Abstract
Hepatitis C virus (HCV)-related liver disease is a significant cause of morbidity and mortality in patients with end-stage renal disease (ESRD) who is treated with dialysis or kidney transplantation (KT). The survival rate for HCV-infected renal transplant recipients is better than that for HCV-infected hemodialysis patients on transplant waiting lists. Early diagnosis and treatment HCV infection prior to KT prevents complications post-transplantation and reduces mortality. In addition to screening for anti-HCV antibodies and detecting HCV RNA, percutaneous liver biopsy is particularly valuable for assessing the stage of liver damage in HCV-infected patients, because the stage of fibrosis is important determining optimal treatment for HCV. Studies have been demonstrated that with conventional interferon (IFN) monotherapy or pegylated IFN monotherapy are similar efficacy and safety in HCV-infected hemodialysis patients. Sustained viral responses (SVRs) with these monotherapies have ranged approximately 30% to 40%. Limited reports support the use of IFN and ribavirin combination therapy as antiviral treatment for ESRD patients or patients on hemodialysis. Ribavirin can be started at low dose and careful monitoring for side effects. Patients that show SVR after treatment are strong candidates for KT. It is also generally accepted that ESRD patients with decompensated cirrhosis and portal hypertension should be referred to the liver transplant team for consideration of combined liver-KT.
Keywords: Hepatitis C virus, Hemodialysis, End-stage renal disease, Kidney transplantation, Hepatitis C treatment
Core tip: Hepatitis C virus (HCV)-related liver disease is a significant cause of morbidity and mortality in patients with end-stage renal disease who are treated with dialysis or KT. Early diagnosis and treatment of HCV infection prior kidney transplantation (KT) prevent complications after transplantation and reduces mortality. Issues with current mode HCV therapy include lack of tolerability and suboptimal response rates.
INTRODUCTION
Liver disease related to hepatitis C virus (HCV) infection is a significant cause of morbidity and mortality in hemodialysis (HD) patients and kidney transplant recipients. In developed countries, the prevalence of anti-HCV seropositivity among patients on maintenance HD ranges between 5% and 60%. Patients on HD are at high risk for HCV, with frequency of infection several times higher than that in non-uremic patients[1]. The spread of HCV in HD units is declining, but the prevalence of HCV in HD patients remains high[1].
Several observational studies have demonstrated a significant and independent relationship between anti-HCV seropositive status and lower survival rate in patients with end-stage renal disease (ESRD). The two major complications of HCV-related chronic liver disease are cirrhosis and hepatocellular carcinoma, and these are suspected causes of higher mortality among HCV-positive patients.
Management of HCV-positive ESRD patients is complicated. There are unknowns related to prevention of HCV spread in dialysis units, anti-viral therapy in dialysis patients, and effects of HCV infection after renal transplantation. A wide range of studies have been published on HCV in ESRD patients, but clinical evidence is lacking with respect to most of the important issues, and most existing evidence is from uncontrolled clinical trials or retrospective surveys[2-4].
EPIDEMIOLOGY OF HCV INFECTION
Serologic testing has clearly demonstrated that HCV infection is highly prevalent among ESRD patients and is a serious cause of increased morbidity and mortality in this group. Failures of HCV screening, excessive exposure to blood and blood products, nosocomial transmission of HCV in HD units, and long dialysis duration are the main determinants of increased risk of HCV infection in the HD patient group[5]. The worldwide prevalence of HCV infection among HD patients varies widely, with estimates ranging from 5% to approximately 60% depending on geographic location[6-9]. In 2002, the prevalence of HCV infection across HD centers of the United States was approximately 8%, nearly five times greater than that of the general population in that country[10,11]. In some European dialysis centers, the yearly incidence of HCV infection reportedly ranges from 0.4% to 16.0%[12]. In 2011, the Turkish Society of Nephrology documented 7.9% anti-HCV seropositivity and 1.7% HCV-RNA seropositivity among Turkish HD patients[13]. Introduction of stricter blood bank screening rules, widespread use of erythropoiesis-stimulating agents instead of blood transfusions, and stronger adherence to infection control practices in dialysis units have reduced the prevalence of HCV infection in the HD patient group[14]. For example, Spain observed a decline from 24% in 1992 to 9.2% in 2002[15]. Anti-HCV prevalence among patients on chronic HD in the United States decreased from 10.4% in 1995 to 7.8% (i.e., 164632 Americans) in 2002[2]. A recent multicenter survey revealed that prevalence of anti-HCV positivity for a Belgian cohort of HD patients (n = 1710) dropped steadily from 13.5% in 1991 to 6.8% in 2000, and the same survey revealed significant drops in many other countries including France (42% to 30%), Italy (27% to 16%), and Sweden (16% to 9%)[2].
IMPACT OF HCV ON SURVIVAL
One study that involved the database of more than 13000 HD patients in the United States revealed that HCV infection was more strongly associated with all causes of mortality than with HCV-negative status[16]. Lee et al[17] identified HCV infection as an independent risk factor for the transition from chronic kidney disease to ESRD. A meta-analysis by Fabrizi et al[18] revealed that HD can negatively modify the course of HCV infection. The authors found that the estimated relative risk of liver-related mortality in anti-HCV-positive patients on HD was 1.57 times (95%CI: 1.33-1.86; P < 0.001) than that observed for anti-HCV-negative counterparts. The authors concluded that, in HD patients, the presence of anti-HCV antibodies is an independent risk factor for death, because of increased risk of cirrhosis and hepatocellular carcinoma. Other research has shown that kidney transplantation (KT) improves the long-term survival of ESRD patients with HCV infection[19,20]. While there is considerable evidence that HCV infection threatens the success of KT, the survival of HCV-infected renal transplant recipients is better than that for HCV-infected HD patients who are on transplant waiting lists[21]. This survival advantage may reflect systemic effects of well-functioning renal allografts that is clearing uremic toxins, and may also reflect reduced inflammatory responses and oxidative stress. HCV-related deterioration of renal transplant recipients may be linked to the immunosuppressive treatment that is required after KT. This can result in flares of HCV infection and can increase liver- and kidney-related morbidity and mortality from conditions such as cirrhosis, hepatocellular carcinoma, transplant glomerulopathy, and graft dysfunction[22].
PREVENTION OF HCV TRANSMISSION
Health care procedures related to nosocomial infections, unsafe drug injection practices, and blood transfusions are key factors in HCV transmission. In HD facilities, the most common lapses of healthcare quality are contamination of dialysis systems, inadequate disinfection and cleaning of environmental surfaces, improper contact of health care staff with equipment and patients, and mishandling of parenteral medications[23,24]. The guidelines for preventing HCV infection in HD settings recommend fundamental infection control practices, and routine screening of HD patients for HCV. Isolating-HCV-infected patients or using dedicated machines for such patients are not advocated, except as necessary during local outbreaks[12,24].
DIAGNOSIS OF HCV INFECTION IN PATIENTS WITH ESRD
Infection with HCV normally leads to increased serum alanine aminotransferase (ALT), and laboratory blood testing for ALT is used to screen for liver disease in the general population. However, this test has weak diagnostic value in ESRD patients because ALT tends to be below reference range in this patient group. The potential causes of this are vitamin B6 deficiency, presence of uremic toxins, or presence of blood components that absorb ultraviolet light[25]. To detect HCV viremia in HD patients, new thresholds for serum ALT have been proposed that are less than half (approximately 0.4 to 0.45 times) the conventional threshold[26]. Serial testing of serum ALT level might be valuable for monitoring patients on HD with known HCV infection.
Enzyme-linked immunoassays (EIAs) are commonly used to detect HCV antibodies. Third-generation EIAs for anti-HCV antibody detection have high sensitivity and specificity because these tests are based on antigens in the core, non-structural 3-4-5 proteins of the virus. Screening for anti-HCV antibodies by EIA remains a simple method, but this type of test is only meaningful for ruling out HCV infection in ESRD patients in low-prevalence settings. In the ESRD patient group, the proposed interval for HCV screening via antibody test is 6 to 12 mo[27].
One disadvantage of this serologic test is false-negative result, which can present challenges for distinguishing acute from chronic HCV infection[2]. In a case where HCV infection is strongly suspected but the HCV antibody EIA is negative, blood testing for HCV RNA should be done directly using polymerase chain reaction technique[28]. Detection of HCV RNA indicates HCV replication. In populations with known higher prevalence of HCV infection, a negative EIA result does not rule out HCV infection, and testing for HCV RNA is appropriate in such cases to avoid missing HCV infections[27]. When EIA reveals that an ESRD patient is anti-HCV positive, the next step is quantitative determination of viral load. This helps confirm the antibody test result and is also useful for assessing the patient’s prognostic risk stratification prior to antiviral treatment[28]. There are two main reasons why blood for HCV RNA testing should be drawn prior to a dialysis procedure: (1) presence of heparin in the blood sample can lead to false-positive PCR result for HCV; and (2) a patient’s HCV RNA level can decrease during the HD session (though it will return to baseline within 48 h). Adsorption of HCV to dialysis membrane, destruction of HCV particles during the HD session, and, rarely, escape of HCV into the dialysate are other reasons why a patient’s HCV RNA could be falsely low[29].
In addition to detecting HCV RNA, HCV genotyping is also required to predict response to treatment and to specify the duration and dosage of treatment. HCV genotypes 1, 4, 5, and 6 are more resistant to treatment and require longer courses of therapy. One study identified HCV genotype 1b as the most prevalent subtype in patients receiving HD or continuous ambulatory peritoneal dialysis in Turkey[1]. In study of Perez et al[30] reported findings that HCV genotype 1a was the most prevalent subtype in patients receiving HD, with genotype 1b the next most frequent, followed by genotype 3, and other less prevalent genotypes (genotypes 2, 4, and 5). This observation may reflect differences in the epidemiology of HCV infection, viral characteristics, and host factors in ESRD patients[31].
Percutaneous liver biopsy constitutes the most reliable tool for examining the effects of HCV infection (i.e., stage of liver disease) and ruling out possible other concomitant liver diseases[31]. Neither HCV RNA viral load nor liver enzymes reflect the severity of liver injury decisively[32,33]. Liver enzyme activity and quantity of HCV RNA can fluctuate during HCV infection, whereas fibrosis is progressive and largely irreversible. There is strong evidence that the stage of liver fibrosis during HCV infection predicts survival for kidney transplant candidates and for renal grafts. In addition, determining stage of fibrosis is important for planning the treatment of HCV[34]. According to one report, up to 25% of HCV-infected patients exhibit bridging fibrosis or cirrhosis on liver biopsy[31]. Presence of advanced fibrosis does not exclude a candidate from KT, but it is inevitable that such patients will develop the comorbidities and complications of portal hypertension after transplantation[35].
Although liver biopsy is reliable tool, it has significant limitations including serious bleeding events, and sampling and interpretation errors. Coagulopathy, thrombocytopenia, platelet dysfunction, anticoagulation during HD and anti-platelet therapy all pose increased bleeding risk in ESRD patients or patients on HD. In these individuals with increased bleeding risk, a transjugular or transfemoral route for liver biopsy may be recommended. Note that even when an experienced physician performs the biopsy and an experienced pathologist interprets the findings, this gold-standard technique is associated with up to 20% error in staging liver disease[34,35]. Ahmad et al[36] compared the results for 46 HD patients with chronic liver disease who underwent transjugular liver biopsy and 32 HD patients who had undergone percutaneous liver biopsy at the same institution. The authors found that both techniques obtained adequate specimens for histological diagnosis in all patients; however, the complications differed. There were no major complications in the transjugular liver biopsy group, whereas 12% of the patients in the percutaneous liver biopsy developed bleeding complications[36]. Some authors have reported the progression of HCV-related liver disease in HD patients who are on transplantation waiting lists. One study concluded that patients whose liver biopsy show Metavir fibrosis score 1 or 2 should undergo a repeat liver biopsy every 5 years, whereas those with Metavir score 3 should be followed more intensely, with biopsies every 3 years[14].
There are also novel non-invasive techniques for assessing liver fibrosis in ESRD patients with HCV infection. Two of these are the aspartate aminotransferase to-platelet ratio index (APRI) and transient elastography (TE) which is performed with a Fibroscan® machine. Studies have demonstrated that APRI and TE are effective for evaluating hepatic fibrosis in HD patients with chronic HCV infection, but both methods have some limitations[37,38]. The predictive values and cut-off values for these methods as optimized for the healthy population may not be valid for ESRD patients[39]. As well, the HD procedure and the presence of uremic toxins may alter blood levels of apolipoprotein A1 and α-2 macroglobulin, which can influence Fibroscan® results[37]. An APRI score cannot precisely predict the histological severity of liver disease, particularly the intermediate fibrosis stage[38].
NATURAL COURSE OF HCV IN ESRD PATIENTS
In patients with ESRD, chronic HCV infection usually takes an insidious clinical course. Early diagnosis and identification of individuals at greater risk for fibrosis progression should be the clinician’s main concern. Evidence clearly indicates that ESRD patients who undergo KT have more favorable results than those who do not, and that this also applies for ESRD patients with HCV infection. Every ESRD patient should be evaluated as a candidate for KT as soon as possible after diagnosis. This is important because during the interval between diagnosis and transplantation there is increased mortality risk due to higher incidence of hepatic and non-hepatic complications. It is essential that each dialysis center carefully follows every HCV-infected ESRD patient to determine viral load, do HCV genotyping, assess the extent of hepatic fibrosis, and establish optimal treatment strategies. A decision tree for the follow-up process is shown in Figure 1. Treating HCV infection prior to KT helps to prevent post-transplantation complications and reduce mortality[3,39-42].
Figure 1.
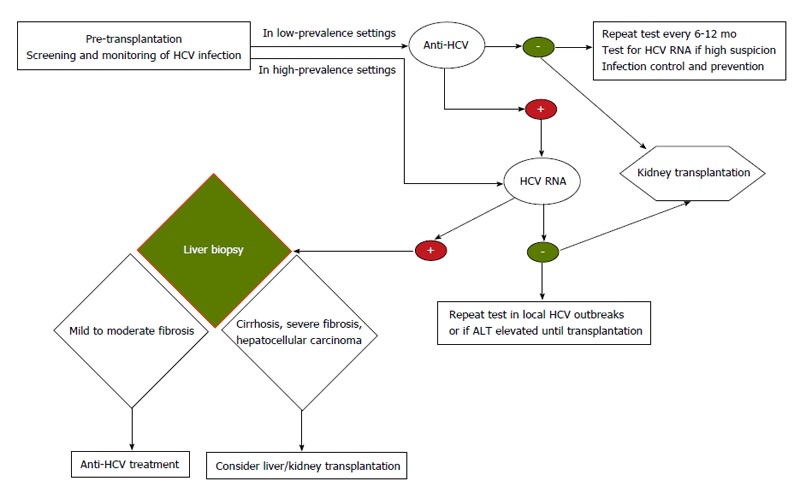
Decision tree for follow-up of hepatitis C virus-positive end-stage renal disease patients. HCV: Hepatitis C virus; ALT: Alanine aminotransferase.
There is general agreement that ESRD patients with decompensated cirrhosis and portal hypertension should be removed from the KT list and referred to the liver transplant team to be considered for combined liver-KT[43].
It is also generally accepted that every HCV-positive ESRD patient who is being evaluated for KT should undergo a liver biopsy to assess the stage of liver disease, unless there is clear radiological or clinical evidence of portal hypertension or decompensated cirrhosis[35]. Patients with Metavir fibrosis score ≤ 3 on histologic examination should be considered for antiviral treatment. Patients with successful sustained viral response (SVR) are definite candidates for KT, and those on the waiting list should be tested at least annually to confirm the durability of the SVR prior to transplantation[35,41,44]. Those who have ready living-related donors could be referred for KT immediately. There is some largely anecdotal evidence that inducing an SVR prior to KT reduces the risk of post-transplantation complications. For patients who have attained SVR, some authors consider the probability of HCV reactivation to be extremely low, however, this is controversial, especially when aggressive immunosuppression is administered[45].
The growing number of ESRD patients with HCV-related compensated cirrhosis is a major problem. In this group, treatment is a dilemma because interferon and ribavirin carry potential risk of decompensation and serious adverse effects[3,45]. Some authors consider these patients “too healthy” for liver transplantation, and yet their limited liver reserve, poor nutritional status, and increased susceptibility to infections puts these individuals at risk for increased perioperative morbidity and mortality if they undergo KT[44]. These patients require comprehensive and detailed evaluation. Presence of portal hypertension should be carefully evaluated to decide whether KT alone or combined kidney and liver transplantation is appropriate[42,43]. It is recommended that patients with wedge hepatic vein pressure > 10 mmHg may be listed for combined kidney and liver transplantation[43]. Some transplantation centers use different criteria to make this decision, such as platelet count or presence of varices on endoscopy[44]. Making the correct treatment decision in these cases in this challenging. To improve decision-making in future, transplant centers need to report incremental experiences and long-term post-transplantation observations.
The other problematic patient group with respect to treatment decisions is ESRD patients with HCV who do not respond to antiviral treatment or who have been assessed as ineligible for antiviral treatment. As HCV replication progresses, there is greater risk of accelerated liver disease, including hepatic failure and hepatocellular carcinoma[46]. KT is not contraindicated for these individuals, but each such candidate must be informed of all difficulties (i.e., those related to progression of HCV and restricted antiviral treatment) after transplantation[47]. It should also be kept in mind that extending waiting time for KT will likely result in poorer liver status for these individuals[46,47]. However, any ESRD patient with HCV who remains viremic while on a KT waiting list should be placed on hold status. These patients should be evaluated carefully and frequently to assess HCV disease status and their suitability for KT as time on the waiting list extended[44].
HCV infection after KT
The natural history of HCV infection after non-liver solid organ transplantation is still not fully understood. One study revealed that HCV-positive kidney transplant recipients had lower survival than HCV-negative patients[42]. As noted previously, KT is associated with better long-term survival for ESRD patients, even those diagnosed with HCV infection. In other words, anti-HCV positivity must not preclude HD patients from KT[48].
After KT: Progression and treatment of HCV-related liver disease
Patients with HCV infection who undergo KT can experience progression of liver disease after the operation. This has been identified as the fourth most frequent cause of mortality (reported range, 8% to 28%) in long-term survivors of KT. Increased risk of cardiovascular disease, post-transplantation diabetes mellitus, and sepsis are considered the primary causes of death after KT[49].
A patient’s HCV viral load will increase in the setting of immunosuppression, which usually develops within the first months after KT[50], but there is no evidence that progression of liver disease is correlated with HCV RNA load.
Kamar et al[47] investigated natural course of HCV-related liver fibrosis after KT. They observed that liver biopsy samples from HCV-positive patients after KT showed progression of fibrosis in 21 patients, stable phase in 21 patients, and regression of fibrosis in 10 patients. The risk factor most strongly associated with progression of hepatic fibrosis was severity of liver disease prior to KT[47].
In HD patients with HCV who undergo KT, antiviral treatment is mandatory after renal transplantation only if the individual develops advanced fibrosis or severe cholestatic hepatitis. Both these conditions are associated with high mortality, and antiviral therapy can be lifesaving[46,51]. For such patients, the decision regarding when to provide antiviral therapy should be made based on a risk-benefit assessment for each individual case[51]. The main concern about interferon (IFN) treatment is that it can trigger acute graft rejection[45,46]. The optimal approach for antiviral treatment of HCV after KT is unclear. A recent meta-analysis by Fabrizi et al[52] evaluated 12 clinical trials. In three of these, a combination of IFN and ribavirin was used, and the estimated rates of SVR and dropout were 18% (95%CI: 7.0%-29%) and 35% (95%CI: 20%-50%), respectively. The most frequent reason for discontinuing the combination treatment was graft dysfunction (71.7%). There is no evidence that the IFN-ribavirin combination is superior to IFN monotherapy[52]. Overall, Fabrizi et al[52] agree that treating these patients with anti-viral therapy before KT is safer and more effective than administering this treatment post-transplantation.
The risk for acute rejection in HCV-infected patients is higher during the first year after KT. In cases where anti-viral therapy is necessary after KT, some authors recommend waiting at least 1 year after the surgery to initiate this treatment[51]. In contrast, other research has shown that anti-viral treatment may be more effective during the first year after transplantation if the patient has stable renal function and no acute rejection occurs[53].
Renal transplant recipients with HCV who have stable renal and liver function should be carefully monitored, as should those receiving anti-viral therapy. In all cases liver function tests should be done every 3 mo and viral load should be measured every 6 mo. Liver biopsy should be repeated every 3 years[54].
After KT: Immunosuppression in patients with HCV-related liver disease
In patients with HCV who undergo KT, immunosuppressive therapy is required to prevent rejection of the renal graft, but this may cause a more rapid and aggressive course of HCV infection[55]. In addition to the severity and duration of HCV infection, at the time of KT, the choice of immunosuppressive drug combination and doses can significantly affect the course of HCV infection after KT. There is still controversy regarding what constitutes the most appropriate immunosuppressive combination.
Induction immunosuppressive therapies for kidney transplant recipients typically contain T-cell-depleting antibodies (OKT3, anti-thymocyte globulin). Induction therapies with non-depleting antibodies (i.e., antibodies that block interleukin-2) have also been debated. There are conflicting reported outcomes regarding the hazardous or beneficial effects of these induction immunosuppressive drugs on the course of HCV infection and on survival of the renal graft. Earlier studies suggested that OKT3 was not a good choice for induction[56], whereas a recent report by Roth et al[21] indicated that patients who received OKT3 had better liver fibrosis scores than those who received daclizumab (an agent that binds the interleukin-2 receptors of T-cells). At minimum, HCV-infected patients who have undergone KT should receive a short course of induction therapy.
Glucocorticosteroids are given in wide range of doses to prevent rejection after KT. This treatment is associated with increased HCV replication[55]; however, Luan et al[57] demonstrated no significant difference in mortality between patients who received steroids and those who did not. Akalin et al[58] pointed out that rapid discontinuation of steroid treatment was not associated with worse outcome in HCV-positive renal transplant recipients.
Cyclosporin might inhibit HCV replication through mediating a blockage of interaction between cyclophilins and non-structural protein 5B (HCV-RNA polymerase)[59,60]. Kahraman et al[61] investigated kidney recipients with HCV, and observed no significant differences between a group that received tacrolimus and a group that received cyclosporin with respect to viral replication and development of hepatic fibrosis.
Mycophenolate mofetil is known to have anti-viral effects in HCV patients, and acts by inducing the expression of anti-viral IFN-related genes[59,62]. In, there is no evidence of a specific destructive effect on either the renal graft or the HCV infection[55].
There are scarce data on the influence of sirolimus and everolimus [both inhibitors of the mammalian target of rapamycin (mTOR)] in renal transplant recipients with HCV. Luan et al[57] found that mTOR inhibitors were associated with 13% increased risk of mortality in this patient group. These drugs are not yet recommended as standard regimen for renal transplant recipients with HCV[55].
After KT: Infections
Several studies have confirmed that HCV-positive kidney recipients are at increased risk for infections of the central nervous system, respiratory system, urinary tract and bloodstream[41,63]. Research has identified a significant relationship between development of tuberculosis and presence of HCV infection in renal transplant recipients[64], but it is not clear why HCV-positive recipients are more susceptible to this infection. Immunosuppression and diabetes are two possible explanations[63].
After KT: HCV-related glomerular disease
HCV infection has been directly linked to glomerular disease in both native and transplanted kidneys[65]. The most common renal diseases associated with HCV infection are membranoproliferative glomerulonephritis with or without cryoglobulinemia and membranous glomerulonephritis[66]. Meyers et al[65] hypothesized that these relationships are explained by increased HCV viral load and decreased immunoglobulin synthesis in the setting of immunosuppression, and an imbalance of antigen and antibody complex status and deposition of these complexes in the allograft. Kamar et al[67] proposed that these diseases were explained by higher cytokine production rather than direct cytotoxic effects of HCV on kidney cells.
After KT: HCV-related new-onset diabetes mellitus
HCV-related new-onset diabetes after KT in patients with chronic HCV is an interesting and relatively frequent complication[35]. Reported prevalences of this in HCV-positive and HCV-negative kidney transplant recipients are 39.4% and 9.8% respectively[68]. Unfortunately, it has been shown that new onset of diabetes after KT impairs graft function[69]. The mechanisms proposed for HCV-related diabetes mellitus include increased insulin resistance, direct cytopathic action of HCV on beta cells, and side effects of immunosuppressive drugs[70,71].
After KT: HCV-related extrahepatic neoplasia
The role of HCV in the pathogenesis of post-transplantation hematologic malignancies is obvious[72]. Post-transplant lymphoproliferative disorder was found to be significantly more frequent in HCV-positive kidney recipients than in HCV-negative kidney recipients[73].
TREATMENT OF HCV INFECTION IN PATIENTS WITH ESRD
Carefully treating HCV and achieving SVR prior to KT should be primary goals to reduce the likelihood of HCV-related complications in the liver and other organs/systems[74]. Another reason it is important to attain SVR before KT relates to the concern that anti-viral therapy administered post-transplantation is associated with high risk of graft rejection[75].
The document entitled Improving Global Outcomes recommended that HCV-infected HD patients awaiting KT should be treated for HCV, and that attending clinicians should decide whether to treat other HCV-infected patients (i.e., those not on the KT waiting list) on case-by-case basis. However, HD patients with HCV infection rarely receive antiviral therapy[76].
Monotherapy with standard interferon or pegylated interferon
Several forms of IFN are available for therapeutic use, including α-2a, α-2b, α-n1. In ESRD patients with HCV, the recommended administration of IFN ranges from 1-6 mU as a/daily dose or up to three times weekly. A long-acting IFN α, namely pegylated IFN (pegIFN), has been used safely and effectively for more than a decade. Peg IFN α-2a administered at 135 μg weekly and peg IFN α-2b administered at 0.5-1 μg/kg are currently approved for HCV treatment and are administered weekly in stage 3-5 ESRD. Treatment duration is 24 wk for HCV genotypes 2 or -3 and 48 wk for HCV genotypes 1-4[77,78].
Three recently published meta-analyses have indicated that SVR, side effects and withdrawal rates in patients with ESRD vary according to treatment with IFN and pegIFN.
The meta-analysis by Fabrizi et al[79] evaluated results from 645 patients the overall SVR rate was 40%; in the subset with HCV genotype 1, the SVR rate was 33% and dropout rates were 19% in the group that received IFN and 27% in the group that received pegIFN. A typical flu-like syndrome was the most common side effect. This occurred in 41% of patients and required withdrawal of anti-viral treatment in 11%. However, the meta-analysis by Fabrizi et al[79] was criticized because the studies examined were somewhat heterogeneous with regard to viral response and dropout rates.
A meta-analysis by Gordon et al[80] in 2008, involved 546 chronic HD patients with HCV infection who were either treated with IFN or pegIFN, with or without ribavirin. Only 49 individuals received pegIFN and ribavirin. The overall SVR rates were 41% for the IFN group (95%CI: 33%-49%) and 37% for the pegIFN group (95%CI: 9%-77%). The frequencies of treatment discontinuation were 26% for the IFN group (95%CI: 20%-34%) and 28% for the pegIFN group (95%CI: 12%-53%). The main side effects were fatigue/weakness and loss of appetite. The authors also found that higher dose of IFN, lower HCV RNA load prior to treatment, early stage of hepatic fibrosis, and HCV genotype other than genotype 1 were associated with higher SVR rates[80].
A more recent meta-analysis evaluated data from 770 HD patients with chronic HCV infection, 491 of whom received IFN-alfa2a or IFN-alfa2b and 279 of whom received pegIFN-alfa2a or PegIFN-alfa2b. The corresponding SVR rates for these two groups were 39.1% (95%CI: 32.1%-46.1%) and 39.3% (95%CI: 26.5%-52.1%), and the corresponding dropout rates were 22.6% (95%CI: 10.4%-34.8%) and 29.7% (95%CI: 21.7%-37.7%). Age younger than 40 years was significantly associated with SVR (OR = 2.17; 95%CI: 1.03-4.50)[81].
Although the above three meta-analyses suggest that conventional IFN treatment and pegIFN therapy have similar efficacy and safety, many studies have shown that pegIFN is superior[27]. For example, one study revealed that patients with renal dysfunction who were treated with pegIFN had a higher HCV eradication rate than HCV patients with normal kidney function who received this treatment. This can be attributed to decreased renal clearance of pegIFN in the setting of ESRD. In practice once weekly dosing of pegIFN is more convenient for the patients with renal dysfunction[82].
Combination therapies: Interferon or pegIFN with ribavirin
The combination of pegIFN and ribavirin is considered the gold standard therapy for patients with chronic HCV who have normal renal function[77]. Some physicians are reluctant to use ribavirin in patients with ESRD or in those who are on HD due to fear of hemolytic anemia which can be exacerbated in the presence of kidney dysfunction. Because ribavirin is filtered by the kidneys, its clearance is impaired in patients with ESRD, and this agent is not removed by dialysis. Despite the fact that ribavirin is contraindicated in the setting of renal failure, this drug can be used at markedly reduced daily doses and with careful monitoring for anemia. Patients can be started on a low dose of ribavirin, and doses can be increased gradually as long as side effects are manageable[82-84].
Only a few reports support the combined use of pegIFN and ribavirin in ESRD patients or patients on HD (Table 1). Rendina et al[88] published the largest series to date on the combined use of pegIFN α-2a (135 μg/wk) plus ribavirin (200 mg daily to every other day) for 48 wk in 35 HD patients. They observed an SVR rate of 97% (34 of the/35 patients) and a dropout rate of 14%. Only one patient developed severe anemia and had to be weaned off treatment.
Table 1.
Results from trials: Interferon-ribavirin combined treatments in patients with end-stage renal disease and hepatitis C virus ınfection
| Ref. | Year | No. of patients | Proportion with HCV genotype 1 (%) | Treatment | SVR (%) | Dropout (%) |
| Tan et al[85] | 2001 | 5 | NA | IFN-3.0 MU/d to 3 times/wk + RBV 200 mg/d, 3 times/wk for 40 wk | NA | 40 |
| Mousa et al[86] | 2004 | 20 | 60-66 | IFN-3.0 MU/d to 3 times/wk + RBV 200 mg/d, 3 times/wk | ||
| For 24 wk | 66 | 0 | ||||
| For 48 wk | 55 | 0 | ||||
| Bruchfeld et al[87] | 2006 | 6 | 66 | PegIFN - 50 μg/wk + RBV 200-400 mg/d for 24-48 wk (depending on genotype) | 50 | 50 |
| Rendina et al[88] | 2007 | 35 | 45.7 | PegIFN-α2a 135 μg/wk + RBV 200 mg/d for 24-48 wk (depending on genotype) | 97 | 15 |
IFN: İnterferon; NA: Not available; PegIFN: Pegylated IFN; RBV: Ribavirin; SVR: Sustained virological response; HCV: Hepatitis C virus.
The dose of ribavirin should be adjusted based on target plasma level which has been identified as 10-15 mcmol/L in patients with normal kidney function. For patients with ESRD, the average dose of ribavirin can be 200 mg daily, but some individuals can only tolerate 200 mg every other day[46]. Assays for monitoring plasma ribavirin levels are not routinely available. Even when therapeutic ribavirin levels are maintained the potential for anemia in HD patients cannot be ruled out. Recombinant human erythropoietin or blood transfusions can be while maintaining the desired ribavirin dosage, for these patients, as these measures can correct anemia and improve quality of life during treatment[83].
Direct-acting anti-viral agents
Direct-acting anti-viral agents (DAA) have yielded exciting results in ESRD patients. When combined with IFN and ribavirin, DAA increase SVR rates in patients with intact kidney function. However, anemia is an important potential side effect of DAA even in patients with normal glomerular filtration rate. Further information and experience are needed with respect to using DAA-based therapy in patients with ESRD[89]. More aggressive therapy maybe considered for HD patients who are eligible for KT, as HCV eradication prior to transplantation can improve outcomes[90].
Two new HCV drugs were approved in 2011: telaprevir and boceprevir. Both these are first-wave, first-generation NS3-4A protease inhibitors. Two other drug were approved in 2013/2014: simeprevir, a second-wave, first-generation NS3-4A protease inhibitor, and sofosbuvir, a nucleotide analogue inhibitor of viral polymerase[91].
Triple therapy for HCV infection currently may have several opportunities including a better therapeutic schedule in patients with renal failure, which apparently do not require dose adjustments to the kidney function, yet the detailed data regarding the new drugs in these patients are not available[92].
Sofosbuvir and simeprevir are not recommended for patients with ESRD or patients who require HD. While no dose reduction is necessary when sofosbuvir or simeprevir are administered to patients mild to moderate HCV infection, a lower dose is needed when this drug is administered to patients with severe HCV infection. Renal insufficiency has no impact on the pharmacokinetic profile of asunaprevir[93].
The development of IFN-free or IFN-sparing regimens represents a breakthrough in the history of anti-HCV treatment. It ıs expected that treatment scenarios for chronic HCV patients will change radically in the next few years, as safe and potent therapies become more accessible. This will simplify the mangement of these cases, and will open possibilities to include patient populations for which pegIFN is currently contraindicated[94].
In conclusion, HCV infection remains a major health problem that can cause substantial liver-related morbidity and mortality in patients with ESRD. Various forms of IFN with or without ribavirin can be used to treat ESRD patients with HCV infection prior to KT; however, only approximately one-third of these patients will achieve SVR. After an HCV-infected patient has undergone KT, IFN-based treatments are generally not recommended owing to the high risk of graft rejection. Recently introduced IFN- free treatment options are promising, but data are lacking regarding their use in HD patients with HCV infection. Well-designed prospective studies are needed to evaluate the efficacy and safety of the new IFN- free regimes in this patient group.
Footnotes
P- Reviewer: Jin B, Komatsu H, Trigka K S- Editor: Tian YL L- Editor: A E- Editor: Liu SQ
Conflict-of-interest: The authors declare that they have no competing interests.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: August 15, 2014
First decision: November 27, 2014
Article in press: February 9, 2015
References
- 1.Selcuk H, Kanbay M, Korkmaz M, Gur G, Akcay A, Arslan H, Ozdemir N, Yilmaz U, Boyacioglu S. Distribution of HCV genotypes in patients with end-stage renal disease according to type of dialysis treatment. Dig Dis Sci. 2006;51:1420–1425. doi: 10.1007/s10620-005-9025-9. [DOI] [PubMed] [Google Scholar]
- 2.Mangia A, Burra P, Ciancio A, Fagiuoli S, Guido M, Picciotto A, Fabrizi F. Hepatitis C infection in patients with chronic kidney disease. Int J Artif Organs. 2008;31:15–33. doi: 10.1177/039139880803100104. [DOI] [PubMed] [Google Scholar]
- 3.Perico N, Cattaneo D, Bikbov B, Remuzzi G. Hepatitis C infection and chronic renal diseases. Clin J Am Soc Nephrol. 2009;4:207–220. doi: 10.2215/CJN.03710708. [DOI] [PubMed] [Google Scholar]
- 4.Aoufi Rabih S, García Agudo R. Management of HCV infection in chronic kidney disease. Nefrologia. 2011;31:260–267. doi: 10.3265/Nefrologia.pre2011.Jan.10768. [DOI] [PubMed] [Google Scholar]
- 5.Goodkin DA, Bragg-Gresham JL, Koenig KG, Wolfe RA, Akiba T, Andreucci VE, Saito A, Rayner HC, Kurokawa K, Port FK, et al. Association of comorbid conditions and mortality in hemodialysis patients in Europe, Japan, and the United States: the Dialysis Outcomes and Practice Patterns Study (DOPPS) J Am Soc Nephrol. 2003;14:3270–3277. doi: 10.1097/01.asn.0000100127.54107.57. [DOI] [PubMed] [Google Scholar]
- 6.Fissell RB, Bragg-Gresham JL, Woods JD, Jadoul M, Gillespie B, Hedderwick SA, Rayner HC, Greenwood RN, Akiba T, Young EW. Patterns of hepatitis C prevalence and seroconversion in hemodialysis units from three continents: the DOPPS. Kidney Int. 2004;65:2335–2342. doi: 10.1111/j.1523-1755.2004.00649.x. [DOI] [PubMed] [Google Scholar]
- 7.Di Napoli A, Pezzotti P, Di Lallo D, Petrosillo N, Trivelloni C, Di Giulio S. Epidemiology of hepatitis C virus among long-term dialysis patients: a 9-year study in an Italian region. Am J Kidney Dis. 2006;48:629–637. doi: 10.1053/j.ajkd.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 8.Sun J, Yu R, Zhu B, Wu J, Larsen S, Zhao W. Hepatitis C infection and related factors in hemodialysis patients in china: systematic review and meta-analysis. Ren Fail. 2009;31:610–620. doi: 10.1080/08860220903003446. [DOI] [PubMed] [Google Scholar]
- 9.Alavian SM, Kabir A, Ahmadi AB, Lankarani KB, Shahbabaie MA, Ahmadzad-Asl M. Hepatitis C infection in hemodialysis patients in Iran: a systematic review. Hemodial Int. 2010;14:253–262. doi: 10.1111/j.1542-4758.2010.00437.x. [DOI] [PubMed] [Google Scholar]
- 10.Finelli L, Miller JT, Tokars JI, Alter MJ, Arduino MJ. National surveillance of dialysis-associated diseases in the United States, 2002. Semin Dial. 2002;18:52–61. doi: 10.1111/j.1525-139X.2005.18108.x. [DOI] [PubMed] [Google Scholar]
- 11.Patel PR, Thompson ND, Kallen AJ, Arduino MJ. Epidemiology, surveillance, and prevention of hepatitis C virus infections in hemodialysis patients. Am J Kidney Dis. 2010;56:371–378. doi: 10.1053/j.ajkd.2010.01.025. [DOI] [PubMed] [Google Scholar]
- 12.Zampieron A, Jayasekera H, Elseviers M, Lindley E, DeVos JY, Visser R, Harrington M. European study on epidemiology and management of hepatitis C virus (HCV) infection in the haemodialysis population. Part 3: prevalence and incidence. EDTNA ERCA J. 2006;32:42–44. doi: 10.1111/j.1755-6686.2006.tb00445.x. [DOI] [PubMed] [Google Scholar]
- 13.Süleymanlar G, Altıparmak MR, Seyahi N, Trabulus S. National hemodialysis, transplantation and nephrology registry report of Turkey. İstanbul: Turkish Society of Nephrology; 2012. [Google Scholar]
- 14.Gordon CE, Balk EM, Becker BN, Crooks PA, Jaber BL, Johnson CA, Michael MA, Pereira BJ, Uhlig K, Levin A. KDOQI US commentary on the KDIGO clinical practice guideline for the prevention, diagnosis, evaluation, and treatment of hepatitis C in CKD. Am J Kidney Dis. 2008;52:811–825. doi: 10.1053/j.ajkd.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 15.Espinosa M, Martn-Malo A, Ojeda R, Santamara R, Soriano S, Aguera M, Aljama P. Marked reduction in the prevalence of hepatitis C virus infection in hemodialysis patients: causes and consequences. Am J Kidney Dis. 2004;43:685–689. doi: 10.1053/j.ajkd.2003.12.030. [DOI] [PubMed] [Google Scholar]
- 16.Kalantar-Zadeh K, Kilpatrick RD, McAllister CJ, Miller LG, Daar ES, Gjertson DW, Kopple JD, Greenland S. Hepatitis C virus and death risk in hemodialysis patients. J Am Soc Nephrol. 2007;18:1584–1593. doi: 10.1681/ASN.2006070736. [DOI] [PubMed] [Google Scholar]
- 17.Lee JJ, Lin MY, Chang JS, Hung CC, Chang JM, Chen HC, Yu ML, Hwang SJ. Hepatitis C virus infection increases risk of developing end-stage renal disease using competing risk analysis. PLoS One. 2014;9:e100790. doi: 10.1371/journal.pone.0100790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fabrizi F, Martin P, Dixit V, Bunnapradist S, Dulai G. Meta-analysis: Effect of hepatitis C virus infection on mortality in dialysis. Aliment Pharmacol Ther. 2004;20:1271–1277. doi: 10.1111/j.1365-2036.2004.02290.x. [DOI] [PubMed] [Google Scholar]
- 19.Sezer S, Ozdemir FN, Akcay A, Arat Z, Boyacioglu S, Haberal M. Renal transplantation offers a better survival in HCV-infected ESRD patients. Clin Transplant. 2004;18:619–623. doi: 10.1111/j.1399-0012.2004.00252.x. [DOI] [PubMed] [Google Scholar]
- 20.Ruhı Ç, Süleymanlar İ, Koçak H, Yilmaz VT, Çolak D, Dınçkan A, Gürkan A, Ersoy F, Yakupoğlu G, Süleymanlar G. The impact of hepatitis C virus infection on long-term outcome in renal transplant patients. Turk J Gastroenterol. 2011;22:165–170. doi: 10.4318/tjg.2011.0236. [DOI] [PubMed] [Google Scholar]
- 21.Roth D, Gaynor JJ, Reddy KR, Ciancio G, Sageshima J, Kupin W, Guerra G, Chen L, Burke GW. Effect of kidney transplantation on outcomes among patients with hepatitis C. J Am Soc Nephrol. 2011;22:1152–1160. doi: 10.1681/ASN.2010060668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ingsathit A, Kamanamool N, Thakkinstian A, Sumethkul V. Survival advantage of kidney transplantation over dialysis in patients with hepatitis C: a systematic review and meta-analysis. Transplantation. 2013;95:943–948. doi: 10.1097/TP.0b013e3182848de2. [DOI] [PubMed] [Google Scholar]
- 23.Recommendations for preventing transmission of infections among chronic hemodialysis patients. MMWR Recomm Rep. 2001;50:1–43. [PubMed] [Google Scholar]
- 24.Bianco A, Bova F, Nobile CG, Pileggi C, Pavia M. Healthcare workers and prevention of hepatitis C virus transmission: exploring knowledge, attitudes and evidence-based practices in hemodialysis units in Italy. BMC Infect Dis. 2013;13:76. doi: 10.1186/1471-2334-13-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang S, Lai KN. Chronic viral hepatitis in hemodialysis patients. Hemodial Int. 2005;9:169–179. doi: 10.1111/j.1492-7535.2005.01129.x. [DOI] [PubMed] [Google Scholar]
- 26.Lopes EP, Gouveia EC, Albuquerque AC, Sette LH, Mello LA, Moreira RC, Coelho MR. Determination of the cut-off value of serum alanine aminotransferase in patients undergoing hemodialysis, to identify biochemical activity in patients with hepatitis C viremia. J Clin Virol. 2006;35:298–302. doi: 10.1016/j.jcv.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 27.Liu CH, Kao JH. Treatment of hepatitis C virus infection in patients with end-stage renal disease. J Gastroenterol Hepatol. 2011;26:228–239. doi: 10.1111/j.1440-1746.2010.06488.x. [DOI] [PubMed] [Google Scholar]
- 28.European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol. 2011;55:245–264. doi: 10.1016/j.jhep.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 29.Kaiser T, Damerow HC, Tenckhoff S, Finger A, Böttcher I, Hafer C, Schwarz A, Lüth JB, Schmidt Gürtler H, Colucci G, et al. Kinetics of hepatitis C viral RNA and HCV-antigen during dialysis sessions: evidence for differential viral load reduction on dialysis. J Med Virol. 2008;80:1195–1201. doi: 10.1002/jmv.21190. [DOI] [PubMed] [Google Scholar]
- 30.Perez RM, Ferraz ML, Figueiredo MS, Contado D, Koide S, Ferreira AP, Cendoroglo Neto M, Medina Pestana JO, Silva AE. Unexpected distribution of hepatitis C virus genotypes in patients on hemodialysis and kidney transplant recipients. J Med Virol. 2003;69:489–494. doi: 10.1002/jmv.10336. [DOI] [PubMed] [Google Scholar]
- 31.Martin P, Carter D, Fabrizi F, Dixit V, Conrad AJ, Artinian L, Peacock V, Han S, Wilkinson A, Lassman CR, et al. Histopathological features of hepatitis C in renal transplant candidates [see comment] Transplantation. 2000;69:1479–1484. doi: 10.1097/00007890-200004150-00045. [DOI] [PubMed] [Google Scholar]
- 32.Boyacioğlu S, Gür G, Yilmaz U, Korkmaz M, Demirhan B, Bilezikçi B, Ozdemir N. Investigation of possible clinical and laboratory predictors of liver fibrosis in hemodialysis patients infected with hepatitis C virus. Transplant Proc. 2004;36:50–52. doi: 10.1016/j.transproceed.2003.11.066. [DOI] [PubMed] [Google Scholar]
- 33.Canbakan M, Senturk H, Canbakan B, Toptas T, Tabak O, Ozaras R, Tabak F, Balcı H, Sut N, Ozbay G. Validation of biochemical markers for the prediction of liver fibrosis and necroinflammatory activity in hemodialysis patients with chronic hepatitis C. Nephron Clin Pract. 2011;117:c289–c295. doi: 10.1159/000320751. [DOI] [PubMed] [Google Scholar]
- 34.Gürsoy M, Bilezikci B, Colak T, Köksal R, Demirhan B, Karavelioğlu D, Boyacioğlu S, Bilgin N, Arslan G. Histologic outcome of hepatitis C virus infection in renal transplant recipients and the effect of pretransplantation interferon treatment. Transplant Proc. 2000;32:558–560. doi: 10.1016/s0041-1345(00)00889-7. [DOI] [PubMed] [Google Scholar]
- 35.Tang IY, Walzer N, Aggarwal N, Tzvetanov I, Cotler S, Benedetti E. Management of the kidney transplant patient with chronic hepatitis C infection. Int J Nephrol. 2011;2011:245823. doi: 10.4061/2011/245823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ahmad A, Hasan F, Abdeen S, Sheikh M, Kodaj J, Nampoory MR, Johny KV, Asker H, Siddique I, Thalib L, et al. Transjugular liver biopsy in patients with end-stage renal disease. J Vasc Interv Radiol. 2004;15:257–260. doi: 10.1097/01.rvi.0000109403.52762.c4. [DOI] [PubMed] [Google Scholar]
- 37.Liu CH, Liang CC, Huang KW, Liu CJ, Chen SI, Lin JW, Hung PH, Tsai HB, Lai MY, Chen PJ, et al. Transient elastography to assess hepatic fibrosis in hemodialysis chronic hepatitis C patients. Clin J Am Soc Nephrol. 2011;6:1057–1065. doi: 10.2215/CJN.04320510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu CH, Liang CC, Liu CJ, Hsu SJ, Lin JW, Chen SI, Hung PH, Tsai HB, Lai MY, Chen PJ, et al. The ratio of aminotransferase to platelets is a useful index for predicting hepatic fibrosis in hemodialysis patients with chronic hepatitis C. Kidney Int. 2010;78:103–109. doi: 10.1038/ki.2010.74. [DOI] [PubMed] [Google Scholar]
- 39.Kellner P, Anadol E, Hüneburg R, Hundt F, Bös D, Klein B, Woitas RP, Spengler U, Sauerbruch T, Trebicka J. The effect of hemodialysis on liver stiffness measurement: a single-center series. Eur J Gastroenterol Hepatol. 2013;25:368–372. doi: 10.1097/MEG.0b013e32835ad180. [DOI] [PubMed] [Google Scholar]
- 40.Botero RC. Should patients with chronic hepatitis C infection be transplanted? Transplant Proc. 2004;36:1449–1454. doi: 10.1016/j.transproceed.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 41.Morales JM, Bloom R, Roth D. Kidney transplantation in the patient with hepatitis C virus infection. Contrib Nephrol. 2012;176:77–86. doi: 10.1159/000332385. [DOI] [PubMed] [Google Scholar]
- 42.Carbone M, Cockwell P, Neuberger J. Hepatitis C and kidney transplantation. Int J Nephrol. 2011;2011:593291. doi: 10.4061/2011/593291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eason JD, Gonwa TA, Davis CL, Sung RS, Gerber D, Bloom RD. Proceedings of Consensus Conference on Simultaneous Liver Kidney Transplantation (SLK) Am J Transplant. 2008;8:2243–2251. doi: 10.1111/j.1600-6143.2008.02416.x. [DOI] [PubMed] [Google Scholar]
- 44.Roth D, Bloom R. Selection and management of hepatitis C virus-infected patients for the kidney transplant waiting list. Contrib Nephrol. 2012;176:66–76. doi: 10.1159/000333774. [DOI] [PubMed] [Google Scholar]
- 45.Kim E, Ko HH, Yoshida EM. Treatment issues surrounding hepatitis C in renal transplantation: a review. Ann Hepatol. 2011;10:5–14. [PubMed] [Google Scholar]
- 46.Terrault NA, Adey DB. The kidney transplant recipient with hepatitis C infection: pre- and posttransplantation treatment. Clin J Am Soc Nephrol. 2007;2:563–575. doi: 10.2215/CJN.02930806. [DOI] [PubMed] [Google Scholar]
- 47.Kamar N, Rostaing L, Selves J, Sandres-Saune K, Alric L, Durand D, Izopet J. Natural history of hepatitis C virus-related liver fibrosis after renal transplantation. Am J Transplant. 2005;5:1704–1712. doi: 10.1111/j.1600-6143.2005.00918.x. [DOI] [PubMed] [Google Scholar]
- 48.Morales JM, Aguado JM. Hepatitis C and renal transplantation. Curr Opin Organ Transplant. 2012;17:609–615. doi: 10.1097/MOT.0b013e32835a2bac. [DOI] [PubMed] [Google Scholar]
- 49.Pereira BJ, Levey AS. Hepatitis C virus infection in dialysis and renal transplantation. Kidney Int. 1997;51:981–999. doi: 10.1038/ki.1997.139. [DOI] [PubMed] [Google Scholar]
- 50.Izopet J, Rostaing L, Sandres K, Cisterne JM, Pasquier C, Rumeau JL, Duffaut M, Durand D, Puel J. Longitudinal analysis of hepatitis C virus replication and liver fibrosis progression in renal transplant recipients. J Infect Dis. 2000;181:852–858. doi: 10.1086/315355. [DOI] [PubMed] [Google Scholar]
- 51.Du LY, Tang H. Treatment of HCV patients before and after renal transplantation. Hepat Mon. 2011;11:880–886. doi: 10.5812/kowsar.1735143X.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fabrizi F, Lunghi G, Dixit V, Martin P. Meta-analysis: anti-viral therapy of hepatitis C virus-related liver disease in renal transplant patients. Aliment Pharmacol Ther. 2006;24:1413–1422. doi: 10.1111/j.1365-2036.2006.03151.x. [DOI] [PubMed] [Google Scholar]
- 53.Kamar N, Sandres-Saune K, Selves J, Ribes D, Cointault O, Durand D, Izopet J, Rostaing L. Long-term ribavirin therapy in hepatitis C virus-positive renal transplant patients: effects on renal function and liver histology. Am J Kidney Dis. 2003;42:184–192. doi: 10.1016/s0272-6386(03)00422-0. [DOI] [PubMed] [Google Scholar]
- 54.Sharma RK, Bansal SB, Gupta A, Gulati S, Kumar A, Prasad N. Chronic hepatitis C virus infection in renal transplant: treatment and outcome. Clin Transplant. 2006;20:677–683. doi: 10.1111/j.1399-0012.2006.00534.x. [DOI] [PubMed] [Google Scholar]
- 55.Manuel O, Baid-Agrawal S, Moradpour D, Pascual M. Immunosuppression in hepatitis C virus-infected patients after kidney transplantation. Contrib Nephrol. 2012;176:97–107. doi: 10.1159/000332387. [DOI] [PubMed] [Google Scholar]
- 56.Rosen HR, Shackleton CR, Higa L, Gralnek IM, Farmer DA, McDiarmid SV, Holt C, Lewin KJ, Busuttil RW, Martin P. Use of OKT3 is associated with early and severe recurrence of hepatitis C after liver transplantation. Am J Gastroenterol. 1997;92:1453–1457. [PubMed] [Google Scholar]
- 57.Luan FL, Schaubel DE, Zhang H, Jia X, Pelletier SJ, Port FK, Magee JC, Sung RS. Impact of immunosuppressive regimen on survival of kidney transplant recipients with hepatitis C. Transplantation. 2008;85:1601–1606. doi: 10.1097/TP.0b013e3181722f3a. [DOI] [PubMed] [Google Scholar]
- 58.Akalin E, Murphy B, Sehgal V, Ames S, Daly L, Bromberg JS. Rapid steroid withdrawal in hepatitis C virus-positive kidney transplant recipients. Clin Transplant. 2004;18:384–389. doi: 10.1111/j.1399-0012.2004.00177.x. [DOI] [PubMed] [Google Scholar]
- 59.Hsu SH, Yeh ML, Wang SN. New insights in recurrent HCV infection after liver transplantation. Clin Dev Immunol. 2013;2013:890517. doi: 10.1155/2013/890517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nakagawa M, Sakamoto N, Tanabe Y, Koyama T, Itsui Y, Takeda Y, Chen CH, Kakinuma S, Oooka S, Maekawa S, et al. Suppression of hepatitis C virus replication by cyclosporin a is mediated by blockade of cyclophilins. Gastroenterology. 2005;129:1031–1041. doi: 10.1053/j.gastro.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 61.Kahraman A, Witzke O, Scherag A, Pütter C, Miller M, Dechêne A, Ross SR, Gerken G, Hilgard P. Impact of immunosuppressive therapy on hepatitis C infection after renal transplantation. Clin Nephrol. 2011;75:16–25. [PubMed] [Google Scholar]
- 62.Pan Q, de Ruiter PE, Metselaar HJ, Kwekkeboom J, de Jonge J, Tilanus HW, Janssen HL, van der Laan LJ. Mycophenolic acid augments interferon-stimulated gene expression and inhibits hepatitis C Virus infection in vitro and in vivo. Hepatology. 2012;55:1673–1683. doi: 10.1002/hep.25562. [DOI] [PubMed] [Google Scholar]
- 63.López-Medrano F, Fernández-Ruiz M, Morales JM, San-Juan R, Cervera C, Carratalá J, Torre-Cisneros J, Gavaldá J, Muñoz P, Len O, et al. Impact of hepatitis C virus infection on the risk of infectious complications after kidney transplantation: data from the RESITRA/REIPI cohort. Transplantation. 2011;92:543–549. doi: 10.1097/TP.0b013e318225dbae. [DOI] [PubMed] [Google Scholar]
- 64.Torres J, Aguado JM, San Juan R, Andrés A, Sierra P, López-Medrano F, Morales JM. Hepatitis C virus, an important risk factor for tuberculosis in immunocompromised: experience with kidney transplantation. Transpl Int. 2008;21:873–878. doi: 10.1111/j.1432-2277.2008.00694.x. [DOI] [PubMed] [Google Scholar]
- 65.Meyers CM, Seeff LB, Stehman-Breen CO, Hoofnagle JH. Hepatitis C and renal disease: an update. Am J Kidney Dis. 2003;42:631–657. doi: 10.1016/s0272-6386(03)00828-x. [DOI] [PubMed] [Google Scholar]
- 66.Sandri AM, Elewa U, Poterucha JJ, Fervenza FC. Treatment of hepatitis C-mediated glomerular disease. Nephron Clin Pract. 2011;119:c121–129; discussion c129-130. doi: 10.1159/000325220. [DOI] [PubMed] [Google Scholar]
- 67.Kamar N, Rostaing L, Boulestin A, Sandres K, Dubois M, Ribes D, Modesto A, Durand D, Izopet J. Evolution of hepatitis C virus quasispecies in renal transplant patients with de novo glomerulonephritis. J Med Virol. 2003;69:482–488. doi: 10.1002/jmv.10335. [DOI] [PubMed] [Google Scholar]
- 68.Fabrizi F, Martin P, Dixit V, Bunnapradist S, Kanwal F, Dulai G. Post-transplant diabetes mellitus and HCV seropositive status after renal transplantation: meta-analysis of clinical studies. Am J Transplant. 2005;5:2433–2440. doi: 10.1111/j.1600-6143.2005.01040.x. [DOI] [PubMed] [Google Scholar]
- 69.Bloom RD, Lake JR. Emerging issues in hepatitis C virus-positive liver and kidney transplant recipients. Am J Transplant. 2006;6:2232–2237. doi: 10.1111/j.1600-6143.2006.01457.x. [DOI] [PubMed] [Google Scholar]
- 70.Bloom RD, Rao V, Weng F, Grossman RA, Cohen D, Mange KC. Association of hepatitis C with posttransplant diabetes in renal transplant patients on tacrolimus. J Am Soc Nephrol. 2002;13:1374–1380. doi: 10.1097/01.asn.0000012382.97168.e0. [DOI] [PubMed] [Google Scholar]
- 71.Kasiske BL, Snyder JJ, Gilbertson D, Matas AJ. Diabetes mellitus after kidney transplantation in the United States. Am J Transplant. 2003;3:178–185. doi: 10.1034/j.1600-6143.2003.00010.x. [DOI] [PubMed] [Google Scholar]
- 72.Zignego AL, Giannini C, Gragnani L. HCV and lymphoproliferation. Clin Dev Immunol. 2012;2012:980942. doi: 10.1155/2012/980942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Burra P, Buda A, Livi U, Rigotti P, Zanus G, Calabrese F, Caforio A, Menin C, Canova D, Farinati F, et al. Occurrence of post-transplant lymphoproliferative disorders among over thousand adult recipients: any role for hepatitis C infection? Eur J Gastroenterol Hepatol. 2006;18:1065–1070. doi: 10.1097/01.meg.0000231752.50587.ae. [DOI] [PubMed] [Google Scholar]
- 74.Esforzado N, Campistol JM. Treatment of hepatitis C in dialysis patients. Contrib Nephrol. 2012;176:54–65. doi: 10.1159/000332383. [DOI] [PubMed] [Google Scholar]
- 75.Rostaing L, Weclawiak H, Izopet J, Kamar N. Treatment of hepatitis C virus infection after kidney transplantation. Contrib Nephrol. 2012;176:87–96. doi: 10.1159/000333775. [DOI] [PubMed] [Google Scholar]
- 76.Goodkin DA, Bieber B, Gillespie B, Robinson BM, Jadoul M. Hepatitis C infection is very rarely treated among hemodialysis patients. Am J Nephrol. 2013;38:405–412. doi: 10.1159/000355615. [DOI] [PubMed] [Google Scholar]
- 77.Weclawiak H, Kamar N, Ould-Mohamed A, Cardeau-Desangles I, Izopet J, Rostaing L. Treatment of chronic hepatitis C virus infection in dialysis patients: an update. Hepat Res Treat. 2010;2010:267412. doi: 10.1155/2010/267412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ghany MG, Strader DB, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335–1374. doi: 10.1002/hep.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fabrizi F, Ganeshan SV, Lunghi G, Messa P, Martin P. Antiviral therapy of hepatitis C in chronic kidney diseases: meta-analysis of controlled clinical trials. J Viral Hepat. 2008;15:600–606. doi: 10.1111/j.1365-2893.2008.00990.x. [DOI] [PubMed] [Google Scholar]
- 80.Gordon CE, Uhlig K, Lau J, Schmid CH, Levey AS, Wong JB. Interferon treatment in hemodialysis patients with chronic hepatitis C virus infection: a systematic review of the literature and meta-analysis of treatment efficacy and harms. Am J Kidney Dis. 2008;51:263–277. doi: 10.1053/j.ajkd.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 81.Alavian SM, Tabatabaei SV. Meta-analysis of factors associated with sustained viral response in patients on hemodialysis treated with standard or pegylated interferon for hepatitis C infection. Iran J Kidney Dis. 2010;4:181–194. [PubMed] [Google Scholar]
- 82.Okoh EJ, Bucci JR, Simon JF, Harrison SA. HCV in patients with end-stage renal disease. Am J Gastroenterol. 2008;103:2123–2134. doi: 10.1111/j.1572-0241.2008.01981.x. [DOI] [PubMed] [Google Scholar]
- 83.Carrion AF, Fabrizi F, Martin P. Should ribavirin be used to treat hepatitis C in dialysis patients? Semin Dial. 2011;24:272–274. doi: 10.1111/j.1525-139X.2011.00851.x. [DOI] [PubMed] [Google Scholar]
- 84.Liu CH, Huang CF, Liu CJ, Dai CY, Liang CC, Huang JF, Hung PH, Tsai HB, Tsai MK, Chen SI, et al. Pegylated interferon-α2a with or without low-dose ribavirin for treatment-naive patients with hepatitis C virus genotype 1 receiving hemodialysis: a randomized trial. Ann Intern Med. 2013;159:729–738. doi: 10.7326/0003-4819-159-11-201312030-00005. [DOI] [PubMed] [Google Scholar]
- 85.Tan AC, Brouwer JT, Glue P, van Leusen R, Kauffmann RH, Schalm SW, de Vries RA, Vroom B. Safety of interferon and ribavirin therapy in haemodialysis patients with chronic hepatitis C: results of a pilot study. Nephrol Dial Transplant. 2001;16:193–195. doi: 10.1093/ndt/16.1.193. [DOI] [PubMed] [Google Scholar]
- 86.Mousa DH, Abdalla AH, Al-Shoail G, Al-Sulaiman MH, Al-Hawas FA, Al-Khader AA. Alpha-interferon with ribavirin in the treatment of hemodialysis patients with hepatitis C. Transplant Proc. 2004;36:1831–1834. doi: 10.1016/j.transproceed.2004.07.025. [DOI] [PubMed] [Google Scholar]
- 87.Bruchfeld A, Lindahl K, Reichard O, Carlsson T, Schvarcz R. Pegylated interferon and ribavirin treatment for hepatitis C in haemodialysis patients. J Viral Hepat. 2006;13:316–321. doi: 10.1111/j.1365-2893.2005.00680.x. [DOI] [PubMed] [Google Scholar]
- 88.Rendina M, Schena A, Castellaneta NM, Losito F, Amoruso AC, Stallone G, Schena FP, Di Leo A, Francavilla A. The treatment of chronic hepatitis C with peginterferon alfa-2a (40 kDa) plus ribavirin in haemodialysed patients awaiting renal transplant. J Hepatol. 2007;46:768–774. doi: 10.1016/j.jhep.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 89.Lee LY, Tong CY, Wong T, Wilkinson M. New therapies for chronic hepatitis C infection: a systematic review of evidence from clinical trials. Int J Clin Pract. 2012;66:342–355. doi: 10.1111/j.1742-1241.2012.02895.x. [DOI] [PubMed] [Google Scholar]
- 90.Kawaoka T, Aikata H, Miyaki D, Murakami E, Azakami T, Takaki S, Nagaoki Y, Hashimoto Y, Katamura Y, Hiramatsu A, et al. Eradication of hepatitis C virus genotype 1 after liver transplantation by interferon therapy before surgery: Report of three patients with analysis of interleukin-28 polymorphism, hepatitis C virus core region and interferon-sensitivity determining region. Hepatol Res. 2011;41:1126–1131. doi: 10.1111/j.1872-034X.2011.00853.x. [DOI] [PubMed] [Google Scholar]
- 91.Pawlotsky JM. New hepatitis C virus (HCV) drugs and the hope for a cure: concepts in anti-HCV drug development. Semin Liver Dis. 2014;34:22–29. doi: 10.1055/s-0034-1371007. [DOI] [PubMed] [Google Scholar]
- 92.Fabrizi F, Penatti A, Messa P, Martin P. Treatment of hepatitis C after kidney transplant: a pooled analysis of observational studies. J Med Virol. 2014;86:933–940. doi: 10.1002/jmv.23919. [DOI] [PubMed] [Google Scholar]
- 93.Zimner-Rapuch S, Janus N, Deray G, Launay-Vacher V. New therapies for hepatitis C: considerations in patients with renal impairment. Drugs. 2014;74:1307–1313. doi: 10.1007/s40265-014-0268-7. [DOI] [PubMed] [Google Scholar]
- 94.Degasperi E, Aghemo A. Sofosbuvir for the treatment of chronic hepatitis C: between current evidence and future perspectives. Hepat Med. 2014;6:25–33. doi: 10.2147/HMER.S44375. [DOI] [PMC free article] [PubMed] [Google Scholar]


