Abstract
Background
Mind–body therapies such as Tai Chi are widely used by breast cancer survivors, yet effects on inflammation are not known. This study hypothesized that Tai Chi Chih (TCC) would reduce systemic, cellular, and genomic markers of inflammation as compared with cognitive behavioral therapy for insomnia (CBT-I).
Methods
In this randomized trial for the treatment of insomnia, 90 breast cancer survivors with insomnia were assigned to TCC or CBT-I for 2-hour sessions weekly for 3 months. At baseline and postintervention, blood samples were obtained for measurement of C-reactive protein and toll-like receptor-4–activated monocyte production of interleukin-6 (IL-6) and tumor necrosis factor-α (TNF), with a random subsample (n = 48) analyzed by genome-wide transcriptional profiling.
Results
Levels of C-reactive protein did not change in the TCC and CBT-I groups. Levels of toll-like receptor-4–activated monocyte production of IL-6 and TNF combined showed an overall reduction in TCC versus CBT-I (P < .02), with similar effects for IL-6 (P = .07) and TNF (P < .05) alone. For genome-wide transcriptional profiling of circulating peripheral blood mononuclear cells, expression of genes encoding proinflammatory mediators showed an overall reduction in TCC versus CBT-I (P = .001). TELiS promoter-based bioinformatics analyses implicated a reduction of activity of the proinflammatory transcription factor, nuclear factor-κB, in structuring these differences.
Conclusions
Among breast cancer survivors with insomnia, 3 months of TCC reduced cellular inflammatory responses, and reduced expression of genes encoding proinflammatory mediators. Given the link between inflammation and cancer, these findings provide an evidence-based molecular framework to understand the potential salutary effects of TCC on cancer survivorship.
The scientific community is increasingly interested in understanding the safety and efficacy of mind–body therapies such as Tai Chi and meditation, given that nearly 19% of American adults (1) and up to 50% of breast cancer survivors report the use of at least one mind–body therapy in the past 12 months (2). The National Center for Complementary and Alternative Medicine currently designates mind–body therapies as a top research priority (3).
Tai Chi is a multidimensional, mind–body therapy that integrates moderate physical activity, deep breathing, and meditation, and has been found to offer a number of benefits in health and psychological functioning (4) including improvements in sleep quality (5). Despite evidence that Tai Chi targets stress response pathways, there is limited evidence regarding the effects of Tai Chi on physiological processes, especially for breast cancer survivors.
Inflammation is implicated in the onset of a number of chronic diseases including cancer (6), is estimated to contribute to 5%–20% of all deaths from cancer worldwide (7), and is associated with recurrence of breast cancer (8,9). In addition, inflammation is associated with insomnia and other behavioral symptoms in breast cancer survivors (10–12), and sleep disruption activates markers of inflammation at the systemic (ie, circulating levels of C-reactive protein [CRP]) (13), cellular (ie, toll-like receptor [TLR]-4 stimulated production of proinflammatory cytokines), and genomic levels (ie, nuclear factor (NF)-κB; inflammatory gene expression) (14,15). TLR-4 activation mediates innate immune responses to common pathogens and is linked to inflammatory diseases. Whereas cancer treatments are potential inducers of inflammation (16), and insomnia is one of the most prevalent behavioral complaints in breast cancer survivors (10,11), no study has examined whether Tai Chi might impact inflammatory biology in breast cancer survivors with insomnia.
Three randomized controlled trials have examined the effects of Tai Chi on systemic markers of inflammation in noncancer samples. Tai Chi reduced circulating levels of CRP in depressed older adults (17) and in patients with type 2 diabetes mellitus (18), and reduced the proinflammatory cytokine, interleukin-6 (IL-6), in healthy older adults (19). Other studies have used genomics-based methods to assess inflammation, and found that cognitive behavioral stress management (CBSM) as well as two types of meditation can reverse the pattern of leukocyte transcriptional alterations typically found in persons experiencing chronic stress or adversity, including activation of genes regulated by the proinflammatory NF-κB/Rel family transcription factors (TFs) (20–22). Only one of these studies focused on cancer survivors (20), and none have comprehensively captured a vertically integrated assessment of inflammation including systemic levels (eg, CRP), upstream cellular expression of proinflammatory cytokines (eg, TLR-4 activation of monocytic production of proinflammatory cytokines), and gene expression with promoter-based bioinformatics analyses of several specific TFs.
This study sought to determine whether Tai Chi might reduce systemic, cellular, and genomic markers of inflammation from baseline to postintervention within the context of a randomized, relative efficacy trial of Tai Chi versus cognitive behavioral therapy for insomnia (CBT-I); sleep outcomes are to be reported separately once the 1-year follow-up is complete. It is thought that Tai Chi targets stress pathways that activate inflammation (6), whereas CBT-I targets sleep behaviors. Hence, Tai Chi is hypothesized to result in greater reduction of markers of inflammation and inflammatory gene expression as compared with CBT-I in the immediate postintervention period in breast cancer survivors with insomnia, who by virtue of comorbid sleep disturbance are at risk for having increases in inflammation.
Materials and methods
Trial Design
Following a 6-week run-in phase in which subjects underwent two separate baseline assessments to evaluate the stability of sleep and behavioral outcomes, eligible participants (n = 90) who completed both baselines were randomly assigned to either Tai Chi Chih (TCC) or CBT-I. CBT-I is recognized as a standard treatment for the behavioral management of insomnia (23). Sleep education was not used as a control intervention because we had already found that it has limited benefit in the treatment of insomnia (5). Hence, withholding the possibility of an effective treatment for the duration of the primary study (ie, 16 months) raised ethical concerns given the level of clinical impairment and severity of insomnia in the breast cancer survivors. Furthermore, before the initiation of this study, surveys found that many breast cancer survivors had received education-focused treatments for insomnia (eg, sleep hygiene), and did not find them to be credible.
Each group of TCC or CBT-I participated in 120 minutes of class time weekly for 3 months. Assessments included two repeated baselines occurring 6 weeks apart (baseline 1; baseline 2) and post-intervention with follow-up at 7 and 16 months (to be reported separately). Because TCC required 3 months for participants to learn, CBT-I was longer than prior trials (23). There were no changes to the methods after trial commencement.
Study Participants
This randomized controlled trial was conducted from April 2007 to August 2013 following UCLA institutional review board approval. Breast cancer survivors with insomnia disorder were recruited by advertisement, specifying a trial to evaluate the relative benefit of CBT-I versus Tai Chi.
Participants fulfilled criteria for primary insomnia in Diagnostic and Statistical Manual (Fourth Edition, Text Revision) (DSM-IV-TR) (24). In addition, participants (age range: 30–85 years of age) had been diagnosed with breast cancer, completed treatment with surgery, radiation, and/or chemotherapy at least 6 months before the study, and showed no evidence of cancer recurrence or new primary tumor. Exclusion criteria are described in the Supplementary Material (available online).
Interventions
CBT-I as previously described by Morin et al. (23) was used with inclusion of a module to help with management of daytime mood impairments related to insomnia. TCC emphasized control over arousal-related responsiveness, which is thought to contribute to insomnia, through the performance of repetitious, nonstrenuous, slow-paced movement. Additional information about the interventions can be found in the Supplementary Material (available online).
Outcomes
Changes in markers of inflammation from baseline to posttreatment were evaluated using three levels of analysis: systemic (ie, CRP levels), cellular (ie, TLR-4 activation of monocytic production of inflammatory cytokines tumor necrosis factor-α [TNF] and IL-6), and genomic (ie, gene expression and bioinformatics analyses of proinflammatory transcription pathways). Before each blood sampling between 8 am and 10 am, subjects were queried about recent (ie, last month) infection, illness, or vaccination, and sampling was rescheduled if subject reported any of these issues. Given the subject burden of blood sampling, and prior evidence that repeated sampling of CRP and TLR-4 activation of monocytic production of cytokines yields stable results (ie, correlation between samples r > 0.94), samples were obtained only at the second baseline assessment, and then at postintervention. Purified peripheral blood mononuclear cells for genomic assays were obtained in a random subsample (n = 48). The assay methods for CRP, TLR-4–activated monocyte production of proinflammatory cytokines, and gene expression are described in the Supplementary Material (available online).
Because body mass index and physical activity Yale Physical Activity Survey are related to inflammation levels, changes in body weight and physical activity, as indexed by Yale Physical Activity Survey, were evaluated.
Sample Size
For circulating markers of inflammation (ie, IL-6, CRP), TCC reduces IL-6 and CRP with effect sizes ranging from 0.91 (17,25); 40 per treatment group provides statistical power of 80% (α = 0.05). For TLR-4 activation of monocytic production of cytokines, TCC reduces production of proinflammatory cytokines in older adults with an effect size of 0.92–1.26 (MRI; unpublished data); 40 per treatment group provides statistical power of greater than 80% (α = 0.05). For the gene expression outcome, three prior studies have evaluated the effect of meditation or CBSM on transcriptional profiling with effect sizes ranging from 0.68 to 1.32 (20–22); 24 per treatment group provides statistical power of greater than 90%, using an average effect size of .98 (α = 0.05).
Statistical Methods
Intervention effects on CRP levels and TLR-4–activated monocyte production of proinflammatory cytokines were tested on an intention-to-treat basis using a mixed model approach, covarying for baseline; data from all randomized participants were included with no imputation of missing data. The mixed model approach utilizes all available data and generates unbiased estimates under the assumption that data are missing at random; or more restrictively, missing completely at random. Because it would require information that is, by definition, not available, there are no well-established tests of the missing at random assumption; hence, the missing completely at random assumption was tested. A priori contrasts tested group differences in CRP and TLR-4 activation from baseline to postintervention. Detailed methods of analyses for the gene expression results are reported in the Supplementary Material (available online).
All analyses controlled for multiple comparisons. Data were available on more than 99% of subjects among those who completed the intervention. Analyses were carried out with IBM SPSS for Windows, version 22, or R package nlme, TELiS, and TOA software (for gene expression analyses).
Results
Baseline Characteristics of the Participants
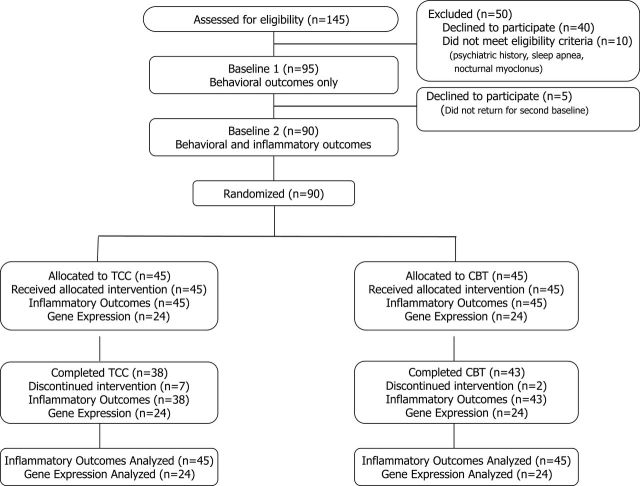
A total of 145 underwent baseline assessment, 129 were eligible, and 90 completed the two baseline assessments (Figure 1). Treatment groups were comparable with regard to demographic and clinical background characteristics, except that that TCC group included significantly more non-white participants (Table 1). For inflammatory outcomes of CRP and TLR-4 activation of monocytes, 81 (90%) participants completed posttreatment assessment. Missing data patterns appeared to fit the missing completely at random assumption (X 2(35) = 11.7; P = .99). None of the clinical and demographic variables were different between completers and noncompleters. Additionally, average rate of session attendance was similar (ie, 80.6% in CBT-I; 75.8% in TCC; t(65) = 1.12; P = .27), with participants perceiving the two interventions as acceptable treatments to improve their insomnia symptoms at baseline (TCC: 94.7% vs CBT-I: 100%; X 2(1) = 0.46; P = .49) and at posttreatment (100% vs 100%). There were no significant between-group changes from baseline to posttreatment in body mass index (F(1,49.0) = 0.01; P = .94), or physical activity (ie, metabolic equivalents per week; F(1,77.0) = 0.38; P = .55).
Figure 1.
Screening, assessment, randomization, and completion of postintervention. Inflammatory outcomes indicate circulating levels of C-reactive protein (CRP)- and toll-like receptor-4 (TLR-4)-activated monocyte production of interleukin-6 (IL-6) and tumor necrosis factor (TNF). Gene expression refers to genome-wide transcriptional profiling and bioinformatic analyses.
Table 1.
Baseline characteristics of participants, by treatment group*
| Variable | TCC (N = 45) | CBT-I (N = 45) | P |
|---|---|---|---|
| Age (42–83 y) | 59.6 (7.9) | 60.0 (9.3) | .82 |
| Race, white, n (%) | 34 (75.6) | 43 (95.6) | .02 |
| Marital status, married/partner, n (%) | 19 (43.2) | 28 (62.2) | .11 |
| Employment, working, n (%) | 28 (60.0) | 27 (62.2) | .99 |
| Education (y) | 15.8 (1.2) | 15.7 (1.4) | .81 |
| Body mass index (kg/m2) | 25.6 (4.5) | 26.2 (5.9) | .60 |
| Cancer history | |||
| Age at Dx (y) | 49.6 (9.0) | 51.4 (9.2) | .38 |
| Time since Dx (y) | 9.4 (8.9) | 8.3 (8.1) | .55 |
| Time since Tx (y) | 7.8 (8.1) | 6.3 (5.8) | .37 |
| Current hormone Tx, n (%) | 10 (28.6) | 18 (42.9) | .20 |
| Comorbidity | |||
| Charlson Comorbidity Index | 1.5 (1.0) | 1.7 (2.0) | .60 |
| Depression history, n (%) | 17 (37.8) | 23 (51.1) | .29 |
| Insomnia and behavioral symptoms | |||
| Sleep quality (PSQI, total score) | 10.9 (2.6) | 10.9 (3.9) | .99 |
| Daytime sleepiness (ESS) | 8.2 (4.7) | 7.5 (5.0) | .54 |
| Depressive symptoms (IDS-C) | 10.3 (6.4) | 9.5 (6.3) | .58 |
* Values are mean (standard deviation), unless otherwise shown. CBT-I = cognitive behavioral therapy for insomnia; Dx = diagnosis; ESS = Epworth Sleepiness Scale; IDS-C = Inventory of Depressive Symptomatology; PSQI = Pittsburgh Sleep Quality Index; TCC = Tai Chi Chih; Tx = treatment.
Outcome of Systemic Inflammation
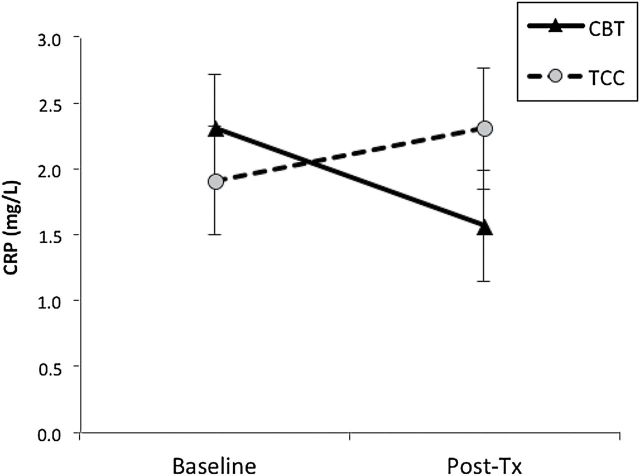
Levels of CRP did not change from baseline to posttreatment in the two groups (CBT-I, P = .13; TCC, P = .44), with similar levels of CRP at posttreatment (F(1,53.6) = 2.60, P = .11; effect size = 0.36; Figure 2).
Figure 2.
Circulating levels of C-reactive protein (CRP) from baseline to posttreatment, by treatment group. Values are mean ± SEM.
Outcome of Cellular Inflammation
Levels of TLR-4–activated monocyte production of proinflammatory cytokines were examined in three unique monocyte populations: cells that produced both IL-6 and TNF together; cells that produced only IL-6; and those that produced only TNF.
In monocyte populations that produced both IL-6 and TNF together in response to TLR-4 activation, the two groups changed differently, with significantly lower levels in the combined production of IL-6 and TNF at posttreatment in TCC as compared with CBT-I (F(1,82.1) = 5.33, P < .02; effect size = 0.50; Figure 3A). Monocytes from participants in the TCC group showed an overall decrease in the combined production of IL-6 and TNF from baseline to posttreatment (P < .005), but not in CBT-I (P = .90).
Figure 3.
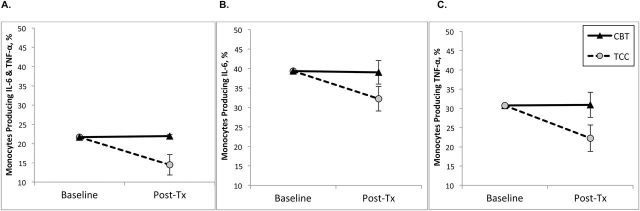
Toll-like receptor-4 (TLR-4) stimulated monocytic production of proinflammatory cytokines, from baseline to posttreatment, by treatment group. Values are SEM percentage of monocytes producing (A) interleukin-6 (IL-6) and tumor necrosis factor (TNF), (B) IL-6 only, (C) TNF only. SEM bars at baseline are within the cognitive behavioral therapy for insomnia (CBT-I) and Tai Chi Chih (TCC) symbols.
In monocyte populations that produced only IL-6 in response to TLR-4 activation, there was a trend for the groups to change differently, with marginally lower levels in the production of IL-6 at postintervention in TCC as compared with CBT-I (F(1,81.9) = 3.35, P = .07; effect size = 0.41; Figure 3B). Monocytes from participants in the TCC group showed an overall decrease in the production of IL-6 from baseline to posttreatment (P < .01), but not in CBT-I (P = .89).
In monocytes populations that produced only TNF in response to TLR-4 activation, the two groups changed differently, with significantly lower levels in the production of TNF at posttreatment in the TCC as compared with CBT-I (F(1,82.2) = 4.2, P < .05; effect size = 0.45; Figure 3C). Monocytes from participants in the TCC also showed an overall decrease in the production of TNF from baseline to posttreatment (P < .01), but not in CBT-I (P = .95).
Covarying for ethnicity did not alter any of the results for changes in CRP- or TLR-4–activated production of IL-6 and TNF. None of the other demographic or clinical characteristics of the two groups differed at baseline, nor were these variables significantly related to markers of inflammation, except for body mass index which did not change during the intervention.
Outcome of Gene Expression
The randomly selected subsample to test gene expression did not differ from the unselected cases for any of the variables in Table 1 (all P’s > .20). In data from genome-wide transcriptional profiling of circulating peripheral blood mononuclear cells, analyses of an a priori-selected set of 19 proinflammatory gene transcripts (25) showed a 9.0% (± 2.5%) greater decline over time in average expression in TCC participants relative to CBT-I participants (Figure 4A; difference, P = .0012). Parallel analyses of a set of 34 genes involved in type I interferon responses and antibody production showed a 3.3% (± 1.5%) greater increase over time in expression in TCC versus CBT-I participants (Figure 4A; difference, P = .0347). To assess the potential role of the proinflammatory TF NF-κB in these differences, we conducted promoter-based bioinformatics analyses of NF-κB–binding elements in the promoters of all genes identified as showing at least a 1.2-fold differential change in expression over time; 68 gene transcripts showed at least 1.2-fold greater downregulation over time in TCC versus CBT-I participants and 19 showed at least 1.2-fold greater upregulation over time (listed in Supplementary Material, available online). Prominent among the transcripts showing greater downregulation in TCC versus CBT-I were genes involved in myelopoiesis (CSF3R) and inflammation (CCR3, GATA2, IL8RB, IL1B, MMP9, PTGES, RELB, and TNF). Consistent with that observation, 1) TELiS promoter-based bioinformatics analyses implicated reduced activity of NF-κB in structuring the greater decline in proinflammatory gene expression in TCC versus CBT-I participants (differential prevalence of NF-κB–binding motifs: −28.7% ± 9.8%, P = .0394), and 2) Transcript origin analyses identified monocytes as the primary cellular context for transcripts showing greater downregulation over time in TCC versus CBT-I participants (Figure 4B; P = .0014). No specific leukocyte subset was linked to genes differentially upregulated in TCC versus CBT-I (Figure 4B; all P > .280).
Figure 4.
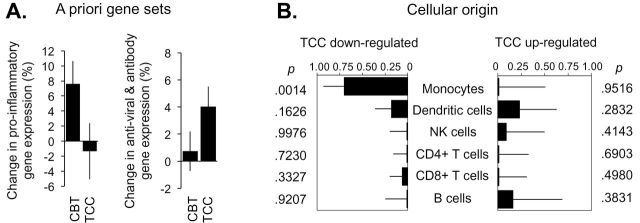
(A) Baseline to postintervention change in average expression of 19 proinflammatory genes and 34 genes involved in type I interferon antiviral responses and antibody production, all selected a priori based on previous research (6,25). (B) Results of transcript origin analyses identifying leukocyte subsets predominately expressing genes showing ≥1.2-fold greater upregulation (right) or downregulation (left) in Tai Chi Chih (TCC) versus cognitive behavioral therapy for insomnia (CBT-I).
Discussion
Among breast cancer survivors with insomnia randomized to 3 months of TCC or CBT-I, posttreatment assessment showed decreased TLR-4–activated monocyte production of IL-6 and TNF, as well as reduced expression of genes encoding proinflammatory mediators and increased expression of genes involved in antiviral responses in peripheral blood mononuclear cells from TCC-treated cancer survivors. These TCC-induced changes in the cellular production of proinflammatory cytokines and in the basal leukocyte transcriptome may have significant implications for cancer-related disease processes. Proinflammatory signaling has been linked breast cancer progression and recurrence (8,9), whereas type I interferon activity has been linked to reduced progression (26).
Sleep disruption has been found to induce a pattern of increases in TLR-4–activated production of proinflammatory cytokines and in the proinflammatory transcriptional bias (14), which is common to populations confronting major life adversities. Such a conserved cellular and transcriptional response may be mediated by common neural responses that ultimately modulate cellular activation and leukocyte gene expression (6). Indeed, β-adrenergic signaling can activate NF-κB and upregulate transcription of proinflammatory cytokine genes while simultaneously repressing innate antiviral responses (6). TCC and other mind–body therapies decrease sympathetic outflow (27), and reduced sympathetic nervous system activity may thus contribute to the relative reduction in inflammatory biology and upregulation of antiviral biology in TCC.
This study highlights the promise that Tai Chi and other mind–body therapies may play in regulating the immune system, and are consistent with other findings that mind–body therapies, as well as CBSM may help to reduce inflammation, including cancer patients. We have previously found that mindfulness-based meditation can reduce proinflammatory response gene profiles (21), and that a yogic meditation reverses increased NF-κB–related transcription of proinflammatory cytokines and decreased IRF1-related transcription of innate antiviral response genes (22) with similar effects reported for CBSM in breast cancer survivors (20). Research also suggests that mind–body therapies may increase virus-specific cell-mediated immune responses and bolster antiviral response to vaccinations (28) with evidence that CBSM also supports cellular immune processes that are implicated in tumor eradication (29,30). Together, the anti-inflammatory and antiviral effects of mind–body therapies, as well as CBSM, provide insight into the potential mechanisms by which these treatments might confer health benefits.
Due possibly to the relatively short duration of assessment, intervention effects on CRP were not detected. CRP is regulated in part by IL-6, and TCC-induced reduction of IL-6 at 16 weeks (19) is followed by reductions of CRP at 24 weeks (17). Although insomnia remission was not evaluated in the present analyses, CBT-I has been found to induce a reduction of CRP at 1 year when insomnia remits in older adults (31). Second, TCC incorporated low levels of physical activity not found in CBT-I, which might have contributed to the effects on inflammation, although neither treatment changed physical activity nor body mass index. Nevertheless, given prior findings that CBSM, similar to CBT-I, has been found to improve psychological and physiological adaptation indicators in cancer patients including markers of inflammation and transcriptional profiling (20,29,30), a larger randomized controlled trial is needed to confirm these results. Third, as is the case with behavior-based treatments, participants were aware of their intervention assignment, which may have introduced bias in the results, although expectancy for benefit was similar in the two groups. Fourth, generalizability of these results to a more diverse sample of cancer survivors is limited by recruitment of breast cancer survivors with comorbid insomnia. Finally, the point estimates of differential expression for individual genes reported in Supplementary Table 1 (available online) should not be taken to be statistically reliable at the level of individual transcripts, and serve only as inputs into higher order bioinformatics analyses based on shared characteristics of aggregate gene sets.
In summary, given the link between inflammation and cancer incidence and progression, these findings provide an evidence-based molecular framework to understand the potential salutary effects of mind–body therapies such as TCC on cancer survivorship. These results provide a compelling justification to use transcriptional analyses, along with assessment of inflammatory biomarkers, to examine the association between behavioral comorbidities and these signaling pathways, and the separate and combined influence of behavioral and biological mechanisms on cancer outcomes including disease progression. Additionally by incorporating innovative inflammatory biomarkers that are linked to behavioral risk factors (ie, sleep disturbance, experienced stress) within the context of a treatment trial, research in personalized integrative oncology has the potential to identify those cancer survivors who are most likely to show reversal of the biological profile (ie, inflammation) with implications for cancer disease progression.
Funding
R01 CA160245-01, and in part by R01-AG034588, R01-AG026364, R01-CA119159, R01 HL095799, R01 DA032922-01, P30-AG028748 to MRI; UCLA (CTSI UL1TR000124); the Cousins Center for Psychoneuroimmunology; UCLA Claude D. Pepper Older Americans Independence Center (P30-AG028748). The National Institutes of Health had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, and approval of the manuscript.
Supplementary Material
We thank the study participants for their support and dedication to this research project; the physicians for providing medical clearance (Drs Lara Kierlin, Hyong Jin Cho, Marissa Caudill); Merrill Collins (www.SpiralingMusic.com) for providing music during the TCC session; and the intervention instructors (Kate Hollister, Jennifer Levin; Roberta Taggert). ClinicalTrials.gov, NCT00690196. The authors report no conflicts of interest.
References
- 1. Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report. 2008;(12):1–23. [PubMed] [Google Scholar]
- 2. DiGianni LM, Garber JE, Winer EP. Complementary and alternative medicine use among women with breast cancer. J Clin Oncol. 2002;20(suppl 18):34S–38S. [PubMed] [Google Scholar]
- 3. National Center for Complementary and Alternative Medicine. Exploring the Science of Complementary and Alternative Medicine: NCCAM Third Strategic Plan 2011–2015. Bethesda, MD: 2011. NIH Publication No. 11-7643. [Google Scholar]
- 4. Wang C, Collet JP, Lau J. The effect of Tai Chi on health outcomes in patients with chronic conditions: a systematic review. Arch Intern Med. 2004;164(5):493–501. [DOI] [PubMed] [Google Scholar]
- 5. Irwin MR, Olmstead R, Motivala SJ. Improving sleep quality in older adults with moderate sleep complaints: a randomized controlled trial of Tai Chi Chih. Sleep. 2008;31(7):1001–1008. [PMC free article] [PubMed] [Google Scholar]
- 6. Irwin MR, Cole SW. Reciprocal regulation of the neural and innate immune systems. Nat Rev Immunol. 2011;11(9):625–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. [DOI] [PubMed] [Google Scholar]
- 8. Cole SW. Chronic inflammation and breast cancer recurrence. J Clin Oncol. 2009;27(21):3418–3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pierce BL, Ballard-Barbash R, Bernstein L, et al. Elevated biomarkers of inflammation are associated with reduced survival among breast cancer patients. J Clin Oncol. 2009;27(21):3437–3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Irwin MR, Olmstead RE, Ganz PA, Haque R. Sleep disturbance, inflammation and depression risk in cancer survivors. Brain Behav Immun. 2013;30(suppl):S58–S67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Irwin MR. Depression and insomnia in cancer: prevalence, risk factors, and effects on cancer outcomes. Curr Psychiatry Rep. 2013;15(11):404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Miller AH, Ancoli-Israel S, Bower JE, Capuron L, Irwin MR. Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. J Clin Oncol. 2008;26(6):971–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Meier-Ewert HK, Ridker PM, Rifai N, et al. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol. 2004;43(4):678–683. [DOI] [PubMed] [Google Scholar]
- 14. Irwin MR, Wang M, Campomayor CO, Collado-Hidalgo A, Cole S. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch Intern Med. 2006;166(16):1756–1762. [DOI] [PubMed] [Google Scholar]
- 15. Irwin MR, Wang M, Ribeiro D, et al. Sleep loss activates cellular inflammatory signaling. Biol Psychiatry. 2008;64(6):538–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mills PJ, Ancoli-Israel S, Parker B, et al. Predictors of inflammation in response to anthracycline-based chemotherapy for breast cancer. Brain Behav Immun. 2008;22(1):98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lavretsky H, Alstein LL, Olmstead RE, et al. Complementary use of tai chi chih augments escitalopram treatment of geriatric depression: a randomized controlled trial. Am J Geriatr Psychiatry. 2011;19(10):839–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen SC, Ueng KC, Lee SH, Sun KT, Lee MC. Effect of t’ai chi exercise on biochemical profiles and oxidative stress indicators in obese patients with type 2 diabetes. J Altern Complement Med. 2010;16(11):1153–1159. [DOI] [PubMed] [Google Scholar]
- 19. Irwin MR, Olmstead R. Mitigating cellular inflammation in older adults: a randomized controlled trial of Tai Chi Chih. Am J Geriatr Psychiatry. 2012;20(9):764–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Antoni MH, Lutgendorf SK, Blomberg B, et al. Cognitive-behavioral stress management reverses anxiety-related leukocyte transcriptional dynamics. Biol Psychiatry. 2012;71(4):366–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Creswell JD, Irwin MR, Burklund LJ, et al. Mindfulness-Based Stress Reduction training reduces loneliness and pro-inflammatory gene expression in older adults: a small randomized controlled trial. Brain Behav Immun. 2012;26(7):1095–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Black DS, Cole SW, Irwin MR, et al. Yogic meditation reverses NF-κB and IRF-related transcriptome dynamics in leukocytes of family dementia caregivers in a randomized controlled trial. Psychoneuroendocrinology. 2013;38(3):348–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Morin CM, Bootzin RR, Buysse DJ, Edinger JD, Espie CA, Lichstein KL. Psychological and behavioral treatment of insomnia:update of the recent evidence (1998-2004). Sleep. 2006;29(11):1398–1414. [DOI] [PubMed] [Google Scholar]
- 24. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR). 4th ed Text Revision ed. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 25. Fredrickson BL, Grewen KM, Coffey KA, et al. A functional genomic perspective on human well-being. Proc Natl Acad Sci USA. 2013;110(33):13684–13689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bielenberg DR, McCarty MF, Bucana CD, et al. Expression of interferon-beta is associated with growth arrest of murine and human epidermal cells. J Invest Dermatol. 1999;112(5):802–809. [DOI] [PubMed] [Google Scholar]
- 27. Motivala SJ, Sollers J, Thayer J, Irwin MR. Tai Chi Chih acutely decreases sympathetic nervous system activity in older adults. J Gerontol A Biol Sci Med Sci. 2006;61(11):1177–1180. [DOI] [PubMed] [Google Scholar]
- 28. Irwin MR, Olmstead R, Oxman MN. Augmenting immune responses to varicella zoster virus in older adults: a randomized, controlled trial of Tai Chi. J Am Geriatr Soc. 2007;55(4):511–517. [DOI] [PubMed] [Google Scholar]
- 29. McGregor BA, Antoni MH, Boyers A, Alferi SM, Blomberg BB, Carver CS. Cognitive-behavioral stress management increases benefit finding and immune function among women with early-stage breast cancer. J Psychosom Res. 2004;56(1):1–8. [DOI] [PubMed] [Google Scholar]
- 30. Antoni MH. Psychosocial intervention effects on adaptation, disease course and biobehavioral processes in cancer. Brain Behav Immun. 2013;30(suppl):S88–S98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Irwin MR, Olmstead R, Carrillo C, et al. Cognitive behavioral therapy vs. Tai Chi for late life insomnia and inflammatory risk: a randomized controlled comparative efficacy trial. Sleep. 2014;37(9):1543–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.