Abstract
Fluids are considered the cornerstone of therapy for many shock states, particularly states that are associated with relative or absolute hypovolemia. Fluids are also commonly used for many other purposes, such as renal protection from endogenous and exogenous substances, for the safe dilution of medications and as “maintenance” fluids. However, a large amount of evidence from the last decade has shown that fluids can have deleterious effects on several organ functions, both from excessive amounts of fluids and from their non-physiological electrolyte composition. Additionally, fluid prescription is more common in patients with systemic inflammatory response syndrome whose kidneys may have impaired mechanisms of electrolyte and free water excretion. These processes have been studied as separate entities (hypernatremia, hyperchloremic acidosis and progressive fluid accumulation) leading to worse outcomes in many clinical scenarios, including but not limited to acute kidney injury, worsening respiratory function, higher mortality and higher hospital and intensive care unit length-of-stays. In this review, we synthesize this evidence and describe this phenomenon as fluid and electrolyte overload with potentially deleterious effects. Finally, we propose a strategy to safely use fluids and thereafter wean patients from fluids, along with other caveats to be considered when dealing with fluids in the intensive care unit.
Keywords: Fluid therapy, Critically Ill, Oliguria, Water-electrolyte balance, Central venous pressure, Resuscitation, Acute kidney injury, Diuretics, Multiple organ dysfunction syndrome, Systemic inflammatory response syndrome
Core tip: Fluids are a cornerstone of the management of critically ill patients with systemic inflammatory response syndrome who are at risk of multiple organ dysfunction syndrome. However, as with any therapy, fluids can be associated with harm, such as added or worsening organ dysfunctions. Therefore, patients should be weaned from fluids when possible, sometimes through an active de-resuscitation strategy.
INTRODUCTION
Fluids have been widely used in critically ill patients to optimize hemodynamics[1], to enhance renal protection from contrast[2], globins[3], and uric acid[4], to offer caloric intake[5] and as an adjunct for medication dilution[6]. During the first hours of shock syndromes, isotonic fluids can stabilize arterial pressure and perfusion and are lifesaving at times. Furthermore, to reach physiological hemodynamic targets, a large amount of intravenous fluids may be required. Some fasting patients receive infusions of one to three liters of dextrose in water with added electrolytes, mainly sodium, chloride and potassium, to avoid hypoglycemia, dehydration and electrolyte deficiency. Renal protection in critically ill patients is of huge importance, and hyper-hydration is frequently used to enhance urine output and to avoid renal tubular cell injury by the retention of toxic substances at high concentrations. Finally, many drugs require a large amount of fluids with electrolytes to be safely administered.
Fluids and electrolytes are responsible for a large amount of volume that is infused in critically ill patients and are commonly associated with reduced urine output and renal electrolyte excretion failure, particularly chloride-rich solutions. This combination is even more accentuated in the first hours of critical illness[1,7] or in the presence of acute kidney injury (AKI)[8-10]. Ultimately, the inadequate management of fluids and electrolytes in critically ill patients culminates in hydroelectrolytic overload, which causes physiological derangements and worse outcomes[11]. This manuscript describes the mechanisms, pathophysiology, and potential consequences of fluid and electrolyte overload and provides a combined bedside approach to avoid it.
ELECTROLYTE OVERLOAD
Primary electrolytes in basic human physiology
Sodium and chloride are the main determinants of colloidosmotic forces in human plasma and interstitial space because they account for 80% of the osmolality of these fluids[12]. Plasma non-permeable proteins attract positively charged ions and repel negatively charged ions, leading to a passive transmembrane distribution of anions to preserve plasma and interstitial space electroneutrality, known as the Gibbs-Donnan effect. A steady-state is achieved when the plasmatic osmolality is 1 mOsm/L greater than the interstitial space, and the capillary hydrostatic pressure opposes the osmotic movement of the water into the intravascular space[13].
Even at a constant interstitial space osmolality, to maintain cell volume, the transport of osmotically active substances across the cell membrane (mainly sodium and potassium) counterbalances the intracellular osmotic forces imposed by high-molecular-weight anionic proteins (Double-Donnan effect)[14].
One of the main roles of electrolytes and their homeostasis is the distribution of fluids throughout the human body. Colloidosmotic and hydrostatic pressures are the main forces influencing the fluid distribution between intravascular and interstitial spaces, whereas changes in intra or extracellular osmolality are in general followed by water movement and determine the changes in cell volume[14].
Sodium overload
Sodium is the main cation of solutions infused into critically ill patients. The 0.9% saline solution has 154 mEq/L of sodium, that is, one liter of 0.9% saline infusion carries 3.4 g of sodium, which represents approximately eight 100 g packages of commercially available potato chips, a huge amount of the electrolyte[15]. Nevertheless, sodium renal excretion is largely impaired in critically ill patients,[10] particularly in patients with AKI[8].
In a sample of septic patients, for instance, the mean isotonic fluid intake was 5000 mL during the first 24 h in the ICU, with a urine output of 2000 mL during the same period and a mean sodium urinary concentration of 55 mEq/L[16]. In this casuistic, the total amount of sodium infused was 770 mEq in the 24 h period analyzed with a concomitant sodium excretion of 110 mEq, resulting in a positive fluid and sodium balance of 3000 mL and 660 mEq, respectively. Because of the large sodium distribution volume in adults (49 L to 70 kg), the expected effect of the remaining 660 mEq of sodium and 3000 mL of water in patients with a pre-infusion sodium concentration of 145 mEq/L is approximately 4.0 mEq/L[17]. Notably, patients with established kidney injuries were excluded from the Stelfox et al[18] study, and consequently the sodium overload effect in patients with AKI is expected to be even more striking. To exemplify this finding, Table 1 shows a water and electrolyte cumulative evolution of a mono-compartment mathematical model of a post-resuscitation phase with low urinary sodium and chloride, similar to the septic patients of Noritomi’s study.
Table 1.
Closed mono-compartment mathematical marginal model simulating the body water space of distribution
| Variables | Day 0 | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 |
| Patient’s resulting variables | ||||||
| Na+ (mEq/L) | 140 | 142 | 148 | 151 | 146 | 144 |
| Cl- (mEq/L) | 100 | 108 | 114 | 117 | 113 | 111 |
| Fluid balance (mL) | - | 6000 | 0 | 800 | 800 | 0 |
| Cumulative fluid balance (mL) | - | 6000 | 6000 | 6800 | 7600 | 7600 |
| Distribution water volume and electrolyte data | ||||||
| Vd (L) | 36 | 42 | 42 | 43 | 44 | 44 |
| Total mass of Na+ (mEq) | 5040 | 5964 | 6216 | 6493 | 6424 | 6336 |
| Total mass of Cl- (mEq) | 3600 | 4536 | 4788 | 5031 | 4972 | 4884 |
| Fluids output | ||||||
| Diuresis (mL) | - | 2000 | 1200 | 1200 | 1200 | 2000 |
| Urinary Na+ (mEq/L) | - | 30 | 50 | 70 | 90 | 110 |
| Urinary Cl- (mEq/L) | - | 30 | 50 | 70 | 90 | 110 |
| Fluids input | ||||||
| Volume | 6000 | 2000 | 2000 | 2000 | 2000 | 2000 |
| Na+ (mEq/L) | 154 | 154 | 154 | 0 | 0 | 0 |
| Cl- (mEq/L) | 154 | 154 | 154 | 0 | 0 | 0 |
The main assumptions of this model are the absence of feces, sudoresis, renal replacement therapy, and the absence of Gibbs-Donnan effect. This patient was resuscitated with 4000 mL of 0.9% saline and received additional 2000 mL of 0.9 fluids during the Day 0. He received an amount of 0.9% saline during day 1 and day 2, afterwards the same 2000 mL of volume was infused without electrolytes due to hypernatremia. Vd: Denotes distribution volume.
Other groups have also described resuscitation fluids as a contributing factor to ICU-acquired hypernatremia with a dose-response effect: the greater the saline infusion, the worse the hypernatremia condition[19]. However, other sources of saline contribute to sodium loading in critically ill patients and may be a modifiable risk factor, such as normal saline used to dilute parenteral drugs and to keep catheters open[20]. Finally, in Australia and New Zealand, a point-prevalence study demonstrated that sodium administration in excess of recommended daily requirements (i.e., 1-2 mmol/kg) was fairly common, with the major sources being maintenance fluids (30.9%), fluid boluses (16.3%) and drug boluses (12.3%)[21].
Hypernatremia is present at hospital admission and at ICU admission in 2% and 7% of patients, respectively[22,23]. By contrast, up to 27% of patients in the ICU develop hypernatremia during their ICU stay[18]. In a speculative view, this higher incidence of hypernatremia in critically ill patients might be explained by sodium overload. Critical illness related hypernatremia is associated with disease severity, kidney injury and dysfunction, mechanical ventilation and ICU length-of-stay[18]. Finally, hypernatremia is associated with higher in-hospital mortality[18,22,24,25] and it has been considered in several ICUs as a quality-of-care marker[26,27]. Recently, a group also retrospectively noted that correcting this abnormality ultimately resulted in better survival[28].
Chloride overload
Chloride is the main anion of fluids used in critical care settings, and its concentration is well correlated to the sodium concentration to maintain the electroneutrality of solutions[29]. The 0.9% saline solution, Ringer’s lactate solution, and Plasmalyte contain 154, 109 and 98 mEq/L of chloride, respectively[15]. Because the serum chloride concentration is approximately 100 mEq/L, it is expected that a 0.9% saline infusion potentially increases the serum chloride concentration. In septic patients, Park et al[30] showed that 2000 ± 300 mL of a 0.9% saline infusion promptly resulted in a disproportionate elevation of serum chloride in comparison to the sodium concentration (Figure 1). Of note, this disproportionate elevation occurred in spite of equal chloride and sodium concentrations in the 0.9% saline solution that was infused[15,30].
Figure 1.
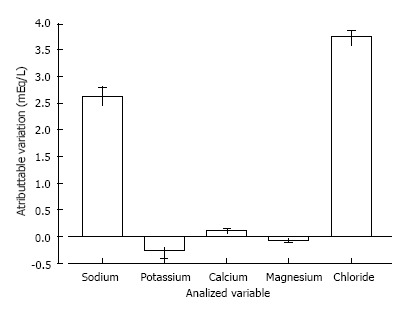
Variation of plasma electrolytes concentration immediately after 2000 ± 300 mL infusion of 0.9% saline in septic patients. Adapted from Park et al[30].
The principle of the unequal chloride and sodium concentration elevations is based on the fact that the initial serum chloride concentration is lower than the initial sodium concentration. Therefore, the same infused amount in mEq/L of sodium and chloride is expected to have a greater effect on the ion with the lower serum concentration - in this case, chloride (Figure 2).
Figure 2.
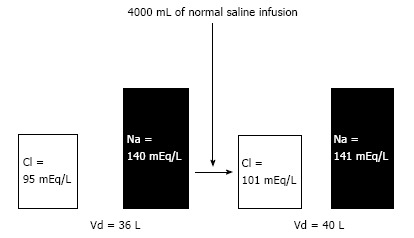
A monocompartimental model of intravascular 0.9% saline infusion, simulating the extracellular volume modification. To the initial distribution volume (Vd) = 36 L (approximately 60% of body mass of 60 kg), the total AMMOUNT of Cl- and Na+ were 3420 and 5040 mEq respectively. The infusion of 4000 mL of 0.9% saline results in additional 616 mEq of Cl- and Na+ to the total QUANTITY of extracellular electrolytes, that is, 4036 and 5656 mEq of Cl- and Na+ respectively. The new total AMMOUNT of electrolytes are distributed in a new Vd of 40 (36 + 4) liters, resulting in the new concentrations, where the chloride elevation was more striking than the sodium elevation.
Kellum et al[31] demonstrated in a canine model of endotoxemia that only one-third of the post-volume infusion of chloride associated acidosis could be explained by exogenous chloride. The authors attributed this fact to an extravascular to intravascular chloride shift that was driven by differences in the transmembrane potential and the Gibbs-Donnan effect secondary to the fluid challenge. This same finding of chloride elevation was observed in humans with severe sepsis and septic shock[16]. Therefore, one can expect an intrinsic chloride elevation in patients with systemic inflammation, which is amplified by a chloride-rich fluid infusion, such as the many previously described sources of normal saline that are infused in critically ill patients.
In addition to these sources of chloride, the renal excretion of chloride is also impaired, similarly to sodium excretion, during the initial phase of critical illness[9,10], particularly in patients with AKI[8]. The net result is a positive balance of chloride (Table 1), and an increased serum chloride concentration results in a hyperchloremic metabolic acidosis[16,32]. This is also called “SID acidosis” and is not related to outcomes in mixed critically ill patients[33]. However, specifically in septic patients, initial hyperchloremic acidosis is associated with a higher mortality[16].
Pathophysiology
Renal consequences: Electrolyte overload may have detrimental effects on renal function, particularly chloride overload. Animal studies suggest that chloride may influence renal blood flow (RBF), which is mediated primarily by its effects on afferent and intrarenal arterial vessels[34,35]. In canine experiments, the renal infusion of solutions containing chloride, such as 0.9% saline or NH4Cl, led to reductions in the total RBF and GFR in both denervated and in situ kidneys[34]. In an animal model of sepsis, unbalanced solutions worsened sepsis-induced AKI[36]. Other experiments confirmed that extracellular chloride is essential for contraction in renal afferent arterioles[37,38]. In humans, an infusion of 2 L of 0.9% saline over 1 h was associated with a reduction in the RBF velocity and renal cortical tissue perfusion measured by magnetic resonance imaging (MRI); these changes were not observed after a similar infusion of a balanced crystalloid[39]. Moreover, studies in healthy volunteers have shown a delayed urine output with saline compared to a balanced solution[40].
An infusion of hypertonic solutions containing chloride into the renal artery causes a biphasic response in renal vascular resistance[34]. Hyperosmolality leads to an abrupt renal vasodilatation and consequent increase in RBF. After 1-5 min, vasodilation is reversed, and RBF and GFR decrease below pre-infusion levels. The second phase is absent in hypertonic solutions that do not contain chloride.
In vitro, the entry of chloride from elevated tubular chloride concentrations into epithelial renal cells causes the depolarization of the basolateral membrane[41]. Increased NaCl concentrations in the macula densa stimulate ATP release, resulting in the extracellular formation of adenosine, which is involved in the signal transmission of the tubule-glomerular feedback response, increasing afferent arteriolar resistance and reducing GFR[35,42].
Clinically, Yunos et al[43] translated this experimental knowledge to a large population of critically ill patients in a prospective open-label sequential period pilot study. After a control period and a wash-out phase, the use of chloride-rich intravenous fluids was restricted; this resulted in decreased chloride administration (694 to 496 mmol/patient) and led to better renal outcomes even after adjustment for covariates, including less high-severity AKI [OR = 0.52 (95%CI: 0.37-0.75); P < 0.001] and the reduced use of renal replacement therapy [OR = 0.52 (95%CI: 0.33-0.81); P = 0.004][43].
Acid-base consequences: Large volume resuscitation is commonly required in patients with sepsis and trauma. These patients may receive crystalloid infusions of many times their plasma volumes. Because the chloride concentration in 0.9% saline is approximately 50% higher than the plasma values, the chloride load associated with these volumes is significant. As previously described in this review, the chloride load associated with normal saline, which is a solution with a strong ion difference (SID) of 0, may be one of the main determinants of acidosis induced by fluids, along with chloride shifts that may occur in patients with sepsis (and other inflammatory states) and the Gibbs-Donnan effect after fluid challenges. These chloride loads and shifts interact with the patient’s renal function, leading to both more AKI and subsequently lower chloride excretion, which might affect the kidney’s recovery.
In surgical patients, when 0.9% saline was used as the primary intraoperative solution, significantly more acidosis was observed on completion of the surgery. These patients required larger amounts of bicarbonate to achieve predetermined measurements of base deficit and were associated with the use of larger amounts of blood products[44]. In another trial, a 0.9% saline infusion was compared to a balanced electrolyte and glucose solution. Two-thirds of the patients in the 0.9% saline group but none of the patients in the balanced fluid group developed hyperchloremic metabolic acidosis, and the hyperchloremic acidosis was associated with reduced gastric mucosal perfusion on gastric tonometry[45].
Although the effects of chloride are well studied, little is known about the potential contributions of sodium to the metabolic acid-base state. In a sample of 51 critically ill patients, a rise in serum sodium levels during the development of hypernatremia was accompanied by an increasing pH, serum bicarbonate, and standard base excess, and consequently metabolic alkalosis[46]. In addition, the development of metabolic alkalosis correlated with the SID but not with the absolute serum sodium concentrations, indicating that the increase in the serum sodium-to-chloride ratio led to the development of metabolic alkalosis[47].
Inflammatory response: Fluid and electrolyte overload may also influence cytokine production and the inflammatory response. In an animal model of hyperchloremic acidosis induced by dilute HCl infusion, moderate (SBE, - 5 to - 10) and severe (SBE, - 10 to - 15) acidosis significantly increased cytokine expression in a dose-dependent fashion in normotensive septic rats[48]. These results are consistent with in vitro studies showing that HCl influences cytokine production in LPS-stimulated cells[49], and pH interferes in nitric oxide[50] and tumor necrosis-α production[51] by macrophages in cell models. Interestingly, acidosis etiology may determine the inflammatory response pattern. Hyperchloremic acidosis is essentially pro-inflammatory as assessed by the increased NO release and the IL-6-to-IL-10 ratio, whereas lactic acidosis is associated with an anti-inflammatory pattern[49].
Hemodynamic consequences: Chloride overload and its consequent hyperchloremic acidosis may have direct and independent deleterious effects on hemodynamics and survival. In an endotoxic shock model in rats, moderate and severe acidosis that was generated by HCl infusion induced a significant drop in blood pressure. This change in blood pressure was correlated with increases in plasma chloride concentrations and to a lesser degree with a decrease in pH[52]. Furthermore, saline solution resuscitation was associated with a significantly shorter survival time compared to a balanced electrolyte solution containing starch in a similar animal model. Survival time was negatively correlated with both the decrease in pH and the increase in serum chloride following the initial resuscitation. The decrease in pH appeared to have been brought on by changes in chloride, lactate, and PaCO2. However, lactate values were not different between the groups, and the changes in PaCO2 were not correlated with survival time. Thus, hyperchloremic acidosis, rather than acidosis in general, was strongly and independently associated with early mortality in these animals[53].
FLUID OVERLOAD
Pathophysiology
The renal compartment syndrome: The human body is composed of different organ systems. Lungs are, perhaps, the most affected organs by fluid overload, followed by encapsulated organs such as the kidneys. Experimental and clinical evidence from more than 30 years ago links the development of renal edema with oliguria and the perpetuation of ischemic AKI[54]. This could be explained by reduced perfusion pressure through the kidneys as a result of higher central venous pressure, which has been better described in the context of cardiorenal syndromes[55,56]. In addition to heart failure, patients with systemic inflammatory response syndrome (SIRS) may also develop interstitial edema and subsequently increases in interstitial pressure, leading to lower perfusion pressure, particularly in encapsulated organs such as the kidney[57].
In animal models, Burnett et al[58] also demonstrated that an increase in renal venous pressure associated with volume expansion led to higher interstitial pressures and decreased sodium excretion in association with a decreased RBF and glomerular filtration rate. Recently, Cruces et al[59] experimentally described a model that provided even more support of the existence of a renal compartment. In their work, pressure had a nonlinear dependence on volume in the intact kidney, whereas the decapsulated kidney followed a linear pressure-volume curve, thus corroborating the hypothesis that kidney hypoperfusion might be explained by a reduced perfusion pressure. Clinical evidence supporting the role of interstitial edema to worse kidney outcomes will be discussed later in this review.
Pulmonary consequences: Derangements in the capillary permeability, which occurs in SIRS, combined with an increased hydrostatic pressure, as induced by aggressive fluid resuscitation, results in major interstitial edema that can lead to important clinical consequences.
Fluid overload increases hydrostatic pressure, leading to fluid accumulation in the lungs. Studies in mice have shown that the leakage occurs in the bronchiole, and the backflow of fluids leads to alveolar edema[60]. There is a reabsorption of fluids in the interstitial space and, because the accumulated fluids are drained across the lymphatic vessels to the thoracic duct and superior vena cava, alterations in systemic venous pressure, which occurs during fluid overload, result in impaired lymphatic drainage and consequently pulmonary edema[61], leading to a gas exchange impairment.
The high hydrostatic pressure not only causes fluid leakage but also generates mechanical stress injury to capillary walls, leading to the impairment of the mechanisms of fluid reabsorption[62] and alveolo-capillary barrier damage[63,64]. This damage causes ultrastructural changes in the capillary, altering permeability to proteins and activating the inflammatory response[65], which compromises gas exchange[66].
Hypoxemia resulting from impaired gas exchange leads to lung regional blood flow redistribution. As demonstrated by Ruff et al[67], fluid overload leads to an inversion of the pulmonary perfusion pattern, with decreased blood flow to the pulmonary dependent regions and increased blood flow to the non-dependent regions, most likely because of hypoxic vasoconstriction.
The clinical features of pulmonary edema are not restricted to oxygenation but are a result of decreased pulmonary ventilation as well. In 1922, Drinker had described a tidal volume reduction of 40%-70% in an induced pulmonary edema animal model[68], and a subsequent study has shown that a negative fluid balance strategy improved lung compliance and arterial oxygenation[69].
Considering Starling’s equation in which pulmonary edema is a result of colloidosmotic and hydrostatic forces, one approach to this clinical problem is to lower filling pressures. Despite concerns regarding lowering cardiac output and oxygen delivery, current evidence shows that a conservative fluid strategy improves the oxygenation index and number of ventilation-free days without compromising hemodynamics or other organ functions[11,70].
Other organ consequences: Other organs might be affected by fluid overload in addition to the lungs and kidneys. Worse outcomes of the skin and the recovery of soft tissue wounds after surgery have been described, and the trial of Brandstrup et al[71] showed that a more conservative approach on fluids achieved better outcomes, particularly regarding surgical complications.
Gastrointestinal complications, such as ileum and anastomotic leakages, can also be increased because of interstitial edema associated with accumulated fluids during critical illness or major surgeries[72]. This might lead to delays in the administration of nutritional needs and worsen the possibility of achieving an adequate enteral nutrition intake.
The liver is also an encapsulated organ, and interstitial edema could lead to a sort of compartment syndrome. In shock states, in addition to hypoperfusion, a high central venous pressure is required for the development of ischemic hepatitis[73]. High venous pressures are usually secondary to a low cardiac output in patients with congestive heart failure, but it can also occur in fluid overloaded patients with SIRS who develop myocardial dysfunction.
From a broader perspective, abdominal compartment syndrome can be seen as another preventable complication of fluid overload. This syndrome would be an extreme situation regarding fluid overload states and can be either primary or secondary. In this case, fluid overload contributes to the development of abdominal compartment syndrome, leading to deleterious effects on many organ systems, including hemodynamic (as a result of reduced venous return), renal (as a result of increased renal venous pressure) and even respiratory system mechanics (by reducing the thoracic wall compliance)[74].
Other organ systems have more limited evidence associating fluid overload with worse outcomes. Although the brain could be considered an encapsulated organ, in general ICU patients whose blood-brain barrier is considered intact, fluid overload will most likely not lead to a significant cerebral edema that will develop into intracranial hypertension. However, it might be associated with an increased incidence of delirium[75], which is associated with worse outcomes.
Acid-base water effect: Despite the effect of electrolytes on acid-base status, water itself might influence the acid-base status. Some experimental evidence from in vitro studies suggests that the dilution of plasma with distilled water changes many electrolyte concentrations, but because the ensuing proportions are maintained regarding the SID, PaCO2 and weak anions, there is no significant difference in the pH[76]. However, in a mathematical modeling approach validated thereafter with human plasma, Gattinoni et al[77] demonstrated that water itself, when in an open system, leads to acidosis, mainly because of the reaction of CO2 with H2O. The same group later described a possible rule that would regulate pH changes during crystalloid infusion, with interesting results. Mainly, the baseline [HCO3-] values would dictate the pH response to a crystalloid solution whose (SID) would be the main determinant of the direction of the pH change[78]. As an example, giving a patient both 0.9% saline [(SID) = 0] or dextrose in water [(SID) = 0] can lead to worsened acidemia, depending on the patient’s renal function and pulmonary function to counteract these effects. However, 0.9% saline comes with an added cost, which is that of electrolyte overload, as we have discussed.
Observational evidence correlating fluid overload with worse outcomes
Fluid overload or cumulative fluid balances have been associated with worse outcomes in many scenarios, including in patients with sepsis[79], cancer[80], and surgical patients[81], and during weaning from mechanical ventilation[82] and at discharge from the ICU[83].
A sub analysis of the VASST trial, which included patients with septic shock who were on vasopressors, reported that a positive fluid balance at both 12 h and 4 d after the onset of shock was associated with worse outcomes. Interestingly, the patients with CVP values below 8 mmHg at 12 h after septic shock onset had improved survival compared to patients with higher values of CVP[79], which are recommended by the surviving sepsis campaign during the first hours of resuscitation[84]. More provocative are the findings of Murphy et al[85], who studied a cohort of patients with septic shock who thereafter developed acute respiratory distress syndrome (ARDS), in which they hypothesized whether an adequate initial fluid resuscitation strategy and a conservative late fluid management strategy were associated with improved survival[85]. In this cohort, the patients who achieved both adequate initial fluid resuscitation and conservative late fluid management had the lowest mortality. Interestingly, the patients who achieved a conservative late fluid management but not adequate initial fluid resuscitation had lower mortality rates than those who achieved adequate initial fluid resuscitation but not conservative late fluid management. This appears to provide a lesson regarding this population: trying to optimize hemodynamics later in the course of the disease is most likely deleterious, whereas achieving negative fluid balances, i.e., actively de-resuscitating patients even if the initial resuscitation was not deemed adequate, appears to be successful, particularly for patients with ARDS[11]. In patients with ARDS, a large observational cohort also demonstrated that more positive fluid balances are associated with worse outcomes[86].
In patients being weaned from mechanical ventilation, data demonstrate that a 24-h negative fluid balance on the day of the spontaneous breathing trial and a cumulative negative fluid balance were associated with better weaning outcomes[82]. In another cohort of elderly patients, both negative fluid balances and decreasing values of central venous pressure were associated with better weaning outcomes[87]. A higher cumulative fluid balance, even after ICU discharge, was also associated with worse outcomes during hospitalization[83].
In patients with AKI, positive fluid balances have also been associated with worse outcomes. In a sub analysis of a European cohort of general ICU patients who developed AKI, a positive mean fluid balance was an independent risk factor for 60-d mortality[88]. In a sub analysis of the RENAL study, the authors investigated the effect of fluid balance on the outcomes of patients with many different statistical approaches, and they consistently found an association between negative mean daily fluid balances and improved clinical outcomes[89].
Many of these conditions in which fluid overload has been shown to be deleterious share a common feature: the presence of SIRS and the risk of multiple organ dysfunction syndrome, which can manifest clinically as shock along with AKI, ARDS and many other possible organ dysfunctions, septic or non-septic in origin[90]. What remains to be established is whether fluid overload is only a biomarker[91] that puts patients under an increased risk of death or an iatrogenic condition from critical care that should be considered in daily care and actively treated and avoided. This is a question to be answered with randomized controlled trials.
Randomized controlled trials correlating fluid overload with worse outcomes
Some randomized controlled trials in critically ill patients attempted to address whether a conservative fluid management approach would result in better outcomes for patients instead of a liberal approach, testing the hypothesis that fluid overload is not only a biomarker but is also a modifiable risk factor for worsening organ dysfunctions and death. In each trial, the fluid-restrictive protocols were different; however, the greatest objective in all of the studies was to withdraw fluids from the patients and/or to avoid giving unnecessary fluids.
In patients admitted to an intensive care unit who required a pulmonary artery catheter, Mitchell et al[70] compared an extravascular lung-water based strategy against a wedge pressure-based strategy for the treatment of 101 patients. Regardless of the protocol used, in the extravascular lung-water based strategy, the cumulative fluid balance was 142 ± 3632 mL compared to 2239 ± 3695 mL in the other group. The conservative strategy led to better outcomes in this group although mortality was unchanged.
In patients undergoing high-risk colorectal surgery, Brandstrup et al[71] also tested another conservative strategy for fluid management during the perioperative state and achieved a significantly lower fluid balance in these patients, yielding a lower rate of complications after surgery, many of which included surgical wound repair and cardiopulmonary complications.
In the landmark FACTT trial, which included two different protocols for the fluid management of patients with ARDS, patients in the conservative group achieved a negative fluid balance throughout the course of the disease, whereas patients in the liberal group progressively accumulated more fluids during their ICU stay. The primary outcome (mortality) was not different between the groups. However, the conservative group achieved a higher number of ventilator-free days and ICU-free days but had less vasopressor free-days, along with a slight trend towards a lower dialysis requirement through day 60 (P = 0.06)[11]. This trial demonstrated an interesting point: when using a conservative fluid management strategy, one will likely require vasopressors for a longer period but paradoxically at the benefit of being able to breathe without the ventilator sooner and being discharged from the ICU sooner. The cumulative fluid balance of both arms of the FACTT study is shown in Figure 3, as is the fluid balance of the ALVEOLI[92] and ARMA[93] studies about protective mechanical ventilation in ARDS patients.
Figure 3.
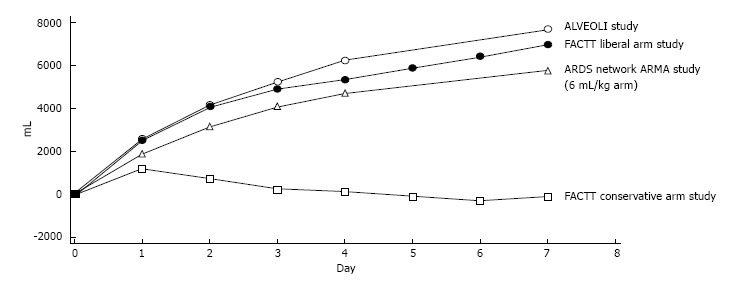
Cumulative fluid balances of the acute respiratory distress syndrome network group studies. The FACTT conservative fluid strategy arm returned to the neutral fluid balance within the first three days after randomization. The former strategy did not result in better survival, however patients were ventilated for less time and spent less time in the ICU in the conservative group. Adapted from Wiedemann et al[11]. ARDS: Acute respiratory distress syndrome; ICU: Intensive care unit.
Finally, during weaning from mechanical ventilation in a mixed ICU population, it has been demonstrated that a brain natriuretic peptide-driven strategy for fluid withdrawal resulted in more negative cumulative fluid balances [median, - 180 (-2556; 2832) vs - 2320 (-4735 to 738), P < 0.001], resulting in more ventilator-free days, although without impact on mortality[94].
These trials, although conducted in different clinical scenarios, share an interesting feature. In the population of patients at risk of developing new organ failures (such as patients during high-risk surgery) or with ongoing organ failures (such as patients with ARDS) or even during recovery of the critical illness, a conservative strategy led to better outcomes. Whether this can be extrapolated to all clinical scenarios remains to be answered.
The kidney paradox (oliguria worsened by fluid challenge)
Oliguria is usually considered a marker of decreased cardiac output, and a fluid challenge is often considered in an attempt to increase the cardiac preload and enhance cardiac output, which would ultimately enhance organ perfusion. However, after the optimization of hemodynamic parameters, some patients will still develop AKI and may have persistent oliguria on the ensuing days. Hence, after the first hours of resuscitation, oliguria should be interpreted as a marker of organ dysfunction but should not be seen as a marker of low cardiac output and does not necessarily indicate the need for volume expansion.
In this context of oliguria and a high risk of fluid overload, an added fluid challenge may worsen urine output and even renal function, as we have discussed previously in this review; most of the time, this will contribute to fluid and electrolyte overload. Clinically, Van Biesen et al[95] demonstrated in a cohort of septic patients with AKI that additional fluid therapy failed to improve renal function, and other studies have shown an association between a positive fluid balance and worse outcomes in patients with AKI[91], in addition to a decreased likelihood of renal recovery[88,96]. Another implication of increasing cumulative fluid balances in this context is the potential underestimation of the diagnosis or severity of AKI because of an increased creatinine distribution volume[97].
Therefore, this kidney paradox should be avoided in which clinicians attempt to enhance urinary output through repeated fluid challenges to avoid worsening kidney injury; these actions may in fact lead to more fluid accumulation and ultimately a worse outcome.
A BEDSIDE PATIENT-TAILORED APPROACH
To avoid potentially deleterious complications associated with fluid and electrolyte overload (Table 2), a patient-tailored approach is necessary to result in better outcomes for the individual patient. This will involve some aspects of fluid resuscitation, maintenance fluids, other sources of electrolytes and water and, ultimately, an active de-resuscitation strategy that may aim at the fluid balance and, if performed adequately will also remove the excessive loads of sodium and chloride. Here we describe how clinicians can address this situation at the bedside.
Table 2.
Potential complications of fluids and electrolytes overload
| Organ system | Complication | Main modifiable risk factor | Pathophysiological mechanism |
| Central nervous system | Delirium | Hypernatremia | Excessive sodium load Kidneys inability to excrete excess sodium load |
| Renal/metabolic | Worse recovery of renal function | Cumulative fluid balance/higher CVP | Renal edema, reduced perfusion pressure |
| Worsening acute kidney injury | Unbalanced solutions | Chloride-induced renal vasoconstriction | |
| Worsening acidemia | Unbalanced solutions | Solution SID relative to plasma SID Kidneys inability to excrete excess chloride load | |
| Respiratory | Impaired gas exchange Altered pulmonar and chest wall mechanics Increased work of breathing | Cumulative fluid balance/higher CVP/higher EVLW | Lung edema |
| Gastrointestinal | Ileum | Cumulative fluid balance | Bowel edema |
| Hepatic congestion | Higher CVP | Hepatic congestion | |
| Increased intra-abdominal pressure (may induce by itself more organ dysfunctions) | Cumulative fluid balance | Visceral edema (bowel, renal, etc.), ascites | |
| Hemostasis | Increased bleeding | Unbalanced solutions | Acidemia secondary to chloride load |
| Wound healing | Impaired wound healing | Cumulative fluid balance | Local edema |
| Hemodynamics | Worse microcirculatory blood flow | Higher CVP | Reduced perfusion pressure |
CVP: Central venous pressure; SID: Strong ion difference; EVLW: Extravascular lung water.
Judicious resuscitation
During the acute phase of resuscitation, fluids should be used judiciously to achieve an adequate perfusion. This encompasses four distinct aspects of fluid resuscitation: timing, type, amount and avoidance of the kidney paradox[98].
Resuscitation with fluids should be performed during the appropriate timing for this action, which occurs during the onset of the injury (intra-operative states) or soon after it (first hours after septic shock, major surgery or other acute physiological insults)[1]. There is no evidence that fluid resuscitation after these first moments will lead to better results. In fact, as we have discussed so far, the evidence points in the opposite direction.
In general, balanced solutions should be the fluids of choice in patients with shock states because they carry a lower chloride load, lead to less acid-base disturbances and most likely to better organ dysfunction outcomes, particularly for the kidneys, as shown in the study by Yunos et al[43].
The proper amount of fluid for acute care resuscitation is another critical component of judicious resuscitation. A recently published cohort of septic patients from Australia and New Zealand, which yielded impressive mortality outcomes, demonstrated that patients received approximately 3 L of fluids during the first hours of resuscitation[99]. Furthermore, in the recently published PROCESS trial, in patients who received more fluids (approximately 1 L more) in one of the three study arms, there were more cases of new onset renal failure than the usual care group[7]. With this in mind, particularly in cases of septic shock, after an initial fluid challenge of 20-30 mL/kg, we favor an earlier use of vasopressors and the avoidance of repeated fluid expansion in patients with vasodilatatory states, particularly in those with adequate perfusion parameters who are no longer fluid-responsive[100].
Another issue to be considered is to avoid the “kidney paradox”. In oliguric patients, one should strongly consider avoiding repeated volume expansions after the first hours of resuscitation because this will most likely lead to higher filling pressures and more fluid accumulation despite a possibly increased urine output. In patients who further develop anuria, fluid challenges might be even more deleterious.
Acid-base monitoring during resuscitation
During the resuscitation phase of critical illness, in addition to usual hemodynamic monitoring, it is important to monitor potentially deleterious effects of fluids and electrolytes on the acid-base status. As previously discussed, both fluid composition and quantity can influence acid-base status[98]. Through acid-base monitoring, one can identify at an earlier stage metabolic complications occurring during resuscitation. If possible, one should attempt to quantify which component of metabolic acidosis is worse during acute resuscitation because they carry different prognostic significances[16].
Active de-resuscitation
After a judicious resuscitation strategy, active de-resuscitation should be considered to avoid the deleterious effects of continuous fluid accumulation when the patient does not passively excrete the excess amount of water and electrolytes. This can be achieved both with diuretics, which have been shown to be safe in the context of AKI, or with an earlier indication of hemodialysis, as defended by some authors, when the former cannot control fluid overload[101]. To achieve a safe withdrawal of fluids and electrolytes, some factors must be considered:
Fluid removal rate: either with dialysis or diuretics, the fluid removal rate should be titrated to the patient hemodynamic status to avoid underfilling during this phase[102]. If tolerated, there will likely be no deleterious effects of fluid withdrawal, even in the presence of vasopressors or inotropic drugs.
Intermittent vs continuous infusion of diuretics: in critically ill patients, the continuous infusion of diuretics was not extensively studied. Better evidence from other clinical situations has not demonstrated any consistent advantage of one way of administering diuretics over another, except for higher doses of diuretics in intermittent therapy for the same fluid balance achievement compared to continuous infusion diuretic therapy[103,104]. As long as a target diuresis is achieved, there will likely be no differences among these treatments.
Association of albumin to furosemide: although albumin was not associated with better outcomes in patients with septic shock[105] or for fluid resuscitation in general ICU patients[106], in patients with ARDS, a better hemodynamic tolerance during fluid withdrawal was achieved with the combination of albumin and furosemide[107] and could be a useful adjunct during the active de-resuscitation phase.
Monitoring and treating metabolic complications: the use of diuretics is associated with more metabolic disturbances, including hypernatremia, hypokalemia and metabolic alkalosis[11]. To counteract these disturbances, we favor the use of acetazolamide if the patient develops metabolic alkalosis, thiazide diuretics and increases in free water reposition if hypernatremia ensues and the aggressive reposition of electrolytes to avoid extreme electrolyte disturbances. We refer the reader to the trial by Mekontso Dessap et al[94], which proposed a method to do this safely with a combination of diuretics.
Consideration for an earlier indication of renal replacement therapy in oliguria should be part of an active de-resuscitation strategy because some patients will develop stage III AKI[108], will be unresponsive to diuretics and, during this process, will accumulate fluids progressively, which has been consistently shown to be associated with worse outcomes in this specific population[88,89,96].
Although pulmonary edema is a common trigger for fluid withdrawal, it is a late and potentially deadly consequence of fluid overload. Hence, during this phase of active fluid withdrawal, some very simple monitoring strategies can be used.
Central venous pressure, although recently receiving discredit as a guide to fluid loading[109], was used in the largest randomized controlled trial on conservative fluid strategies[11]. In this regard, although there are many physiological states that can influence its isolated value, higher CVP values have been associated with worse outcomes in many conditions, including septic shock[79], likely not only because of fluid overload but also because of the associated significant effect of heart dysfunction on its values. Therefore, it can be an adjunct for active de-resuscitation strategies, and a goal towards lower CVP values (e.g., down to < 4 mmHg in adequately perfused patients without vasopressors) is a simple way to monitor this strategy.
An even simpler solution is to focus on fluid balance. Although daily weights would potentially be better, these values are also prone to error when measuring patients on ICU beds[110], and we believe that fluid balance, although imperfect, appears to be a simple bedside target of active de-resuscitation. Therefore, reaching even to negative fluid balances during the first days of critical illness onset and, as soon as possible, aiming at more negative fluid balances until reaching a cumulative fluid balance of approximately zero during the ICU stay appears to be a good approach.
In addition to active fluid withdrawal, one should avoid the unnecessary entrance of fluids and electrolytes in the form of electrolyte reposition, drugs dilution and as caloric intake on a daily basis because as we have previously discussed, these are important sources of sodium, chloride and water. In this regard, we favor using hypertonic glucose solutions if deemed necessary for minimum caloric intake (when the enteral route is not available for feeding) and the avoidance of maintenance fluids when there are no clinically relevant fluid losses (e.g., ileostomy). Finally, electrolytes, antimicrobials and other drugs should be diluted in the minimum necessary amount of fluids and preferentially in 5% dextrose in water[20].
With all this evidence combined, it appears that a judicious initial fluid resuscitation followed by a conservative fluid management approach will lead to better outcomes in patients in many different shock states, including in patients with sepsis and patients with other causes of non-septic SIRS. As we have discussed, hypernatremia is associated with worse outcomes, along with hyperchloremic acidosis in some scenarios and also progressive cumulative fluid balances. Many of the studies in this regard examined these issues separately, whereas these may be different aspects of the same problem: that of fluid and electrolyte overload.
CONCLUSION
Although there are no adequate prospective experimentally designed studies that have shown that fluids are essential in the treatment of critically ill patients, fluids are used liberally among ICU patients, and fluid accumulation is very common throughout the course of a patient’s ICU stay. In this review, we provided evidence of the potentially deleterious effects of fluids and electrolytes on many organ systems, how to monitor for these complications and how to manage this increasingly recognized clinical problem. Currently, we favor a more conservative approach regarding fluid management strategies in general ICU patients, although a randomized controlled trial addressing this issue is mandatory to shed light on this discussion, along with deeper mechanistic studies to understand the relative contributions of each component of fluid therapy.
Footnotes
P- Reviewer: Markic D, Su M, Shou ZF, Trkulja V S- Editor: Song XX L- Editor: A E- Editor: Lu YJ
Conflict-of-interest: The authors have no conflicts of interest to declare.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: October 2, 2014
First decision: November 14, 2014
Article in press: March 5, 2015
References
- 1.Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 2.Eisenberg RL, Bank WO, Hedgock MW. Renal failure after major angiography can be avoided with hydration. AJR Am J Roentgenol. 1981;136:859–861. doi: 10.2214/ajr.136.5.859. [DOI] [PubMed] [Google Scholar]
- 3.Gunal AI, Celiker H, Dogukan A, Ozalp G, Kirciman E, Simsekli H, Gunay I, Demircin M, Belhan O, Yildirim MA, et al. Early and vigorous fluid resuscitation prevents acute renal failure in the crush victims of catastrophic earthquakes. J Am Soc Nephrol. 2004;15:1862–1867. doi: 10.1097/01.asn.0000129336.09976.73. [DOI] [PubMed] [Google Scholar]
- 4.Davidson MB, Thakkar S, Hix JK, Bhandarkar ND, Wong A, Schreiber MJ. Pathophysiology, clinical consequences, and treatment of tumor lysis syndrome. Am J Med. 2004;116:546–554. doi: 10.1016/j.amjmed.2003.09.045. [DOI] [PubMed] [Google Scholar]
- 5.van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345:1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 6.Nemec K, Kopelent-Frank H, Greif R. Standardization of infusion solutions to reduce the risk of incompatibility. Am J Health Syst Pharm. 2008;65:1648–1654. doi: 10.2146/ajhp070471. [DOI] [PubMed] [Google Scholar]
- 7.Yealy DM, Kellum JA, Huang DT, Barnato AE, Weissfeld LA, Pike F, Terndrup T, Wang HE, Hou PC, LoVecchio F, et al. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014;370:1683–1693. doi: 10.1056/NEJMoa1401602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maciel AT, Park M, Macedo E. Physicochemical analysis of blood and urine in the course of acute kidney injury in critically ill patients: a prospective, observational study. BMC Anesthesiol. 2013;13:31. doi: 10.1186/1471-2253-13-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maciel AT, Park M. Urine assessment in the critically ill: a matter of both quantity and quality. Rev Bras Ter Intensiva. 2013;25:184–185. doi: 10.5935/0103-507X.20130032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maciel AT, Park M, Macedo E. Urinary electrolyte monitoring in critically ill patients: a preliminary observational study. Rev Bras Ter Intensiva. 2012;24:236–245. [PubMed] [Google Scholar]
- 11.National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network; Wiedemann HP, Wheeler AP, Bernard GR,Thompson BT, Hayden D, deBoisblanc B, Connors AF Jr, Hite RD, Harabin AL. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354:2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 12.Guyton AC, Hall JE. The body fluid compartments: extracellular and intracellular fluids; interstitial fluid and edema. In: Guyton AC, Hall JE, editors. Textbook of Medical Physiology. 10th ed. Philadelphia, PA: Saunders; 2000. pp. 250–264. [Google Scholar]
- 13.Nguyen MK, Kurtz I. Quantitative interrelationship between Gibbs-Donnan equilibrium, osmolality of body fluid compartments, and plasma water sodium concentration. J Appl Physiol (1985) 2006;100:1293–1300. doi: 10.1152/japplphysiol.01274.2005. [DOI] [PubMed] [Google Scholar]
- 14.Lang F, Busch GL, Ritter M, Völkl H, Waldegger S, Gulbins E, Häussinger D. Functional significance of cell volume regulatory mechanisms. Physiol Rev. 1998;78:247–306. doi: 10.1152/physrev.1998.78.1.247. [DOI] [PubMed] [Google Scholar]
- 15.Guidet B, Soni N, Della Rocca G, Kozek S, Vallet B, Annane D, James M. A balanced view of balanced solutions. Crit Care. 2010;14:325. doi: 10.1186/cc9230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noritomi DT, Soriano FG, Kellum JA, Cappi SB, Biselli PJ, Libório AB, Park M. Metabolic acidosis in patients with severe sepsis and septic shock: a longitudinal quantitative study. Crit Care Med. 2009;37:2733–2739. doi: 10.1097/ccm.0b013e3181a59165. [DOI] [PubMed] [Google Scholar]
- 17.Adrogué HJ, Madias NE. Aiding fluid prescription for the dysnatremias. Intensive Care Med. 1997;23:309–316. doi: 10.1007/s001340050333. [DOI] [PubMed] [Google Scholar]
- 18.Stelfox HT, Ahmed SB, Khandwala F, Zygun D, Shahpori R, Laupland K. The epidemiology of intensive care unit-acquired hyponatraemia and hypernatraemia in medical-surgical intensive care units. Crit Care. 2008;12:R162. doi: 10.1186/cc7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van De Louw A, Shaffer C, Schaefer E. Early intensive care unit-acquired hypernatremia in severe sepsis patients receiving 0.9% saline fluid resuscitation. Acta Anaesthesiol Scand. 2014;58:1007–1014. doi: 10.1111/aas.12368. [DOI] [PubMed] [Google Scholar]
- 20.Choo WP, Groeneveld AB, Driessen RH, Swart EL. Normal saline to dilute parenteral drugs and to keep catheters open is a major and preventable source of hypernatremia acquired in the intensive care unit. J Crit Care. 2014;29:390–394. doi: 10.1016/j.jcrc.2014.01.025. [DOI] [PubMed] [Google Scholar]
- 21.Bihari S, Peake SL, Seppelt I, Williams P, Bersten A; George Institute for Global Health; Australian and New Zealand Intensive Care Society Clinical Trials Group. Sodium administration in critically ill patients in Australia and New Zealand: a multicentre point prevalence study. Crit Care Resusc. 2013;15:294–300. [PubMed] [Google Scholar]
- 22.Arampatzis S, Frauchiger B, Fiedler GM, Leichtle AB, Buhl D, Schwarz C, Funk GC, Zimmermann H, Exadaktylos AK, Lindner G. Characteristics, symptoms, and outcome of severe dysnatremias present on hospital admission. Am J Med. 2012;125:1125.e1–1125.e7. doi: 10.1016/j.amjmed.2012.04.041. [DOI] [PubMed] [Google Scholar]
- 23.Funk GC, Lindner G, Druml W, Metnitz B, Schwarz C, Bauer P, Metnitz PG. Incidence and prognosis of dysnatremias present on ICU admission. Intensive Care Med. 2010;36:304–311. doi: 10.1007/s00134-009-1692-0. [DOI] [PubMed] [Google Scholar]
- 24.Lindner G, Funk GC, Schwarz C, Kneidinger N, Kaider A, Schneeweiss B, Kramer L, Druml W. Hypernatremia in the critically ill is an independent risk factor for mortality. Am J Kidney Dis. 2007;50:952–957. doi: 10.1053/j.ajkd.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 25.Vandergheynst F, Sakr Y, Felleiter P, Hering R, Groeneveld J, Vanhems P, Taccone FS, Vincent JL. Incidence and prognosis of dysnatraemia in critically ill patients: analysis of a large prevalence study. Eur J Clin Invest. 2013;43:933–948. doi: 10.1111/eci.12123. [DOI] [PubMed] [Google Scholar]
- 26.Lindner G, Funk GC. Hypernatremia in critically ill patients. J Crit Care. 2013;28:216.e11–216.e20. doi: 10.1016/j.jcrc.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 27.Lindner G. “Hypernatremia in the intensive care unit--an iatrogenic complication?”. J Crit Care. 2013;28:214–215. doi: 10.1016/j.jcrc.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 28.Darmon M, Pichon M, Schwebel C, Ruckly S, Adrie C, Haouache H, Azoulay E, Bouadma L, Clec’h C, Garrouste-Orgeas M, et al. Influence of early dysnatremia correction on survival of critically ill patients. Shock. 2014;41:394–399. doi: 10.1097/SHK.0000000000000135. [DOI] [PubMed] [Google Scholar]
- 29.Berend K, van Hulsteijn LH, Gans RO. Chloride: the queen of electrolytes? Eur J Intern Med. 2012;23:203–211. doi: 10.1016/j.ejim.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 30.Park M, Calabrich A, Maciel A, Zampieri F, Taniguchi L, Souza C, Barboza C, Nassar Junior AP, Azevedo L. Physicochemical characterization of metabolic acidosis induced by normal saline resuscitation of patients with severe sepsis and septic shock. Rev Bras Ter intensiva. 2011;23:176–182. [PubMed] [Google Scholar]
- 31.Kellum JA, Bellomo R, Kramer DJ, Pinsky MR. Etiology of metabolic acidosis during saline resuscitation in endotoxemia. Shock. 1998;9:364–368. doi: 10.1097/00024382-199805000-00009. [DOI] [PubMed] [Google Scholar]
- 32.Maciel AT, Park M. Differences in acid-base behavior between intensive care unit survivors and nonsurvivors using both a physicochemical and a standard base excess approach: a prospective, observational study. J Crit Care. 2009;24:477–483. doi: 10.1016/j.jcrc.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 33.Boniatti MM, Cardoso PR, Castilho RK, Vieira SR. Is hyperchloremia associated with mortality in critically ill patients? A prospective cohort study. J Crit Care. 2011;26:175–179. doi: 10.1016/j.jcrc.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 34.Wilcox CS. Regulation of renal blood flow by plasma chloride. J Clin Invest. 1983;71:726–735. doi: 10.1172/JCI110820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Imig JD, Passmore JC, Anderson GL, Jimenez AE. Chloride alters renal blood flow autoregulation in deoxycorticosterone-treated rats. J Lab Clin Med. 1993;121:608–613. [PubMed] [Google Scholar]
- 36.Zhou F, Peng ZY, Bishop JV, Cove ME, Singbartl K, Kellum JA. Effects of fluid resuscitation with 0.9% saline versus a balanced electrolyte solution on acute kidney injury in a rat model of sepsis*. Crit Care Med. 2014;42:e270–e278. doi: 10.1097/CCM.0000000000000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jensen BL, Ellekvist P, Skøtt O. Chloride is essential for contraction of afferent arterioles after agonists and potassium. Am J Physiol. 1997;272:F389–F396. doi: 10.1152/ajprenal.1997.272.3.F389. [DOI] [PubMed] [Google Scholar]
- 38.Hansen PB, Jensen BL, Skott O. Chloride regulates afferent arteriolar contraction in response to depolarization. Hypertension. 1998;32:1066–1070. doi: 10.1161/01.hyp.32.6.1066. [DOI] [PubMed] [Google Scholar]
- 39.Chowdhury AH, Cox EF, Francis ST, Lobo DN. A randomized, controlled, double-blind crossover study on the effects of 2-L infusions of 0.9% saline and plasma-lyte® 148 on renal blood flow velocity and renal cortical tissue perfusion in healthy volunteers. Ann Surg. 2012;256:18–24. doi: 10.1097/SLA.0b013e318256be72. [DOI] [PubMed] [Google Scholar]
- 40.Reid F, Lobo DN, Williams RN, Rowlands BJ, Allison SP. (Ab)normal saline and physiological Hartmann’s solution: a randomized double-blind crossover study. Clin Sci (Lond) 2003;104:17–24. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- 41.Bell PD, Lapointe JY, Cardinal J. Direct measurement of basolateral membrane potentials from cells of the macula densa. Am J Physiol. 1989;257:F463–F468. doi: 10.1152/ajprenal.1989.257.3.F463. [DOI] [PubMed] [Google Scholar]
- 42.Ren Y, Garvin JL, Liu R, Carretero OA. Role of macula densa adenosine triphosphate (ATP) in tubuloglomerular feedback. Kidney Int. 2004;66:1479–1485. doi: 10.1111/j.1523-1755.2004.00911.x. [DOI] [PubMed] [Google Scholar]
- 43.Yunos NM, Bellomo R, Hegarty C, Story D, Ho L, Bailey M. Association between a chloride-liberal vs chloride-restrictive intravenous fluid administration strategy and kidney injury in critically ill adults. JAMA. 2012;308:1566–1572. doi: 10.1001/jama.2012.13356. [DOI] [PubMed] [Google Scholar]
- 44.Waters JH, Gottlieb A, Schoenwald P, Popovich MJ, Sprung J, Nelson DR. Normal saline versus lactated Ringer’s solution for intraoperative fluid management in patients undergoing abdominal aortic aneurysm repair: an outcome study. Anesth Analg. 2001;93:817–822. doi: 10.1097/00000539-200110000-00004. [DOI] [PubMed] [Google Scholar]
- 45.Wilkes NJ, Woolf R, Mutch M, Mallett SV, Peachey T, Stephens R, Mythen MG. The effects of balanced versus saline-based hetastarch and crystalloid solutions on acid-base and electrolyte status and gastric mucosal perfusion in elderly surgical patients. Anesth Analg. 2001;93:811–816. doi: 10.1097/00000539-200110000-00003. [DOI] [PubMed] [Google Scholar]
- 46.Lindner G, Schwarz C, Grüssing H, Kneidinger N, Fazekas A, Funk GC. Rising serum sodium levels are associated with a concurrent development of metabolic alkalosis in critically ill patients. Intensive Care Med. 2013;39:399–405. doi: 10.1007/s00134-012-2753-3. [DOI] [PubMed] [Google Scholar]
- 47.Hofmann-Kiefer KF, Chappell D, Jacob M, Schülke A, Conzen P, Rehm M. [Hypernatremic alkalosis. Possible counterpart of hyperchloremic acidosis in intensive care patients?] Anaesthesist. 2009;58:1210–1215. doi: 10.1007/s00101-009-1640-y. [DOI] [PubMed] [Google Scholar]
- 48.Kellum JA, Song M, Almasri E. Hyperchloremic acidosis increases circulating inflammatory molecules in experimental sepsis. Chest. 2006;130:962–967. doi: 10.1378/chest.130.4.962. [DOI] [PubMed] [Google Scholar]
- 49.Kellum JA, Song M, Li J. Lactic and hydrochloric acids induce different patterns of inflammatory response in LPS-stimulated RAW 264.7 cells. Am J Physiol Regul Integr Comp Physiol. 2004;286:R686–R692. doi: 10.1152/ajpregu.00564.2003. [DOI] [PubMed] [Google Scholar]
- 50.Bellocq A, Suberville S, Philippe C, Bertrand F, Perez J, Fouqueray B, Cherqui G, Baud L. Low environmental pH is responsible for the induction of nitric-oxide synthase in macrophages. Evidence for involvement of nuclear factor-kappaB activation. J Biol Chem. 1998;273:5086–5092. doi: 10.1074/jbc.273.9.5086. [DOI] [PubMed] [Google Scholar]
- 51.Heming TA, Davé SK, Tuazon DM, Chopra AK, Peterson JW, Bidani A. Effects of extracellular pH on tumour necrosis factor-alpha production by resident alveolar macrophages. Clin Sci (Lond) 2001;101:267–274. [PubMed] [Google Scholar]
- 52.Kellum JA, Song M, Venkataraman R. Effects of hyperchloremic acidosis on arterial pressure and circulating inflammatory molecules in experimental sepsis. Chest. 2004;125:243–248. doi: 10.1378/chest.125.1.243. [DOI] [PubMed] [Google Scholar]
- 53.Kellum JA. Fluid resuscitation and hyperchloremic acidosis in experimental sepsis: improved short-term survival and acid-base balance with Hextend compared with saline. Crit Care Med. 2002;30:300–305. doi: 10.1097/00003246-200202000-00006. [DOI] [PubMed] [Google Scholar]
- 54.Stone HH, Fulenwider JT. Renal decapsulation in the prevention of post-ischemic oliguria. Ann Surg. 1977;186:343–355. doi: 10.1097/00000658-197709000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bock JS, Gottlieb SS. Cardiorenal syndrome: new perspectives. Circulation. 2010;121:2592–2600. doi: 10.1161/CIRCULATIONAHA.109.886473. [DOI] [PubMed] [Google Scholar]
- 56.Damman K, van Deursen VM, Navis G, Voors AA, van Veldhuisen DJ, Hillege HL. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J Am Coll Cardiol. 2009;53:582–588. doi: 10.1016/j.jacc.2008.08.080. [DOI] [PubMed] [Google Scholar]
- 57.Legrand M, Dupuis C, Simon C, Gayat E, Mateo J, Lukaszewicz AC, Payen D. Association between systemic hemodynamics and septic acute kidney injury in critically ill patients: a retrospective observational study. Crit Care. 2013;17:R278. doi: 10.1186/cc13133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Burnett JC, Knox FG. Renal interstitial pressure and sodium excretion during renal vein constriction. Am J Physiol. 1980;238:F279–F282. doi: 10.1152/ajprenal.1980.238.4.F279. [DOI] [PubMed] [Google Scholar]
- 59.Cruces P, Salas C, Lillo P, Salomon T, Lillo F, Hurtado DE. The renal compartment: a hydraulic view. Intensive Care Medicine Experimental. 2014;2:26. doi: 10.1186/s40635-014-0026-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yoneda K. Anatomic pathway of fluid leakage in fluid-overload pulmonary edema in mice. Am J Pathol. 1980;101:7–16. [PMC free article] [PubMed] [Google Scholar]
- 61.Laine GA, Allen SJ, Katz J, Gabel JC, Drake RE. Effect of systemic venous pressure elevation on lymph flow and lung edema formation. J Appl Physiol (1985) 1986;61:1634–1638. doi: 10.1152/jappl.1986.61.5.1634. [DOI] [PubMed] [Google Scholar]
- 62.West JB, Mathieu-Costello O. Vulnerability of pulmonary capillaries in heart disease. Circulation. 1995;92:622–631. doi: 10.1161/01.cir.92.3.622. [DOI] [PubMed] [Google Scholar]
- 63.West JB. Invited review: pulmonary capillary stress failure. J Appl Physiol (1985) 2000;89:2483–2489; discussion 2497. doi: 10.1152/jappl.2000.89.6.2483. [DOI] [PubMed] [Google Scholar]
- 64.Tsukimoto K, Mathieu-Costello O, Prediletto R, Elliott AR, West JB. Ultrastructural appearances of pulmonary capillaries at high transmural pressures. J Appl Physiol (1985) 1991;71:573–582. doi: 10.1152/jappl.1991.71.2.573. [DOI] [PubMed] [Google Scholar]
- 65.De Pasquale CG, Arnolda LF, Doyle IR, Grant RL, Aylward PE, Bersten AD. Prolonged alveolocapillary barrier damage after acute cardiogenic pulmonary edema. Crit Care Med. 2003;31:1060–1067. doi: 10.1097/01.CCM.0000059649.31659.22. [DOI] [PubMed] [Google Scholar]
- 66.Guazzi M. Alveolar-capillary membrane dysfunction in heart failure: evidence of a pathophysiologic role. Chest. 2003;124:1090–1102. doi: 10.1378/chest.124.3.1090. [DOI] [PubMed] [Google Scholar]
- 67.Ruff F, Caubarrere I, Salem A, Dubois F, Duroux P. [Regional distribution of pulmonary perfusion during fluid overload in man] Ann Anesthesiol Fr. 1975;16 Spec No 2-3:164–168. [PubMed] [Google Scholar]
- 68.Drinker CK, Peabody FW, Blumgart HL. The effect of pulmonary congestion on the on the ventilation of the lungs. J Exp Med. 1922;35:77–95. doi: 10.1084/jem.35.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bone RC. Treatment of adult respiratory distress syndrome with diuretics, dialysis, and positive end-expiratory pressure. Crit Care Med. 1978;6:136–139. doi: 10.1097/00003246-197805000-00002. [DOI] [PubMed] [Google Scholar]
- 70.Mitchell JP, Schuller D, Calandrino FS, Schuster DP. Improved outcome based on fluid management in critically ill patients requiring pulmonary artery catheterization. Am Rev Respir Dis. 1992;145:990–998. doi: 10.1164/ajrccm/145.5.990. [DOI] [PubMed] [Google Scholar]
- 71.Brandstrup B, Tønnesen H, Beier-Holgersen R, Hjortsø E, Ørding H, Lindorff-Larsen K, Rasmussen MS, Lanng C, Wallin L, Iversen LH, et al. Effects of intravenous fluid restriction on postoperative complications: comparison of two perioperative fluid regimens: a randomized assessor-blinded multicenter trial. Ann Surg. 2003;238:641–648. doi: 10.1097/01.sla.0000094387.50865.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Macafee DA, Allison SP, Lobo DN. Some interactions between gastrointestinal function and fluid and electrolyte homeostasis. Curr Opin Clin Nutr Metab Care. 2005;8:197–203. doi: 10.1097/00075197-200503000-00015. [DOI] [PubMed] [Google Scholar]
- 73.Seeto RK, Fenn B, Rockey DC. Ischemic hepatitis: clinical presentation and pathogenesis. Am J Med. 2000;109:109–113. doi: 10.1016/s0002-9343(00)00461-7. [DOI] [PubMed] [Google Scholar]
- 74.Kirkpatrick AW, Roberts DJ, De Waele J, Jaeschke R, Malbrain ML, De Keulenaer B, Duchesne J, Bjorck M, Leppaniemi A, Ejike JC, et al. Intra-abdominal hypertension and the abdominal compartment syndrome: updated consensus definitions and clinical practice guidelines from the World Society of the Abdominal Compartment Syndrome. Intensive Care Med. 2013;39:1190–1206. doi: 10.1007/s00134-013-2906-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Prowle JR, Echeverri JE, Ligabo EV, Ronco C, Bellomo R. Fluid balance and acute kidney injury. Nat Rev Nephrol. 2010;6:107–115. doi: 10.1038/nrneph.2009.213. [DOI] [PubMed] [Google Scholar]
- 76.Haskins SC, Hopper K, Rezende ML. The acid-base impact of free water removal from, and addition to, plasma. J Lab Clin Med. 2006;147:114–120. doi: 10.1016/j.lab.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 77.Gattinoni L, Carlesso E, Maiocchi G, Polli F, Cadringher P. Dilutional acidosis: where do the protons come from? Intensive Care Med. 2009;35:2033–2043. doi: 10.1007/s00134-009-1653-7. [DOI] [PubMed] [Google Scholar]
- 78.Carlesso E, Maiocchi G, Tallarini F, Polli F, Valenza F, Cadringher P, Gattinoni L. The rule regulating pH changes during crystalloid infusion. Intensive Care Med. 2011;37:461–468. doi: 10.1007/s00134-010-2095-y. [DOI] [PubMed] [Google Scholar]
- 79.Boyd JH, Forbes J, Nakada TA, Walley KR, Russell JA. Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med. 2011;39:259–265. doi: 10.1097/CCM.0b013e3181feeb15. [DOI] [PubMed] [Google Scholar]
- 80.de Almeida JP, Palomba H, Galas FR, Fukushima JT, Duarte FA, Nagaoka D, Torres V, Yu L, Vincent JL, Auler JO, et al. Positive fluid balance is associated with reduced survival in critically ill patients with cancer. Acta Anaesthesiol Scand. 2012;56:712–717. doi: 10.1111/j.1399-6576.2012.02717.x. [DOI] [PubMed] [Google Scholar]
- 81.Walsh SR, Walsh CJ. Intravenous fluid-associated morbidity in postoperative patients. Ann R Coll Surg Engl. 2005;87:126–130. doi: 10.1308/147870805X28127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Upadya A, Tilluckdharry L, Muralidharan V, Amoateng-Adjepong Y, Manthous CA. Fluid balance and weaning outcomes. Intensive Care Med. 2005;31:1643–1647. doi: 10.1007/s00134-005-2801-3. [DOI] [PubMed] [Google Scholar]
- 83.Lee J, de Louw E, Niemi M, Nelson R, Mark RG, Celi LA, Mukamal KJ, Danziger J. Association between fluid balance and survival in critically ill patients. J Intern Med. 2014;277:468–477. doi: 10.1111/joim.12274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41:580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 85.Murphy CV, Schramm GE, Doherty JA, Reichley RM, Gajic O, Afessa B, Micek ST, Kollef MH. The importance of fluid management in acute lung injury secondary to septic shock. Chest. 2009;136:102–109. doi: 10.1378/chest.08-2706. [DOI] [PubMed] [Google Scholar]
- 86.Sakr Y, Vincent JL, Reinhart K, Groeneveld J, Michalopoulos A, Sprung CL, Artigas A, Ranieri VM; Sepsis Occurence in Acutely Ill Patients Investigators. High tidal volume and positive fluid balance are associated with worse outcome in acute lung injury. Chest. 2005;128:3098–3108. doi: 10.1378/chest.128.5.3098. [DOI] [PubMed] [Google Scholar]
- 87.Epstein CD, Peerless JR. Weaning readiness and fluid balance in older critically ill surgical patients. Am J Crit Care. 2006;15:54–64. [PubMed] [Google Scholar]
- 88.Payen D, de Pont AC, Sakr Y, Spies C, Reinhart K, Vincent JL; Sepsis Occurrence in Acutely Ill Patients (SOAP) Investigators. A positive fluid balance is associated with a worse outcome in patients with acute renal failure. Crit Care. 2008;12:R74. doi: 10.1186/cc6916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.RENAL Replacement Therapy Study Investigators; Bellomo R, Cass A, Cole L, Finfer S, Gallagher M, Lee J, Lo S, McArthur C, McGuiness S, Norton R, et al. An observational study fluid balance and patient outcomes in the Randomized Evaluation of Normal vs. Augmented Level of Replacement Therapy trial. Crit Care Med. 2012;40:1753–1760. doi: 10.1097/CCM.0b013e318246b9c6. [DOI] [PubMed] [Google Scholar]
- 90.Dulhunty JM, Lipman J, Finfer S; Sepsis Study Investigators for the ANZICS Clinical Trials Group. Does severe non-infectious SIRS differ from severe sepsis? Results from a multi-centre Australian and New Zealand intensive care unit study. Intensive Care Med. 2008;34:1654–1661. doi: 10.1007/s00134-008-1160-2. [DOI] [PubMed] [Google Scholar]
- 91.Bagshaw SM, Brophy PD, Cruz D, Ronco C. Fluid balance as a biomarker: impact of fluid overload on outcome in critically ill patients with acute kidney injury. Crit Care. 2008;12:169. doi: 10.1186/cc6948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Brower RG, Lanken PN, MacIntyre N, Matthay MA, Morris A, Ancukiewicz M, Schoenfeld D, Thompson BT. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004;351:327–336. doi: 10.1056/NEJMoa032193. [DOI] [PubMed] [Google Scholar]
- 93.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 94.Mekontso Dessap A, Roche-Campo F, Kouatchet A, Tomicic V, Beduneau G, Sonneville R, Cabello B, Jaber S, Azoulay E, Castanares-Zapatero D, et al. Natriuretic peptide-driven fluid management during ventilator weaning: a randomized controlled trial. Am J Respir Crit Care Med. 2012;186:1256–1263. doi: 10.1164/rccm.201205-0939OC. [DOI] [PubMed] [Google Scholar]
- 95.Van Biesen W, Yegenaga I, Vanholder R, Verbeke F, Hoste E, Colardyn F, Lameire N. Relationship between fluid status and its management on acute renal failure (ARF) in intensive care unit (ICU) patients with sepsis: a prospective analysis. J Nephrol. 2005;18:54–60. [PubMed] [Google Scholar]
- 96.Bouchard J, Soroko SB, Chertow GM, Himmelfarb J, Ikizler TA, Paganini EP, Mehta RL; Program to Improve Care in Acute Renal Disease (PICARD) Study Group. Fluid accumulation, survival and recovery of kidney function in critically ill patients with acute kidney injury. Kidney Int. 2009;76:422–427. doi: 10.1038/ki.2009.159. [DOI] [PubMed] [Google Scholar]
- 97.Macedo E, Bouchard J, Soroko SH, Chertow GM, Himmelfarb J, Ikizler TA, Paganini EP, Mehta RL; Program to Improve Care in Acute Renal Disease Study. Fluid accumulation, recognition and staging of acute kidney injury in critically-ill patients. Crit Care. 2010;14:R82. doi: 10.1186/cc9004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.McDermid RC, Raghunathan K, Romanovsky A, Shaw AD, Bagshaw SM. Controversies in fluid therapy: Type, dose and toxicity. World J Crit Care Med. 2014;3:24–33. doi: 10.5492/wjccm.v3.i1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Peake SL, Bailey M, Bellomo R, Cameron PA, Cross A, Delaney A, Finfer S, Higgins A, Jones DA, Myburgh JA, et al. Australasian resuscitation of sepsis evaluation (ARISE): A multi-centre, prospective, inception cohort study. Resuscitation. 2009;80:811–818. doi: 10.1016/j.resuscitation.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 100.Marik PE. Early management of severe sepsis: concepts and controversies. Chest. 2014;145:1407–1418. doi: 10.1378/chest.13-2104. [DOI] [PubMed] [Google Scholar]
- 101.Uchino S, Doig GS, Bellomo R, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Nacedo E, Gibney N, Tolwani A, Ronco C, Kellum JA; Beginning and Ending Supportive Therapy for the Kidney (B. E.S.T. Kidney) Investigators. Diuretics and mortality in acute renal failure. Crit Care Med. 2004;32:1669–1677. doi: 10.1097/01.ccm.0000132892.51063.2f. [DOI] [PubMed] [Google Scholar]
- 102.Prowle JR, Kirwan CJ, Bellomo R. Fluid management for the prevention and attenuation of acute kidney injury. Nat Rev Nephrol. 2014;10:37–47. doi: 10.1038/nrneph.2013.232. [DOI] [PubMed] [Google Scholar]
- 103.Ostermann M, Alvarez G, Sharpe MD, Martin CM. Frusemide administration in critically ill patients by continuous compared to bolus therapy. Nephron Clin Pract. 2007;107:c70–c76. doi: 10.1159/000108641. [DOI] [PubMed] [Google Scholar]
- 104.Felker GM, Lee KL, Bull DA, Redfield MM, Stevenson LW, Goldsmith SR, LeWinter MM, Deswal A, Rouleau JL, Ofili EO, et al. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med. 2011;364:797–805. doi: 10.1056/NEJMoa1005419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Caironi P, Tognoni G, Masson S, Fumagalli R, Pesenti A, Romero M, Fanizza C, Caspani L, Faenza S, Grasselli G, et al. Albumin replacement in patients with severe sepsis or septic shock. N Engl J Med. 2014;370:1412–1421. doi: 10.1056/NEJMoa1305727. [DOI] [PubMed] [Google Scholar]
- 106.Finfer S, Bellomo R, Boyce N, French J, Myburgh J, Norton R; SAFE Study Investigators. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med. 2004;350:2247–2256. doi: 10.1056/NEJMoa040232. [DOI] [PubMed] [Google Scholar]
- 107.Martin GS, Moss M, Wheeler AP, Mealer M, Morris JA, Bernard GR. A randomized, controlled trial of furosemide with or without albumin in hypoproteinemic patients with acute lung injury. Crit Care Med. 2005;33:1681–1687. doi: 10.1097/01.ccm.0000171539.47006.02. [DOI] [PubMed] [Google Scholar]
- 108.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A; Acute Kidney Injury Network. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Marik PE, Cavallazzi R. Does the central venous pressure predict fluid responsiveness? An updated meta-analysis and a plea for some common sense. Crit Care Med. 2013;41:1774–1781. doi: 10.1097/CCM.0b013e31828a25fd. [DOI] [PubMed] [Google Scholar]
- 110.Schneider AG, Baldwin I, Freitag E, Glassford N, Bellomo R Estimation of fluid status changes in critically ill patients: fluid balance chart or electronic bed weight? J Crit Care. 2012;27:745.e7–745.12. doi: 10.1016/j.jcrc.2011.12.017. [DOI] [PubMed] [Google Scholar]


