Abstract
Background
In this randomized trial, Project CARE, we examined whether participation in a cognitive-behavioral stress management and breast cancer wellness and education program improved psychological outcomes among a sample of underserved black breast cancer survivors.
Methods
Both complementary medicine interventions were 10-sessions, manualized, group-based, and were culturally adapted for black women in the community from evidence-based interventions. Participants were 114 black women (mean age = 51.1, 27–77 years) who had completed breast cancer treatment 0–12 months before enrollment (stages 0–IV, mean time since cancer diagnosis = 14.1 months). Women were enrolled upon completion of curative treatment (ie, surgical, chemotherapy, radiation oncology) and randomized to receive cognitive-behavioral stress management or cancer wellness and education program.
Results
There was a remarkable 95% retention rate from baseline to 6-month follow-up. Participants in both conditions showed statistically significant improvement on indices of psychological well-being, including overall quality of life (Functional Assessment of Cancer Therapy-Breast), intrusive thoughts (Impact of Event Scale-Revised), depressive symptoms (Center for Epidemiologic Studies-Depression), and stress levels (Perceived Stress Scale) over the 6-month postintervention follow-up (all repeated measures analysis of variance within-subjects time effects: P < .05, except for overall mood; Profile of Mood States-Short Version). Contrary to hypotheses, however, condition × time effects were not statistically significant.
Conclusions
Findings suggest that improvements in multiple measures over time may have been due to intensive training in stress management, extensive provision of breast cancer information, or participation in an ongoing supportive group of individuals from a similar racial background. Implications bear on decisions about appropriate control groups, the timing of intervention delivery during the treatment trajectory, and perceived support from the research team.
Despite lower incidence of disease, black women have higher breast cancer mortality than other racial and ethnic groups (1). Black women with breast cancer are more likely to be diagnosed with later stage disease, imparting a poorer prognosis. This necessitates more invasive treatments, thereby resulting in diminished quality of life and greater physical discomfort (2,3). Black and low-income women are traditionally underserved in all aspects of cancer care (4) including psychosocial support (5) and integrative medicine modalities (6).
Black breast cancer survivors report several unique concerns, while sharing many of the issues that plague breast cancer survivors in general, such as distress, anxiety, fatigue, and sleep disruption (7,8). Black women have specific concerns about keloid scarring and the unavailability of prosthetic devices that match skin tone. They also report the erosion of social support after surgery due to stigma (9,10). The latter may be of great importance. Among black and white women with breast cancer, lower perceived emotional support at diagnosis predicted higher risk of death over 10-year follow-up (11). Such findings suggest that black women with breast cancer might benefit greatly from interventions designed to maintain and augment their social support networks.
Within the field of integrative oncology, researchers are developing interventions to mitigate the symptoms caused by disease and treatment for cancer (12). Often called “complementary medicine,” psychosocial approaches are used alongside traditional medical care and are designed to reduce emotional distress, improve quality of life, foster social support, enhance coping, support the adoption of healthy behaviors (eg, dietary changes, increased physical activity, quitting smoking), and encourage stress management [eg, (13,14); for a meta-analysis, see (15)]. Unfortunately, the samples in published studies typically contain little racial/ethnic diversity.
The 10-week cognitive-behavioral stress management (CBSM) intervention developed at the University of Miami utilizes cognitive-behavioral techniques and relaxation skills training in a group setting (14). A wealth of data show that CBSM intervention is effective in enhancing adaptation to illness among varied groups of individuals living with life-threatening and chronic diseases, including breast cancer. Colloquially known as Project CARE, the current project tested whether a culturally specific adaptation of CBSM imparted benefits to a sample of minority women at a particularly vulnerable time: the end of active curative treatment (16). This phase of cancer treatment is a particularly troubling time for many women, who often voice concerns about posttreatment survivorship (17). CBSM was compared with a group-based, time-matched breast cancer wellness and education condition (CW) that focused on highly salient topics for cancer survivors.
In accordance with NCI’s call to reduce health disparities, the sample included black women in South Florida who came from diverse population subgroups, including African American, Haitian, Jamaican, and other Caribbean Islanders. Given their shared experiences as women of color, they had a common point of reference despite differing cultural practices. To meet recruitment goals, the research team partnered with a community consultant and engaged more than 30 strategic partners, including churches, cancer-serving organizations, and local community organizations that provide resources specifically to black women.
In summary, this clinical trial examined whether participation in a CBSM or CW program improved psychological adaptation outcomes among a sample of black breast cancer survivors. We hypothesized that participants randomized to the CBSM intervention would show better adaptation to breast cancer survivorship on multiple indices of adjustment over the study period (baseline, postintervention, 6-month follow-up) as compared with women assigned to the CW condition.
Methods
This was a single center, randomized (1:1), parallel-group, superiority trial. The study took place in community-based locations in South Florida from 2008–2013. The primary endpoints were quality of life, mood disturbance, intrusive thoughts about breast cancer, and perceived stress. Sample size was based on previous work (14). There were no interim analyses and no a priori stopping rules. To account for the small amount of missing data, multiple imputation was used. Five imputed data sets were created, and pooled estimates across data sets were used because missingness was not systematic (eg, 18). Thus, all participants were represented in the analyses (reflecting intention-to-treat).
Participants
Participants were 114 women who met the following criteria: age more than 21 years; English-speaking; self-identify as black (eg, African American, African, Caribbean black, black Hispanic); definitively diagnosed with breast cancer (any stage and any type); received at least one type of traditional medical treatment (ie, surgery, chemotherapy, radiation therapy); completed treatment within 12 months before enrollment; had no prior history of cancer; reported moderate stress or distress (rating of 4/10); no hospitalizations for severe mental illness within the past year; no active suicidality; and denied substance dependence within the past year.
Recruitment and Retention
Given that breast cancer survivors generally have numerous competing demands and black breast cancer survivors are a particularly difficult population to access, special efforts were made to recruit and retain women (16). Recruitment was intensive and took place through a variety of venues, including community-based breast cancer programs, local churches of several denominations, community centers, health fairs, hospitals, private physicians within the community, public service announcements in local media, and local cultural activities. Participants were self-referred and did not require medical provider referral to enroll. Participants were compensated for completing assessments, received transportation reimbursement, and enjoyed healthy meals during the intervention sessions. Between timepoints, intensive contact was maintained by phone calls, mailings, and newsletters.
Procedures
All study procedures were approved and monitored by the institutional review board at the University of Miami. Screening for eligibility and in-person assessments were conducted by a black female assessor who was supervised by a licensed clinical psychologist. Assessments occurred at prerandomization (baseline), immediately postintervention (T2), and 6-month follow-up (T3) and were conducted in the participant’s home or location of her choice (eg, private room in a community center). The assessor explained the study procedures, played a videorecording of the informed consent document, and verified participants’ complete understanding. Written informed consent was obtained. Given the wide range of literacy and educational levels within the sample, assessments were conducted as interviews, with printed prompts for each of the response sets. All assessments were conducted over the course of two visits to reduce participant burden and to allow for saliva collection in the interim (salivary cortisol findings are not reported here).
Randomization
Once a cohort was formed (ie, 10–14 women in the eligible pool), women were randomly assigned to one of two groups. The groups were then randomly assigned to one of the conditions. Our randomization procedures created groups with equal characteristics with respect to disease-related and medical variables, as well as social and economic backgrounds (Table 1). Assessors were blind to participants’ assigned condition.
Table 1.
Demographic and medical variables by condition (CBSM vs CW) at baseline including means, SDs, F statistic, and P value for continuous variables and Ns, percentages, chi-square statistic (χ 2), and P value for categorical variables*
| Variable | CBSM mean/N (SD/%) | CW mean/N (SD/%) | F/χ 2, P |
|---|---|---|---|
| Age, y | 50.16 (7.89) | 52.07 (9.93) | 1.30, >.2 |
| Years of education | 13.60 (2.56) | 13.68 (2.04) | 0.04, >.8 |
| Income (thousands of US dollars) | 37.05 (44.16) | 29.65 (26.15) | 1.19, >.2 |
| Partnered | 20 (35%) | 15 (26%) | 1.03, >.3 |
| Current experience of poverty | 64.47 (12.5) | 65.26 (15.0) | .08, >.8 |
| Employment status | |||
| Employed | 26 (46%) | 29 (51%) | 2.33, >.5 |
| Disability/leave | 12 (21%) | 7 (12%) | |
| Retired | 4 (7%) | 7 (12%) | |
| Unemployed | 15 (26%) | 14 (24%) | |
| Integrative oncology modality use | |||
| Used prayer for health | 26 (46%) | 33 (58%) | 1.72, >.2 |
| Used other integrative oncology modalities as treatment | 14 (25%) | 16 (28%) | .18, >.6 |
| Attended therapy or counseling for cancer | 10 (18%) | 13 (23%) | 0.49, >.4 |
| Cancer stage, TNM | |||
| Stage 0/DCIS/LCIS | 6 (10%) | 4 (7%) | 7.67, >.1 |
| Stage I | 8 (14%) | 18 (32%) | |
| Stage II | 22 (38%) | 24 (42%) | |
| Stage III | 20 (35%) | 10 (18%) | |
| Stage IV | 1 (2%) | 1 (2%) | |
| Months since diagnosis | 13.72 (6.22) | 14.12 (5.67) | 0.13, >.7 |
| Months since end of treatment | 3.30 (2.97) | 3.81 (3.19) | 0.78, >.3 |
| Underwent surgery | 55 (96%) | 57 (100%) | 2.04, >.1 |
| Received chemotherapy | 51 (93%) | 41 (72%) | 7.45, <.01† |
| Received radiation | 40 (77%) | 39 (71%) | 0.50, >.4 |
| Received hormone therapy | 35 (70%) | 34 (67%) | 0.13, >.7 |
| Received trastuzumab | 14 (30%) | 12 (30%) | 0.21, >.6 |
| Underwent reconstructive surgery | 12 (21%) | 10 (17%) | 0.22, >.6 |
| Quality of life, baseline | 137.05 (25.92) | 135.82 (26.87) | 0.06, >.8 |
| Mood, baseline | 13.65 (9.29) | 15.14 (10.84) | 0.62, >.4 |
| Intrusive thoughts, baseline | 11.14 (7.62) | 12.98 (7.14) | 1.77, >.1 |
| Depressive symptoms, baseline | 37.09 (11.26) | 36.49 (12.51) | 0.07, >.7 |
| Perceived stress, 4-item, baseline | 6.95 (2.92) | 6.37 (3.42) | 0.94, >.3 |
* CBSM = cognitive-behavioral stress management; CW = cancer wellness and education; DCIS = ductal carcinoma in situ; LCIS = lobular carcinoma in situ; SD = standard deviation † = P<.01.
CBSM Condition
The CBSM intervention is a structured group intervention with 10 consecutive weekly sessions. Groups were led by a licensed clinical psychologist who was also a black woman. Participants received a workbook that contained the session content, short out-of-session exercises, and the content of the CW condition. Relaxation techniques included progressive muscle relaxation, visual imagery, deep breathing, and meditation. The cognitive stress management component was designed to teach strategies to reduce anxiety, promote cognitive restructuring, provide coping skills training, build interpersonal skills (eg, communication skills, anger management, assertiveness training), and enhance social networks (eg, identifying sources of support matched to needs). Lechner et al. (16) provides the details of the process of adapting the CBSM intervention (19) into a culturally targeted version.
Breast Cancer Wellness and Education Condition
The comparison condition was time- and attention-matched, and designed to be an enriching experience. Participants completed 10-weekly 90-minute sessions with a graduate level black female interventionist. This condition used structured PowerPoint slides to present culturally targeted information on breast cancer, cancer treatment, side effects (eg, pain; fatigue), communication with the health-care team, navigating the health-care system, physical activity, nutrition, social support, quality of life, taking time for self, fear of recurrence, sexuality, heredity and family issues, goal setting in posttreatment survivorship, the tyranny of positive thinking, and “the new normal.” Women in both conditions received a workbook containing the same slides.
Measures
Sociodemographic factors—age, education, family income, partnership status, current burdens associated with poverty, employment status, integrative oncology modality use, and use of psycho-oncology support services—were examined to determine if the groups differed in systematic ways. In addition, disease-related characteristics were examined, including: categorical TNM staging, time since diagnosis, time since treatment offset, and treatments received (surgery, chemotherapies, radiation therapy, endocrine therapy, and immunotherapy).
Functional Assessment of Cancer Therapy-Breast measured quality of life (20). The Functional Assessment of Cancer Therapy-Breast (version 4) assesses well-being across four broad domains (ie, physical, social/family, emotional, and functional) and one specific domain of breast cancer symptoms. The instructions ask participants to indicate to what degree each of 44 statements has been “true” during the past 7 days (on a 5-point scale). Consistent with the wide range of content of the scale, Cronbach’s α was .66 in this sample.
Profile of Mood States-Short Version (21) is a measure of psychological distress that has been widely used among individuals with breast cancer. The short form consists of 14 adjectives that respondents are asked to rate on a Likert scale of 1–5 indicating the degree to which the adjective describes them during the last week. Consistent with the wide range of content of the scale, Cronbach’s α was .67 in this sample.
Cancer-Related Thought Intrusions were measured by the 8-item Intrusive Thoughts subscale of the Impact of Event Scale-Revised (22). Intrusions are unwanted thoughts about cancer, its treatment, and recurrence. The items are answered on 5-point scales, ranging from “not at all” (0) to “often (4).” Internal consistency was high (α = .90).
Center for Epidemiologic Studies-Depression (23) measured the current level of depressive symptoms. Its 20 items assess the frequency of depressive symptoms over the previous week. Each participants rated the degree to which she experienced each statement on a scale of 1 “rarely or none of the time (<1 day)” to 4 “most or all of the time (5–7 days).” Internal consistency was acceptable (α = .82).
The Perceived Stress Scale (24) is 10-item scale that assesses one’s overall sense of being overwhelmed by stress. The five response options range from “never” to “very often.” Internal consistency was poor; Cronbach’s α for the 10-item version was .46 in this sample. A 4-item version was also computed per the scale authors’ instructions (α = .69); it was used in subsequent analyses due to its higher reliability.
Results
We screened 204 women, of whom 114 met eligibility criteria. Of those who did not enroll, 28% declined to participate, 33% met medical exclusion criteria (eg, previous cancer), 29% were diagnosed more than 12 months before, and 10% were excluded for other reasons (eg, geographical issues). Per CONSORT (25), Figure 1 shows participant allocation to study conditions and study attrition.
Figure 1.
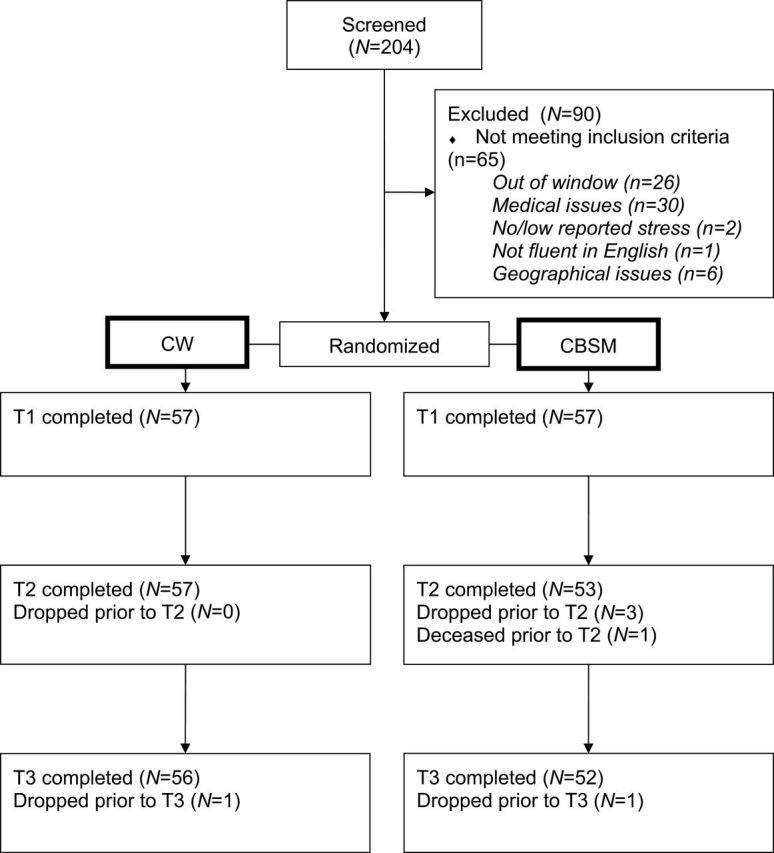
CONSORT diagram.
There were no baseline differences between CBSM and CW groups on demographic factors, disease-related variables or outcome variables, with the exception of chemotherapy treatment (see Table 1). Women assigned to CBSM were more likely to have undergone chemotherapy than participants in CW (χ 2 (1) = 7.45, P < .01). Retention rates were outstanding over the course of the study (95% at 6-month follow-up). On average, women in both conditions attended 7 sessions out of a possible 10. Demographic and disease-related variables were investigated as potential covariates, but none met criteria for inclusion as control variables in the models (all Ps > .1). Use of integrative oncology modalities was low, with the exception of prayer as a method to treat cancer, which was endorsed by 52% of the sample and did not differ by condition.
We conducted a series of two-way repeated measures analysis of variance tests (time by condition) to examine changes from baseline to 6-month follow-up on outcome variables in each condition. The sphericity assumption was violated for the Functional Assessment of Cancer Therapy-Breast, thus the Huynh-Feldt correction was used. Contrary to hypotheses, there was no time by condition effect for mood, quality of life, intrusive thoughts, depressive symptoms, or perceived stress. However, statistically significant within-subjects effects occurred on four of the five outcomes: quality of life, intrusive thoughts, depressive symptoms, and perceived stress (Table 2), mood disturbance being the exception. Thus psychosocial well-being improved from baseline to 6-month follow-up including higher ratings of quality of life (Figure 2), and lower ratings of intrusive thoughts (Figure 3), depressive symptoms (Figure 4), and perceived stress four-item (not depicted) among participants randomized to either condition. Planned post hoc analyses for all analyses revealed that there were no differences between CBSM and CW at any of the three time points for any of the five outcome measures (all Ps < .05). These statistics and observed effect sizes and confidence intervals for each analysis are reported in Table 2.
Table 2.
Estimated marginal means and SEs of outcome measures at baseline, postintervention, and 6-month follow-up by condition with test statistics (F), degrees of freedom, P values, and partial eta-squared (η2 p) for RMANOVA time and time by condition effects with adjusted difference and CIs from Time 1 to Time 3 (across both conditions)*
| Condition | Time 1, mean (SE) | Time 2, mean (SE) | Time 3, mean (SE) | RMANOVA time effect | Adjusted difference (SE), CI | RMANOVA time × condition effect | |
|---|---|---|---|---|---|---|---|
| Quality of life: FACT-B | CBSM | 137.05 (3.50) | 140.20 (3.38) | 142.96 (3.16) | F(1.94, 216.98) = 5.79, P < .01, η2 p = 0.05 | 5.15 (1.67), 1.84 to 8.46 | F(1.94, 216.98) = 0.29, P > .7, η2 p = 0.00 |
| CW | 135.82 (3.50) | 139.57 (3.38) | 140.21 (3.16) | ||||
| Total mood disturbance: POMS-SV | CBSM | 13.65 (1.34) | 13.32 (1.41) | 12.39 (1.13) | F(2, 224) = 1.38, P > .2, η2 p = 0.01 | −1.26 (0.77), −2.78 to 0.27 | F(2, 224) = 0.24, P > .7, η2 p = 0.00 |
| CW | 15.14 (1.34) | 13.96 (1.41) | 13.89 (1.13) | ||||
| Intrusive thoughts: IES-R-Int | CBSM | 12.23 (1.13) | 11.47 (1.19) | 9.90 (1.08) | F(1.99, 222.83) = 5.86, P < .01, η2 p = 0.05 | −2.37 (0.64), −3.63 to −1.11 | F(1.99, 222.83) = 0.09, P > .9, η2 p = 0.00 |
| CW | 14.60 (1.13) | 13.40 (1.19) | 12.19 (1.08) | ||||
| Depressive symptoms: CES-D | CBSM | 37.09 (1.58) | 33.83 (1.54) | 33.58 (1.30) | F(2, 224) = 5.63, P < .01, η2 p = 0.05 | −2.85 (0.93), −4.70 to −1.00 | F(2, 224) = 0.45, P > .6, η2 p = 0.00 |
| CW | 36.49 (1.58) | 34.81 (1.54) | 34.30 (1.30) | ||||
| Perceived stress: PSS, 4-item | CBSM | 6.95 (0.42) | 5.39 (0.40) | 5.57 (0.37) | F(2, 224) = 14.78, P < .01, η2 p = 0.12 | −1.24 (0.26), −1.76 to −0.72 | F(2, 224) = 1.15, P > .3, η2 p = 0.01 |
| CW | 6.37 (0.42) | 5.56 (0.40) | 5.27 (0.37) |
* CBSM = cognitive-behavioral stress management; CES-D = Center for Epidemiologic Studies-Depression; CI = confidence interval; CW = cancer wellness and education; FACT-B = Functional Assessment of Cancer Therapy-Breast; IES-R-Int = Impact of Event Scale-Revised—Intrusive Thoughts; POMS-SV = Profile of Mood States Short Version; PSS = Perceived Stress Scale; RMANOVA = repeated measures analysis of variance; SE = standard error; Time 1 = baseline; Time 2 = postintervention; Time 3 = 6-month follow-up.
Figure 2.
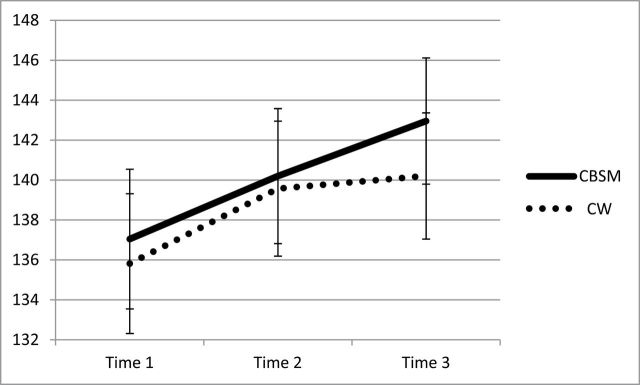
Change over time for quality of life across baseline (Time 1), postintervention (Time 2), and 6-month follow-up (Time 3) by condition (cognitive-behavioral stress management [CBSM] versus cancer wellness and education [CW]) with standard error bars.
Figure 3.
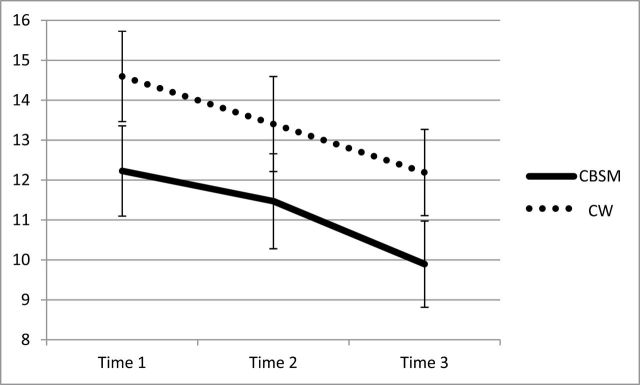
Change over time for intrusive thoughts across baseline (Time 1), post-intervention (Time 2), and 6-month follow-up (Time 3) by condition (cognitive-behavioral stress management [CBSM] versus cancer wellness and education [CW]) with standard error bars.
Figure 4.
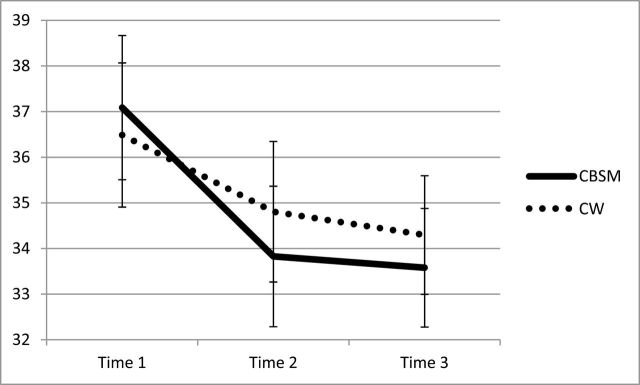
Change over time for depressive symptoms across baseline (Time 1), post-intervention (Time 2), and 6-month follow-up (Time 3) by condition (cognitive-behavioral stress management [CBSM] versus cancer wellness and education [CW]) with standard error bars.
To better explain the repeated measures analysis of variance findings, unplanned post hoc latent growth modeling analyses compared trajectories of participants who attended at least one session to nonattenders (to approximate a pseudo-natural history group). As summarized in Table 3, attending more than one session significantly predicted the T1–T3 slope of Functional Assessment of Cancer Therapy-Breast and Center for Epidemiologic Studies-Depression with reasonable model fit. The nonattender group had statistically better adjustment at baseline; however over the course of the 6-month follow-up, the nonattenders adjustment deteriorated compared with women who attended sessions.
Table 3.
Results of post hoc analyses of trajectories of change for the slope of outcome variables using latent growth curve modeling from baseline, postintervention, and 6-month follow-up, with a least one session attended as a moderator for all enrolled participants*
| Outcome Variable | Model Fit | Group Assignment Effect on Slope | Model Fit Indices |
|---|---|---|---|
| Quality of life: FACT-B | Reasonable model fit | b = 0.024, z = 3.727, P < .01 | Model fit: χ2 (2) = 4.009, P = .14, CFI = 0.99, RMSEA = 0.094, SRMR = 0.027 |
| Total mood disturbance: POMS-SV | Poor model fit | b = −.67, z = −1.51, ns | Model fit: χ2 (3) = 1.25, P =.74, CFI = 1.00, RMSEA = 0.000, SRMR = 0.016 |
| Intrusive thoughts: IES-R-Int | Poor model fit | b = .56, z = 1.47, ns | Model fit: χ2 (3) = 3.08, P = .38, CFI = .999, RMSEA = 0.015, SRMR = 0.04 |
| Depressive symptoms: CES-D | Reasonable model fit | b =−1.218, z = −2.30, P = .02 | Model fit: χ2 (3) = 5.365, P = .147, CFI = 0.98, RMSEA = 0.083, SRMR = 0.22 |
| Perceived stress: PSS, 4-item | Poor model fit | b = −.35, z = −2.43, P = .02 | Model fit: χ2 (3) = 16.82, P = .001, CFI = 0.88, RMSEA = 0.20, SRMR = 0.055 |
*Model fit is evaluated by a combination of nonsignificant chi-square, CFI > .95, RMSEA < .06, and SRMR < .08. The Z-statistic of the unstandardized coefficients was examined to interpret direct and indirect associations. CFI = comparative fit index; CES-D = Center for Epidemiologic Studies-Depression; FACT-B = Functional Assessment of Cancer Therapy-Breast; IES-R-Int = Impact of Event Scale-Revised—Intrusive Thoughts; ns = not significant; POMS-SV = Profile of Mood States Short Version; PSS = Perceived Stress Scale; RMSEA = root mean square error of approximation; SRMR = standardized root mean square residual; Time 1 = baseline; Time 2 = postintervention; Time 3 = 6-month follow-up.
Discussion
In this trial, we conducted a translational study to test the effects of a relatively brief, culturally competent, psychosocial intervention. Women in both conditions (CBSM and CW) showed improvements on multiple indices of psychosocial adaptation to cancer survivorship, including quality of life, perceived stress, depressive symptoms, and intrusive thoughts related to breast cancer.
The absence of condition by time effects was surprising. It could be argued that these results were a reflection of the natural history of the course of psychosocial improvement over the first year after breast cancer treatment, an indication of actual improvement resulting from psychosocial intervention, or a result of nonspecific factors. First, the magnitude of the change in quality of life and intrusive thoughts is similar to our previous work with newly diagnosed women (unpublished data, Antoni R01CA64710), suggestive of potential intervention effects. Second, placing our post hoc analyses within the context of studies that examined trajectories of change in the posttreatment survivorship period (26,27), we would expect the majority of women in Project CARE to show a plateau of QOL and distress during the posttreatment phase, with about 25% of the sample showing improvement followed by decline. The pattern we observed (eg, Figures 2–4) differed from the trajectory that would be predicted by natural history studies. Lastly, when the session videotapes were coded for intervention fidelity, our clinical judgment suggested that the interventions led to transformative benefits for participants. Without a no-treatment control group, however, reasons for the observed improvements are speculative.
Yet, the observed effects may have resulted from important nonspecific factors, such as cultural relevance of content, delivery in community settings, facilitation by a female black professional with community credibility, extensive provision of breast cancer information, a supportive group of individuals from a similar racial background experiencing a shared stressful experience (7,8). For many participants, this was their first opportunity to interact with other black breast cancer survivors in a group setting, which they attributed to ongoing stigma of breast cancer in the black community, and participants reported a strong healing effect of coming together as a sisterhood of black women. This project was also designed to provide maximal interaction with the research team who provided encouragement and empowerment.
There are several strengths that warrant mention. This trial successfully recruited and enrolled women from a very hard-to-reach population using a community-based approach to outreach and retention. Attendance rates, an objective indicator of satisfaction, were quite good; this is remarkable when placed in the context of the women’s daily burdens. Weekly reports, end-of-intervention satisfaction ratings, and retention rates reflected participants’ extremely positive experience and high perceived value of the sessions.
There are notable limitations as well. First, by including all black population subgroups in the study, there was a trade-off between specificity in favor of generalizability. There are too few members of each subgroup to allow us to directly analyze cultural effects on outcomes. Additionally, this study examined a population of primarily low-income breast cancer survivors. In our work with this population, we have identified several hardships that women face. Many participants commented that “breast cancer was the least of their worries,” and the study was limited by the fact that attempts to influence a woman’s sense of well-being in light of all of her other stressors would be challenging. We knew a priori that this factor could attenuate the findings.
Finally, the choice of “control” group for this trial hampered our ability to observe differences over time between conditions. In planning the study, we opted for an ethically defensible, attention-matched, comparison group rather than a wait list or no-treatment control group (28). The CW condition used here had a strong focus on useful and actionable information. It appears that CW intervention may have had stronger than expected effects, perhaps by increasing empowerment and decreasing uncertainty.
In future work, it will be important to address whether the intervention(s) could be enhanced and to consider whether there are specific subsets of women who may be in greatest need of intervention. This trial limited enrollment to women who endorsed moderate stress/distress, but there may be other ways to target women who will show a more robust treatment response. In addition, though the behavioral randomized controlled trial is still the gold standard, our field now has a great opportunity to employ methodological approaches that will allow us to research the effectiveness of our interventions in innovative ways [eg, MOST and SMART (29)].
We see great promise in the future of integrative oncology approaches in attenuating disparities in cancer morbidities, including quality of life, among racial/ethnic groups. With their focus on a tailored approach to health and thriving, integrative medicine interventions are attractive to women across the broad spectrum of races, ethnicities, and income groups [though black individuals may be less likely to seek out integrative modalities other than prayer for health (30)]. It will be critical for future research to delineate the economic and social influences that contribute to integrative oncology acceptance and use. Armed with an understanding of attitudes toward health, access, and usage within communities of color (31), this burgeoning field can implement high-quality, culturally appropriate integrative oncology interventions among vulnerable populations.
Funding
National Cancer Institute of the National Institutes of Health (R01CA131451). This research was also funded by the Sylvester Comprehensive Cancer Center Braman Family Breast Cancer Institute Developmental Awards Program and the Papanicolaou Corps for Cancer Research Health Disparities Awards Program. Technical support was provided by the Non-Therapeutic Research Support Core and the Disparities and Community Outreach Core of Sylvester Comprehensive Cancer Center.
We are grateful to our participants for their dedication to this program. We are deeply grateful for support provided by: Bonnie Blomberg, PhD, Alain Diaz, PhD, Andrea Vinard, MSEd, Arnetta Phillips, Cassaundra Wimes, PhD, Chelsea Greaves, MPH, Dina Dumercy, PharmD, Gabrielle Hazan, MA, Madeline Krause, MSEd, Midian Ambo, MPH, Natasha Colas, Rhonda Smith, MBA, Suresh Atapattu, PhD, and Sylvester Comprehensive Cancer Center Research Administration.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1. American Cancer Society. Cancer Facts & Figures 2013. Atlanta, GA: American Cancer Society; 2013. [Google Scholar]
- 2. Aziz NM, Rowland JH. Cancer survivorship research among ethnic minority and medically underserved groups. Oncol Nurs Forum. 2002;29(5):789–801. [DOI] [PubMed] [Google Scholar]
- 3. Chlebowski RT, Chen Z, Anderson GL, et al. Ethnicity and breast cancer: factors influencing differences in incidence and outcome. J Natl Cancer Inst. 2005;97(6):439–448. [DOI] [PubMed] [Google Scholar]
- 4. Shavers VL, Brown ML. Racial and ethnic disparities in the receipt of cancer treatment. J Natl Cancer Inst. 2002;94(5):334–357. [DOI] [PubMed] [Google Scholar]
- 5. Forsythe LP, Kent EE, Weaver KE, et al. Receipt of psychosocial care among cancer survivors in the United States. J Clin Oncol. 2013;31(16):1961–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Graham RE, Ahn AC, Davis RB, O’Connor BB, Eisenberg DM, Phillips RS. Use of complementary and alternative medical therapies among racial and ethnic minority adults: results from the 2002 National Health Interview Survey. J Natl Med Assoc. 2005;97(4):535–545. [PMC free article] [PubMed] [Google Scholar]
- 7. Ashing-Giwa KT, Padilla GV, Tejero JS, Kim J. Breast cancer survivorship in a multiethnic sample: challenges in recruitment and measurement. Cancer. 2004;101(3):450–465. [DOI] [PubMed] [Google Scholar]
- 8. Taylor KL, Lamdan RM, Siegel JE, Shelby R, Hrywna M, Moran-Klimi K. Treatment regimen, sexual attractiveness concerns and psychological adjustment among African American breast cancer patients. Psychooncology. 2002;11(6):505–517. [DOI] [PubMed] [Google Scholar]
- 9. Spencer SM, Lehman JM, Wynings C, et al. Concerns about breast cancer and relations to psychosocial well-being in a multiethnic sample of early-stage patients. Health Psychol. 1999;18(2):159–168. [DOI] [PubMed] [Google Scholar]
- 10. Wilmoth MC, Sanders LD. Accept me for myself: African American women’s issues after breast cancer. Oncol Nurs Forum. 2001;28(5): 875–879. [PubMed] [Google Scholar]
- 11. Soler-Vila H, Kasl SV, Jones BA. Prognostic significance of psychosocial factors in African-American and white breast cancer patients: a population-based study. Cancer. 2003;98(6):1299–1308. [DOI] [PubMed] [Google Scholar]
- 12. Deng GE, Frenkel M, Cohen L, et al. Evidence-based clinical practice guidelines for integrative oncology: complementary therapies and botanicals. J Soc Integr Oncol. 2009;7(3):85–120. [PubMed] [Google Scholar]
- 13. Andersen BL. Psychological interventions for cancer patients to enhance the quality of life. J Consult Clin Psychol. 1992;60(4):552–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Antoni MH, Lechner SC, Kazi A, et al. How stress management improves quality of life after treatment for breast cancer. J Consult Clin Psychol. 2006;74(6):1143–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tatrow K, Montgomery GH. Cognitive behavioral therapy techniques for distress and pain in breast cancer patients: a meta-analysis. J Behav Med. 2006;29(1):17–27. [DOI] [PubMed] [Google Scholar]
- 16. Lechner SC, Ennis-Whitehead N, Robertson BR, et al. Adaptation of a psycho-oncology intervention for black breast cancer survivors: Project CARE. Couns Psychol. 2013;41:286–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Holland JC, Reznik I. Pathways for psychosocial care of cancer survivors. Cancer. 2005;104:2624–2637. [DOI] [PubMed] [Google Scholar]
- 18. Rubin DB. Multiple imputation after 18+ years. J Am Stat Assoc. 1996; 91(434):473–489. [Google Scholar]
- 19. Antoni MH. Stress Management for Women with Breast Cancer. Washington, DC: American Psychological Association; 2003. [Google Scholar]
- 20. Brady MJ, Cella DF, Mo F, et al. Reliability and validity of the Functional Assessment of Cancer Therapy-Breast quality-of-life instrument. J Clin Oncol. 1997;15(3):974–986. [DOI] [PubMed] [Google Scholar]
- 21. Guadagnoli E, Mor V. Measuring cancer patients’ affect: revision and psychometric properties of the profile of mood states (POMS). Psychol Assess. 1989;1:150–154. [Google Scholar]
- 22. Weiss DS, Marmar CR. The impact of event scale – revised. In: Wilson JP, Keane TM, eds. Assessing Psychological Trauma and PTSD. New York, NY: Guilford Press; 1997:399–411. [Google Scholar]
- 23. Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- 24. Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–396. [PubMed] [Google Scholar]
- 25. Schulz KF, Altman DG, Moher D; CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med. 2010;152(11):726–732. [DOI] [PubMed] [Google Scholar]
- 26. Henselmans I, Helgeson VS, Seltman H, de Vries J, Sanderman R, Ranchor AV. Identification and prediction of distress trajectories in the first year after a breast cancer diagnosis. Health Psychol. 2010;29(2):160–168. [DOI] [PubMed] [Google Scholar]
- 27. Helgeson VS, Snyder P, Seltman H. Psychological and physical adjustment to breast cancer over 4 years: identifying distinct trajectories of change. Health Psychol. 2004;23(1):3–15. [DOI] [PubMed] [Google Scholar]
- 28. Schwartz CE, Chesney MA, Irvine MJ, Keefe FJ. The control group dilemma in clinical research: applications for psychosocial and behavioral medicine trials. Psychosom Med. 1997;59(4):362–371. [DOI] [PubMed] [Google Scholar]
- 29. Collins LM, Murphy SA, Strecher V. The multiphase optimization strategy (MOST) and the sequential multiple assignment randomized trial (SMART): new methods for more potent eHealth interventions. Am J Prev Med. 2007;32:S112–S118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mao JJ, Palmer CS, Healy KE, Desai K, Amsterdam J. Complementary and alternative medicine use among cancer survivors: a population-based study. J Cancer Surviv. 2011;5(1):8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kronenberg F, Cushman LF, Wade CM, Kalmuss D, Chao MT. Race/ethnicity and women’s use of complementary and alternative medicine in the United States: results of a national survey. Am J Public Health. 2006;96(7):1236–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]


