Abstract
Receptors coupled to G proteins have many effects on the heart. Enhanced signaling by Gαs and Gαq leads to cardiac injury and heart failure, while Gαi2 signaling in cardiac myocytes can protect against ischemic injury and β-adrenergic-induced heart failure. We asked whether enhanced Gαi2 signaling in mice could protect against heart failure using a point mutation in Gαi2 (G184S), which prevents negative regulation by regulators of G protein signaling. Contrary to our expectation, it worsened effects of a genetic dilated cardiomyopathy (DCM) and catecholamine-induced cardiac injury. Gαi2G184S/+/DCM double heterozygote mice (TG9+Gαi2G184S/+) had substantially decreased survival compared to DCM animals. Furthermore, heart weight/body weight ratios (HW/BW) were significantly greater in TG9+Gαi2G184S/+ mice as was expression of natriuretic peptide genes. Catecholamine injury in Gαi2G184S/G184S mutant mice produced markedly increased isoproterenol-induced fibrosis and collagen III gene expression vs WT mice. Cardiac fibroblasts from Gαi2G184S/G184S mice also showed a serum-dependent increase in proliferation and ERK phosphorylation, which were blocked by pertussis toxin and a mitogen-activated protein/extracellular signal-regulated kinase kinase inhibitor. Gαi2 signaling in cardiac myocytes protects against ischemic injury but enhancing Gαi2 signaling overall may have detrimental effects in heart failure, perhaps through actions on cardiac fibroblasts.
Keywords: Gαi2, RGS, Cardiac fibroblasts, Isoproterenol, Angiotensin
Introduction
The primary pharmacologic therapies that prolong life in congestive heart failure target beta adrenergic (βAR) and angiotensin (AT1) receptor signaling (Dzau 1992; Lechat et al. 1998; MacLellan 2000). The sympathetic and renin–angiotensin systems activate Gs- and Gq-type G proteins linked to cAMP and Ca++ signaling. There is abundant clinical and animal model data for the role of βAR and AT1R signaling and Gs and Gq to cause cardiac hypertrophy and heart failure (Schnabel and Bohm 1996).
In contrast to the deleterious effects of Gs and Gq signaling, there is significant literature on protection by Gi family G proteins, predominantly Gαi2. Activation of many Gi-coupled receptors (e.g., adenosine, sphingosine-1-phosphate, estrogen, and opioid) can reduce ischemia– reperfusion injury. Loss of Gαi2 function also worsens ischemic insults (DeGeorge et al. 2008), myocyte apoptosis (Chesley et al. 2000; DeGeorge et al. 2008), and heart failure [Gαi2 knockout mice die prematurely in a model of βAR-induced heart failure (Foerster et al. 2003)]. These observations have prompted efforts to enhance signaling through Gαi-family G proteins as a means to protect the heart.
Regulator of G protein signaling (RGS) proteins inhibit Gi and Gq protein signaling by increasing their GTPase activity (Hollinger and Hepler 2002). Blocking RGS action (Zhong and Neubig 2001; McEwen et al. 2002) could enhance protective effects of Gαi2 in the heart. As a test of this idea, we used a point mutation (G184S) in Gαi2 that blocks the binding of RGS proteins, prevents the RGS-mediated suppression of Gαi2 signaling (Lan et al. 2000; Fu et al. 2004), and enhances Gαi2 signaling in vitro and in vivo (Huang et al. 2006). We recently showed that Gαi2G184S mutant mice have reduced myocardial injury in a Langendorff ischemia– reperfusion model (Waterson et al. 2011).
While Gαi2 signaling in myocytes can protect the heart from injury and failure, increased Gαi signaling also, produces negative inotropic effects, functions in inflammatory mechanisms (Fan et al. 2005; Pero et al. 2007; Zarbock et al. 2007), and may increase fibroblast proliferation and fibrosis (Liu et al. 2006). Consequently, enhancing Gαi signaling as a means to cardioprotection may need to be selective to achieve the desired effect.
Here, we assess, in two distinct models of cardiac injury and heart failure, the effect of increased Gαi2 signaling in mice with an RGS-insensitive Gαi2 subunit. A mouse model of dilated cardiomyopathy (Tg9-DCM), like human heart failure, shows improved survival with angiotensin-converting enzyme inhibition and β1AR blockade (Buerger et al. 2006) and worsens with abnormal glucose homeostasis (Hruz et al. 2008; Vyas et al. 2011). Second, isoproterenol injections are used to induce injury and subsequent heart failure (Ozaki et al. 2002). In both models, loss of RGS action at Gαi2 led to worsened outcomes. In the DCM model, active mutant Gαi2 produced early deaths and increased hypertrophy, and in the Iso-induced injury model, markedly increased fibrosis was seen. Furthermore, cardiac fibroblasts from the Gαi2G184S/ G184S mutant mice show a pertussis toxin sensitive increase in ERK activation and proliferation in vitro and enhanced collagen gene expression in vivo. Consequently, any manipulations to enhance Gαi2 signaling in the heart should be selective for myocytes as Gαi2 signaling in other tissues, such as fibroblasts may be detrimental.
Methods
Animals
Animal care was supervised by the University of Michigan Unit for Lab Animal Medicine and all animal study protocols were approved by the University Committee on Use and Care of Animals. Animals were maintained under a 12:12-h light–dark cycle with ad libitum access to standard chow and water. Mice with a genomic knock-in of the Gαi2G184S RGS-insensitivity allele were described previously (Huang et al. 2006). All studies utilized mice crossed at least five times onto the appropriate genetic background (C57BL/6J or FVB/NJ).
The transgenic DCM model, which results from cardiac-specific over-expression of cre-recombinase, was previously described and the transgene is referred to as TG9+ (Buerger et al. 2006). Heterozygous Gαi2G184S/+ females on an FVB/NJ background were crossed with TG9+ (DCM) males also on an FVB/NJ background, yielding four genotypes: Gαi2+/+, Gαi2G184S/+, TG9+Gαi2+/+, and TG9+Gαi2G184S/+. For the isoproterenol studies, homozygous RGS-insensitive Gαi2G184S/G184S mice and littermate controls (WT) mice were on the C57Bl/6J background.
Chronic drug treatment in the DCM model
Losartan (0.6 g/L) was added to drinking water at 3 weeks of age in TG9+Gαi2+/+ vs TG9+Gαi2G184S/+ mice. Doses were chosen from a previous report showing protective effects in a vascular model (Habashi et al. 2006). Survival was assessed up to 95 days for various genotypes and treatments.
Catecholamine-induced cardiac injury
Male, Gαi2G184S/ G184S and WT mice (14–16 weeks old) were treated with Iso (30 mg/kg) or saline via intraperitoneal injection daily for 7 days (Ozaki et al. 2002).
Cardiac tissue analysis
Animals were sacrificed at 10 weeks of age (DCM study) or 24 h after the last Iso or saline injection. Hearts were removed, washed with cold phosphate-buffered saline (PBS), and fixed for histology or frozen in liquid nitrogen for biochemical studies.
Fibrosis and cell size
Samples for histology were fixed in 10% formalin for 24 h, paraffin embedded, sectioned, stained with Masson’s trichrome, imaged using light microscopy, and photographed (Zeiss Axioplan, Carl Zeiss IMT Corporation, Maple Grove, MN, USA). The percentage with collagen (blue) and without (red) was quantified using Adobe Photoshop (Dahab et al. 2004). For myocyte cross-sectional area, 10–20 randomly selected myoctyes per image were measured. Cells were outlined manually (total n=251–333 cells per condition) using the polygon selection tool ImageJ (http://rsb.info.nih.gov/nih-image). Due to the non-normal distribution of cell sizes, statistical analysis was done on the logarithm of cell area values.
mRNA analysis by qPCR
RNA isolated from frozen myocardial tissue using RNA STAT-60 reagent (Iso-Tex Diagnostics, Friendswood, TX, USA) was treated with DNase for 15 min at room temperature (Qiagen, Valencia, CA, USA) then quantitative PCR (qPCR) performed after first-strand complementary DNA (cDNA) synthesis (Taq-Man Kit, Applied Biosystems, Branchburg, NJ, USA). cDNA was then subjected to qPCR with SYBR green master mix (Stratagene, La Jolla, CA, USA) and 100 pM each of sense and antisense primer. Primers for genes relevant to cardiac hypertrophy (ANP, BNP, SMA, and Col III) and β-actin (an internal control) were described previously (Schoenfeld et al. 1998). PCR conditions were denaturation at 94°C and 10 min, then 40 cycles of denaturation at 94°C and 30 s, annealing at 60°C and 60 s, then extension at 72°C for 10 min. No-template controls and no-RT controls were run during each experiment to detect RNA and/or DNA contamination. Results are expressed as relative gene expression normalized against β-actin.
Adult cardiac fibroblast culture
Cardiac fibroblasts were isolated from 20- to 26-week-old female mice as described by O’Connell et al. (2007) with minor modifications. Briefly, after quick removal, hearts were washed in icecold PBS, then retrogradely perfused through the aorta for 5 min with calcium-free perfusion buffer (NaCl, 120.4 mM; KCl, 5 mM; MgSO4·H2O, 1.2 mM; Na2HPO4, 0.6 mM; KH2PO4, 0.6 mM; Na–HEPES, 10 mM; NaHCO3, 4.6 mM; taurine, 30 mM; glucose, 10 mM; and butanedione monoxime, 10 mM, pH 7.4). Hearts were then perfused for 10 min with the same buffer containing 0.5 mg/ml collagenase II (Worthington Biochemicals, Lakewood, NJ, USA, lot no. 46M9065). Hearts were removed from the apparatus and atria were discarded. Ventricles were suspended in perfusion buffer containing 10% serum and 1.25 µmol/L calcium to stop the digestion. Cells were separated from the tissue by gentle pipetting of the digested heart using sterile plastic transfer pipettes. Samples were allowed to settle under gravity for 15 min to remove debris and myocytes. The remaining suspended fibroblasts were centrifuged at 300×g for 10 min and resuspended in Dulbecco's modified Eagle’s medium supplemented with 1% penicillin/streptomycin, insulin/transferrin/selenium (ITS from Sigma), and 10% fetal bovine serum (full medium). Cells isolated by this method all stain positively for α-SMA (data not shown).
Fibroblast proliferation
Cardiac fibroblasts (passages 3–5) were plated in 24-well dishes (104/well) and allowed to grow in 10% “fetal bovine serum (FBS)-containing medium for the specified time in the presence or absence of pertussis toxin (PTX) (100 ng/ml). At the end of the growth period (1–4 days), medium was aspirated, cells were rinsed with Ca2+/Mg2+ free PBS, and 150 µL of 0.05% trypsin was added to each well. Cells were incubated with trypsin at 37°C for 5 min and then shaken on a plate shaker at room temperature for 5 min. Then, 50 µL of FBS (100%) was added to stop trypsin action, and 200 µL of 10% formalin was added to fix cells. Cells were collected in microcentrifuge tubes, and 200 µL of this cell suspension was used for cell counting using a flow cytometer (Accuri Cytometers, Inc. Ann Arbor, MI, USA). To study the role of ERK, the mitogen-activated protein/extracellular signal-regulated kinase kinase (MEK) inhibitor PD 098059 (30 µM, Cayman Chemical, Ann Arbor, MI, USA) was added to the medium on day 1, and cells were counted on day 3.
ERK phosphorylation measurements
Western blotting was performed to assess ERK activation using a phospho-p44/ 42 Erk1/2 Thr202/Tyr204 antibody (no. 9101, Cell Signaling Technology, Inc., Danvers, MA, USA) and a total ERK antibody (no. 9102, Cell Signaling Technology, Inc., Danvers, MA, USA). Cells were seeded at an initial density of 2×105 cells/well in 12-well plates and grown in serumcontaining medium for 24 h in the absence or presence of PTX. Cells were washed with cold PBS and lysed with RIPA buffer (Tris, 25 mM; NaCl, 150 mM; NP-40, 1%; sodium deoxycholate, 1%; sodium dodecyl sulfate, 0.1%). The cell lysates (20 µg) were then subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis, immunostained, then visualized using enhanced chemiluminescence (Thermo Scientific, Rockford, IL, USA). The band intensities (p42+p44) were quantified densitometrically, and ERK phosphorylation was expressed as the intensity of the pERK bands normalized to the intensity of the total ERK bands.
Statistical analyses
Comparisons of individual group means used a two-tailed Student’s t test. One- or two-way analysis of variance (ANOVA) with Bonferroni post-test was used to compare multiple data sets. Survival curves were compared by log-rank (Mantel–Cox) test. All statistical calculations were done using GraphPad Prism version 5 (GraphPad Software Inc., San Diego, CA, USA), and p< 0.05 was considered significant.
Results
Effect of Gαi2G184S/+ mutation on survival in DCM mice
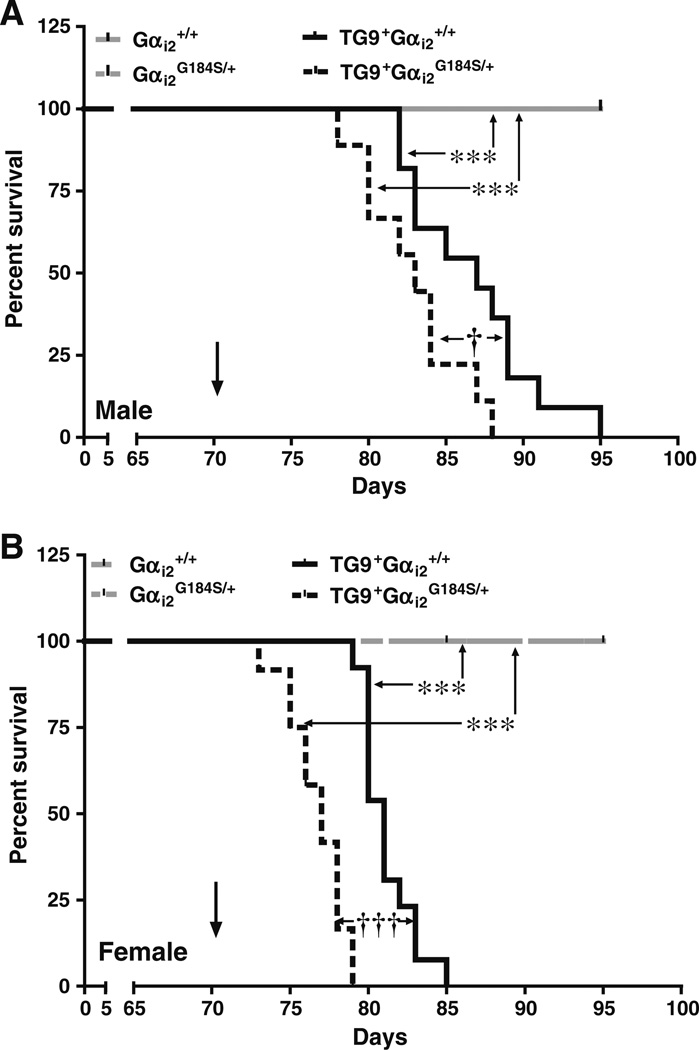
The TG9 DCM model leads to the development of congestive heart failure and death (Buerger et al. 2006). As seen previously, the maximum life span for TG9+Gαi2+/+ males was 13.5 weeks (95 days, Fig. 1a), and for females, it was about 12 weeks (85 days, Fig. 1b). There were no deaths in non-TG9 mice during the 95-day duration of the study, regardless of their Gαi2 genotype. Combining the RGS-insensitive Gαi2 mutation (Huang et al. 2006) with the TG9+ mutation significantly worsened survival. TG9+Gαi2G184S/+ male mice survived for maximum of 88 days, and females survived for only 79 days (Fig. 1a and Table 1, p<0.05 for male and Fig. 1b, p< 0.001 for female).
Fig. 1.
Effect of RGS-insensitive Gαi2G184S/+ mutation on survival of DCM mice. Kaplan–Meier survival curves in a males and b females for Gαi2+/+ (WT); Gαi2G184S/+; TG9+Gαi2+/+ (DCM); TG9+Gαi2G184S/+ (DCM/Gαi2G184S/+) were compared by log-rank (Mantel–Cox) test. ***p<0.001 compared to respective TG9-control. †p<0.05; †††p<0.001 compared to respective Gαi2+/+control. n=9–13. Arrows indicate the time at which tissues were collected in subsequent studies
Table 1.
Effect of RGS-insensitive Gαi2 mutation (Gαi2G184S/+) on average age day of death in mice with cardiac-specific overexpression of Cre (TG9+)
| Gαi2+/+ | Gαi2G184S/+ | TG9+ Gαi2+/+ | TG9+ Gαi2G184S/+ | |
|---|---|---|---|---|
| Male | ND | ND | 86.7±1.3 (n = 11) | 82.9±1.1* (n=9) |
| Female | ND | ND | 81.2±0.5 (n = 13) | 76.7±0.5**(n=12) |
Values are mean±standard error of the mean, n represents animals in each group (9–13). Differences were determined by Mantel–Cox log rank test from the survival curve analysis
ND none died up to 95 days
p<0.05
p<0.001, indicate a significant effect on survival as compared to respective Gαi2+/+ control
Effect of the Gαi2G184S/+ mutation on cardiac hypertrophy in TG9+ mice
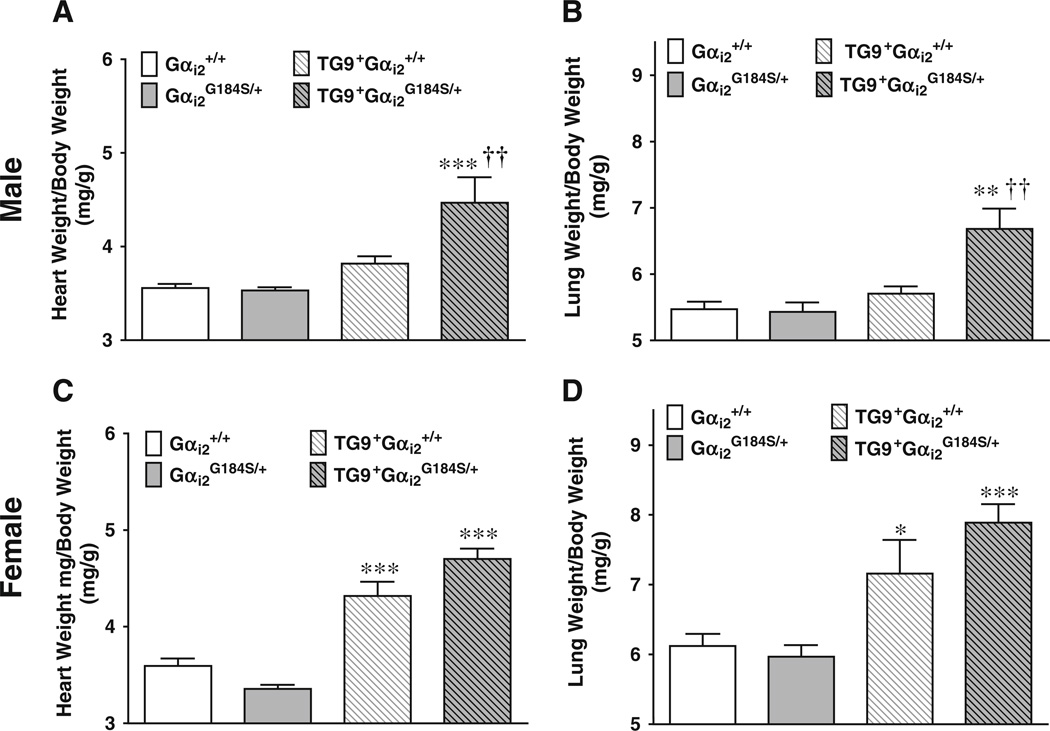
At 10 weeks of age (i.e., prior to any deaths), male DCM mice with WT Gαi2 showed a modest increase in HW/BW, which was markedly greater in the double-mutant TG9+ Gαi2G184S/+ mice (Fig. 2a, p<0.001, two-way ANOVA vs Gαi2G184S/+ controls and p<0.01 vs DCM Gαi2+/+ mice). Consistent with the earlier onset of heart failure, 10-week-old TG9+Gαi2G184S/+ male mice also showed a significant increase in lung weight to body weight ratio (LW/BW, Fig. 2b) compared to both TG9− controls (p<0.01) and TG9+ mice with WT Gαi2 (p<0.01).
Fig. 2.
Effect of Gαi2G184S/+ on the heart weight and lung weight in DCM mice. a, c HW/BW, b, d LW/BW in males (a, b) and females (c, d) at 10 weeks of age. Values are mean±standard error of the mean, n=4–6 in each group. *p<0.05 compared to non-DCM-control. †p< 0.05 compared to Gαi2+/+. Differences were determined by two-way ANOVA with Bonferroni post-test
Female mice of both Gαi2 genotypes showed significant hypertrophy at 10 weeks, 20.2±4.1% and 40.1±3.2% increase in HW/BW, respectively, for DCM Gαi2 and Gαi2G184S/+ compared to their non-DCM controls (Fig. 2c, p<0.001). The difference between DCM Gαi2G184S/+ and DCM Gαi2+/+ was not statistically significant most likely due to the more advanced progression of heart failure in females at 10 weeks. TG9+Gαi2++ and TG9+Gαi2G184S/+ female mice both had significant increases in LW/BW (17.0± 7.9% vs 32.2±4.5%, p<0.05 and p<0.001, respectively).
Effect of Gαi2G184S/+ mutation on marker gene expression and fibrosis
Heterozygous Gαi2G184S/+ mutant mice had normal expression of all four of the marker genes studied (ANP, BNP, SMA, and Col III, Figs. ESM1 and ESM2), while DCM mice had marked increases. As seen for HW/BW, male DCM mice carrying the Gαi2G184S/+ mutation showed a greater increase in ANP and BNP expression at 10 weeks vs DCM alone (ANP, p<0.001; BNP, p< 0.01). In contrast, DCM females showed major increases in natriuretic peptide gene expression, but there was no further effect of the Gαi2G184S/+ mutation (Fig. ESM2). The effect of the Gαi2G184S/+ mutation appears to have been overshadowed by the earlier onset of heart failure in the females.
Both male and female DCM mice exhibited a modest increase in fibrosis compared to non-DCM mice, but there was no augmentation when they were carrying the Gαi2G184S/+ mutation vs WT Gαi2 (data not shown). This result was in agreement with the levels of expression seen for Col III (Figs. ESM1D and ESM2D).
Effect of losartan treatment
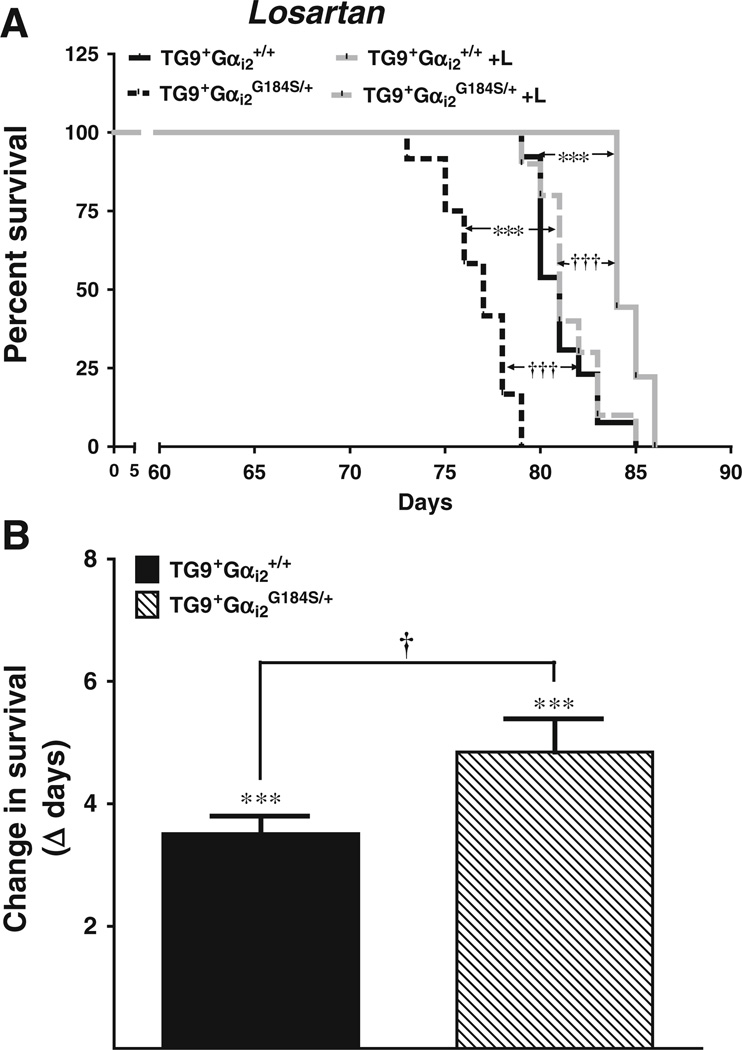
To assess signaling pathways that might be involved in worsened cardiac failure with the Gαi2G184S/+ mutation, we tested pharmacological blockade of angiotensin II signaling. Female mice, chosen due to the greater effect of the Gαi2G184S/+ mutation, were given drinking water containing losartan starting at 3 weeks of age. Losartan treatment significantly improved survival. The difference between the control and losartan treatment groups was highly significant for both genotypes (Fig. 3a, p<0.001). The losartan-induced change in survival (4.85±0.54 days for TG9+Gαi2G184S/+ vs 3.51±0.30 days for TG9+Gαi2+/+) was significantly greater for double-mutants compared to the TG9+/Gαi2+/+ mice (Fig. 3b, p<0.05).
Fig. 3.
Effect of losartan treatment on survival. Losartan (a) was included in drinking water (see “Methods”). ***p<0.001 for effect of drug treatment compared to non-drug control. †††p<0.001 compared to WT Gαi2 control. n=9–13 in each group. b Change in survival days after losartan treatment. Values are mean±standard error of the mean, n=9–13. ***p<0.001 for drug treatment compared to non-drug control. †p<0.05 compared to respective Gαi2+/+ control. Differences were determined by one-way ANOVA with Bonferroni post-test
Catecholamine injury model: effect of Gαi2 G184S mutation
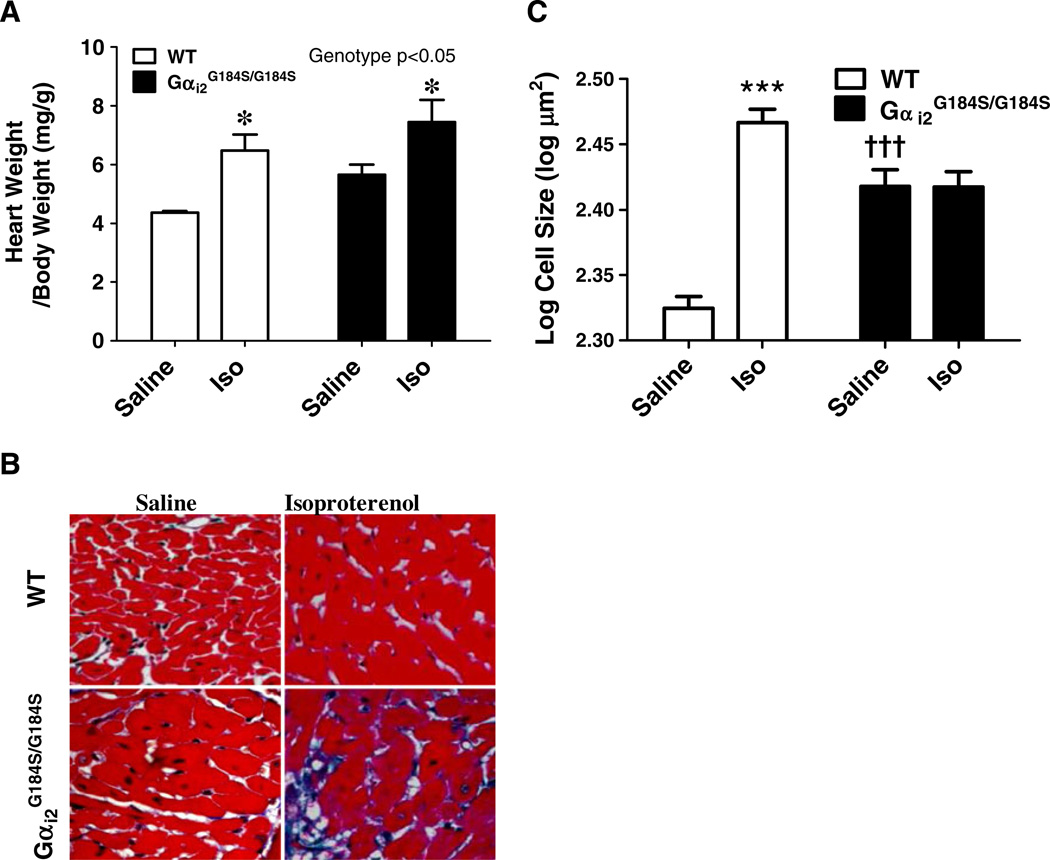
Seven days of Iso treatment resulted in one death among the nine WT mice (on day 4) while three of the nine RGS-insensitive Gαi2G184S/G184S mice (or G184S/G184S) mice died on the second day of Iso treatment. As reported previously (Huang et al. 2006), there was a modestly greater HW/BW in homozygous Gαi2G184S/G184S mice than in WT mice (p<0.01, unpaired, t test). Iso treatment increased HW/BW in both WT and mutant mice (Fig. 4a). There were significant effects of both treatment (p<0.001) and genotype (p<0.05) by two-way ANOVA and a Bonferroni post-test showed significant Iso effects (Fig. 5a, p<0.05) for both WT and Gαi2G184S/G184S mice.
Fig. 4.
Iso-induced injury in WT and RGS-insensitive Gαi2G184S/G184S mice. Mice were injected with saline or isoproterenol (30 mg/kg) daily for 7 days then tissues collected, weighed, and processed for microscopy as described in “Methods.” a HW/BW after saline or Iso treatment in WT and RGS-insensitive Gαi2G184S/G184S mice. b Images of transverse myocardial sections. c Myocyte cross sectional area measured using NIH-Image as described in “Methods.” Values are mean±SEM, n =6–8 for HW/BW and >250 for myocyte size in each group. *p<0.05 or ***p<0.001 for Iso- vs saline-treated animals or †††p<0.001 for WT vs Gαi2G184S/G184Sby ANOVA
Fig. 5.
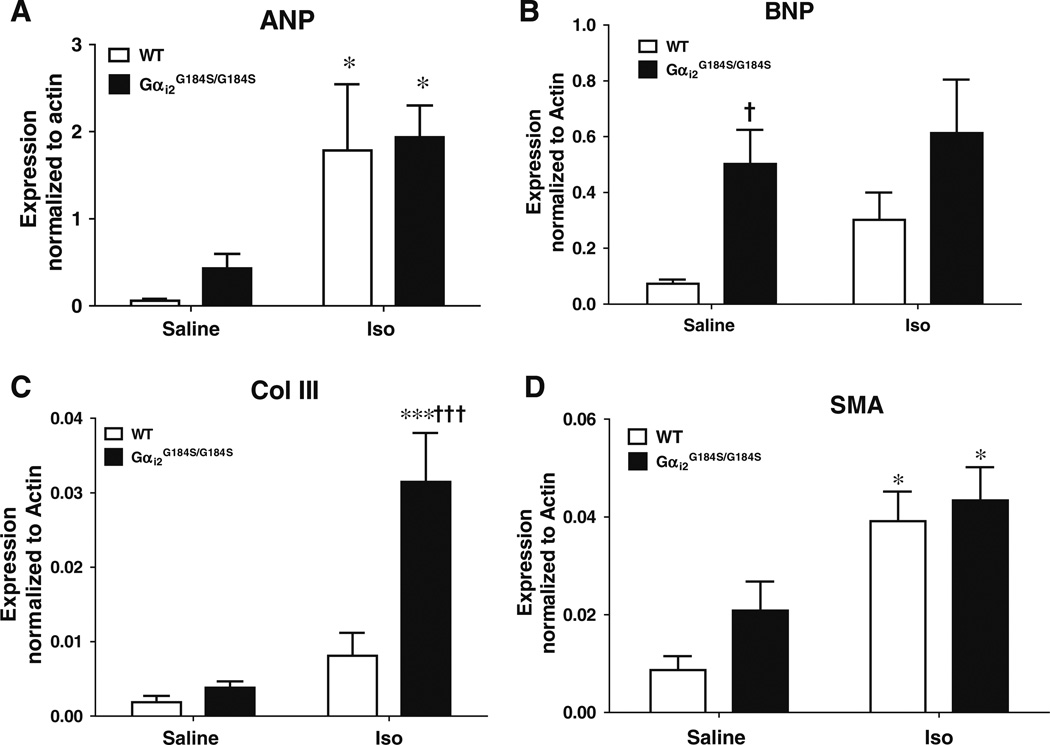
Expression of hypertrophy-associated genes. Real-time RT-PCR was performed on extracts of ventricular muscle from WT or Gαi2G184S/G184S mice after 7 days of saline or Iso treatment. Expression was normalized to β-actin. a Atrial natriuretic peptide (ANP), b brain natriuretic peptide (BNP), c type III collagen (Col III), and d smooth muscle actin (SMA). Values are mean±SEM, n=6 in each group. *p<0.05 and ***p<0.001 Iso- vs saline-treated animals. †p<0.05 and †††p<0.001, Gαi2G184S/G184S vs WT animals
Since heart weight is determined by both myocyte and non-myocyte elements, we also assessed myocyte size (Fig. 4b, c), which was 24% greater in the hearts from saline-treated mutant mice (262 µm2) compared to those from WT mice (211 µm2), consistent with the 30% greater HW/BW. Iso treatment increased myocyte size in WT mice (293 vs 211 µm2), while for Gαi2G184S/G184S, cell size was unchanged (261 vs 262 µm2) despite an increase in HW/ BW of 32%. The lack of effect on myocyte size for mutants in the face of increased HW/BW suggests a role of non-myocyte elements in the Iso-induced cardiac hypertrophy in mutant mice (see below).
Effect of Gαi2G184S mutation on marker gene expression and fibrosis
Mutant mice had significantly higher basal expression of BNP (Fig. 5, p<0.05). ANP in saline-treated Gαi2G184S/G184S mice also was significantly greater (750±290% of WT values, p< 0.05, by non-paired t test). Iso treatment significantly increased the expression of ANP and SMA in both WT and Gαi2 mutant mice (Fig. 5, p<0.05); however, the magnitude was not different between the WT and mutant mice.
Mutants, however, showed a markedly increased expression of Col III messenger RNA (mRNA; 725±172%, p<0.01) after Iso with no effect in WT mice. The difference between WT and Gαi2G184S/G184S mice after Iso treatment was also highly significant (p<0.001, Fig. 5).
Effect of Gαi2G184S mutation on fibrosis
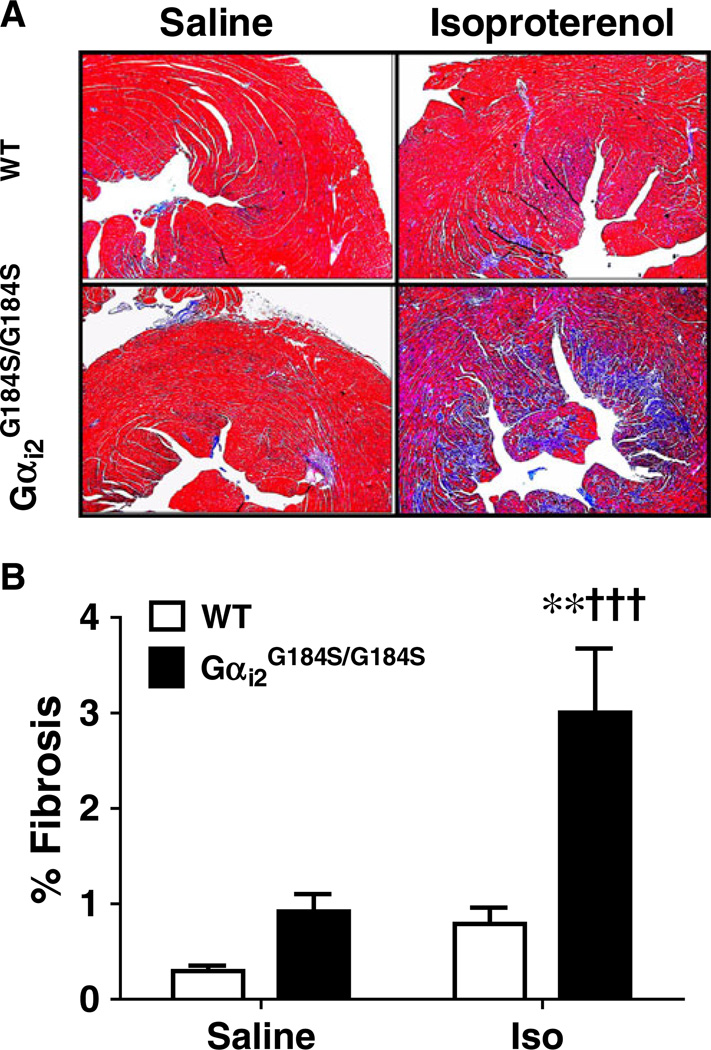
To understand the functional relevance of this increase in Col III mRNA expression, we analyzed cardiac fibrosis by trichrome staining (Dahab et al. 2004). Consistent with the gene expression results, there was a marked increase in fibrosis in mutant mice after Iso treatment (p<0.01), and this level of fibrosis was much greater than that seen in WT hearts after Iso treatment (p<0.001, Fig. 6a, b). The effect of Iso treatment on fibrosis in WT mice (264±58% of saline), was not statistically significant.
Fig. 6.
Increased fibrosis in Gαi2G184S/G184S mutant hearts after Iso. a Cardiac sections were stained with Masson’s trichrome after saline or isoproterenol treatment of wild-type (WT) or Gαi2G184S/G184S mice. b Digital quantification of the tissue fibrosis was done as described in “Methods.” Values are mean±SEM, n=6 in each group. **p<0.01 Iso- vs saline-treated animals. †††p<0.001 Gαi2G184S/G184S vs WT animals
Effect of Gαi2G184S mutation on cardiac fibroblast proliferation
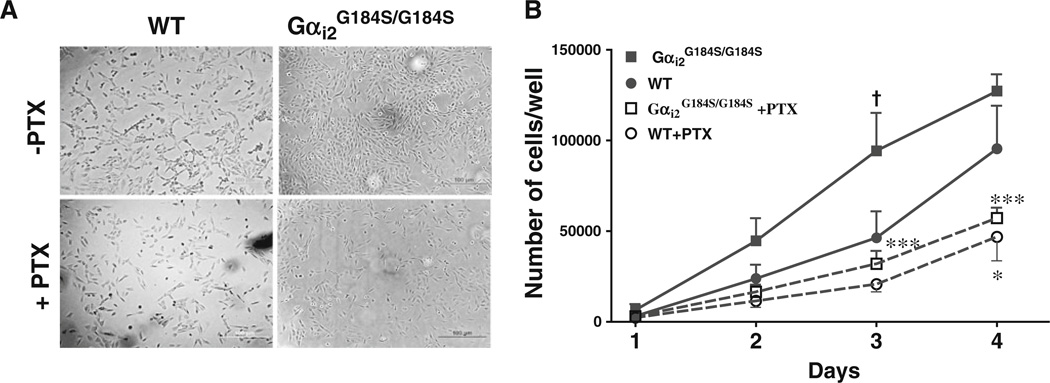
We examined cardiac fibroblasts and found a marked difference in their growth properties under normal growth conditions (i.e., 10% FBS). Cardiac fibroblast were isolated from three WT and three Gαi2G184S/G184S mice and mutant cells grew significantly faster than WT (Fig. 7a, b); 104±45% more at 3 days in culture (p<0.05). There was no difference between WT and mutant fibroblast proliferation in low serum medium (Table ESM1). Fibroblast numbers were significantly decreased by PTX treatment for both mutant (p<0.001) and WT (p<0.05). After PTX, the difference in the fibroblast numbers between WT and mutant was not significant, so ongoing signaling through PTX-sensitive mechanisms (presumably via Gαi2 coupled receptors) contributes to increased proliferation/survival of the Gαi2G184S/G184S cardiac fibroblasts.
Fig. 7.
Gαi2-dependent cardiac fibroblast proliferation. Cardiac fibro-blasts from wild-type (WT) and RGS-insensitive Gαi2G184S/G184S hearts were grown for 3 days in 10% FBS without (PTX-) or with (PTX+) PTX. a DIC micrographs of cells. b Cell number was determined as described in “Methods.” Values are mean±SEM for three different animals with each assay done in duplicate. †p<0.05 Gαi2G184S/G184S vs WT cells *p<0.05 or ***p<0.001 for the effect of PTX
Effect of Gαi2G184S mutation on cardiac fibroblast ERK signaling
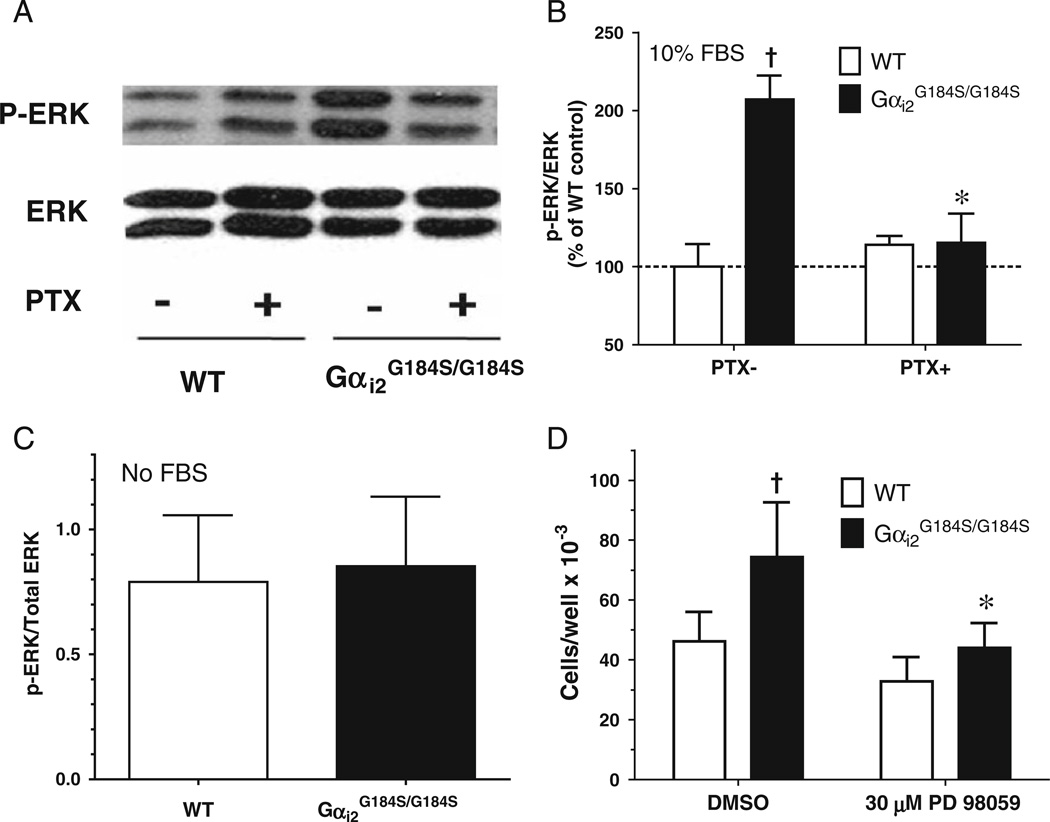
Mutant cardiac fibroblasts grown in serum-containing medium had increased phospho-ERK levels (207±34% of WT, Fig. 8b, p<0.05). This increased ERK phosphorylation was abolished by PTX treatment (p<0.05) and was absent in mutant cardiac fibroblast grown in serum free medium (Fig. 8c). To determine whether this might contribute to the enhanced proliferation/survival of Gαi2G184S/G184S mutant fibroblasts, we evaluated treatment with the MEK inhibitor, PD 98059, which significantly decreased the number of mutant fibroblasts (Fig. 8d, p<0.05), while it had no effect on WT fibroblasts.
Fig. 8.
Increased ERK activity controls proliferation of RGS-insensitive Gαi2 cardiac fibroblasts. Cardiac fibroblasts from wild-type (WT) or Gαi2G184S/G184S mice were grown for 24 h in complete medium without (PTX-) or with (PTX+) PTX. a Representative blot for phospho-ERK and total ERK assessed as in “Methods.” b Densitometric analysis of phospho-ERK/total ERK normalized to the value of the PTX-WT cells. c After growing for 24 h in complete medium, cardiac fibroblasts were starved with serum-free medium overnight, and samples were analyzed for phospho-ERK and total ERK expression. d The effect of the MEK inhibitor PD 98059 on fibroblast number was assessed after 3 days in culture with DMSO or 30 µM PD 98059. Values are mean±SEM for fibroblasts from three different animals with each assay done in duplicate. †p<0.05 Gαi2G184S/G184S vs WT cells. *p<0.05 vehicle- vs PTX- or PD 98059-treated cells
Discussion
The unclear significance of Gαi2 signaling in myocardial injury complicates efforts to modulate its function as a therapeutic strategy. Early reports showing increased Gαi2 expression in heart failure patients (Feldman et al. 1988) did not differentiate between cause and effect regarding complications of heart failure. Recent studies on protective roles of Gαi2 signaling (Chesley et al. 2000; Foerster et al. 2003; DeGeorge et al. 2008) studied myocytes or used in vivo genetic manipulations specific to myocytes perhaps missing a role for other cell types. Here, we used a Gαi2G184S RGS-insensitive mouse model of enhanced Gαi2 signaling cardiac pathophysiology. We recently showed that these mice are protected from myocardial ischemia/reperfusion injury (Waterson et al. 2011). However, in heart failure models examined here, detrimental effects were seen. Gαi2G184S mutant mice show early mortality in the DCM model, which is partially ameliorated by angiotensin receptor blockade. In addition, they have markedly increased fibrosis after Iso-induced injury in vivo and increased cardiac fibroblast ERK activity and cell proliferation. These results further emphasize the growing realization that signaling in fibroblasts, as well as that in myocytes, may be key to understanding cardiac pathophysiology (Baudino et al. 2006).
The best evidence for a protective role of Gαi in heart failure comes from studies of the β2-AR. Overexpression of the β1-AR causes severe heart failure (Engelhardt et al. 1999) but similar expression of the β2-AR does not (Foerster et al. 2003). This has been attributed to a Gi-dependent anti-apoptotic effect in cardiac myocytes (Chesley et al. 2000; DeGeorge et al. 2008). Knockout of Gαi2 (Foerster et al. 2003; DeGeorge et al. 2008) or pertussis toxin treatment of myocytes in vitro (Chesley et al. 2000) reverses the apparent protection afforded by Gi signaling. Consequently, the adverse outcomes seen here with RGSi mutation-induced enhancement of Gαi2 signaling were surprising.
Fibroblasts are key cells in cardiac remodeling, but we are not able to discriminate with this study whether they initiatiated the pathophysiological response or if they were simply responding to the myocyte dysfunction and death occasioned by cardiac injury. Future studies will be needed to determine that. In the context of the Gαi2G184S mice, catecholamine-induced cardiac injury did lead to more fibrosis, while in the DCM model the mutation seems to primarily facilitate hypertrophy. Although there was no a direct link between the Iso-induced fibrosis and the decline in DCM survival, end points for both models show a common detrimental role of enhanced Gαi2 signaling.
The fibrosis after high-dose Iso treatment is consistent with known Gi signaling in cardiac fibroblasts by thrombin (Sabri et al. 2002) and lysophosphatidic acid (LPA) (Epperson et al. 2009). RGS-insensitive Gαi2 MEFs show increased LPA-stimulated ERK and Akt phosphorylation (Huang et al. 2006), so LPA may be the serum factor causing the enhanced our Gi- and ERK-pathway-dependent proliferation. The Gi-linked LPA3 receptor induces proliferation and collagen synthesis in cardiac fibroblasts (Chen et al. 2006), and LPA levels in serum are markedly increased after myocardial infarction (Chen et al. 2003).
The modest increases in HW/BW in the Gαi2G184S/G184S mice [Huang et al. (2006) and Fig. 4a] could have been either physiologic hypertrophy, protective against heart failure, or pathologic hypertrophy that would worsen heart failure. Our present data now point to pathological hypertrophy. Earlier death in the DCM transgenic heart failure model provides one of the hallmarks of pathological hypertrophy (i.e., exacerbation of other hypertrophic insults). Furthermore, the increased expression of “hypertrophy genes” such as BNP and ANP is characteristic of pathologic hypertrophy. The Gαi2G184S/G184S homozygotes showed increases in natriuretic peptide expression at baseline, while both Iso and DCM insults led to substantially higher hypertrophy gene expression in the mutants.
Angiotensin AT1 receptors may contribute in the DCM model. Losartan produces improved survival of DCM mice, and this was significantly greater for mice with the Gαi2G184S/+ mutant (Fig. 3b, c). In addition to Gq signaling, AT1 receptors can activate Raf-1 and ERK in liver epithelial cells (Tsygankova et al. 1998) and increase proliferation and ERK activation with cardiac fibroblasts (Zou et al. 1998) via PTX-sensitive signals. Enhanced AT1 receptor activation of Gαi2, perhaps in fibroblasts, may contribute to the worsened heart failure in Gαi2G184S mice.
Our results raise a number of questions for future consideration. First, which RGS proteins control Gαi2 signaling relevant to the worsened injury, and do they differ from those in myocytes that suppress Gαi2-mediated protective effects seen in the ischemia-reperfusion model (Waterson et al. 2011)? If so, one could target the latter pharmacologically (Neubig and Siderovski 2002; Riddle et al. 2005; Blazer et al. 2011) to improve outcomes in myocardial infarction with less risk of complications from subsequent heart failure. Second, which receptors signal through Gαi2 to induce the worsened heart failure and fibrosis? Identification of these receptors could reveal potential targets of therapeutic intervention. While angiotensin blockade improves survival in the DCM model, we cannot say that this is a direct effect of AT1R on Gαi2 as it could be a downstream action such as from released chemokines, S1P, etc., which act through Gicoupled pathways. Studies to assess these questions are ongoing.
In summary, we asked whether the whole-body knock-in of the RGS-insensitive Gαi2G184S mutation could protect against cardiac injury and heart failure. We show, however, that in two animal models, RGS proteins through their action on Gαi2 appear to play a protective role perhaps in part by reducing ERK activity and proliferation of cardiac fibroblasts.
Supplementary Material
Acknowledgments
This study supported by National Institutes of Health grant R01-GM39561 (R.R.N.) and the University of Michigan Comprehensive Cancer Center (National Institutes of Health grant P30-CA46592). PYJ is a Scholar of the Child Health Research Center of Excellence in Developmental Biology at Washington University School of Medicine (National Institutes of Health K12-HD001487).
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00210-011-0705-z) contains supplementary material, which is available to authorized users.
Contributor Information
Kuljeet Kaur, Department of Pharmacology, The University of Michigan Medical School, Ann Arbor, MI 48109-0632, USA.
Sergio Parra, Department of Pharmacology, The University of Michigan Medical School, Ann Arbor, MI 48109-0632, USA.
Rong Chen, Department of Pharmacology, The University of Michigan Medical School, Ann Arbor, MI 48109-0632, USA.
Raelene A. Charbeneau, Department of Pharmacology, The University of Michigan Medical School, Ann Arbor, MI 48109-0632, USA
Susan M. Wade, Department of Pharmacology, The University of Michigan Medical School, Ann Arbor, MI 48109-0632, USA
Patrick Y. Jay, Departments of Pediatrics and Genetics, Washington University School of Medicine, St. Louis, MO 63110, USA
Richard R. Neubig, Email: rneubig@umich.edu, Department of Pharmacology, The University of Michigan Medical School, Ann Arbor, MI 48109-0632, USA; Department of Internal Medicine (Cardiovascular Medicine), The University of Michigan Medical School, 1150 W. Medical Center Drive 1303 MSRB III, Ann Arbor, MI 48109-0632, USA; Center for Chemical Genomics, The University of Michigan Medical School, Ann Arbor, MI 48109-0632, USA.
References
- Baudino TA, Carver W, Giles W, Borg TK. Cardiac fibroblasts: friend or foe? Am J Physiol Heart Circ Physiol. 2006;291:H1015–H1026. doi: 10.1152/ajpheart.00023.2006. [DOI] [PubMed] [Google Scholar]
- Blazer LL, Zhang H, Casey EM, Husbands SM, Neubig RR. A nanomolar-potency small molecule inhibitor of regulator of G protein signaling (RGS) proteins. Biochemistry. 2011;50:3181–3192. doi: 10.1021/bi1019622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buerger A, Rozhitskaya O, Sherwood MC, Dorfman AL, Bisping E, Abel ED, Pu WT, Izumo S, Jay PY. Dilated cardiomyopathy resulting from high-level myocardial expression of Cre-recombinase. J Card Fail. 2006;12:392–398. doi: 10.1016/j.cardfail.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Chen X, Yang XY, Wang ND, Ding C, Yang YJ, You ZJ, Su Q, Chen JH. Serum lysophosphatidic acid concentrations measured by dot immunogold filtration assay in patients with acute myocardial infarction. Scand J Clin Lab Invest. 2003;63:497–503. doi: 10.1080/00365510310003265. [DOI] [PubMed] [Google Scholar]
- Chen J, Han Y, Zhu W, Ma R, Han B, Cong X, Hu S, Chen X. Specific receptor subtype mediation of LPA-induced dual effects in cardiac fibroblasts. FEBS Lett. 2006;580:4737–4745. doi: 10.1016/j.febslet.2006.07.061. [DOI] [PubMed] [Google Scholar]
- Chesley A, Lundberg MS, Asai T, Xiao RP, Ohtani S, Lakatta EG, Crow MT. The beta(2)-adrenergic receptor delivers an antiapoptotic signal to cardiac myocytes through G(i)-dependent coupling to phosphatidylinositol 3′-kinase. Circ Res. 2000;87:1172–1179. doi: 10.1161/01.res.87.12.1172. [DOI] [PubMed] [Google Scholar]
- Dahab GM, Kheriza MM, El-Beltagi HM, Fouda AM, El-Din OA. Digital quantification of fibrosis in liver biopsy sections: description of a new method by Photoshop software. J Gastroenterol Hepatol. 2004;19:78–85. doi: 10.1111/j.1440-1746.2004.03183.x. [DOI] [PubMed] [Google Scholar]
- DeGeorge BR, Jr, Gao E, Boucher M, Vinge LE, Martini JS, Raake PW, Chuprun JK, Harris DM, Kim GW, Soltys S, Eckhart AD, Koch WJ. Targeted inhibition of cardiomyocyte Gi signaling enhances susceptibility to apoptotic cell death in response to ischemic stress. Circulation. 2008;117:1378–1387. doi: 10.1161/CIRCULATIONAHA.107.752618. [DOI] [PubMed] [Google Scholar]
- Dzau VJ. Autocrine and paracrine mechanisms in the pathophysiology of heart failure. Am J Cardiol. 1992;70:4C–11C. doi: 10.1016/0002-9149(92)91352-5. [DOI] [PubMed] [Google Scholar]
- Engelhardt S, Hein L, Wiesmann F, Lohse MJ. Progressive hypertrophy and heart failure in beta1-adrenergic receptor transgenic mice. Proc Natl Acad Sci U S A. 1999;96:7059–7064. doi: 10.1073/pnas.96.12.7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epperson SA, Brunton LL, Ramirez-Sanchez I, Villarreal F. Adenosine receptors and second messenger signaling pathways in rat cardiac fibroblasts. Am J Physiol Cell Physiol. 2009;296:C1171–C1177. doi: 10.1152/ajpcell.00290.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H, Zingarelli B, Peck OM, Teti G, Tempel GE, Halushka PV, Spicher K, Boulay G, Birnbaumer L, Cook JA. Lipopolysaccharide-and gram-positive bacteria-induced cellular inflammatory responses: role of heterotrimeric Galpha(i) proteins. Am J Physiol Cell Physiol. 2005;289:C293–C301. doi: 10.1152/ajpcell.00394.2004. [DOI] [PubMed] [Google Scholar]
- Feldman AM, Cates AE, Veazey WB, Hershberger RE, Bristow MR, Baughman KL, Baumgartner WA, Van Dop C. Increase of the 40,000-mol wt pertussis toxin substrate (G protein) in the failing human heart. J Clin Invest. 1988;82:189–197. doi: 10.1172/JCI113569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foerster K, Groner F, Matthes J, Koch WJ, Birnbaumer L, Herzig S. Cardioprotection specific for the G protein Gi2 in chronic adrenergic signaling through beta 2-adrenoceptors. Proc Natl Acad Sci U S A. 2003;100:14475–14480. doi: 10.1073/pnas.1936026100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Zhong H, Nanamori M, Mortensen RM, Huang X, Lan K, Neubig RR. RGS-insensitive G-protein mutations to study the role of endogenous RGS proteins. Methods Enzymol. 2004;389:229–243. doi: 10.1016/S0076-6879(04)89014-1. [DOI] [PubMed] [Google Scholar]
- Habashi JP, Judge DP, Holm TM, Cohn RD, Loeys BL, Cooper TK, Myers L, Klein EC, Liu G, Calvi C, Podowski M, Neptune ER, Halushka MK, Bedja D, Gabrielson K, Rifkin DB, Carta L, Ramirez F, Huso DL, Dietz HC. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science. 2006;312:117–121. doi: 10.1126/science.1124287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollinger S, Hepler JR. Cellular regulation of RGS proteins: modulators and integrators of G protein signaling. Pharmacol Rev. 2002;54:527–559. doi: 10.1124/pr.54.3.527. [DOI] [PubMed] [Google Scholar]
- Hruz PW, Yan Q, Struthers H, Jay PY. HIV protease inhibitors that block GLUT4 precipitate acute, decompensated heart failure in a mouse model of dilated cardiomyopathy. FASEB J. 2008;22:2161–2167. doi: 10.1096/fj.07-102269. [DOI] [PubMed] [Google Scholar]
- Huang X, Fu Y, Charbeneau RA, Saunders TL, Taylor DK, Hankenson KD, Russell MW, D’Alecy LG, Neubig RR. Pleiotropic phenotype of a genomic knock-in of an RGS-insensitive G184S Gnai2 allele. Mol Cell Biol. 2006;26:6870–6879. doi: 10.1128/MCB.00314-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan KL, Zhong H, Nanamori M, Neubig RR. Rapid kinetics of regulator of G-protein signaling (RGS)-mediated Galphai and Galphao deactivation. Galpha specificity of RGS4 AND RGS7. J Biol Chem. 2000;275:33497–33503. doi: 10.1074/jbc.M005785200. [DOI] [PubMed] [Google Scholar]
- Lechat P, Packer M, Chalon S, Cucherat M, Arab T, Boissel JP. Clinical effects of beta-adrenergic blockade in chronic heart failure: a meta-analysis of double-blind, placebo-controlled, randomized trials. Circulation. 1998;98:1184–1191. doi: 10.1161/01.cir.98.12.1184. [DOI] [PubMed] [Google Scholar]
- Liu X, Sun SQ, Hassid A, Ostrom RS. cAMP inhibits transforming growth factor-beta-stimulated collagen synthesis via inhibition of extracellular signal-regulated kinase 1/2 and Smad signaling in cardiac fibroblasts. Mol Pharmacol. 2006;70:1992–2003. doi: 10.1124/mol.106.028951. [DOI] [PubMed] [Google Scholar]
- MacLellan WR. Advances in the molecular mechanisms of heart failure. Curr Opin Cardiol. 2000;15:128–135. doi: 10.1097/00001573-200005000-00002. [DOI] [PubMed] [Google Scholar]
- McEwen DP, Gee KR, Kang HC, Neubig RR. Fluorescence approaches to study G protein mechanisms. Methods Enzymol. 2002;344:403–420. doi: 10.1016/s0076-6879(02)44730-1. [DOI] [PubMed] [Google Scholar]
- Neubig RR, Siderovski DP. Regulators of G-protein signalling as new central nervous system drug targets. Nat Rev Drug Discov. 2002;1:187–197. doi: 10.1038/nrd747. [DOI] [PubMed] [Google Scholar]
- O’Connell TD, Rodrigo MC, Simpson PC. Isolation and culture of adult mouse cardiac myocytes. Meth Mol Biol. 2007;357:271–296. doi: 10.1385/1-59745-214-9:271. [DOI] [PubMed] [Google Scholar]
- Ozaki M, Kawashima S, Yamashita T, Hirase T, Ohashi Y, Inoue N, Hirata K, Yokoyama M. Overexpression of endothelial nitric oxide synthase attenuates cardiac hypertrophy induced by chronic isoproterenol infusion. Circ J. 2002;66:851–856. doi: 10.1253/circj.66.851. [DOI] [PubMed] [Google Scholar]
- Pero RS, Borchers MT, Spicher K, Ochkur SI, Sikora L, Rao SP, Abdala-Valencia H, O’Neill KR, Shen H, McGarry MP, Lee NA, Cook-Mills JM, Sriramarao P, Simon MI, Birnbaumer L, Lee JJ. Galphai2-mediated signaling events in the endothelium are involved in controlling leukocyte extravasation. Proc Natl Acad Sci U S A. 2007;104:4371–4376. doi: 10.1073/pnas.0700185104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle EL, Schwartzman RA, Bond M, Insel PA. Multi-tasking RGS proteins in the heart: the next therapeutic target? Circ Res. 2005;96:401–411. doi: 10.1161/01.RES.0000158287.49872.4e. [DOI] [PubMed] [Google Scholar]
- Sabri A, Short J, Guo J, Steinberg SF. Protease-activated receptor-1-mediated DNA synthesis in cardiac fibroblast is via epidermal growth factor receptor transactivation: distinct PAR-1 signaling pathways in cardiac fibroblasts and cardiomyocytes. Circ Res. 2002;91:532–539. doi: 10.1161/01.res.0000035242.96310.45. [DOI] [PubMed] [Google Scholar]
- Schnabel P, Bohm M. Heterotrimeric G proteins in heart disease. Cell Signal. 1996;8:413–423. doi: 10.1016/s0898-6568(96)00087-3. [DOI] [PubMed] [Google Scholar]
- Schoenfeld JR, Vasser M, Jhurani P, Ng P, Hunter JJ, Ross J, Jr, Chien KR, Lowe DG. Distinct molecular phenotypes in murine cardiac muscle development, growth, and hypertrophy. J Mol Cell Cardiol. 1998;30:2269–2280. doi: 10.1006/jmcc.1998.0787. [DOI] [PubMed] [Google Scholar]
- Tsygankova OM, Peng M, Maloney JA, Hopkins N, Williamson JR. Angiotensin II induces diverse signal transduction pathways via both Gq and Gi proteins in liver epithelial cells. J Cell Biochem. 1998;69:63–71. doi: 10.1002/(sici)1097-4644(19980401)69:1<63::aid-jcb7>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Vyas AK, Yang KC, Woo D, Tzekov A, Kovacs A, Jay PY, Hruz PW. Exenatide improves glucose homeostasis and prolongs survival in a murine model of dilated cardiomyopathy. PLoS One. 2011;6:e17178. doi: 10.1371/journal.pone.0017178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterson RE, Thompson CG, Mabe NW, Kaur K, Talbot JN, Neubig RR, Rorabaugh BR. G{alpha}i2-mediated protection from ischaemic injury is modulated by endogenous RGS proteins in the mouse heart. Cardiovasc Res. 2011;91:45–52. doi: 10.1093/cvr/cvr054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarbock A, Deem TL, Burcin TL, Ley K. Galphai2 is required for chemokine-induced neutrophil arrest. Blood. 2007;110:3773–3779. doi: 10.1182/blood-2007-06-094565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong H, Neubig RR. Regulator of G protein signaling proteins: novel multifunctional drug targets. J Pharmacol Exp Ther. 2001;297:837–845. [PubMed] [Google Scholar]
- Zou Y, Komuro I, Yamazaki T, Kudoh S, Aikawa R, Zhu W, Shiojima I, Hiroi Y, Tobe K, Kadowaki T, Yazaki Y. Cell type-specific angiotensin II-evoked signal transduction pathways: critical roles of Gbetagamma subunit, Src family, and Ras in cardiac fibroblasts. Circ Res. 1998;82:337–345. doi: 10.1161/01.res.82.3.337. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.