Abstract
Early in embryogenesis, the heart begins its rhythmic contractions as a tube that helps perfuse the nascent vasculature, but the embryonic heart soon changes shape and mechanical properties like many other developing organs. A key question in the field is whether stresses in development impact the underlying gene circuits, and if so, how? Here we attempt to address this question as we review the mechanical maturation of heart – and to a limited extent lung and blood – with a focus on a few key abundant structural proteins for which emerging evidence suggests a direct sensitivity to mechanical stress in expression dynamics. In heart maturation, proliferating fibroblasts deposit increasing amounts of collagenous matrix in parallel with cardiomyocytes expressing more sarcomeric proteins that increase the contractile stress and strength of the tissue, which in turn pumps more blood at higher stress throughout the developing vasculature. Feedback of beating cardiomyocytes on fibroblast expression of matrix seems a reasonable model, with both synthesis and turnover of matrix and contractile elements achieving a suitable balance. Based on emerging evidence for coiled-coil biopolymers that are Tension-stabilized against degradation, a minimal network model of a dynamic cell-cell-matrix interaction is proposed. This same concept is extended to nuclear mechanics as regulated by stress on the Lamins, which are examined in part because of the prominence of mutations in these coiled-coil proteins in diseases of heart among other tissues. Variations in Lamin levels during development and across adult tissue are to some extent known and appear systematic with extracellular matrix mechanics – which we illustrate across heart, lung, and blood development. The formal perspective here on the mechanochemistry of tissue development and homeostasis could provide a useful framework for ‘big data’ quantitative biology, particularly of stress-sensitive differentiation, maturation, and disease processes.
Keywords: cardiogenesis, Cardiac fibroblast, cardiomyocyte, collagen, lamins, mechanosensitivity, systems biology
Introduction
The development of tissue with mechanical function such as heart, lung, muscle, and bone could be based entirely on pre-programmed expression profiles and the self-assembly of components. However, evidence is mounting for the important influence of mechanics in sculpting cell and matrix structure and function, and it seems likely that mechanosensitive pathways help direct tissue growth with feedback that modulates normal gene and or protein levels. The heart is the first functional organ to form, and recent studies document a stiffness that changes daily but matches the contractile optimum of the cardiomyocytes at each stage[1]. Extracellular matrix (ECM) density and/or stiffness indeed regulates embryonic and neonatal cardiomyocyte structure and function(reviewed[2, 3]) as well as early embryonic or embryonic stem-cell derived cardiomyocyte differentiation[4, 5]. Brain in contrast always remains soft, consistent with a relative lack of mechanical function.
The cardiomyocyte’s increasing ability to contract enough to pump blood in heart also parallels daily enrichment in the levels of both Excitation-Contraction proteins and Collagen-I relative to protein mass. However, while cardiomyocytes express key excitation-contraction proteins, they do not make significant matrix[6]; cardiac fibroblasts(CF) secrete collagen-I and other matrix proteins but do not express significant muscle contractility proteins or take part in active beating. A contraction-matrix balance must therefore be achieved between two distinct cell populations, i.e. cardiomyocytes and fibroblasts (Fig. 1A). Biochemical signaling no doubt occurs between cardiomyocytes and CFs[7], and a host of mechonsensitive and matrix-sensitive pathways have been identified and reviewed as important to the function of both cardiomyocytes and CFs[8]. Here we focus on the strictly essential physical interactions of cardiomyocytes that strain cardiac tissue throughout tissue strengthening in development. We try to briefly summarize current descriptions of fibroblast and cardiomyocyte population dynamics during development, and we propose a simple network model that could provide a useful framework for understanding and further exploring how contraction against tissue stiffness contributes to a functional balance between the two cell types during development. Our analyses extend to the nuclear structure proteins called lamins, and the approach seems to provide new mechanistic insight into some of the otherwise unrelated human genes most often linked to dilated cardiac myopathies, namely lamin-A and the myosin-II motors[9].
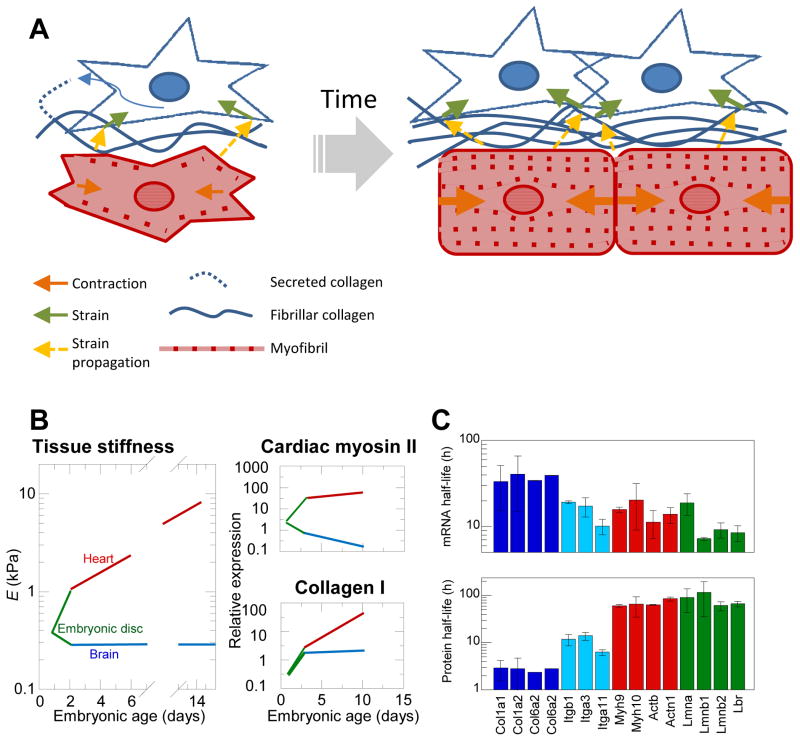
Fig. 1. Cardiomyocytes and Fibroblasts create a balance between contractile ability and ECM abundance during development.
(A) Schema illustrating concept of how such a balance could be struck. Early in development, cardiomyocytes are relatively small with unorganized and relatively spare myofibril content. Cardiac fibroblasts feel strain from passive and active contraction of surrounding cells (orange arrows) propagated through the ECM and cell-cell adhesions (yellow arrows) prompting them to divide and produce ECM in a strain and growth-factor responsive manner. The increased ECM due to increased CF population prompts increased production of myofibril proteins and encourages myofibril organization, which in turn increases contractile strain on the cardiac fibroblasts. We propose that fibroblast population growth is at least in part limited by stiffness conferred significantly by collagenous matrix production, leading to an ultimate steady state of cardiomyocyte to CF volume fractions in normal adult tissue. (B) Tissue stiffness of embryonic chick heart and brain tissue was measured to increase throughout embryonic development in a way that is paralleled with both collagen-I and cardiac myosin-II expression. (C) Half-lives of collagens (dark blue) and collagen-binding integrins (light blue), actomyosin contractility (red), and nuclear Lamin mRNAs and proteins measured coincidently in NIH3T3 mouse fibroblasts. Half-lives are fairly constant within functional groups suggesting similar dynamics within groups.
Flow stresses in heart development
As the first functional organ in the developing vertebrate embryo, the heart begins contracting to pump blood throughout the rapidly growing organism, allowing for transport of oxygen and various nutrients and other biochemical signals throughout the no longer diffusively accessible tissue. In addition to transport, blood flow imposes both pressures and shear stresses on the developing heart and vasculature, which has important developmental consequences to cellular differentiation, proliferation and growth as well as tissue-scale morphological events[10]. For example, initial looping of the earliest heart tube occurs independently of blood flow[11], but subsequent vascularization and importantly valvulogenesis are highly sensitive to blood flow[12]. Expression of important regulators of cardiomyocyte differentiation (NOS-3, ET-1, and KLF-2) demonstrate mechanosensitivity to flow, increasing or decreasing in regions of the developing heart with higher or lower shear stress[13]. TGFβ, a growth factor important to fibroblast differentiation to more contractile phenotype, also contributes to many events in cardiogenesis[12], but TGFβ binds ECM and is mechanically activated and released by cellular tension and also by fluid shear forces that stretch the large latent complex of TGFβ in plasma[14, 15]. Such release likely contributes to cardiac fibrosis in pressure-overload disease[16]. In normal development and aging, the heart must continuously produce and respond to adequate blood flow both for the maintenance of physiological function and for the biomechanical and biochemical cues it provides. Thus, as the heart grows to pump more blood, it must provide a greater driving pressure so that both contractile capacity of the cardiomyocytes and mechanical integrity of the ECM must increase.
Cardiac fibroblast and collagen content in development increase but saturate
Cardiac fibroblasts are mesenchymal cells that arise primarily from the proepicardial region of the developing heart tube [17] and are the primary ECM-producing cells in the heart[6]. They also play roles in electrochemical and mechanical signaling [18]. In injuries such as infarcts that cause cardiomyocyte death, CF’s rapidly proliferate and contribute to a collagen-rich scar at the site of injury, in contrast with adult cardiomyocytes that primarily hypertrophy to compensate for the altered mechanics and contractility of scar tissue [19].
Quantifying the CF population has been difficult due to a lack of reliable markers[20]. Discoidin Domain Receptor 2 (DDR2), periostin, cadherin-11 in combination with mesenchymal proteins vimentin and fibronectin have recently been used as CF markers and have allowed for more precise studies of when and where CFs arise in the heart and how this cell population develops[21, 22]. However, quantitative studies of cellular populations by numbers or volume fraction in tissue have thus-far focused on late-embryonic to adult and aging hearts in various organisms[21, 23] with little understanding of early development and underlying growth/secretion activity. Relative fibroblast populations in adult hearts across species are typically attributed to requirements of increased collagen needed to withstand greater pressures in larger organisms, for example[21, 17]. Similarly, increased collagen and fibroblast population are associated with periods of significant growth and postnatal developmental events that involve stiffening from a few hundred Pascal to a dozen kilo Pascals or higher[24, 21]. Pathological stiffening in response to disease or injury is also associated with increased collagen and local fibroblast population[25].
While most of the markers discussed above are not expressed until later in development[23], collagen content across species and through development has been measured for decades. Collagen-I is first detected at low but measurable levels in embryonic day 3 (E3) chick heart tube[26]. Recent proteomic measurements of heart and brain during embryonic chick development show that this increase in matrix is matched by contractile proteins and parallels the increase in tissue mechanics (Fig. 1B). By the time the heart tube first begins beating, the cellular make-up is thought to be fairly homogenous and primarily composed of early cardiomyocytes[27]. How the cellular make-up of the early heart tube develops to that of adult tissue remains unclear, but CFs must increase to relatively stable levels in adulthood[21].
Systems biology and structural gene modules in cardiac physiology and development
To address the problem of how these complicated mechanical and chemical effects ultimately affect fibroblast proliferation, collagen deposition and the associated increase in contractile capacity of the myocardium would likely benefit from an integrative analysis of known contributory factors. A systems biology approach in which the relevant gene message and protein dynamics are explicit in an appropriate model that captures both ECM and contractile protein behavior could prove useful in predicting and understanding perturbations, especially the diseases that affect the many structural proteins.
Systems biology approaches in cardiac physiology and pathophysiology have the potential to help build an integrated understanding of the electrophysiological and physical processes involved in cardiac function[28]. Such approaches might also help identify drug targets[29]. Cardiac fibroblasts should be included and appear mechanosensitive, at least in culture. Static or cyclic, uniaxial or biaxial strain has been found to modulate ECM production by such fibroblasts in a strain-dependent manner, with moderate strain inducing ECM production and large strain decreasing ECM production[30]. In vivo, such responses are likely complicated by mechanically stimulated paracrine signaling molecules that are also known to influence ECM production and proliferation rates of CFs[7]. Nonetheless, collagens are the most abundant proteins by mass in animals and have clear structural functions.
Fully understanding all of the details of how the balance of mechanical stiffness and contractile ability of myocardium is achieved and yet changes with age and pathology ultimately requires a systems level model to guide hypotheses. Such a model should explicitly include the various components of the developing heart matrix and cytoskeleton as well as any other functionally relevant signaling proteins integrated with a realistic physical model of the associated mechanics. However, as myocardial stiffening parallels expression of actomyosin contractility proteins and collagen I (Fig. 1B) among hundreds of the most abundant proteins [1], we consider initially a simplified system focusing on the interaction between the mechanical contribution of collagenous ECM deposited by fibroblasts versus the contractility of cardiomyocytes.
Genome-wide measurements of the production and degradation dynamics of mRNA and protein in NIH3T3 mouse fibroblasts have shown that mRNA and protein half-lives are fairly constant within structural groupings or modules of key collagens, integrins, and actomyosin components [Fig. 1C, data from ref. [31]]. Whereas the collagen and actomyosin modules differ significantly in half-lives, the integrins exhibit intermediate half-lives consistent perhaps with these membrane proteins serving as an intermediary link between matrix and the actomyosin cytoskeleton. Even the nucleus with structural proteins at the envelope called lamins exhibit largely coordinated expression as a module. Moreover, lamina module half-lives approximate those of the actomyosin module, which seems consistent with coordinated responses of the nuclear lamina to actomyosin stresses– as discussed below. Overall, such data for a single fibroblastic cell line in culture suggests a simplified, modular analysis of the molecular systems biology in which we refer to Collagen-I as representative of the extracellular matrix module and Myosin-II as representative of the actomyosin contractility module of the myocardium.
Model for Mechanical coupling between Collagen and Myosin production
Since both static and cyclic strains encourage collagen production by CFs, and both passive and active contraction increases in heart tissue through embryonic development, what mechanism might ultimately create the balance between CFs and cardiomyocytes? As contractility, and therefore myosin expression, must always effectively strain the heart tissue, we postulate that fibroblast proliferation is ultimately limited by the stiffness of their environment, which correlates strongly with collagenous matrix density[32, 1].
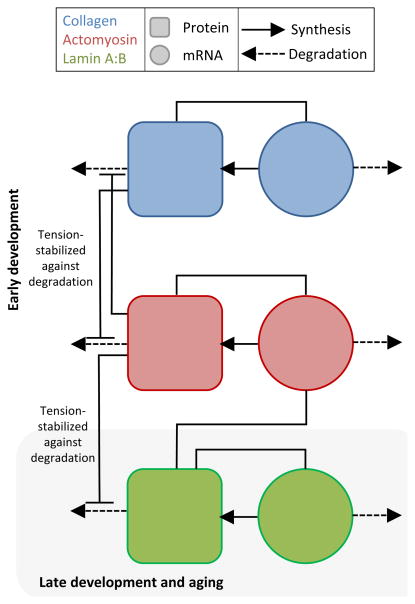
To explore possible general mechanisms, we propose a coupled network model of myosin and collagen mRNAs and proteins within developing cardiac tissue (Fig. 2). With collagen produced primarily by CFs, the rate of collagen mRNA production is assumed proportional to the fibroblast population but also regulated by collagen concentration by our postulate above. Cardiomyocytes are of course the primary contributors of muscle myosin-II in cardiac tissue. Importantly, collagen matrices have been shown to be stabilized from degradation by applied tension[33]; likewise, myosin-IIs under tension remain assembled and abundant [32, 1]– with some evidence of tension-suppressed phosphorylation of nonmuscle myosin-II suggesting an intermediate step [34]. Striated muscle myosin-II is certainly degraded in vivo[35], and disuse no doubt favors degradation and muscle atrophy. The detailed molecular scale mechanism for tension-stabilization for rope-like, coiled-coil polymers is not known, but tension in polymer fibers and network is thought to sterically or conformationally prevent protease binding to collagen fibers or kinase binding that leads to dissociation and digestion of myosin minifilaments[32]. While single molecule studies of collagen suggest tension-enhanced degradation [36], such short polymers would tend to unwind under tension where as rope like polymers would tend to tighten their coils and knots. Regardless, protein turnover appears mechano-regulated.
Fig. 2. Network model of interplay between ECM, actomyosin, and nuclear lamina genetic modules in cardiac tissue development.
Collagen and myosin modules are modeled as simple genetic regulatory network in which the protein is formed proportionally to the amount of mRNA, mRNA is produced at a protein-dependentrate, and both proteins and mRNA degrade in relation to the applied tension that stabilizes the protein networks against degradation. The nuclear lamin module can be coupled in turn to the myosin module with the same mutually applied tension stabilization against degradation. This coupling is an example of the types of possible testable implications of the mechanical interactions between the protein structural networks.
From ECM to Nucleus: Lamins in development and heart disease
Beyond ECM and acto-myosin, a host of extremely important mechanosensitive signaling pathways exist at the ECM-cellular interface. These include the large super family of integrins, but since the integrins as a group have typical half-lives for mRNA and protein between those of collagens and acto-myosins (Fig.1C), integrins are intermediates from a systems viewpoint and do not define or delimit the broader range of expression dynamics. However, implicit in this interplay is communication of extracellular and intracellular mechanics with the expression machinery of the nucleus. Mechanosensitivity at the cell-ECM or cell-cell interface contributes to biochemical signaling pathways that lead to changes in activity in the nucleus[37], but mechanical signals from the extracellular environment can also be transmitted physically by the contractile cytoskeleton to the nucleus via connections through the nuclear membrane to the nuclear lamina[38]. The nuclear lamina is composed of a meshwork of filamentous proteins that confer mechanical stability to the nucleus and interact with chromatin and various proteins that regulate transcription. Laminsare intermediate filaments found in all metazoans, and in adult vertebrates, A and B type lamins are expressed with a lamin-A:B ratio that scales with tissue stiffness and also enhances mechanically directed differentiation[32].
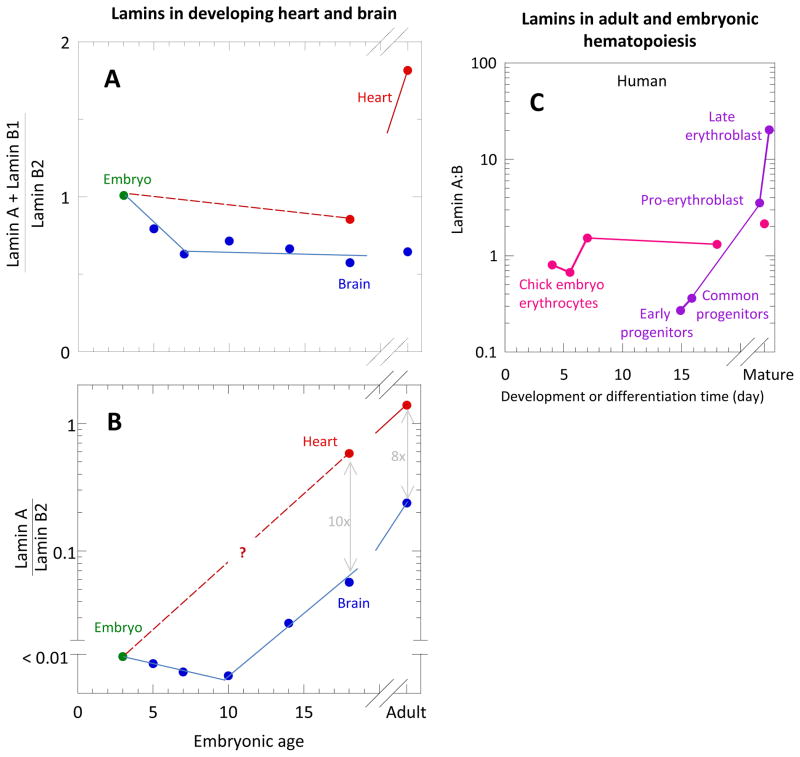
Lamin expression is developmentally regulated and plays a role in tissue-specific maturation. Developmental studies in mouse[39], frog[40], and chicken[41]show that lamin-A is typically expressed first in muscle and not until late embryogenesis or shortly after birth, but continues to increase into adulthood. Quantitative immunoblotting measurements in developing chick embryos demonstrated differential expression of laminsB2, B1 and A in various tissues[41] in what seem to be the only such measurements throughout early embryonic chick development. Lamin-B2 was constitutively expressed at relatively stable levels, but lamin-B1 and lamin-A were variably expressed in tissues through time. In brain, the total lamin-A plusB1 normalized by lamin-B2 remained relatively constant (Fig. 3A). This total amount is dominated by lamin B1 throughout development and aging. Although the ratio of lamin-A to lamin-B2 was negligible before E10, it then increases to a non-negligible level into adulthood (Fig. 3B). Data is lacking for heart embryogenesis, but by late embryogenesis and into adulthood, lamin-A is the major isoform and constitutes much more of the heart cell nuclear lamina than in the brain, consistent with recent Mass Spectrometry studies on mouse [32].
Fig. 3. Lamin levels in developing heart and brain.
[41, 47]. (A) Total variable lamins A plusB1 normalized by constant lamin-B2 in brain (blue) and heart (red). In brain, this total remained relatively constant; in heart, this total increases from late embryogenesis to adult levels. (B) Lamin-A to lamin-B2 for brain (blue) and heart (red). In brain, the variable lamins is dominated by lamin-B1 throughout the development and aging, although, while the ratio of lamin-A:B was negligible before E10, it then increases to a non-negligible level adulthood. In heart, measurements are more sparse and missing during early embryogenesis. However, by late embryogenesis and into adulthood, lamin-A is the major variable isoform and constitutes much more of the heart cell nuclear lamina than in the brain. (C) Lamin-A:B for embryonic chick erythrocytes as measured by Lehner et al. (1987) compared to the same for adult mouse hematopoietic cells as measured by Shin et al. (2013) (inset).
In a broad review of genetic mechanisms underlying dilated cardiac myopathy (DCM), mutations in the lamin-A gene LMNA were were among the most common DCM mutations[9]. Families with autosomal dominant DCM and conduction-system disease show defects in Lamin-A’s coiled rod-domain and C-terminal domain [42]. LMNA gene defects account for 33% of DCM with atrioventricular block, a common conduction disorder[43]. In a later broader study of unrelated patients with DCM, LMNA mutations occurred in 6% of patients with a general absence of a broader muscular dystrophy phenotype[44]. Myocyte nuclei appeared damaged, which could lead to myocyte death and could also mislocalize and dysregulate muscle specific genes[45]. Altered lamin-A assembly and interaction with another nuclear protein, emerin, could lead to dysregulation of nuclear actin and nuclear-cytoplasmic shuttling of MKL1, a critical transcription factor incardiac development and function[46]. Proper Lamin-A expression in developing and mature cardiac tissue is critical to tissue maintenance from a structural to transcriptional level.
Lamins in the stressful process of blood development
In development of the incessantly beating heart, mechanical linkage of ECM to the contractile cytoskeleton and to the nucleus seems critical, and should physically impact nuclear integrity. However, cells need not be adherent for the cytoskeletal-nuclear mechanical interplay to have significant consequences. Hematopoiesis of stem cells and progenitors in adult humans, for example, is accompanied by large, systematic changes in lamin-A:B[47]. These changes likely reflect the mechanical stress requirements of the various cell types, including those that differentiate and migrate across small pores in the marrow endothelium to ultimately circulate and survive the shear stresses of blood flow(Fig. 3C). Externally imposed shear stresses regulate embryonic hematopoiesis[48], and in mouse embryos the transcriptional regulators of hematopoiesis are expressed in the vascular endothelium soon after blood flow begins[49]. Interestingly, avian blood cells are all nucleated, and so the lamin stoichiometry of maturing blood cells can be measured[41] even after the cells have transitioned from adherent cells in soft tissue to a fluid environment. Such measurements are perhaps usefully compared to the human hematopoietic cells that eventually enucleate in final erythropoiesis[47] (Fig 3C).
It seems an interesting proposition that the diverse white cells found in all sorts of tissues, the nucleated red cells, and nucleated thrombocytes found in the circulation of birds and lower species, and the early hematopoietic stem cells and progenitors all might have lamin levels that are relate to the local stresses in their microenvironment. The feedback effect of lamins on gene expression adds to the intriguing possibilities.
Lung development and the protective lamina
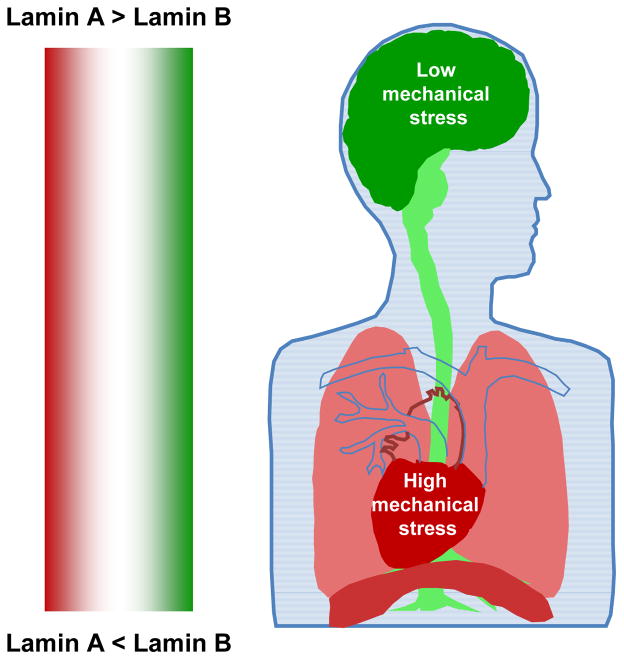
Branching morphogenesis in avian lung depends on apical constriction of the epithelial cells in the original bronchial tube, and the branching process requires fibronectin [50] as well as moderate cell tension and cell shape changes[51]. Any associated strains and distortions of lung nuclei would be sustained only by lamin-B because it is expressed in all embryonic tissue whereas lamin-A appears in mouse lung at postnatal day-1 even though lamin-A appears elsewhere by embryonic day-12 [39]. Adult lung, on the other hand, has more lamin-A than lamin-B (Fig. 4) with a stoichiometry fitting a general power law versus tissue stiffness in which lung is softer than heart and muscle but stiffer than bone marrow and neural tissue[32]. Stresses in and on the lung and its nuclei thus seem to increase from birth to adulthood and seem likely to reflect inflation of the lung driven by the diaphragm muscle. Indeed, mouse embryos with mutated lamin-B exhibit abnormal development of neural tissue and lung, with fewer and smaller alveoli and a thicker surrounding mesenchyme than wild-type mice[52]. Lamin-B null mice fail to breathe at birth and likely die (in part) because the diaphragm (which should have a high muscle-like lamin-A:B stoichiometry) is abnormally thin and poorly innervated [53]. Excessive apoptosis of neural progenitor cells within lamin-B mutant embryos[54, 53]is likely to occur during migration of such cells through dense tissue because the lamins protect against cell death in 3D migration as they confer nuclear stiffness and thereby regulate migration[55]. These functions of the lamins as well as the mechano responsiveness of the lamins [32]can thus help explain the development of lung as well as some defects.
Fig. 4. Schema of adult tissue Lamin A:B stoichiometries.
Summary figure of the lamin-A:B stoichiometries of the heart (high, red), brain (low, green) and the intermediate Lung, with heterogeneous lung tissue, diaphragm muscle (high), and nerves(low).
Model of Lamin levels in response to Myosin
Mechanical coupling between the nuclear lamins and myosin in the model of Fig. 2 represents the physical interplay between the nuclear lamina and the cytoskeletal contractile proteins, respectively. The mechanosensitive response of lamins are largely downstream of the cytoskeleton-ECM module since Lamin A is expressed later in embryonic development than most early organoagenesis and seem to continue change in expression level even into neonatal development of animal models. Lamin-mutants are typically born with all lineages, and laminopathy related diseases are often characterized by late onset, indicating that proper lamin expression is important in tissue maintenance and aging response. Descriptions of the communication from ECM to the nuclear lamina are nicely reviewed in [56].
Unlike the myosin-collagen model above, lamin and myosin are produced within the same cell (as every cell has a nucleus!), so relative cell densities in tissue do not need to be taken into account. As with myosins, lamins in interphase cells seem likely to dissociate from their meshwork and be degraded when phosphorylated with the first evidence for such a process obtained recently by Mass Spectrometry of cell lysates [32]. This is not always the case, as in dividing cells phosphorylation of lamins classically precedes envelope disassembly without significant degradation. However, in interphase cells on soft substrates – which exhibit fewer stress fibers and less contractility – show more lamin-A phosphorylation and less lamin-A and no perturbations to mitosis (where cells always round up). Follow-up studies using a phosphospecific antibody for lamin-A and phospho-mimetic constructs of lamin-A (Buxboim et al. unpublished) demonstrate the expected lamin-A mechanosensitivity to matrix stiffness in all interphase cells as well as the expected effects on nuclear rheology. Ultimately, with more measurements of lamin levels in developing tissue, this type of coupled modeling could be extended to include coupling of ECM to cytoskeleton to nucleus in the highly stressful and dynamic processes of development.
Conclusions
During development and aging, the establishment and maintenance of proper mechanics is essential from the cellular to organ scale. Primarily focusing on the heart, this review outlines how a balance is required and struck between contractile components and the stiffening ECM in tissue, and how this mechanical coupling in turn couples mechanically to the nucleus. The network model presented here has the primary aim of succinctly addressing where molecular mechanics might enter into otherwise canonical expressions for synthesis and degradation of key structural factors that dictate cell and tissue structure and mechanics. This is a central issue in mechanobiology since there is no more important response than for a cell to transduce a stress or strain into a change in protein level reflected at the transcript level. It could be that forces directly affect transcription, degradation of transcript, and/or protein synthesis, but if transcription somehow couples to net protein levels, a ‘use it or lose it’ mechanism of protein degradation is a sufficient and likely mechanism for mechanical stresses to control key structural gene circuits. Broader uses of such circuit models are clearly ‘hot topics’ of stem cell differentiation, maturation to a functional lineage, and dysfunction in disease.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Majkut SF, Idema T, Swift J, Krieger C, Liu A, Discher D. Heart stiffening in early embryos parallels matrix and myosin levels to optimize beating. Current Biology. 2013 doi: 10.1016/j.cub.2013.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCain M, Parker K. Mechanotransduction: the role of mechanical stress, myocyte shape, and cytoskeletal architectre on cardiac form and function. Pflugers Arch - Eur J Physiol. 2011;462:89–104. doi: 10.1007/s00424-011-0951-4. [DOI] [PubMed] [Google Scholar]
- 3.Majkut S, Discher D. Cardiomyocytes from late embryos and neonates do optimal work and striate best on substrates with tissue-level elasticity: metris and mathematics. Biomechanics and modeling in mechanobiology. 2012;11(8):1219–1225. doi: 10.1007/s10237-012-0413-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chung C, Pruitt B, Heilshorn S. Spontaneous cardiomyocyte differentiation of mouse embryoid bodies regulated by hydrogel rosslin density. Biomaterials Science. 2013;2013(1):1082–1090. doi: 10.1039/C3BM60139K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Young J, Engler A. Hydrogels with time-dependent material properties enhance cardiomyocyte differentiation in vitro. Biomaterials. 2011;32(4):1002–1009. doi: 10.1016/j.biomaterials.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eghbali M, Czaja M, Zeydel M, Weiner F, Zern M, Seifter S, Blumenfeld O. Collagen chain mRNAs in isolated heart cells from young and adult rats. Journal of Molecular and Cellular Cardiology. 1988;20(3):267–276. doi: 10.1016/s0022-2828(88)80059-2. [DOI] [PubMed] [Google Scholar]
- 7.Kakkar R, Lee R. Intramyocardial fibroblast myocyte communication. Circulation Research. 2010;106:47–57. doi: 10.1161/CIRCRESAHA.109.207456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Samarel A. Costameres, focal adhesions, and cardiomyocyte mechanotransduction. American Journal of Physiology: Heart and Circulatory Physiology. 2005;289:H2291–H2301. doi: 10.1152/ajpheart.00749.2005. [DOI] [PubMed] [Google Scholar]
- 9.McNally E, Golbus J, Puckelwartz M. Genetic mutations and mechanisms in dilated cardiomyopathy. Journal of Clinical Investigation. 2013;123(1):19–26. doi: 10.1172/JCI62862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hierck B, Van der Heiden K, Poelma C, Westerweel J, Poelmann R. Fluid shear stress and inner curvature remodeling of the embryonic heart. Choosing the right lane! The Scientific World Journal. 2008;8:212–222. doi: 10.1100/tsw.2008.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taber LA. Biophysical mechanisms of cardiac looping. International Journal of Developmental Biology. 2006;50:323–332. doi: 10.1387/ijdb.052045lt. [DOI] [PubMed] [Google Scholar]
- 12.Butcher J, Markwald R. Valvulogenesis: the moving target. Phil Trans R Soc B. 2007;362(1484):1489–1503. doi: 10.1098/rstb.2007.2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Groenendijk B, Hierck B, Gittenberger-de Groot A, Poelmann R. Development-related changes in the expression of shear stress responsive genes KLF-2, ET-1, and NoS-3 in the developing cardiovascular system of chicken embryos. Developmental Dynamics. 2004;230(1):57–68. doi: 10.1002/dvdy.20029. [DOI] [PubMed] [Google Scholar]
- 14.Tenney R, Discher D. The interplay between stem cells microenvironment mechanics and growth factor activation. Current Opinion in Cell Biology. 2009;21(5):630–635. doi: 10.1016/j.ceb.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahamed J, Burg N, Yoshinaga KJC, Rifkin D, Coller B. In vitro and in vivo evidence for shear induced activation of latent transforming growth factor-beta-1. Blood. 2008;112(9):3650–3660. doi: 10.1182/blood-2008-04-151753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meyer A, Wang W, Qu J, Croft L, Degen JCB, Ahamed J. Platelet TGF-beta1 contributions to plasma TGF-beta1, cardiac fibrosis, and systolic dysfunction in a mouse model of pressure overload. Blood. 2012;119(4):1064–1074. doi: 10.1182/blood-2011-09-377648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Souders C, Bowers S, Baudino T. Cardiac fibroblast: the Renaissance cell. Circulation Research. 2009;105:1164–1176. doi: 10.1161/CIRCRESAHA.109.209809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bowers S, Banerjee I, Baudino T. The extracellular matrix: At the center of it all. Journal of Molecular and Cellular Cardiology. 2010;48(3):474–482. doi: 10.1016/j.yjmcc.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sadoshima J, Izumo S. The cellular and molecular response of cardiac myocytes to mechanical stress. Annual Review of physiology. 1997;59:551–571. doi: 10.1146/annurev.physiol.59.1.551. [DOI] [PubMed] [Google Scholar]
- 20.Goldsmith E, Hoffman A, Morales M, Potts J, Price R, McFadden A, Rice M, Borg T. Organization of the fibroblasts in the heart. Developmental Dynamics. 2004;230(4):787–794. doi: 10.1002/dvdy.20095. [DOI] [PubMed] [Google Scholar]
- 21.Banerjee I, Fuseler J, Price R, Borg T, Baudino T. Determination of cell types and numbers during cardiac development in the neonatal and adult rat and mouse. American Journal of Physiology: Heart and Circulatory Physiology. 2007;293(3):H1883–H1891. doi: 10.1152/ajpheart.00514.2007. [DOI] [PubMed] [Google Scholar]
- 22.Snider P, Standley K, Wang J, Azhar M, Doetschman T, Conway S. Origin of cardiac fibroblasts and the role of Periostin. Circulation Research. 2009;105:934–047. doi: 10.1161/CIRCRESAHA.109.201400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruiz-Villalba A, Ziogas A, Ehrbar M, Perez-Pomares JM. Characterization of epicardial-derived cardiac interstitial cells: differentiation and mobilization of heart fibroblast progenitors. PLoS ONE. 2013;8(1):e52694. doi: 10.1371/journal.pone.0053694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacot J, Martin J, Hunt D. Mechanobiology of cardiomyocyte development. Journal of Biomechanics. 2010;43(1):93–98. doi: 10.1016/j.jbiomech.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jalil J, Doering C, Janicki J, Pick R, Shroff S, Weber K. Fibrillar collagen and myocardial stiffness in the intact hypertrophied rat left ventricle. Circulation Research. 1989;64:1041–1050. doi: 10.1161/01.res.64.6.1041. [DOI] [PubMed] [Google Scholar]
- 26.Woessner J, Jr, Bashey R, Boucek R. Collagen development in the heart and skin of the chick embryo. Biochimica et Biophysica Acta - Protein Structure. 1967;140(2):329–338. doi: 10.1016/0005-2795(67)90473-4. [DOI] [PubMed] [Google Scholar]
- 27.Gittenberger-de Groot A, Vrancken Peeters M, Mentink M, Gourdie R, Poelmann R. Epicardium-derived cells contribute a novel population to the myocardial wall and the atrioventricular cushions. Circulation Research. 1998;82(10):1043–1052. doi: 10.1161/01.res.82.10.1043. [DOI] [PubMed] [Google Scholar]
- 28.McCulloch A, Paternostro G. Cardiac Systems Biology. Annals of the New York Academy of Sciences. 2005;1047:283–295. doi: 10.1196/annals.1341.025. [DOI] [PubMed] [Google Scholar]
- 29.Ryall K, Holland D, Delaney K, Kraeutler M, Parker A, Saucerman J. Network reconstruction and systems analysis of cardiac myocyte hypertrophy signalling. Journal of Biological Chemistry. 2012;287(50):42259–42268. doi: 10.1074/jbc.M112.382937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.MacKenna D, Summerour S, Villarreal F. Role of mechanical factors in modulating cardiac fibroblast function and extracellular matrix synthesis. Cardiovascular Research. 2000;46(2):257–263. doi: 10.1016/s0008-6363(00)00030-4. [DOI] [PubMed] [Google Scholar]
- 31.Schwanhausser B, Busse D, Li N, Dittmar G, Schuchhardt J, Wolf J, Chen W, Selbach M. Global quantification of mammalian gene expression control. Nature. 2011;473:337–342. doi: 10.1038/nature10098. [DOI] [PubMed] [Google Scholar]
- 32.Swift J, Ivanovska I, Buxboim A, Harada T, Dingal P, Pinter J, Pajerowski J, Spinler K, Shin J, Tewari M, Rehfeldt F, Speicher D, Discher D. Nuclear Lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science. 2013;341:1240104-1–15. doi: 10.1126/science.1240104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Flynn B, Bhole A, Saeidi N, Liles M, DiMarzio C, Ruberti J. Mechanical strain stabilizes reconstituted collagen fibrils against enzymatic degradation by mammalian collagenase matrix metalloproteinase 8 (MMP-8) PLoS ONE. 2010;5(8):e12337. doi: 10.1371/journal.pone.0012337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raab M, Swift J, Dingal P, Shah P, Shin J, Discher D. Crawling from soft to stiff matrix polarizes the cytoskeleton and phosphoregulates myosin-II heavy chain. Journal of Cell Biology. 2012;199(4):669–683. doi: 10.1083/jcb.201205056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ball R, Krus D, Alizadeh B. Myosin degradation fragments in skeletal muscle. Journal of Molecular Biology. 1987;193(1):47–56. doi: 10.1016/0022-2836(87)90625-5. [DOI] [PubMed] [Google Scholar]
- 36.Adhikari A, Chai J, Dunn A. Mechanical load induces a 100-fold increase in the rate of collagen proteolysis by MMP-1. Journal of the American Chemical Society. 2011;133(6):1686–1689. doi: 10.1021/ja109972p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Discher D, Janmey P, Wang Y. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310(5751):1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 38.Haque FLD, Smallwood Dent D, Shanahan Fry CLC, Trembath AMR, Shackleton S. SUN1 interacts with nuclear lamin A and cytoplasmic nesprins to provide a physical connection between the nuclear lamina and the cytoskeleton. Mollecular and Cellular Biology. 2006;26(10):3738–3751. doi: 10.1128/MCB.26.10.3738-3751.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rober R, Weber K, Osborn M. Differential timing of nuclear lamin A/C expression in the various organs of mouse embryo and young animal: a developmental study. Development. 1989;105:365–378. doi: 10.1242/dev.105.2.365. [DOI] [PubMed] [Google Scholar]
- 40.Benavente R, Krohne G, Franke WW. Cell type-specific expression of nuclear lamina proteins during development of Xenopus laevis. Cell. 1985;41(1):177–190. doi: 10.1016/0092-8674(85)90072-8. [DOI] [PubMed] [Google Scholar]
- 41.Lehner C, Stick R, Eppenberger H, Nigg E. Differential expression of nuclear lamin proteins during chicken development. Journal of Cell Biology. 1987;105(1):577–587. doi: 10.1083/jcb.105.1.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fatkin D, MacRae C, Sasaki T, Wolff MpM, Frenneaux M, Atherton J, Vidaillet H, Spudich SGU, Seidman J, seidman C. Missense mutations in the rod domain of the Lamin A/C gene as causes of dilated cardiomyopathy and conduction-system disease. New England Journal of Medicine. 1999;341(23):1716–1724. doi: 10.1056/NEJM199912023412302. [DOI] [PubMed] [Google Scholar]
- 43.Arbustini E, Pilotto A, Repetto A, Grasso M, Negri A, Diegoli M, Campana C, Scelsi L, Baldini E, Gavazzi A, Tavazzi L. Autosomal dominant dilated cardiomyopathy with atrioventricular block: a Lamin A/C defect-related disease. Journal of the American College of Cardiology. 2002;39(6):981–990. doi: 10.1016/s0735-1097(02)01724-2. [DOI] [PubMed] [Google Scholar]
- 44.Parks S, Kushner J, Nauman D, Burgess DLS, Peterson A, Li D, Jakobs P, Litt M, Porter C, Rahko P, Hershberger R. Lamin A/C mutation analysis in a cohort of 324 unrelated patients with idiopathic or familial dilated cardiomyopathy. American Heart Journal. 2008;156(1):161–169. doi: 10.1016/j.ahj.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zuela N, Bar DA, Gruenbaum Y. Lamins in development, tissue maintenance and stress. EMBO reports. 2012;13:1070–1078. doi: 10.1038/embor.2012.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ho CY, Jaalouk DE, Vartiainen MK, Lammerding J. Lamin A/C and emerin regulate MKL1-SRF activity by modulating actin dynamics. Nature. 2013;497:507–511. doi: 10.1038/nature12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shin J, Spinler K, Swift J, Chasis J, Mohandas N, Discher D. Lamins regulate cell trafficking and lineage maturation of adult human hematopoietic cells. PNAS. 2013;110(47):188892–18897. doi: 10.1073/pnas.1304996110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Adamo LNO, Wenzel P, McKinney-Freeman S, Mack P, Gracia-Sancho J, Suchy-Dicey A, Yoshimoto Biomechanical forces promote embryonic haematopoiesis. Nature. 2009;459:1131–1135. doi: 10.1038/nature08073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Banjo T, Grajcarek J, Yoshino D, Osada H, Miyasaka K, Kida Y, Ueki Y, Nagayama K, Kawakami K, Matsumoto T, Sato M, Ogura T. Haemodynamically dependent valvulogenesis of zebrafish heart is mediated by flow-dependent expression of miR-21. Nature Communications. 2012;4 doi: 10.1038/ncomms2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sakai T, Larsen M, Yamada K. Fibronectin requirement in branching morphogenesis. Nature. 2003;423(6942):876–881. doi: 10.1038/nature01712. [DOI] [PubMed] [Google Scholar]
- 51.Kim H, Varner V, Nelson C. Apical constriction initiates new bud formation during monopodial branching of the embryonic chicken lung. Development. 2013;140:3146–3155. doi: 10.1242/dev.093682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vergnes L, Peterfy M, Bergo M, Young S. Lamin B1 is required for mouse development and nuclear integrity. PNAS. 2004;101(28):10428–10433. doi: 10.1073/pnas.0401424101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim Y, Sharov AA, McDole K, Cheng M, Hao H, Fan CM, Gaiano N, Ko MS, Zheng Y. Mouse B-type lamins are required for proper organogenesis but not by embryonic stem cells. Science. 2011;334(6063):1706–1710. doi: 10.1126/science.1211222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Coffinier C, Jung H, Nobumori C, Chang S, Tu Y, Barnes R2, Yoshinaga Y, de Jong P, Vergnes L, Reue K, Fong L, Young S. Deficiencies of lamin B1 and lamin B2 cause neurodevelopmental defects and distinct nuclear shape and abnormalities in neurons. Molecular Biology of the Cell. 2011;22(23):4683–4693. doi: 10.1091/mbc.E11-06-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Harada T. Doctoral Dissertation. University of Pennsylvania; 2013. From engineering of tumor shrinking DDS to engineering the nucleus for tumor growth. [Google Scholar]
- 56.Wang N, Tytell J, Ingber D. Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nature Reviews Molecular Cell Biology. 2009;10:75–82. doi: 10.1038/nrm2594. [DOI] [PubMed] [Google Scholar]