotypes, and habitats.
Fourth, the current tools have not incorporated some recent advancements in prokaryotic genomics, such as the so-called genome-organization frameworks (GOFs), which are not only unique to each species but also provide guidance for sequence assembly and finishing [32]. Other annotation information, such as that of non-coding RNAs, pseudogenes, and epigenetic elements, remains to be implemented into the relevant software packages. Finally, a never-ending improvement of pangenomic tools is visualization that provides not only better displays but also quality graphics for publication.
Concluding remarks
We provide an overview on the existing pangenomic analysis tools and hope to see improvements of the software tools from their original developers. We certainly express our enthusiasm for new tools to join the competition, and after all, for a piece of bioinformatic work, a database or a toolkit, the survival or winning game is in its long-term maintenance and constant improvement.
Competing interests
The authors declared that there are no competing interests.
Acknowledgements
This study was supported by the National High-tech R&D Program (863 Program; Grant No. 2012AA020409) from the Ministry of Science and Technology of China, the Key Program of the Chinese Academy of Sciences (Grant No. KSZD-EW-TZ-009-02), and the National Natural Science Foundation of China (Grant Nos. 31471248 and 31271386).
Handled by Vladimir Bajic
Footnotes
Peer review under responsibility of Beijing Institute of Genomics, Chinese Academy of Sciences and Genetics Society of China.
References
- 1.Tettelin H., Masignani V., Cieslewicz M.J., Donati C., Medini D., Ward N.L., et al. Genome analysis of multiple pathogenic isolates of Streptococcus agalactiae: implications for the microbial “pan-genome”. Proc Natl Acad Sci U S A. 2005;102:13950–13955. doi: 10.1073/pnas.0506758102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vernikos G., Medini D., Riley D.R., Tettelin H. Ten years of pan-genome analyses. Curr Opin Microbiol. 2014;23C:148–154. doi: 10.1016/j.mib.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 3.Rasmussen T.B., Danielsen M., Valina O., Garrigues C., Johansen E., Pedersen M.B. Streptococcus thermophilus core genome: comparative genome hybridization study of 47 strains. Appl Environ Microbiol. 2008;74:4703–4710. doi: 10.1128/AEM.00132-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Willenbrock H., Hallin P.F., Wassenaar T.M., Ussery D.W. Characterization of probiotic Escherichia coli isolates with a novel pan-genome microarray. Genome Biol. 2007;8:R267. doi: 10.1186/gb-2007-8-12-r267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang A., Yang M., Hu P., Wu J., Chen B., Hua Y., et al. Comparative genomic analysis of Streptococcus suis reveals significant genomic diversity among different serotypes. BMC Genomics. 2011;12:523. doi: 10.1186/1471-2164-12-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaas R.S., Friis C., Ussery D.W., Aarestrup F.M. Estimating variation within the genes and inferring the phylogeny of 186 sequenced diverse Escherichia coli genomes. BMC Genomics. 2012;13:577. doi: 10.1186/1471-2164-13-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park J., Zhang Y., Buboltz A.M., Zhang X., Schuster S.C., Ahuja U., et al. Comparative genomics of the classical Bordetella subspecies: the evolution and exchange of virulence-associated diversity amongst closely related pathogens. BMC Genomics. 2012;13:545. doi: 10.1186/1471-2164-13-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D’Auria G., Jimenez-Hernandez N., Peris-Bondia F., Moya A., Latorre A. Legionella pneumophila pangenome reveals strain-specific virulence factors. BMC Genomics. 2010;11:181. doi: 10.1186/1471-2164-11-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu P., Yang M., Zhang A., Wu J., Chen B., Hua Y., et al. Comparative genomics study of multi-drug-resistance mechanisms in the antibiotic-resistant Streptococcus suis R61 strain. PLoS One. 2011;6:e24988. doi: 10.1371/journal.pone.0024988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fang Y., Li Z., Liu J., Shu C., Wang X., Zhang X., et al. A pangenomic study of Bacillus thuringiensis. J Genet Genomics. 2011;38:567–576. doi: 10.1016/j.jgg.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Konstantinidis K.T., Ramette A., Tiedje J.M. The bacterial species definition in the genomic era. Philos Trans R Soc Lond B Biol Sci. 2006;361:1929–1940. doi: 10.1098/rstb.2006.1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aherfi S., Pagnier I., Fournous G., Raoult D., La Scola B., Colson P. Complete genome sequence of Cannes 8 virus, a new member of the proposed family “Marseilleviridae”. Virus Genes. 2013;47:550–555. doi: 10.1007/s11262-013-0965-4. [DOI] [PubMed] [Google Scholar]
- 13.Cao J., Schneeberger K., Ossowski S., Gunther T., Bender S., Fitz J., et al. Whole-genome sequencing of multiple Arabidopsis thaliana populations. Nat Genet. 2011;43:956–963. doi: 10.1038/ng.911. [DOI] [PubMed] [Google Scholar]
- 14.Read B.A., Kegel J., Klute M.J., Kuo A., Lefebvre S.C., Maumus F., et al. Pan genome of the phytoplankton Emiliania underpins its global distribution. Nature. 2013;499:209–213. doi: 10.1038/nature12221. [DOI] [PubMed] [Google Scholar]
- 15.Li Y.H., Zhou G., Ma J., Jiang W., Jin L.G., Zhang Z., et al. De novo assembly of soybean wild relatives for pan-genome analysis of diversity and agronomic traits. Nat Biotechnol. 2014;32:1045–1052. doi: 10.1038/nbt.2979. [DOI] [PubMed] [Google Scholar]
- 16.Dunn B., Richter C., Kvitek D.J., Pugh T., Sherlock G. Analysis of the Saccharomyces cerevisiae pan-genome reveals a pool of copy number variants distributed in diverse yeast strains from differing industrial environments. Genome Res. 2012;22:908–924. doi: 10.1101/gr.130310.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laing C., Buchanan C., Taboada E.N., Zhang Y.X., Kropinski A., Villegas A., et al. Pan-genome sequence analysis using Panseq: an online tool for the rapid analysis of core and accessory genomic regions. BMC Bioinformatics. 2010;11:461. doi: 10.1186/1471-2105-11-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bayjanov J.R., Siezen R.J., van Hijum S.A.F.T. PanCGHweb: a web tool for genotype calling in pangenome CGH data. Bioinformatics. 2010;26:1256–1257. doi: 10.1093/bioinformatics/btq103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wozniak M., Wong L., Tiuryn J. CAMBer: an approach to support comparative analysis of multiple bacterial strains. BMC Genomics. 2011;12:S6. doi: 10.1186/1471-2164-12-S2-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brittnacher M.J., Fong C., Hayden H.S., Jacobs M.A., Radey M., Rohmer L. PGAT: a multistrain analysis resource for microbial genomes. Bioinformatics. 2011;27:2429–2430. doi: 10.1093/bioinformatics/btr418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bayjanov J.R., Wels M., Starrenburg M., van Hylckama Vlieg J.E., Siezen R.J., Molenaar D. PanCGH: a genotype-calling algorithm for pangenome CGH data. Bioinformatics. 2009;25:309–314. doi: 10.1093/bioinformatics/btn632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao Y., Wu J., Yang J., Sun S., Xiao J., Yu J. PGAP: pan-genomes analysis pipeline. Bioinformatics. 2012;28:416–418. doi: 10.1093/bioinformatics/btr655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Contreras-Moreira B., Vinuesa P. GET_HOMOLOGUES, a versatile software package for scalable and robust microbial pangenome analysis. Appl Environ Microbiol. 2013;79:7696–7701. doi: 10.1128/AEM.02411-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ernst C., Rahmann S. In: German conference on bioinformatics 2013. Beißbarth T., Kollmar M., Leha A., Morgenstern B., Schultz A., editors. Schloss Dagstuhl-Leibniz-Zentrum fuer Informatik; Dagstuhl, Germany: 2013. PanCake: a data structure for pangenomes; pp. 35–45. [DOI] [Google Scholar]
- 25.Santos A.R., Barbosa E., Fiaux K., Zurita-Turk M., Chaitankar V., Kamapantula B., et al. PANNOTATOR: an automated tool for annotation of pan-genomes. Genet Mol Res. 2013;12:2982–2989. doi: 10.4238/2013.August.16.2. [DOI] [PubMed] [Google Scholar]
- 26.Benedict M.N., Henriksen J.R., Metcalf W.W., Whitaker R.J., Price N.D. ITEP: an integrated toolkit for exploration of microbial pan-genomes. BMC Genomics. 2014;15:8. doi: 10.1186/1471-2164-15-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Treangen T.J., Ondov B.D., Koren S., Phillippy A.M. The Harvest suite for rapid core-genome alignment and visualization of thousands of intraspecific microbial genomes. Genome Biol. 2014;15:524. doi: 10.1186/s13059-014-0524-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao Y., Jia X., Yang J., Ling Y., Zhang Z., Yu J., et al. PanGP: a tool for quickly analyzing bacterial pan-genome profile. Bioinformatics. 2014;30:1297–1299. doi: 10.1093/bioinformatics/btu017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ozer E.A., Allen J.P., Hauser A.R. Characterization of the core and accessory genomes of Pseudomonas aeruginosa using bioinformatic tools Spine and AGEnt. BMC Genomics. 2014;15:737. doi: 10.1186/1471-2164-15-737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koren S., Harhay G.P., Smith T.P., Bono J.L., Harhay D.M., Mcvey S.D., et al. Reducing assembly complexity of microbial genomes with single-molecule sequencing. Genome Biol. 2013;14:R101. doi: 10.1186/gb-2013-14-9-r101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim M., Oh H.S., Park S.C., Chun J. Towards a taxonomic coherence between average nucleotide identity and 16S rRNA gene sequence similarity for species demarcation of prokaryotes. Int J Syst Evol Microbiol. 2014;64:346–351. doi: 10.1099/ijs.0.059774-0. [DOI] [PubMed] [Google Scholar]
- 32.Kang Y., Gu C., Yuan L., Wang Y., Zhu Y., Li X., et al. Flexibility and symmetry of prokaryotic genome rearrangement reveal lineage-associated core-gene-defined genome organizational frameworks. MBio. 2014;5:e01867. doi: 10.1128/mBio.01867-14. [DOI] [PMC free article] [PubMed] [Google Scholar]