Abstract
Herpes simplex virus (HSV) is a common causative agent of genital ulceration and can lead to subsequent neurological disease in some cases. Here, using a genital infection model, we tested the efficacy of vinegar-processed flos of Daphne genkwa (vp-genkwa) to modulate vaginal inflammation caused by HSV-1 infection. Our data revealed that treatment with optimal doses of vp-genkwa after, but not before, HSV-1 infection provided enhanced resistance against HSV-1 infection, as corroborated by reduced mortality and clinical signs. Consistent with these results, treatment with vp-genkwa after HSV-1 infection reduced viral replication in the vaginal tract. Furthermore, somewhat intriguingly, treatment of vp-genkwa after HSV-1 infection increased the frequency and absolute number of CD3-NK1.1+NKp46+ natural killer (NK) cells producing interferon (IFN)-γ and granyzme B, which indicates that vp-genkwa treatment induces the activation of NK cells. Supportively, secreted IFN-γ was detected at an increased level in vaginal lavages of mice treated with vp-genkwa after HSV-1 infection. These results indicate that enhanced resistance to HSV-1 infection by treatment with vp-genkwa is associated with NK cell activation. Therefore, our data provide a valuable insight into the use of vp-genkwa to control clinical severity in HSV infection through NK cell activation.
Keywords: Daphne genkwa, Vinegar-processed flos, Herpes simplex virus, Genital infection, NK cells
INTRODUCTION
Herpes simplex viruses (HSV) are members of the Alphaherpesvirinae subfamily within the Herpesviridae family. There are typically two serotypes of HSV: HSV type 1 (HSV-1), which is more frequently found in the oral mucosal and ocular areas and HSV type 2 (HSV-2), which is most commonly encountered as the causative agent of genital infection (1). However, confused infection of HSV-1 and HSV-2 are currently detected due to disorganized habits (2,3). Thus, both HSV-1 and 2 have been regarded as the most common cause of genital ulceration in humans worldwide (4,5). Moreover, HSV infection via the genital route results in the establishment of a lifelong latent infection, which subsequently provides potential transmission to neighbor hosts in response to reactivation (6). In addition, increased acquisition of human immunodeficiency virus (HIV) has been reported in HSV-infected individuals (7,8,9). Therefore, it is necessary to develop effective therapeutic strategies for HSV-infected patients.
Because the virus is prevalent in the human population, the development of therapeutic strategies rests on an understanding of how each component of the innate and adaptive immune systems responds to HSV infection. Using multiple animal models, the role of innate and adaptive immune cells has been defined. It is believed that HSV replication is initially limited to the epithelium of the mucosa (10,11), and subsequently spreads into the central nervous system (CNS) upon retrograde transport of virions into the sacral ganglia, resulting in a fatal paralysis (11). The majority of research demonstrates that innate immune responses, including natural killer (NK) and NKT cells as well as monocytes, play an important role in conferring protective immunity against genital HSV infection (11,12). Moreover, effectively generated CD4+ Th1 immunity is essentially required for protection against primary or secondary HSV infection via the mucosal route (13,14,15). Therefore, orchestrated infiltration of effector innate and adaptive immune cells, such as NK and T cells, in mucosal tissues appears to be essential for effective protection against HSV infection. In addition, soluble factors, such as type I IFNs (IFN-α/β), IL-12, and IL-18, produced from innate and adaptive immune cells reportedly play a crucial role in conferring protection against HSV infection (16,17,18). Specifically, NK cells are critical for HSV control owing to their direct or indirect mechanisms of recognizing and killing infected cells through the secretion of granzyme B, perforin, or the antiviral cytokines interferon (IFN)-γ and tumor necrosis factor (TNF)-α (4,5). The activity of antiviral NK cells is regulated by endogenous cytokines, such as type I IFNs and IL-2 (19,20,21), and exogenous materials including synthesized and natural compounds (22,23).
The flower (flos) buds of Daphne genkwa (Thymelaeaceae) are commonly used in traditional folk medicine as a diuretic for the treatment of ascites, edema, and asthma in Korea and China, although their specific biological activities have not been defined yet (24,25). The medicine also was found to have anti-cancer effects on malignant ascites and tumors (26,27). The phenolic constituents of D. genkwa exhibit anti-complementary activities (28). Furthermore, the flower buds of D. genkwa were recently reported to induce the functional recovery of exhausted CD4+ and CD8+ T cells generated during chronic viral infection (29), suggesting that D. genkwa is useful in the treatment of chronic diseases. The experiments presented here were undertaken to determine whether vinegar-processed flos of D. genkwa (vp-genkwa) regulate the mortality and clinical signs associated with mucosal HSV-1 infection. Our data indicate that oral treatment of vp-genkwa after mucosal HSV-1 infection alleviates the mortality and clinical signs induced by viral infection through enhancement of NK cell activity. Therefore, this result provides a valuable insight into the use of vp-genkwa to control clinical severity in HSV infection.
MATERIALS AND METHODS
Animals and ethical considerations
BALB/c (H-2d) mice (6- to 8-weeks old) were purchased from Samtako Co. (O-San, Korea). All experimental procedures were pre-approved by the Institutional Animal Care and Use Committees (IACUC), Chonbuk National University (Permission code 2013-0040), and adhered to the guidelines of the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC). The animal facility of Chonbuk National University is fully accredited by the Korea Association for Laboratory Animal Sciences (KALAS).
Plant materials and preparation of vinegar-processed sample
The Genkwa Flos was collected from Huan, one of the native cultivating provinces in China. The vinegar-processed Genkwa Flos (vp-genkwa) was produced, as described elsewhere (29). Briefly, the dried and powdered Genkwa Flos was equally mixed with 4% vinegar diluted in H2O (v/v), placed in a moist closed container for one night, transferred to a drug-parching machine to dry at 180℃, and removed promptly thereafter (30). The dried powder (3 kg) was extracted twice with 3 volumes of room-temperature ethanol. The extract (199 g) was evaporated, freeze dried, and stored at 4℃ before use. The analysis of the vp-genkwa components was performed by UPLC-QTOF-MS chromatogram. Ultra performance liquid chromatography (UPLC) analysis was performed using an ACQUITY UPLCTM system (Waters Corporation, Milford, MA, USA) equipped with a binary solvent delivery manager and photodiode array (PDA). The organic solvents used for extraction were of first grade, and acetonitrile and water for UPLC were purchased as analytical grade from Honeywell (Morristown, NJ, USA). The chromatographic separations were performed on a 2.1×100 mm, 1.7-µm ACQUITY HSS T3 C18 chromatography column. The column temperature was maintained at 35oC, and the mobile phases A and B comprised water with 0.1% formic acid and acetonitrile with 0.1% formic acid, respectively. The gradient duration program was as follows: 0 min, 20% B; 0~1 min, 20% B; 1~6 min, 20~80% B; 6~8 min, 80~98% B; 1-min wash with 98% B; and a 2-min recycle time. The flow rate was 0.4 ml/min.
Cells and viruses
The HSV-1 McKrae strain was propagated in Vero cells (CCL81; ATCC, Manassas, VA, USA) using DMEM supplemented with 2.5% FBS, penicillin (100 U/ml), and streptomycin (100 U/ml). The Vero cells were infected with HSV-1 at a multiplicity of infection (MOI) of 0.01, and then incubated in a humidified CO2 incubator for 1 h at 37℃. After absorption, the inoculums were removed, and 10 mL of maintenance medium containing 2.5% FBS was added. Approximately 48~72 h after infection, a culture of host cells that showed an 80~90% cytopathic effect was harvested. The virus stocks were then concentrated by centrifugation at 50,000×g, titrated using a plaque assay, and stored in aliquots at -80℃ until needed.
Animal infection
The mice were treated with progesterone to synchronize their estrous cycles (31) prior to intravaginal (i.vag.) infection with the HSV-1 McKrae strain. Briefly, BALB/c mice were injected subcutaneously (s.c.) with Depo-Provera (DP) (Upjohn Co., Kalamazoo, MI, USA) at 2 mg per mouse. Five days following DP injection, the mice were i.vag. infected with 106 PFU of HSV-1 McKrae strain (20 µl) per mouse. The infected mice were examined daily for vaginal inflammation, neurological illness, and death, as described previously (31). They were scored from 1 to 5 depending on clinical disease severity (0, no inflammation; 1, mild inflammation; 2, moderate inflammation; 3, severe inflammation; 4, paralysis; 5, death).
Viral titration in vaginal lavages
Vaginal lavage fluid was obtained by introducing 100 µl of PBS (pH 7.2) into the vaginal canals and then recovering it with a micropipette. The samples were stored at -80℃ until used. Individual subsamples (50 µl from each sample) were further diluted, and viral titers were determined by a plaque assay performed on Vero cells as described elsewhere (31).
FACS analysis of NK cell activity
The mice were treated with vp-genkwa before and after HSV-1 infection, and the splenocytes were prepared from infected mice 3 days post-infection (dpi). NK cells were enumerated by FACS staining using a cocktail of CD3 (145-2C11), NK1.1 (PK136), and NKp46 (29A1.4.9) antibodies (eBioscience, San Diego, CA, USA). To determine the activity of NK cells, we used intracellular staining for IFN-γ and granzyme B (GrB) in response to stimulation of PMA (750 ng/ml; Sigma-Aldrich, St. Louis, MO, USA) plus ionomycin (50 ng/ml; Sigma-Aldrich). Briefly, prepared splenocytes were cultured with PMA and ionomycin in the presence of monensin (2 µM; Sigma-Aldrich) for 2 h for IFN-γ and 4 h for GrB. Cells were harvested and labeled with CD3, NK1.1, and NKp46 antibodies, and subsequently stained with intracellular IFN-γ and GrB after fixation and permeabilization. All samples were acquired on FACS Calibur (BD Bioscience, Mountain View, CA, USA), and analyzed by FlowJo software (ver. 7.6.5; Tree Star, San Carlos, CA, USA). Forward scatter and side scatter were used to identify lymphocytes, which were further used to define NK cell and intracellular expression of IFN-γ and GrB.
Determination of vaginal IFN-γ secretion
Vaginal lavage fluid for IFN-γ secretion was collected 3 dpi by introducing 100 µl of PBS (pH 7.2) into the vaginal canal and then recovering it with a micropipette following infection of synchronized mice with the HSV-1 McKrae strain. The vaginal mucus was subsequently removed from the fluid by centrifugation at 10,000 rpm for 1 min. IFN-γ levels in vaginal lavages were determined by ELISA using IFN-γ anti-mouse Ab (R4-6A2) and biotinylated IFN-γ Ab (XMG1.2). The ELISA plates were washed and incubated with peroxidase-conjugated streptavidin for 1 h, and then the color was developed. The IFN-γ concentration was calculated with an automated ELISA reader.
Statistical analysis
All data were expressed as the average±standard deviation, and statistically significant differences between groups were analyzed by unpaired two-tailed Student's t-test. Kaplan-Meier survival curves were analyzed by the log-rank test. A p-value≤0.05 was considered significant. All data were analyzed using Prism software (GraphPadPrism4, San Diego, CA, USA).
RESULTS
Reduction of mortality and clinical signs by treatment of vp-genkwa after HSV-1 infection
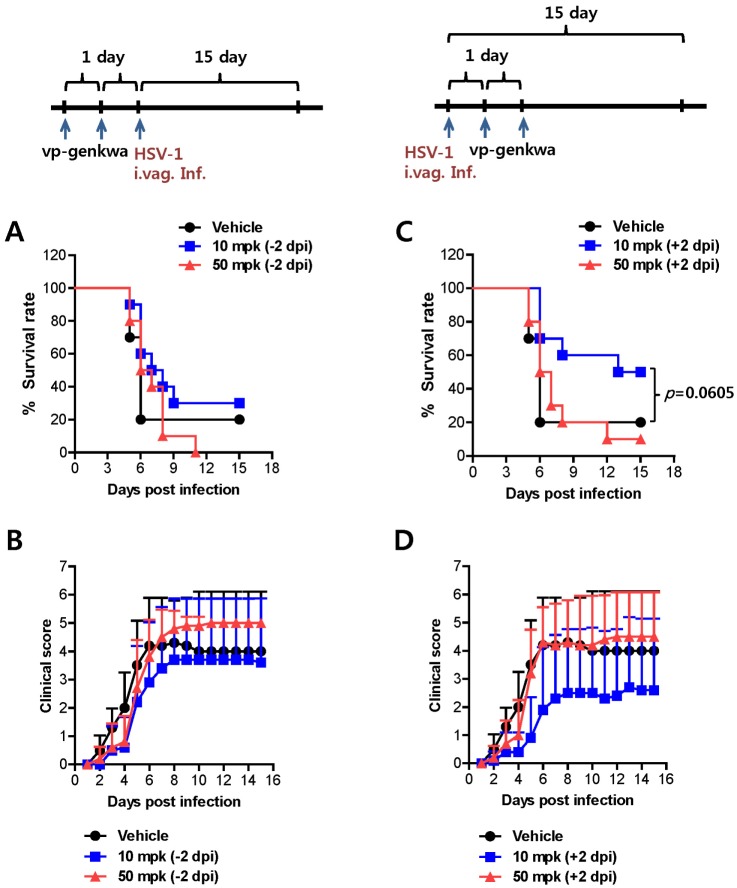
In order to assess the effect of vp-genkwa on the mortality and clinical signs associated with mucosal HSV-1 infection, we orally administered 10 and 50 mg/kg (mpk) vp-genkwa twice prior to HSV-1 infection. Subsequently, we examined infected mice daily until 15 dpi. Our results revealed that pre-treatment of vp-genkwa had no effect on mortality and clinical signs, compared to the group treated with vehicle (Fig. 1A and B). However, somewhat intriguingly, mice that were treated with vp-genkwa (10 mpk) after HSV-1 infection showed enhanced resistance (p=0.0605), as well as alleviated clinical signs, compared to mice that received vehicle (Fig. 1C and D). Furthermore, higher doses of vp-genkwa (50 mpk) did not provide increased protection against mucosal HSV-1 infection, indicating that enhanced protective immunity against HSV-1 infection may be achieved with treatment of vp-genkwa at optimal doses after infection. Therefore, these results suggest that treatment of vp-genkwa at an optimal dose after HSV-1 infection provides enhanced protection, while pre-treatment of vp-genkwa before HSV-1 infection does not affect the associated mortality and clinical signs.
Figure 1. Vp-genkwa treatment following mucosal HSV-1 infection reduces mortality and ameliorates clinical signs. (A and B) Effect of vp-genkwa treatment prior to HSV-1 infection. Groups of BALB/c mice received oral vp-genkwa twice, and were infected i.vag. with HSV-1 the next day. The infected mice were then examined daily until 15 dpi for survival (A) and for vaginal inflammation, neurological illness, and death (B). (C and D) Effect of vp-genkwa treatment after HSV-1 infection. Groups of BALB/c mice were infected i.vag. with HSV-1 and treated orally with vp-genkwa twice with a 1-day interval. The infected mice were examined daily until 15 dpi to assess survival (C), and vaginal inflammation, neurological illness, and death (D). Kaplan-Meiers survival curves were computed and analyzed using the chi-square test. Clinical severity was graded as follows: 0, no inflammation; 1, mild inflammation; 2, moderate swelling; 3, severe inflammation; 4, paralysis; 5, death. The graph of clinical scores represents the average clinical score of 10 mice per group.
Treatment of vp-genkwa reduces viral burden following HSV-1 infection
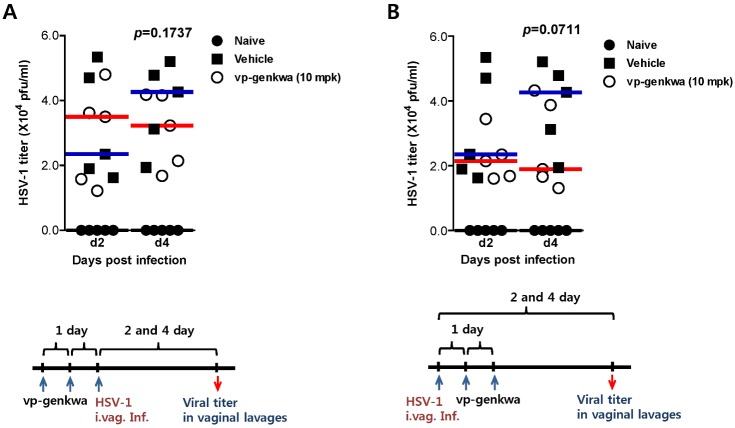
Because treatment of vp-genkwa (10 mpk) after infection offered increased protection against mucosal HSV-1 infection, we examined viral secretion in vaginal lavages of mice that received vp-genkwa (10 mpk) before and after HSV-1 infection. Consistent with the previous results, vp-genkwa treatment before HSV-1 infection showed no regulatory role in the control of viral secretion in vaginal lavages, whereas vp-genkwa treatment after HSV-1 infection reduced viral titers in vaginal lavages (Fig. 2). Thus, this result indicates that vp-genkwa treatment after HSV-1 infection might control HSV-1 replication in the vaginal tract.
Figure 2. Treatment with vp-genkwa after HSV-1 infection reduces viral replication. (A) Effect of vp-genkwa pretreatment on HSV-1 replication. (B) Effect of vp-genkwa posttreatment on HSV-1 replication. Groups of BALB/c mice (n=5) were infected i.vag. with HSV-1 after (A) and before (B) oral administration of vp-genkwa (10 mpk). Viral titers in vaginal lavages collected at 2 and 4 dpi were determined by plaque assay.
Increased NK cell activity in vp-genkwa-treated mice following HSV-1 infection
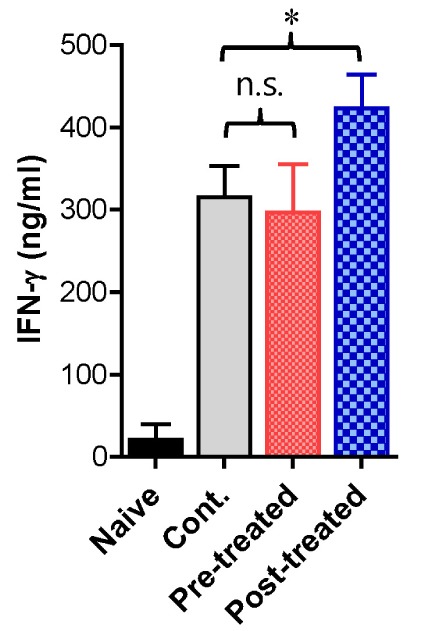
Vaginal infiltration of innate leukocytes, such as granulocytes and monocytes/macrophages, are thought to contribute to HSV infection resistance via direct and indirect mechanisms (10,11). Moreover, NK cells reportedly play a crucial role in regulating vaginal inflammation caused by HSV-1 infection (4,5). Thus, we decided to explore the regulatory role of vp-genkwa treatment on NK cell activity. First, we determined the frequency and absolute number of CD3-NK1.1+NKp46+NK cells in the spleen of mice treated with vp-genkwa before and after HSV-1 infection 3 dpi. In spite of vp-genkwa treatment before and after HSV-1 infection, our results did not indicate any changes in the frequency or absolute number of NK cells, except that vp-genkwa treatment before HSV-1 infection slightly increased the percentage of NK cells in the spleen (Fig. 3A). However, intriguingly, vp-genkwa treatment after HSV-1 infection increased the frequency and absolute number of IFN-γ- and granzyme B-producing NK cells, whereas pre-treatment with vp-genkwa before HSV-1 infection did not affect the frequency or absolute number of IFN-γ- and granzyme B-producing NK cells (Fig. 3B and C). These data indicate that vp-genkwa treatment after HSV-1 infection increases NK cell activity because IFN-γ and granzyme B expressed on activated NK cells are involved in eradicating virions and killing virus-infected cells. Supportively, secreted IFN-γ was detected at higher levels in vaginal lavages of mice treated with vp-genkwa after HSV-1 infection, compared to that of mice treated with vehicle and vp-genkwa before HSV-1 infection (Fig. 4). Collectively, these results indicate that the enhanced resistance of mice treated with vp-genkwa after HSV-1 infection is closely associated with increased NK cell activity.
Figure 3. Enhancement of NK cell activity in vp-genkwa-treated mice following HSV-1 infection. (A) The frequency and absolute number of NK cells in the spleen of mice treated with vp-genkwa. The splenocytes were prepared from mice that were pre- and post-treated with vp-genkwa (10 mpk) 3 dpi, and used to enumerate NK cells (CD3+NK1.1+NKp46+) using FACS analysis. The left graph denotes the average percentage of NK cells in the spleen, and the right graph shows the absolute number of NK cells in the spleens of four mice per group. (B and C) The frequency and absolute number of NK cells producing IFN-γ and granzyme B. The splenocytes were prepared from mice pre- and post-treated with vp-genkwa 3 dpi and stimulated with PMA plus ionomycin. The frequency and absolute number of IFN-γ- (B) and granzyme B (C; GrB)-producing cells in NK cells (CD3-NK1.1+NKp46+) were determined by intracellular FACS staining. *,p<0.05; **, p<0.01; n.s., not significant between the indicated groups.
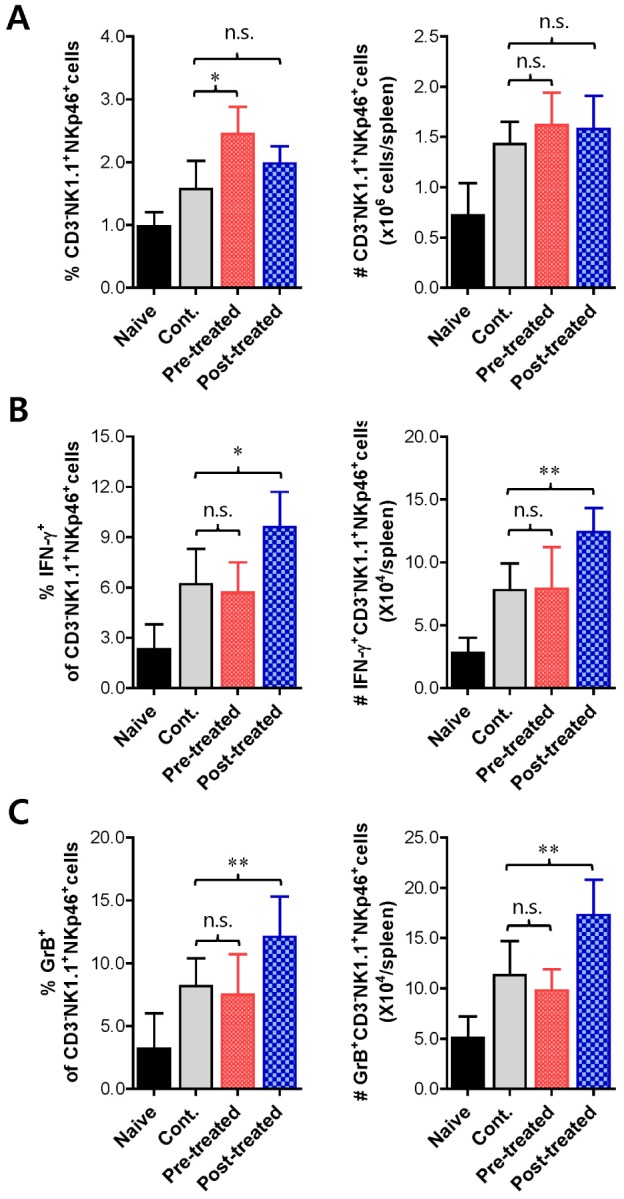
Figure 4. Treatment with vp-genkwa after HSV-1 infection increases vaginal IFN-γ. Groups of mice were treated with vp-genkwa (10 mpk) before (pre-treated) and after HSV-1 infection (post-treated). The levels of IFN-γ protein in vaginal lavages collected at 3 dpi were determined by ELISA. *, p<0.05; n.s., not significant between the indicated groups.
DISCUSSION
Examination of genital HSV infection using a murine model has significantly contributed to our understanding of the role of innate and adaptive immune cells during vaginal inflammation and CNS-invasion. In the present study, we demonstrated using a murine infection model that treatment with vp-genkwa at optimal doses after, but not before, HSV-1 infection provides enhanced resistance, as reflected by reduced mortality and clinical severity. Further, the reduction in viral secretion in vaginal lavages of mice treated with vp-genkwa after infection supported the observed decrease in the clinical signs caused by mucosal HSV-1 infection. Interestingly, the reduction in mortality and clinical signs in mice treated with vp-genkwa after viral infection was closely associated with enhanced NK cell activation, which indicates the involvement of NK cell activation in vp-genkwa efficacy in the HSV-1 infection model. Therefore, our data suggest that vp-genkwa is a valuable tool for controlling the clinical severity caused by mucosal HSV-1 infection.
NK cells are innate immune cells effective against different kinds of viruses, including influenza virus, HSV, and hepatitis B virus, thereby indicating a general role of these cells in potent antiviral immune responses (32,33). The complex function of NK cells is achieved by the production of several immunomodulatory cytokines, such as IFN-γ and TNF-α, as an indirect mechanism, and the direct killing of virus-infected cells through secretion of granzyme B and perforin in cell-to-cell contact (32,33). Specifically, the induction of IFN-γ is an important feature of activated NK cells because IFN-γ plays a crucial role in various immunological processes such as the generation of CD4+ Th1 cells (34). There have been various attempts to activate NK cells using immunomodulatory molecules derived from endogenous bioactive molecules, such as interleukin (IL)-12 and IL-15 (19,20,21). In addition, several materials derived from chemically synthesized or natural resources were found to be effective forms of drug therapy for chronic diseases, such as cancer and viral infections, through NK cell activation (22,23). Although the molecular networks through which NK cells are activated by vp-genkwa are not clear, our data is strengthened by recent findings that genkwadaphnin, derived from the flower buds of D. genkwa, induces IFN-γ production from NK cells through activation of protein kinase D (PKD), a member of the PKC family (35). However, our data does not exclude the role of Toll-like receptor (TLR) molecules in NK cell activation because NK cells express several TLR molecules on cell and endosomal membranes (36,37). It is assumed that some components derived from vp-genkwa could trigger the activation of TLRs, thereby inducing phosphorylation of mitogen-activated protein kinase (MAPK), such as extracellular signal-regulated kinase (ERK), p38 and mitogen-activated protein kinase kinase (MEK), and subsequently nuclear factor (NF)-κB activation. Therefore, we are currently investigating the role of TLR and downstream molecular networks in the induction of NK cell activation using TLR- and MyD88-deficient mice. However, one incongruous finding is that higher doses of vp-genkwa administered after HSV-1 infection do not increase resistance. It was presumed that excessive triggering of TLR and other innate immune receptors on NK cells could result in a form of innate immunity paralysis characterized by reduced pro- and anti-inflammatory cytokine release and hypo-activation of NK cells (38).
In addition, NK cell activation can enhance the function of dendritic cells (DCs) for tailored adaptive immune responses, since crosstalk between NK cells and DCs plays a role in inducing immune responses to vaccine antigens (39). Also, IFN-γ produced from activated NK cells may elicit various effects that confer protection against HSV-1 genital infection because IFN-γ is important for immune resistance to HSV infection, as well as macrophage activation (10,11). Furthermore, IFN-γ may promote the generation of CD4+ Th1 and CD8+ T cell responses that provide protective immunity against HSV-1 infection (34). Therefore, it is speculated that treatment of vp-genkwa at optimal doses after HSV-1 infection fosters an environment conducive to the provision of effective protection against HSV-1 infection via IFN-γ production from activated NK cells.
Acyclovir is a commonly used drug for treating HSV genital and skin infections, but an increasing number of resistant strains have emerged owing to the drug's wide clinical applications. This situation compels us to seek a drug that can act against acyclovir-resistant strain. Because genital inflammation caused by HSV infection is considered an immune response orchestrated by innate and adaptive immune cells, a drug that can enhance innate and adaptive immunity against HSV infection may be a promising candidate for the treatment of genital inflammation. Although the specific molecules activated by vp-genkwa that increase protective immunity were not defined, our data provide a valuable insight into the use of vp-genkwa to control clinical severity in HSV infection.
ACKNOWLEDGEMENTS
This study was supported by a National Research Foundation of Korea (NRF) grant, funded by the Korea Government (MISP) (2012R1A2A1A03670284). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of manuscript.
Abbreviations
- NK
natural killer
- PKD
protein kinase D
- vp-genkwa
vinegar-processed flos of Daphne genkwa
Footnotes
CONFLICTS OF INTEREST: The authors have no financial conflict of interest.
References
- 1.Shahin V, Hafezi W, Oberleithner H, Ludwig Y, Windoffer B, Schillers H, Kuhn JE. The genome of HSV-1 translocates through the nuclear pore as a condensed rod-like structure. J Cell Sci. 2006;119:23–30. doi: 10.1242/jcs.02705. [DOI] [PubMed] [Google Scholar]
- 2.Daniels D, Mortlock S. Mixed HSV-1 and HSV-2 infection in a patient attending a GUM clinic. Br J Biomed Sci. 2008;65:203–204. doi: 10.1080/09674845.2008.11978131. [DOI] [PubMed] [Google Scholar]
- 3.Pereira VS, Moizeis RN, Fernandes TA, Araujo JM, Meissner RV, Fernandes JV. Herpes simplex virus type 1 is the main cause of genital herpes in women of Natal, Brazil. Eur J Obstet Gynecol Reprod Biol. 2012;161:190–193. doi: 10.1016/j.ejogrb.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 4.Lee AJ, Ashkar AA. Herpes simplex virus-2 in the genital mucosa: insights into the mucosal host response and vaccine development. Curr Opin Infect Dis. 2012;25:92–99. doi: 10.1097/QCO.0b013e32834e9a56. [DOI] [PubMed] [Google Scholar]
- 5.Chentoufi AA, Benmohamed L. Mucosal herpes immunity and immunopathology to ocular and genital herpes simplex virus infections. Clin Dev Immunol. 2012;2012:149135. doi: 10.1155/2012/149135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grinde B. Herpesviruses: latency and reactivation - viral strategies and host response. J Oral Microbiol. 2013;5:22766. doi: 10.3402/jom.v5i0.22766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Auvert B, Ballard R, Campbell C, Carael M, Carton M, Fehler G, Gouws E, MacPhail C, Taljaard D, Van DJ, Williams B. HIV infection among youth in a South African mining town is associated with herpes simplex virus-2 seropositivity and sexual behaviour. AIDS. 2001;15:885–898. doi: 10.1097/00002030-200105040-00009. [DOI] [PubMed] [Google Scholar]
- 8.Mugo N, Dadabhai SS, Bunnell R, Williamson J, Bennett E, Baya I, Akinyi N, Mohamed I, Kaiser R. Prevalence of herpes simplex virus type 2 infection, human immunodeficiency virus/herpes simplex virus type 2 coinfection, and associated risk factors in a national, population-based survey in Kenya. Sex Transm Dis. 2011;38:1059–1066. doi: 10.1097/OLQ.0b013e31822e60b6. [DOI] [PubMed] [Google Scholar]
- 9.Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS. 2006;20:73–83. doi: 10.1097/01.aids.0000198081.09337.a7. [DOI] [PubMed] [Google Scholar]
- 10.Parr MB, Kepple L, McDermott MR, Drew MD, Bozzola JJ, Parr EL. A mouse model for studies of mucosal immunity to vaginal infection by herpes simplex virus type 2. Lab Invest. 1994;70:369–380. [PubMed] [Google Scholar]
- 11.Uyangaa E, Patil AM, Eo SK. Prophylactic and therapeutic modulation of innate and adaptive immunity against mucosal infection of herpes simplex virus. Immune Netw. 2014;14:187–200. doi: 10.4110/in.2014.14.4.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gill N, Rosenthal KL, Ashkar AA. NK and NKT cell-independent contribution of interleukin-15 to innate protection against mucosal viral infection. J Virol. 2005;79:4470–4478. doi: 10.1128/JVI.79.7.4470-4478.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harandi AM, Svennerholm B, Holmgren J, Eriksson K. Differential roles of B cells and IFN-γ-secreting CD4+ T cells in innate and adaptive immune control of genital herpes simplex virus type 2 infection in mice. J Gen Virol. 2001;82:845–853. doi: 10.1099/0022-1317-82-4-845. [DOI] [PubMed] [Google Scholar]
- 14.Milligan GN, Bernstein DI. Interferon-gamma enhances resolution of herpes simplex virus type 2 infection of the murine genital tract. Virology. 1997;229:259–268. doi: 10.1006/viro.1997.8441. [DOI] [PubMed] [Google Scholar]
- 15.Parr MB, Parr EL. The role of gamma interferon in immune resistance to vaginal infection by herpes simplex virus type 2 in mice. Virology. 1999;258:282–294. doi: 10.1006/viro.1999.9739. [DOI] [PubMed] [Google Scholar]
- 16.Harandi AM, Svennerholm B, Holmgren J, Eriksson K. Interleukin-12 (IL-12) and IL-18 are important in innate defense against genital herpes simplex virus type 2 infection in mice but are not required for the development of acquired gamma interferon-mediated protective immunity. J Virol. 2001;75:6705–6709. doi: 10.1128/JVI.75.14.6705-6709.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conrady CD, Jones H, Zheng M, Carr DJ. A functional type I interferon pathway drives resistance to cornea herpes simplex virus type 1 infection by recruitment of leukocytes. J Biomed Res. 2011;25:111–119. doi: 10.1016/S1674-8301(11)60014-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conrady CD, Zheng M, Mandal NA, van RN, Carr DJ. IFN-α-driven CCL2 production recruits inflammatory monocytes to infection site in mice. Mucosal Immunol. 2013;6:45–55. doi: 10.1038/mi.2012.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hansen ML, Woetmann A, Krejsgaard T, Kopp KL, Sokilde R, Litman T, Straten PT, Geisler C, Wasik MA, Odum N, Eriksen KW. IFN-α primes T- and NK-cells for IL-15-mediated signaling and cytotoxicity. Mol Immunol. 2011;48:2087–2093. doi: 10.1016/j.molimm.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 20.Beuneu H, Deguine J, Bouvier I, Di Santo JP, Albert ML, Bousso P. Cutting Edge: A dual role for type I IFNs during polyinosinic-polycytidylic acid-induced NK cell activation. J Immunol. 2011;187:2084–2088. doi: 10.4049/jimmunol.1004210. [DOI] [PubMed] [Google Scholar]
- 21.Baranek T, Manh TP, Alexandre Y, Maqbool MA, Cabeza JZ, Tomasello E, Crozat K, Bessou G, Zucchini N, Robbins SH, Vivier E, Kalinke U, Ferrier P, Dalod M. Differential responses of immune cells to type I interferon contribute to host resistance to viral infection. Cell Host Microbe. 2012;12:571–584. doi: 10.1016/j.chom.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 22.Kovalova A, Ledvina M, Saman D, Zyka D, Kubickova M, Zidek L, Sklenar V, Pompach P, Kavan D, Bily J, Vanek O, Kubinkova Z, Libigerova M, Ivanova L, Antolikova M, Mrazek H, Rozbesky D, Hofbauerova K, Kren V, Bezouska K. Synthetic N-acetyl-D-glucosamine based fully branched tetrasaccharide, a mimetic of the endogenous ligand for CD69, activates CD69+ killer lymphocytes upon dimerization via a hydrophilic flexible linker. J Med Chem. 2010;53:4050–4065. doi: 10.1021/jm100055b. [DOI] [PubMed] [Google Scholar]
- 23.Nielsen CH, Balachandran P, Christensen O, Pugh ND, Tamta H, Sufka KJ, Wu X, Walsted A, Schjorring-Thyssen M, Enevold C, Pasco DS. Enhancement of natural killer cell activity in healthy subjects by Immulina®, a Spirulina extract enriched for Braun-type lipoproteins. Planta Med. 2010;76:1802–1808. doi: 10.1055/s-0030-1250043. [DOI] [PubMed] [Google Scholar]
- 24.Jiangsu New Medical College. The Encyclopedia of Traditional Chinese medicine. 2nd ed. Shanghai Science and Technology: Shanghai; 1985. pp. 2573–2574. [Google Scholar]
- 25.Zhan ZJ, Fan CQ, Ding J, Yue JM. Novel diterpenoids with potent inhibitory activity against endothelium cell HMEC and cytotoxic activities from a well-known TCM plant Daphne genkwa. Bioorg Med Chem. 2005;13:645–655. doi: 10.1016/j.bmc.2004.10.054. [DOI] [PubMed] [Google Scholar]
- 26.Li HS, Xiao ML. Research progress of the Shi-Zao-Tang to treat malignant pleural effusion and ascites. Res Integr Tradit Chin West Med. 2012;4:93–94. [Google Scholar]
- 27.Zheng W, Gao X, Gu Q, Chen C, Wei Z, Shi F. Antitumor activity of daphnodorins from Daphne genkwa roots. Int Immunopharmacol. 2007;7:128–134. doi: 10.1016/j.intimp.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 28.Park BY, Min BS, Oh SR, Kim JH, Bae KH, Lee HK. Isolation of flavonoids, a biscoumarin and an amide from the flower buds of Daphne genkwa and the evaluation of their anti-complement activity. Phytother Res. 2006;20:610–613. doi: 10.1002/ptr.1915. [DOI] [PubMed] [Google Scholar]
- 29.Uyangaa E, Choi JY, Patil AM, Kim JH, Kim SB, Kim K, Ryu HW, Oh SR, Eo SK. Functional restoration of exhausted CD4+ and CD8+ T cells in chronic viral infection by vinegar-processed flos of Daphne genkwa. Comp Immunol Microbiol Infect Dis. 2015;39:25–37. doi: 10.1016/j.cimid.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 30.Geng L, Sun H, Yuan Y, Liu Z, Cui Y, Bi K, Chen X. Discrimination of raw and vinegar-processed Genkwa Flos using metabolomics coupled with multivariate data analysis: a discrimination study with metabolomics coupled with PCA. Fitoterapia. 2013;84:286–294. doi: 10.1016/j.fitote.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 31.Uyangaa E, Lee HK, Eo SK. Glutamine and leucine provide enhanced protective immunity against mucosal infection with herpes simplex virus type 1. Immune Netw. 2012;12:196–206. doi: 10.4110/in.2012.12.5.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lanier LL. Evolutionary struggles between NK cells and viruses. Nat Rev Immunol. 2008;8:259–268. doi: 10.1038/nri2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferlazzo G, Munz C. NK cell compartments and their activation by dendritic cells. J Immunol. 2004;172:1333–1339. doi: 10.4049/jimmunol.172.3.1333. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y, Zhang Y, Gu W, Sun B. TH1/TH2 cell differentiation and molecular signals. Adv Exp Med Biol. 2014;841:15–44. doi: 10.1007/978-94-017-9487-9_2. [DOI] [PubMed] [Google Scholar]
- 35.Kang HB, Ahn KS, Oh SR, Kim JW. Genkwadaphnin induces IFN-gamma via PKD1/NF-κB/STAT1 dependent pathway in NK-92 cells. PLoS One. 2014;9:e115146. doi: 10.1371/journal.pone.0115146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.dib-Conquy M, Scott-Algara D, Cavaillon JM, Souza-Fonseca-Guimaraes F. TLR-mediated activation of NK cells and their role in bacterial/viral immune responses in mammals. Immunol Cell Biol. 2014;92:256–262. doi: 10.1038/icb.2013.99. [DOI] [PubMed] [Google Scholar]
- 37.Millard AL, Spirig R, Mueller NJ, Seebach JD, Rieben R. Inhibition of direct and indirect TLR-mediated activation of human NK cells by low molecular weight dextran sulfate. Mol Immunol. 2010;47:2349–2358. doi: 10.1016/j.molimm.2010.05.284. [DOI] [PubMed] [Google Scholar]
- 38.Chen S, Lin G, Lei L, You X, Wu C, Xu W, Huang M, Luo L, Wang Z, Li Y, Zhao X, Ya Fn. Hyperlipidemia modifies innate immune responses to lipopolysaccharide via the TLR-NF-κB signaling pathway. Inflammation. 2013;36:968–976. doi: 10.1007/s10753-013-9628-9. [DOI] [PubMed] [Google Scholar]
- 39.Langers I, Renoux V, Reschner A, Touze A, Coursaget P, Boniver J, Koch J, Delvenne P, Jacobs N. Natural killer and dendritic cells collaborate in the immune response induced by the vaccine against uterine cervical cancer. Eur J Immunol. 2014;44:3585–3595. doi: 10.1002/eji.201444594. [DOI] [PubMed] [Google Scholar]