Abstract
In growing leaves, lack of isoprene synthase is considered responsible for delayed isoprene emission, but competition for dimethylallyl diphosphate (DMADP), the substrate for both isoprene synthesis and prenyltransferase reactions in photosynthetic pigment and phytohormone synthesis, can also play a role. We used a kinetic approach based on postillumination isoprene decay and modeling DMADP consumption to estimate in vivo kinetic characteristics of isoprene synthase and prenyltransferase reactions, and determine the share of DMADP use by different processes through leaf development in Populus tremula. Pigment synthesis rate was also estimated from pigment accumulation data, and distribution of DMADP use from isoprene emission changes due to alendronate, a selective inhibitor of prenyltransferases. Development of photosynthetic activity and pigment synthesis occurred with the greatest rate in 1-5 days old leaves when isoprene emission was absent. Isoprene emission commenced on days 5-6 and increased simultaneously with slowing down of pigment synthesis. In vivo Michaelis-Menten constant (Km) values obtained were 265 nmol m−2 (20 μM) for DMADP-consuming prenyltransferase reactions and 2560 nmol m−2 (190 μM) for isoprene synthase. Thus, despite decelerating pigment synthesis reactions in maturing leaves, isoprene emission in young leaves was limited by both isoprene synthase activity and competition for DMADP by prenyltransferase reactions.
Keywords: competition within isoprenoid synthesis pathway, geranyl diphosphate synthase, isoprene emission, leaf development, photosynthesis development, pigment synthesis, prenyltransferase reactions
Introduction
Isoprene (2-methyl-1,3-butadiene) is a volatile leaf hydrocarbon importantly modulating the balance of photochemically induced oxidative processes in troposphere, in particularly contributing to ozone formation in atmospheres enriched with nitrogen oxides (e.g., Atkinson & Arey 2003; Sanderson et al. 2003; Pike & Young 2009). Isoprene is produced through the chloroplastic 2-C-methylerythritol 5-phosphate (MEP) isoprenoid synthesis pathway, and there is major interest in understanding the regulation of the isoprene synthesis pathway to predict isoprene emissions from biosphere (Sharkey, Wiberley & Donohue 2008; Sharkey 2009; Niinemets et al. 2010b; Monson et al. 2012; Grote, Monson & Niinemets 2013; Guenther 2013; Li & Sharkey 2013b; Niinemets & Monson 2013). Not all plant species emit isoprene, and in the emitters, the rate of isoprene emission can vary orders of magnitude during foliage life span (Grinspoon, Bowman & Fall 1991; Monson et al. 1992; Kuzma & Fall 1993; Sharkey et al. 1996).
Leaf development comprises an important part of leaf lifetime (Miyazawa, Satomi & Terashima 1998; Miyazawa, Makino & Terashima 2003; Niinemets, García-Plazaola & Tosens 2012), but the factors controlling the induction of isoprene emission and variation during leaf ontogeny are still not fully understood and empirical approaches are used in models predicting isoprene emission during leaf development (Niinemets et al. 2010a; Monson et al. 2012; Grote et al. 2013). Development of leaf photosynthetic activity is a highly orchestrated process characterized by coordinated accumulation of photosynthetic pigments, proteins and membrane lipids in developing chloroplasts regulated by phytohormones, especially cytokinins (Skoog & Armstrong 1970; Fletcher & McCullagh 1971; Schmülling, Schafer & Romanov 1997; Nakano et al. 2001) Shesták, 1985 #45309} and gibberellins (Nelissen et al. 2012). As carotenoids and gibberellins, the phytyl residue of chlorophylls, plastoquinone (Lichtenthaler 2007; 2009), and the 4-hydroxy-3-methyl-2-butenyl-diphosphate side chain of cytokinins (Kakimoto 2003; Hwang & Sakakibara 2006) are formed by the MEP pathway, the MEP pathway is central in leaf development.
Despite the important role of the MEP pathway in photosynthetic machinery development, delays between the development of photosynthetic activity and isoprene emission have been observed in previous studies (Grinspoon et al. 1991; Sharkey & Loreto 1993; Harley et al. 1994; Monson et al. 1994; Wiberley et al. 2005; Niinemets et al. 2010a; Guidolotti, Calfapietra & Loreto 2011). The delay in isoprene emission has been associated with the time-lag between the development of photosynthetic activity and expression of isoprene synthase (IspS) (Schnitzler et al. 1996; Fall & Wildermuth 1998; Wiberley et al. 2005; Sharkey et al. 2008; Cinege et al. 2009; Wiberley et al. 2009; Vickers et al. 2010), suggesting that the first step towards induction of isoprene emission is the activation of the promoter of IspS (Sharkey et al. 2008; Cinege et al. 2009; Wiberley et al. 2009). Increases in isoprene synthase activity are strongly correlated with time-dependent increases in isoprene emission in developing leaves (Kuzma & Fall 1993; Monson et al. 1994; Schnitzler et al. 1996; Lehning et al. 1999; Lehning et al. 2001; Vickers et al. 2010), further emphasizing that the induction of IspS protein synthesis is the point of no return for the onset of isoprene emission in developing leaves.
Although IspS is needed for the onset of isoprene, it is characterized by a very high Michaelis-Menten constant (Km) for its immediate substrate, dimethylallyl diphosphate (DMADP) with estimates varying between 0.3-9 mM (for recent reviews see Li & Sharkey 2013b; Rajabi Memari, Pazouki & Niinemets 2013; Weise et al. 2013). Especially high estimates corresponding to in vitro studies are likely overestimates (Rasulov et al. 2009a; Rasulov et al. 2009b; Weise et al. 2013), but the available data collectively do demonstrate that high DMADP concentrations are needed for high isoprene emission rates. Given that isoprene emission relies on carbon, ATP and NADPH provided by photosynthesis (Sanadze & Dzhaiani 1972; Loreto & Sharkey 1990; Sharkey, Loreto & Delwiche 1991; Rasulov et al. 2009b; Li & Sharkey 2013b), low isoprene emission rates in young leaves can reflect limited supply of carbon and energetic and reductive co-factors in young leaves with low photosynthetic activity, reducing the key substrate, DMADP, availability for isoprene formation. On the other hand, synthesis of the components of the photosynthetic machinery (carotenoids, chlorophylls, plastoquinone), and gibberellins and cytokinins during the initial period of leaf development may also reduce the availability of DMADP for isoprene synthesis (Owen & Peñuelas 2005). Given that the first reaction downstream of DMADP, synthesis of geranyl diphosphate (GDP) by geranyl diphosphate synthase has a much lower Km value for DMADP (Orlova et al. 2009; Wang & Dixon 2009; Chang et al. 2010) than isoprene synthase (Schnitzler et al. 2005; Rasulov et al. 2009a; Rasulov et al. 2009b; Köksal et al. 2010), competition for DMADP may constrain isoprene emission rate in developing leaves. This trade-off between the synthesis of “essential” isoprenoids with well-known structural and protective functions, and “non-essential” isoprenoids such as isoprene with still partly unknown functions has been postulated (Rosenstiel et al. 2004; Owen & Peñuelas 2005), but to our knowledge, has not been experimentally confirmed.
We investigated kinetics of isoprene emission and photosynthetic machinery development from leaf unfolding to maturation in aspen (Populus tremula) using a recently developed method to estimate chloroplastic DMADP pool responsible for isoprene emission and isoprene synthase kinetics (Rasulov et al. 2009a; Rasulov et al. 2009b; Li, Ratliff & Sharkey 2011). The nondestructive method based on integration of postillumination isoprene release provides estimates of DMADP pool size that are well-correlated with estimates by alternative destructive methods (Rasulov et al. 2009a; Weise et al. 2013). We hypothesized that isoprene synthesis during leaf development is simultaneously limited by isoprene synthase activity (protein content) and competition by other DMADP consuming reactions. Model- and inhibitor-based methods were developed to determine simultaneously the chloroplastic DMADP pools used for isoprene and for other competing reactions and determine the extent to which synthesis of “essential” isoprenoids limits isoprene emission during leaf development. The results demonstrate that the control of isoprene synthesis in developing leaves is shared between isoprene synthase and DMADP pool size, and overall support the hypothesis of a trade-off between “essential” and “non-essential” isoprenoid synthesis.
Material and methods
Plant material
Aspen (Populus tremula L.) trees (age 6-7 yrs, height 7-8 m) growing in the field in the vicinity of Tartu, Estonia (58.39°N, 26.70°E, elevation 41 m) were used in these experiments (Sun, Copolovici & Niinemets 2012a for the site description). During bud-burst (May 18) and rapid leaf expansion in May, average air temperature was 13.8 °C (average day-time air temperature of 16.6 °C), while average air temperature during foliage maturation in June was 18.8 °C (average day-time air temperature of 21.3 °C) according to the weather station of the Laboratory of Environmental Physics, University of Tartu (http://meteo.physic.ut.ee, 58.37°N, 26.73°E, elevation 76 m, inset in Fig. 1a).
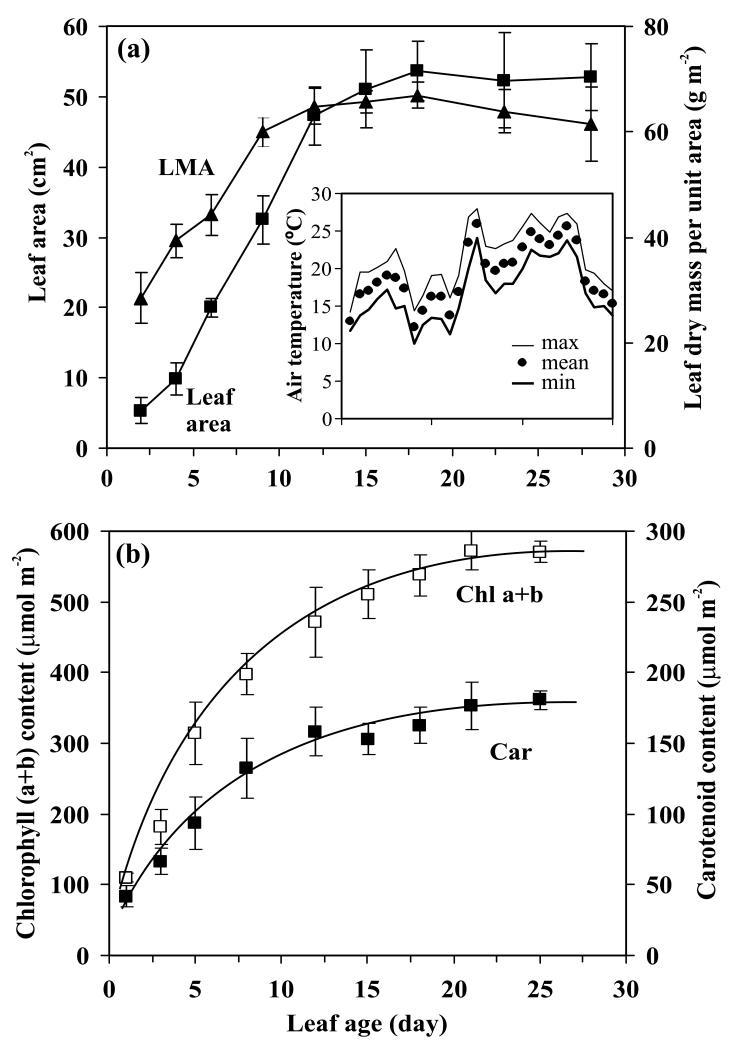
Figure 1.
Age-dependent changes in (a) the size of main stem leaves, leaf dry mass per unit area (LMA), and (b) contents of total chlorophyll (Chl a+b) and total carotenoids (Car) in aspen (Populus tremula L.). In (b), the data were fitted by exponential relationships by minimizing the sum of the squares between predicted and measured values (Eq. 8; r2 = 0.994 for chlorophyll and r2 = 0.986 for carotenoids, P < 0.001 for both). The fitting coefficients (Eq. 8) obtained were for chlorophyll (a+b): a1 = 601 μmol m−2, a2 = 0.443 d and a3 = 0.125 d−1 ; for carotenoids: a1 = 185 μmol m−2, a2 = 0.775 d and a3 = 0.130 d−1. Data are means ± SE of at least four replicate experiments. Day 0 corresponds to the day of bud-burst (May 18). Data in the inset demonstrate variation in daytime minimum, maximum and mean temperature during the study period (derived from the data of the weather station of the Laboratory of Environmental Physics, University of Tartu, http://meteo.physic.ut.ee).
For experiments, leaves were taken from young fully exposed long shoots where new leaves developed continuously. Leaf age and rate of leaf formation were determined by marking the leaves and frequently monitoring new leaf formation. Gas-exchange measurements were conducted with detached leaves sampled in the morning to assure full hydration of leaves. The leaf petiole was cut under water and the leaf with petiole in water was immediately transported to the laboratory where it was stabilized in dim light of 50 μmol m−2 s−1 and room temperature of 22-25 °C until the measurements, 1-2 hr after sampling.
Gas-exchange system for CO2, water vapor and isoprene measurements
A fast-response gas-exchange system with a circular clip-on type thermostatted leaf chamber (8.04 cm2 area and 0.3 cm height) was used (Laisk et al. 2002). Given the chamber volume and gas flow rate maintained at 0.5 mmol s−1, the system full response time is ca. 1 s, belonging to the fastest currently available gas-exchange systems (Niinemets 2012 for a review). The upper surface of the leaf was glued by a starch paste to the glass window of the leaf chamber water jacket. This strongly enhanced the heat exchange such that leaf temperature was maintained within 0.5 °C of the thermostatted water jacket.
The system has two identical gas lines (channels) that allow for rapid alternate measurements of reference and sample lines. While the leaf was in the gas stream of channel 1, gas analyzers connected to channel 2 recorded the reference (zero) line. The channels could be switched in less than in 1 s, and as soon as the leaf chamber was connected to channel 2, the analyzers immediately measured water vapor, CO2 and isoprene mole fractions at the gas concentrations preadjusted in channel 2. This setup is especially useful for fast measurement of transient responses, avoiding delays in the detection of leaf responses due to stabilization of gas concentrations.
A Schott KL 1500 halogen light source with a heat-reflecting filter (Optical Coating Laboratory, Santa Rosa, CA, USA) was used for actinic light. Measurement air was mixed from pure N2, O2 and CO2 by computer-controlled manostatic mixers (Laisk & Oja 1998). The air was humidified to maintain a water vapor pressure deficit of 1.7 kPa. A LI-6251 (Li-Cor, Inc., Lincoln, NE, USA) infra-red gas analyzer was used to measure chamber CO2 concentration, while water vapor concentration was gauged by a micropsychrometer integrated in the gas-exchange system (Laisk & Oja 1998). Isoprene concentration (m/z = 69) was measured by a proton transfer reaction mass spectrometer (PTR-MS; high-sensitivity version; Ionicon Analytik GmbH, Innsbruck, Austria) with a response time of approximately 0.1 s and a limit of detection of ca. 10 pmol mol−1 for isoprene (Hansel et al. 1995; Lindinger, Hansel & Jordan 1998). In addition, masses corresponding to monoterpenes (m/z = 137) and lipoxygenase pathway volatiles (LOX), main fragments of hexenals (m/z = 81) and other C6 volatiles (m/z = 83, sum of hexenols, hexanal, and hexenyl acetates) were routinely recorded following Graus et al. (2004). PTR-MS was calibrated with a standard gas containing all key volatiles (Ionimed GmbH, Innsbruck, Austria).
Chlorophyll fluorescence estimations
Chlorophyll fluorescence characteristics were measured together with foliage gas-exchange characteristics by a PAM 101 fluorimeter (Walz GmbH, Effeltrich, Germany) operated at 1.6 kHz for darkened leaves and at 100 kHz for illuminated leaves and during saturation pulses. Two Schott KL 1500 light sources were employed for chlorophyll fluorescence measurements. One light source was for 2 s saturated pulses of white light of 14,000 μmol m−2 s−1 to estimate either the dark-adapted (Fm) or light-adapted (Fm’) maximum fluorescence yields of PSII. The other light source with a 720-nm narrow-pass interference filter (Andover Corp) was for far-red light (50 μmol m−2 s−1) needed to obtain the true dark-adapted minimum fluorescence yield (F0).
Measurement protocol
After leaf enclosure in the chamber, standard experimental conditions were established. These included gas concentrations of 21% O2 and 360 μmol mol−1 CO2, and relative humidity of 60%, and leaf temperature of 30 °C in darkness. When leaf gas-exchange rates had stabilized, but at least 20 min. after leaf enclosure, dark respiration rate (Rd) and dark-adapted minimum (F0) and maximum (Fm) fluorescence yields were measured. After these measurements, actinic light of 650 μmol m−2 s−1 was switched on and the leaf was stabilized again until stomata opened and gas-exchange rates reached a steady state, typically in 20-30 min. Steady-state rates of net assimilation, transpiration and isoprene emission, and light-adapted steady-state (F) and maximum (Fm’) fluorescence yields were recorded and the leaf was subject to a light-dark transient to measure DMADP pool size.
To determine the initial quantum yield of net assimilation, net assimilation rate was measured in separate leaves at three light intensities between 15 to 55 μmol m−2 s−1 where Kok effect is absent (Sharp, Matthews & Boyer 1984). After completion of the gas-exchange and chlorophyll fluorescence measurements, leaf absorptance (ζ) was measured by an Ulbricht-type integrated sphere.
All gas-exchange rates were calculated according to von Caemmerer and Farquhar (1981) and Niinemets et al. (2011). The dark-adapted quantum yield of PSII was computed as (Fm – F0)/Fm and the light-adapted quantum yield (ΦPSII) as (Fm’ – F)/Fm’. The rate of photosynthetic electron transport from chlorophyll fluorescence was further calculated as 0.5QζΦPSII (Genty, Briantais & Baker 1989; Schreiber, Bilger & Neubauer 1994). The maximum quantum yield of photosynthesis for an absorbed light was found as the slope of the linear regression of net assimilation vs. ζQ over low Q values of 15 to 55 μmol m−2 s−1.
Determination of the apparent dimethylallyl diphosphate (DMADP) pool size
The size of the immediate precursor pool responsible for isoprene emission in steady state was estimated by the method of Rasulov et al. (2009a; 2010). This method is based on integrating the fast, 200–400 s after darkening, dark decay of isoprene release, providing the amount of substrate available for isoprene emission (Rasulov et al. 2009a; Rasulov et al. 2010; Li et al. 2011; Weise et al. 2013). Typically, application of this method requires correction for chamber responsiveness (Rasulov et al. 2009a; Li et al. 2011; Sun et al. 2012b), but this was not required with the ultra-fast system used here (Rasulov et al. 2010; Rasulov et al. 2011). As chloroplastic DMADP/IDP ratios are high in mature chloroplasts (Ramos-Valdivia, Heijden & Verpoorte 1997), the integral of the fast isoprene release has been generally assumed to mainly consist of the primary isoprene precursor DMADP, with minor contribution of isopentenyl diphosphate (IDP) converted to DMADP by IDP isomerase (Rasulov et al. 2009a; Li et al. 2011). However, recently a relatively low ratio of whole-leaf DMADP to IDP of ca. 2 was reported for kudzu leaves (Zhou et al. 2013). Possible dark-conversion of IDP to DMADP and use by isoprene synthase would result in moderate overestimation of DMADP pool size prior to the dark transient. Nevertheless, only a slight overestimation of the chloroplastic DMADP pool by the postillumination method compared with a mass-spectrometric method has been reported for poplar leaves (Weise et al. 2013). On the other hand, a secondary dark-rise of isoprene emission between 400-1200 s has been attributed to MEP pathway metabolites upstream of DMADP and IDP that are slowly converted to DMADP in darkness (Li et al. 2011; Rasulov et al. 2011; Li & Sharkey 2013a).
Apart from the assumption that DMADP/IDP ratio is high in chloroplasts, implicit in this method is also that isoprene synthesis is the only process consuming DMADP. This latter assumption is generally satisfied in mature leaves where other processes competing for DMADP are operating only at a low level, but may not be necessarily valid for young leaves. Therefore, we denote the pool of DMADP determined here as an apparent DMADP pool.
Determination of isoprene synthase rate constant and apparent Km and Vm values
The time-courses of postillumination isoprene emission were further employed to determine the isoprene emission rate vs. DMADP pool size relationships and derive the kinetic characteristics of isoprene synthase as in Rasulov et al. (2010; 2011). This was done by integrating the dark decay curves of isoprene emission rate at different times after leaf darkening, resulting in paired values of isoprene emission rate vs. remaining DMADP pool size.
The initial slope of isoprene emission rate vs. DMADP pool size (s−1) provides the first order rate constant of isoprene synthase (ka). The Michaelis-Menten constant for isoprene synthase (Km,iso) and the capacity (Vm,iso) were derived from Hanes-Wolff plot (DMADP pool size per emission rate vs. apparent DMADP pool size). The slope of this plot is 1/Vm,iso and the intercept is Km,iso/Vm,iso. As with the DMADP pool size, when isoprene is not the only process consuming DMADP, the derived values of Km,iso and Vm,iso are apparent values (Km,iso,app and Vm,iso,app). We note that dark-conversion of IDP to DMADP would only moderately affect the estimates of Km,iso,app and Vm,iso,app by this method. For example, for a relatively low DMADP to IDP ratio of 2 (Zhou et al. 2013), and DMADP pool size of 1500 nmol m−2 and assuming an IDP dark-conversion rate constant of 0.002 s−1 (chloroplastic IDP pool reaches to half of the initial value in ca. 350 s), Km,iso,app is only overestimated by ca. 20% with the effect becoming progressively less with decreasing the conversion rate.
To convert Km,iso to molar units, a leaf water content of 0.84 g g−1 fresh mass for young and 0.79 g g−1 for mature leaves was used, and the fraction of chloroplast water of total leaf water was taken as 0.15 (Wolfertz et al. 2004). The results of these calculations are demonstrated in Supplemental Material S1 (Figs. S1.1 and S1.2)
Derivation of true DMADP pool size, and Km and Vm values for isoprene synthase and geranyl diphosphate synthase in vivo
When multiple processes compete for chloroplastic DMADP, the change in DMADP pool size (SDMADP, nmol m−2 ) is driven by all of these processes. Provided isoprene (rate νIso, nmol m−2 s−1) and pigment synthesis (νPig, sum of the rates of chlorophyll and carotenoid synthesis, νChl + νCar, nmol m−2 s−1) are the dominant DMADP-consuming processes (with some additional share for isoprenoid-containing phytohormone and plastoquinone synthesis), their rate at time t depends on SDMADP as:
| (1) |
where νDMADP is the rate of DMADP synthesis. In a steady-state, νDMADP = νIso + νPig. When light is switched off, νDMADP becomes zero, at least for a time-period of 200-400 s followed switching off the light, until NADPH becomes available again and phosphorylated intermediates of MEP pathway are converted to DMADP (Li et al. 2011; Rasulov et al. 2011; Li & Sharkey 2013a). Thus, the change in DMADP pool corresponding to a light-dark transient is given as:
| (2) |
Assuming Michaelis-Menten kinetics, we write Eq. 2 as:
| (3) |
where νm,iso is the capacity of isoprene synthase reaction and νm,pig that for pigment synthesis and, Km,iso is the Michaelis-Menten constant for isoprene synthase and Km,pig that for pigment synthesis. As geranyl diphosphate (GDP) synthase is generally considered as the main enzyme consuming DMADP in the pathway leading to pigment synthesis (Li & Sharkey 2013b; Rajabi Memari et al. 2013), νm,pig and Km,pig most likely reflect the kinetics of geranyl diphosphate synthase. However, in addition to GDP synthase, some forms of geranylgeranyl diphosphate (GGDP) synthase (Tholl et al. 2004; Orlova et al. 2009; Wang & Dixon 2009) may also use DMADP as a donor. As in such a case, GDP is formed as an intermediate, νm,pig and Km,pig correspond to the GDP synthase activity of GGDP. According to literature data, GDP (and DMADP-consuming GGDP synthases) has a very high affinity towards DMADP (Orlova et al. 2009; Wang & Dixon 2009; Chang et al. 2010), while isoprene synthase has a low affinity (Schnitzler et al. 2005; Rasulov et al. 2009a; Rasulov et al. 2009b; Köksal et al. 2010). On the other hand, as GDP synthase activity can be feedback-inhibited by GDP (e.g., Tholl et al. 2004), reactions downstream of GDP synthesis can alter the νm,pig and Km,pig values, and thus, they characterize the effective kinetics of DMADP consumption by processes other than isoprene synthesis.
Integrating Eq. 3 from the start of darkening (t0) when the DMADP pool has a maximum value, SDMADP,0, to a given time of the light-dark transient, t1, provides the size of the DMADP pool size corresponding to the rate of the sum of DMADP consuming reactions at time t1:
| (4) |
The first part of the integral is equal to the apparent DMADP pool size, SDMADP,app measured by integrating the dark-release of isoprene emission:
| (5) |
where νm,iso,app is the apparent maximum isoprene synthase activity and Km,iso,app is the apparent Km value of isoprene synthase found by fitting the isoprene emission rate vs. SDMADP,app responses. The key implication of Eqs. 4 and 5 is that if Km values for different DMADP consuming processes are different, Km,iso,app values derived according to Eq. 5, will depend both on Km,iso and Km,pig. The value of Km,iso,app will approximate Km,iso the more the greater is the pathway flux towards isoprene synthesis, i.e., the larger is νm,iso relative to νm,pig. Thus, variations in νm,iso relative to νm,pig are expected to result in modified shapes of the responses predicted by Eq. 5 (Fig. 4 for sample responses predicted by Eq. 4 and 5). Such modification in curve shape can be used to estimate the true value of Km,iso and Km,pig and the true pool size of DMADP, and partition DMADP consumption between isoprene and pigment synthesis.
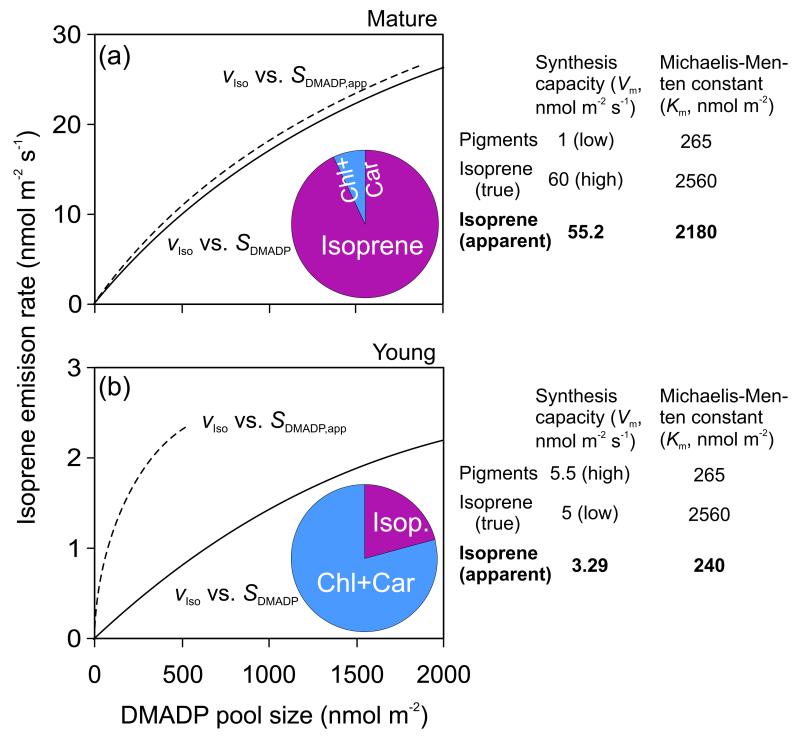
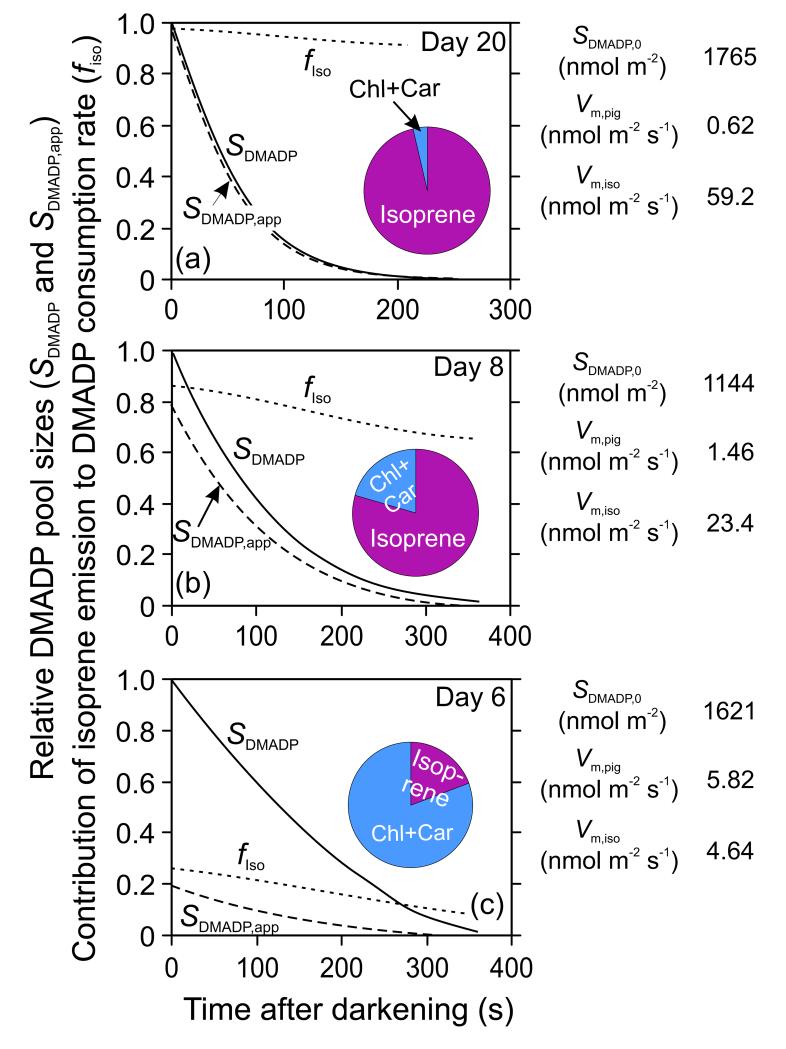
Figure 4.
Simulation of isoprene emission rate (νIso) vs. true (SDAMAP, solid line) and apparent DMADP pool size (SDMADP,app, dashed line) pool size relationships during light-dark transients (Supplemental Material S1, Fig. S1.1a for sample relationships) for a mature leaf (a) with a high capacity for isoprene emission and a low capacity for pigment synthesis and for a young leaf (b) with a low capacity for isoprene emission and a high capacity for pigment synthesis. After switching off the light, isoprene emission rate and DMADP pool size start to decrease until the preexisting pool of DMADP is exhausted. When isoprene emission rate is the only process consuming DMADP, integrating isoprene emission to a certain moment of time provides DMADP pool size that is remaining at that moment of time. However, when other processes compete for chloroplastic DMADP, this integration results in an apparent DMADP pool size (SDMADP,app). In both panels, the relationships of νIso vs. SDAMAP were derived by Eq. 3 and 4 using constant Km values for isoprene and pigments based on simultaneous fitting of all data. SDMADP,app was thereafter derived by integrating the values of isoprene emission (Eq. 5), and the apparent Km,iso values derived from νIso vs. SDMADP,app relationships (Hanes-Wolff plots). The pie diagrams show relative contributions of isoprene emission and pigment synthesis to overall consumption of DMADP pool size accumulated before the light-dark transient.
We used an iterative least-squares technique to estimate all the parameters by fitting equations 4 and 5 to SDMADP,app(t) vs. isoprene emission relationships of all leaf ages pooled. Constant values of Km,iso and Km,pig were fitted to all leaves of different age, while age-specific values of νm,iso, νm,pig and SDMADP,app were used in fitting. The model parameters were found by simultaneously minimizing the weighted sum of squares of [SDMADP,app(measured)-SDMADP,app(predicted)]2 and [νIso(measured)-νIso(predicted)]2. Excellent fits between measured and predicted SDMADP,app (r2 = 0.994 for all leaves pooled) and νIso (r2 = 0.981) values were observed using constant Km,iso and Km,pig values for all leaves of different ages, suggesting that the model reliably described the data (Supplemental Material S2, Fig. S2.1).
Alendronate inhibitor experiments
Alendronate (Fosamax, Merck Sharp & Dohme Corp., Holland), a highly specific inhibitor of geranyl diphosphate (Lange, Ketchum & Croteau 2001; Burke, Klettke & Croteau 2004) and farnesyl diphosphate synthases (Bergstrom et al. 2000; Burke et al. 2004) and less specific inhibitor of geranylgeranyl diphosphate synthase (Szabo et al. 2002) was used to inhibit isoprenoid synthesis pathway downstream of DMADP. The inhibitor was applied by feeding the 10 mM aqueous solution through the cut petiole of leaves enclosed in the gas-exchange system under standard experimental conditions. Feeding times of 20, 40 and 60 min. were employed, and isoprene emission rates, and DMADP pool sizes were estimated as before.
Provided alendronate does not affect the rates of DMADP and isoprene synthesis, Eq. 1 simplifies to:
| (6) |
As pigment synthesis is inhibited, DMADP pool is expected to increase until a new equilibrium is reached. Thus, monitoring time-dependent changes in apparent maximum DMADP pool size (SDMADP,app,0) and steady-state isoprene emission rate in response to alendronate treatment can be used to estimate the rates of DMADP synthesis from the rate of DMADP pool accumulation in alendronate-inhibited leaves. Having determined νDMADP, the rate of pigment synthesis can be estimated as the difference between νDMADP and νIso in control leaves without alendronate treatment. We fitted SDMADP,app,0 and steady-state isoprene emission rate vs. time of alendronate treatment relationships with non-linear regressions, and estimated νDMADP from the rate of DMADP pool size accumulation, allowing us to get an alternative estimate of νPig.
Another implication of alendronate inhibition is that upon leaf darkening equation 2 simplifies to:
| (7) |
Thus, true Km and νm values of isoprene synthase can be determined in alendronate-inhibited leaves, provided pigment synthesis has been fully inhibited and DMADP pool has reached a steady-state before the start of the dark transient. As with non-treated leaves, Km and νm values were determined from Hanes-Wolff plots at different times after alendronate treatment.
Foliage structural analyses and determination of pigment contents
Immediately after gas-exchange measurements, discs of 2-4 cm2 were taken for fresh mass and dry mass estimation and for pigment extraction. The pigment samples were frozen in liquid nitrogen and stored at −80 °C. The fresh mass of the rest of the discs was determined immediately, and dry mass was determined after oven-drying at 70 °C for 48 h.
Pigment samples were ground in liquid N2 and extracted with 80% aqueous acetone. As we were interested in total pigment pool sizes, chlorophylls and carotenoids were measured according to the method of Lichtenthaler & Buschmann (2001) using a Shimadzu UV-Vis UV2550PC spectrophotometer (Shimadzu, Kyoto, Japan).
Modeling the rate of Chl and Car synthesis from changes in pigment contents
We model the time-dependent (t) changes in leaf chlorophyll and carotenoid contents in greening leaves (Fig. 1b) by an asymptotic relationship often used in chemical kinetics to describe the first-order increase of reaction product:
| (8) |
where Si is the content of given leaf pigment pool (μmol m−2) and the pool-specific coefficients are defined as: ai,1 is the asymptotic value of the pigment pool size (the pigment content in mature leaves), ai,2 (d) is the time-offset that corresponds to the time at the start of leaf greening, and ai,3 is the time constant (d−1). This equation was fitted to the data by iteratively minimizing the sum of squares between measured and predicted values.
The rates of Chl and Car synthesis were found by differentiating Eq. 8 with respect to time:
| (9) |
We further assumed that synthesis of both pigments occurs during daytime hours with 12 hour light period. Given that carotenoids contain 40 carbon atoms and the phytyl residue of chlorophyll 20 carbon atoms, and given the corresponding molecular masses of carotenoids and chlorophylls, the rates were converted to nmol C m−2 s−1 (νChl for the rate of phytyl residue synthesis in chlorophylls and νCar for the rate of carotenoid synthesis). The total rate of isoprenoids going into pigment synthesis (nmol C m−2 s−1) was calculated as νChl + νCar.
Data analyses
For all leaf ages, 4 to 15 replicate measurements were conducted and means ± SE were calculated. Whenever pertinent, data among different leaf ages and alendronate treatments were compared by ANOVA and considered different at P < 0.05. Correlations between pigment synthesis rate and isoprene emission rate through leaf ontogeny were analyzed by non-linear regressions. As leaf temperature during isoprene emission measurements differed from the ambient temperature at leaf growth location, this analysis was conducted both with the actual isoprene emission rates and after scaling the emission rates to day-time average temperature during the experimental period (19.2 °C) using the temperature response of isoprene emission from Rasulov et al. (2010).
Results
Ontogenetic modifications in leaf size and pigment content
Leaves on long shoots developed continuously with a time interval of 2-3 days between successive leaves. Full leaf expansion of about 50 cm2 was reached in 12-14 days after bud-burst (Fig. 1a), while leaf dry mass per unit area stabilized a few days later, between 15-20 days after bud-burst.
Foliage pigment contents accumulated with the maximum rate in the youngest leaves (Fig. 1b), and approached maximum values of ca. 570 μmol m−2 for chlorophylls and 180 μmol m−2 for carotenoids at days 20-22. The asymptotic model (Material and Methods, section Modeling the rate of Chl and Car synthesis from changes in pigment contents, Eq. 8) provided excellent fits to the data (Fig. 1b).
Time-dependent changes in photosynthetic characteristics
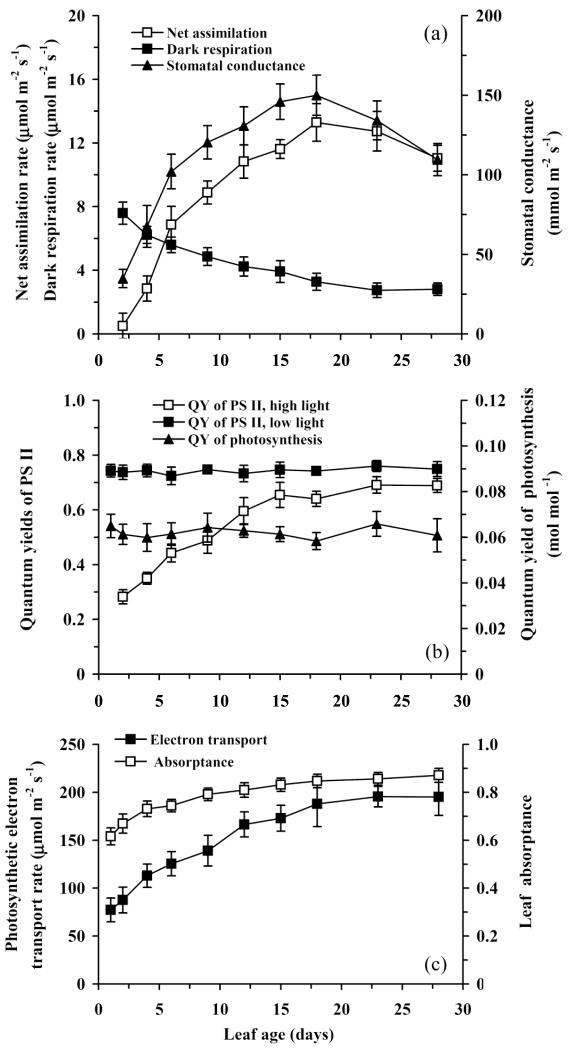
Net CO2 assimilation rate (A) became positive at day 2 and continued to increase until day 20 (Fig. 2a). Stomatal conductance increased in parallel with A, except in older leaves where stomatal conductance decreased somewhat, resulting in reductions in net assimilation rate as well (Fig. 2a). Dark respiration rate (Rd) was high in juvenile leaves, up to 8 μmol m−2 s−1, and exponentially decreased to a stable value of ca. 3 μmol m−2 s−1 in mature leaves (Fig. 2a).
Figure 2.
Dynamics of net CO2 uptake, dark respiration, stomatal conductance to water vapor (a), quantum yields (QY) of Photosystem II (PSII) in dark- and light-adapted leaves and quantum yield of CO2 uptake for an absorbed light (b), and the rate of photosynthetic electron transport and leaf absorptance (c) in aspen leaves. Data presentation as in Fig. 1.
The maximum quantum yield of photosynthesis for an absorbed light, estimated either as the initial slope of the light response curve of net CO2 assimilation or as the dark-adapted quantum yield of PSII from chlorophyll fluorescence, was constant through leaf ontogeny (Fig. 2b). However, quantum-yield for an incident light increased with increasing leaf age (data not shown), reflecting increases in leaf absorptance due to chlorophyll accumulation (Fig. 2c).
The light-adapted quantum yield of PSII (Fig. 2b) and photosynthetic electron transport rate increased simultaneously with net assimilation rate (Fig. 2c). The increase in electron transport rate was less rapid than in A, and no reduction was observed in older leaves with reduced stomatal conductance (cf. Figs. 2a and 2c). These discrepancies reflect the reductions of A by Rd in young leaves and by stomatal conductance in older leaves.
Isoprene emission in relation to leaf age
Isoprene emission was activated with a delay of about 4-5 days after net assimilation rate became positive (Fig. 3a), but it increased fast and approached the maximum rate almost simultaneously with photosynthesis between days 18-20 (cf. Figs. 2a and 3a). Under steady-state conditions, only isoprene emission was observed without any significant monoterpene emissions neither in young nor mature leaves (Supplemental Material S3, Fig. S3.1).
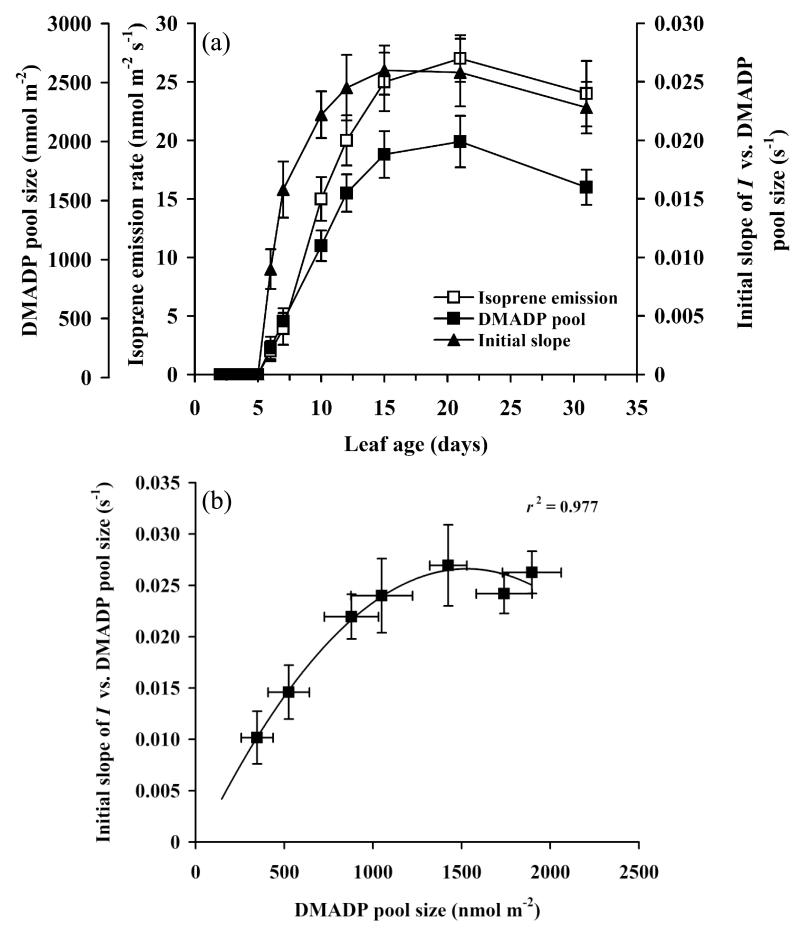
Figure 3.
Age-dependent changes in isoprene emission rate, apparent DMADP pool size and the apparent rate constant of isoprene synthase (a), and the apparent rate constant of isoprene synthase in relation to apparent DMADP pool size (b) in different-aged aspen leaves. Figure S1.1 demonstrates determinations of the apparent DMADP pool size and the rate constant of isoprene synthase (Supplemental Material S1). Data are means ± SE of at least four replicate measurements.
Integration of the first peak of the postillumination isoprene release after turning off the light was employed to estimate the pool of the substrate DMADP that was converted to isoprene in the dark subsequent to illumination (SDMADP,app, Supplemental Material S1, Fig. S1.1). After day 4 following the onset of isoprene emission, this pool consistently increased, approaching a maximum of about 2000 nmol m−2 at day 20 simultaneously with the maximum of isoprene emission rate (Fig. 3a).
From dark-decay data of isoprene emission, isoprene emission rates (νIso) in relation to remaining DMADP pool were derived (Supplemental Material S1, Fig. S1.1), and the apparent characteristics of isoprene synthase were estimated. The initial slope of isoprene emission vs. remaining DMADP pool size (apparent rate constant of isoprene synthase) increased sharply with increasing leaf age, reaching a maximum on day 15, a few days before maximum isoprene emission rate was observed on day 20 (Fig. 3a). The increase was also initially faster than changes in either isoprene emission rate or SDMADP,app (Fig. 3a). The initial slope was positively correlated with both isoprene emission rate and SDMADP,app through leaf development, but the correlation was curvilinear and the initial slope became almost invariable at SDMADP,app values of greater than ca. 1000 nmol m−2 (Fig. 3b).
The shapes of isoprene emission rate vs. SDMADP,app relationships were different among young and mature leaves. Saturation of isoprene emission rate was observed at low values of SDMADP,app of about 400-500 nmol m−2, but no saturation even at maximum observed values of ca. 1500-2000 nmol m−2 was observed for mature leaves (Supplemental Material S1, Fig. S1.1b, Fig. 3a for maximum SDMADP,app values). Thus, both the apparent Michaelis-Menten constant (Km,iso,app) and maximum rate (νm,iso,app) increased with increasing leaf age (Supplemental Material S1, Fig. S1.2). Differences in the isoprene emission vs. SDMADP,app response shapes were reflected in lower Km,iso,app values of ca. 200-300 nmol m−2 (17-26 μM in chloroplast water) in young compared to 2000-2800 nmol m−2 in mature leaves (135-160 μM, Supplemental Material S1, Fig. S1.2).
True kinetic characteristics of isoprene synthase: the role of pigment synthesis
Model analyses (Eq. 1-5) demonstrated that when multiple processes with differing Km values compete for the same chloroplastic DMADP pool size, the bias in Km,iso,app can be large, especially if the second process proceeds with a rate higher than or comparable to the rate of isoprene emission (Fig. 4). Under such circumstances, integration of postillumination isoprene emission results in an apparent DMADP pool that seemingly depletes much faster than the total chloroplastic DMADP pool (Fig. 4). Given the presence of active pigment synthesis in young leaves (Fig. 1b), and low isoprene synthase activity (Fig. 3b) and that pigment synthesis at the level of geranyl diphosphate synthase and isoprene emission compete for the same chloroplastic DMADP pool, competition for DMADP can explain the low Km,iso,app in young leaves.
The data of νIso vs. SDMADP,app were iteratively fitted by Eq. 4 and 5 by adjusting globally constant values of Michaelis-Menten parameters for isoprene (Km,iso) and pigments (Km,pig, mainly reflecting geranyl diphosphate synthase) simultaneously to all leaves, and maximum activities of isoprene synthase (νm,iso) and pigment synthesis (νm,pig) and initial DMADP pool size (SDMADP,0) individually to leaves of different age. The fitted model with a constant Km,iso of 2560 nmol m−2 (188 μM in chloroplast water, converted by using an average water content for young and mature leaves) and Km,pig of 265 nmol m−2 (20 μM) provided excellent fits to the data (r2 > 0.99, Supplemental material S2, Fig. S2.1).
The rate of pigment synthesis was predicted to be minor in mature leaves (νm,pig = 0.62 nmol m−2 s−1) with the overall contribution of pigment synthesis being less than 10% of total DMADP consumption (Fig. 5a). In contrast, in young leaves, νm,pig was predicted to be much higher (5.8 nmol m−2 s−1), and the bulk of DMADP, ca. 80% was used for pigment synthesis (Fig. 5c). Overall, the effect of pigment synthesis on dark-decay patterns of DMADP pool increased with increasing the rate of pigment synthesis (Fig. 5). The predicted DMADP pool sizes in steady-state (SDMADP,0) were similar in leaves of different age as soon as isoprene emission started. However, the predicted decay of DMADP pool size after leaf darkening was slower in young than in mature leaves, reflecting the circumstance that the achieved maximum rate of DMADP consumption (νIso + νPig) was much larger in mature leaves (24.7 nmol m−2 s−1) than in youngest leaves (6.8 nmol m−2 s−1) (Fig. 5) due to high isoprene emission rate in mature leaves.
Figure 5.
Comparison of the decay of DMADP pool size (SDMADP for true pool, and SDMADP,app for the apparent pool) and the contribution of isoprene emission rate to overall rate of DMADP consumption (fIso) following light-dark transients in hybrid aspen leaves of different age (Supplemental Material S1, Fig. S1.1a for corresponding measurements). fIso is defined as the ratio of isoprene emission, νIso, to total rate of DMADP consumption (sum of νIso and pigment synthesis, νPig). For better visual comparison of patterns, DMADP pool sizes were normalized to DMADP pool size at the steady-state before the transient (SDMADP,0). An iterative least squares approach was employed to fit simultaneously isoprene emission rate and DMADP pool size by Eq. 4 and 5 and estimate the Michaelis-Menten constants (Km) for isoprene synthase (Km,iso obtained was 2560 nmol m−2) and pigment synthesis (Km,pig = 265 nmol m−2) for all leaves simultaneously, as well as to fit the capacities for isoprene synthase (Vm,iso) and pigment synthesis (Vm,pig) reactions and SDMADP,0 for leaves of different age (Supplemental material S2, Fig. S2.1 for the overall fit of predicted and measured values). The pie graphs indicate relative contributions of isoprene emission and pigment synthesis to consumption of DMADP pool size accumulated before the light-dark transient.
Effects of alendronate on isoprene emission in leaves of different age
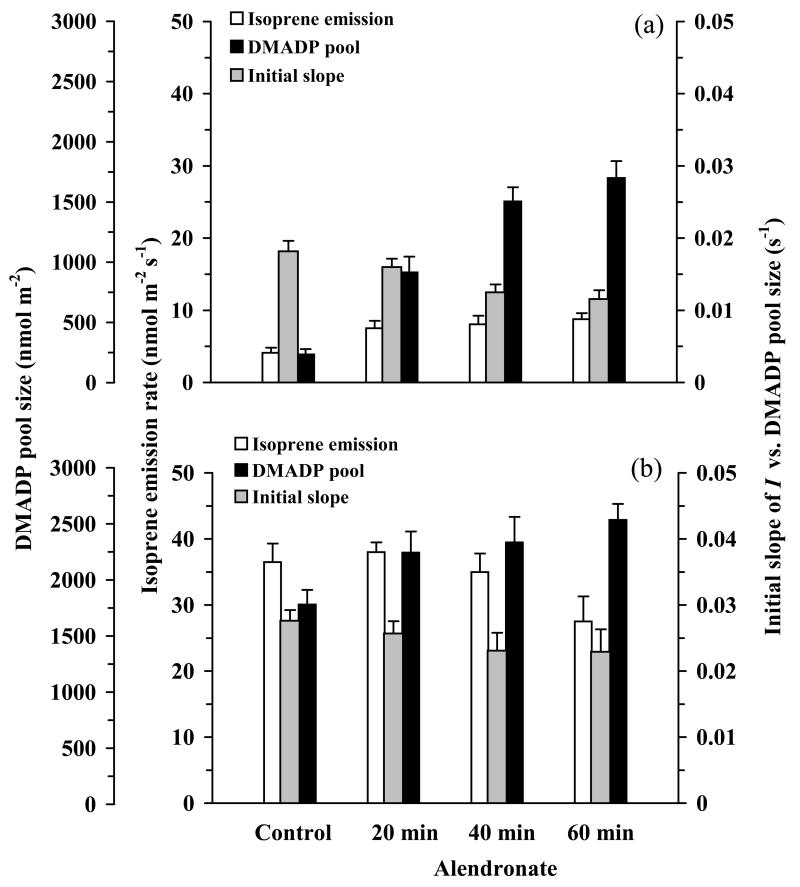
Application of the inhibitor alendronate that blocks the synthesis of isoprenoids downstream of DMADP and IDP resulted in enhanced isoprene emission rate, by ca. 1-8-fold, in young leaves already after 20 min. application (Fig. 6a). The enhancement of isoprene emission was associated with a major increase of DMADP pool size available for isoprene emission, 3-fold increase after 20 min. and 7-8-fold after 60 min. of application (Fig. 6a). Increased time of alendronate application moderately affected isoprene emission by further 16% for 1 h treatment, but the pool size of DMADP available for isoprene emission increased almost 2-fold in 1 h treatment relative to 20 min treatment (Fig. 6a). Assuming that the enhancement of pool size of DMADP results from inhibition of pigment synthesis, the average rate of pigment synthesis in non-inhibited leaves estimated from the change in the apparent DMADP pool size (Eqs. 6-7) was predicted to be 4.6 nmol m−2 s−1.
Figure 6.
Isoprene emission rate, DMADP pool size and the rate constant of isoprene synthase of young 6-day-old (a) and mature 20-day-old leaves (b) of aspen treated with 10 mM solution of alendronate for 0 (control) to 60 min. Alendronate specifically inhibits geranyl diphosphate (Lange et al. 2001; Burke et al. 2004) and farnesyl diphosphate synthase (Bergstrom et al. 2000; Burke et al. 2004) activities. Data are means + SE of at least four replicate measurements.
Differently from young leaves, isoprene emission rate in older leaves was affected to a minor extent by 20 min. treatment with alendronate, but longer-term application of alendronate even appeared somewhat inhibitory to isoprene emission (Fig. 6b). The pool size of DMADP was increased by ca. 20% by 20 min. application of alendronate, and with increasing the time of treatment up to 60 min., DMADP pool size further increased by 10% relative to 20 min. treatment (Fig. 6b). From the initial 20 min. increase of DMADP pool size, the average rate of pigment synthesis (Eq. 6-7) was estimated to be 1.3 nmol m−2 s−1.
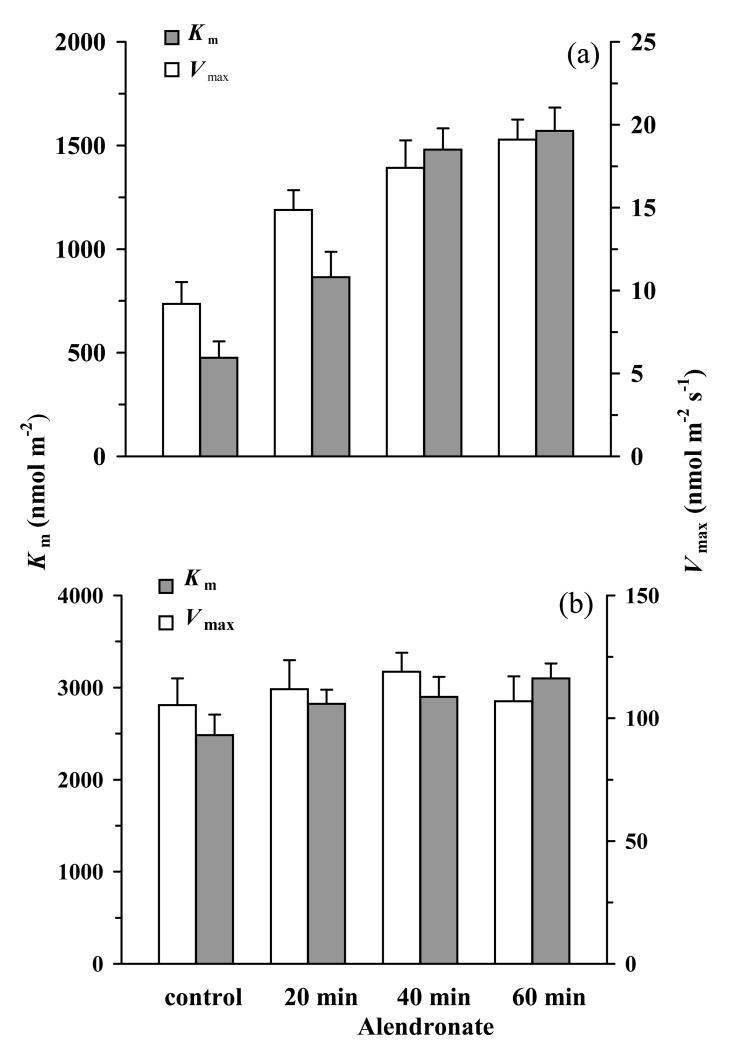
The effects of alendronate treatment on the initial slope of isoprene emission vs. DMADP pool size (apparent rate constant of isoprene synthase) were moderate, ca. 35% reduction for young leaves and 15% reduction for mature leaves (Fig. 6a, b). The apparent νm,iso increased in young leaves by 20 min. alendronate treatment by 1.6-fold and by additional 30% by extending the treatment to 60 min. (Fig. 7a), but no effect of alendronate on νm,iso could be statistically confirmed in mature leaves. In addition, a major increase in Km,iso,app, by 3.3-fold for the longest treatment was observed for young leaves (Fig. 7a). In mature leaves, the enhancement of Km,iso,app was moderate, at most 1.2-fold for the longest alendronate treatment (Fig. 7b). These continued alendronate treatments, however, appeared to constitute a moderate stress to the plant as suggested by somewhat enhanced emissions of typical dark-induced lipoxygenase pathway volatiles (Graus et al. 2004) (Supplemental Material S3, Fig. S3.1).
Figure 7.
Effects of alendronate on the apparent characteristics of isoprene synthase, νm,iso,app and Km,iso,app, in young 6-7-day-old (a) and mature 20-day-old leaves (b) of aspen treated for various times with alendronate. The isoprene synthase characteristics were derived from Hanes-Wolff plots as in Fig. S1.2 (Supplemental Material S1). If isoprene synthesis was the only process consuming DMADP as is expected in alendronate-inhibited leaves, the derived values reflected the true Km and Vm of isoprene synthase. Data presentation and the number of replicates as in Fig. 6.
Interrelationships between isoprene emission and pigment synthesis
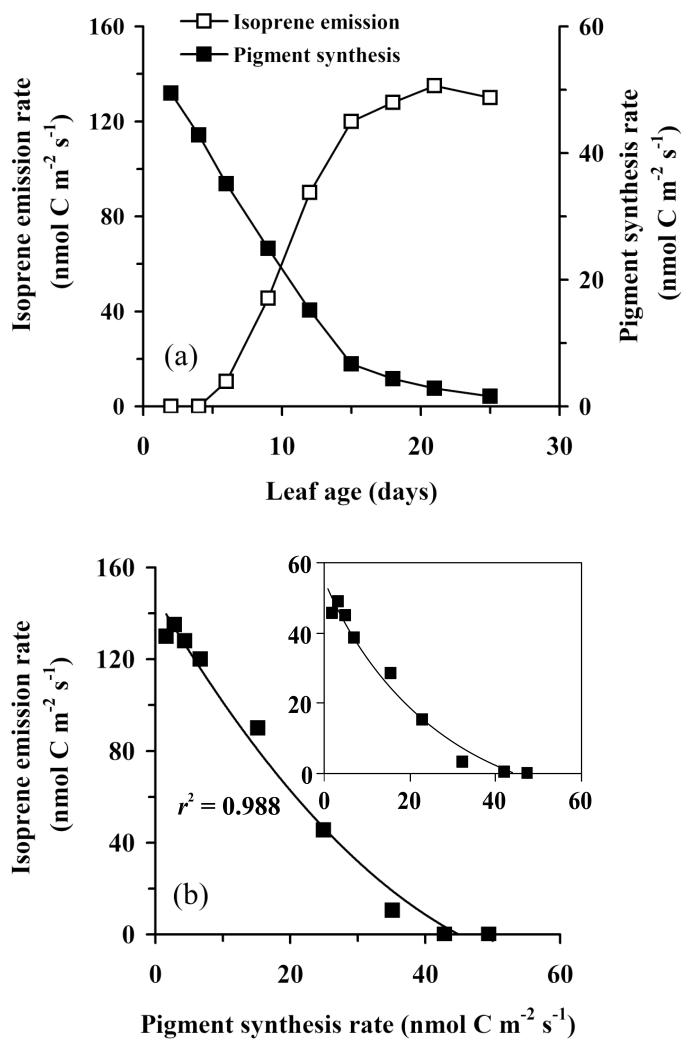
The rate of pigment synthesis predicted on the basis of pigment accumulation (Eq. 9) assuming a constant pigment accumulation rate over 12 h light period yielded pigment synthesis rates between 2.2 nmol m−2 s−1 (mol pigments) for the youngest leaves and ca. 0.2 nmol m−2 s−1 for mature leaves. Considering that a C20 moiety production requires 1 mole DMADP, and a C40 moiety requires 2 mole DMADP and the rest comes from IDP, these rates correspond to 2.7 nmol m−2 s−1 and ca. 0.3 nmol m−2 s−1 in DMADP equivalents. In carbon equivalents, these rates correspond to 54 nmol C m−2 s−1 in youngest leaves and 2 nmol C m−2 s−1 in mature leaves. Isoprene emission started when the rate of pigment synthesis began to slow down after 4-5 days of leaf growth (Fig. 8a). The overall isoprenoid pathway fluxes to isoprene and to pigment synthesis were negatively correlated (Fig. 8b), but there was a positive x-intercept, indicating that isoprene did not evolve until the daily average rate of pigment synthesis decreased to ca. 40 nmol C m−2 s−1. Further decrease in pigment synthesis was accompanied by proportional increases in isoprene emission. When corrected for the average ambient temperature (Fig. 1a), the maximum pigment and isoprene emission rates were almost numerically equivalent (inset in Fig. 8b).
Figure 8.
Dynamic changes in the rates of isoprene emission and synthesis of photosynthetic pigments (Chl+Car) (a) and the correlation between isoprene emission and pigment synthesis rate (b) through the development of aspen leaves. Both the rates of isoprene emission and pigment synthesis are expressed in nmol C m−2 s−1 (isoprene contains 5, phytyl residue of chlorophylls 20 and carotenoids 40 carbon atoms). The rates of pigment synthesis are calculated from the rate of pigment accumulation by Eq. 9 assuming a constant pigment synthesis rate for 12 h light period. The rates of isoprene emission were measured at a light intensity of 650 μmol m−2 s−1 and leaf temperature of 30 °C (data in the main panels). For quantitative comparison, isoprene emissions were also scaled to an average daytime temperature of 19.2 °C (inset in Fig. 1a for variations in temperature during the experiment) using the temperature response from Rasulov et al. (2010, inset in (b)). Data in (b) were fitted by a non-linear regression in the form y = aLn(x)+b.
Discussion
Photosynthetic activity is present since leaf unfolding
Our results on the coordinated development of photosynthetic machinery during leaf ontogeny are in broad agreement with previous findings (for reviews see, Shesták 1985; Suzuki et al. 1987; Niinemets et al. 2012). Photosynthetic pigments, and photosynthetic electron transport were detectable immediately after leaf unfolding, while positive net assimilation rate was observed a few days after leaf unfolding (Fig. 1-2). As observed in previous studies (Osborne & Garrett 1983; Long, Postl & Bolhár-Nordenkampf 1993; Harley et al. 1994; Singsaas, Ort & DeLucia 2001; Yoo et al. 2003; Eichelmann et al. 2004), the maximum quantum yield for an absorbed light was constant from leaf unfolding to full expansion and photosynthetic maturation (Fig. 2b,c), indicating that photosynthetically active chloroplasts with fully coupled light and dark reactions of photosynthesis were present in juvenile leaves (Cai, Slot & Fan 2005). Further changes in photosynthetic activity are mainly due to expansive developments, such as increased size (Hernandez-Gil & Schaedle 1973) and number of chloroplasts, cells and cell layers per unit leaf area (Dickmann & Gordon 1975; Kennedy & Johnson 1981; Yoo et al. 2003; Lichtenthaler & Babani 2004). Increased photosynthetic activity of mesophyll is accompanied by enhanced density of stomata, resulting in greater stomatal conductance (Stitt 1991; Eichelmann et al. 2004; Miyazawa, Livingston & Turpin 2006; Mott, Sibbernsen & Shope 2008; Mott 2009).
The rates of development of the traits characterizing leaf photosynthetic function were the highest during the first eight days after leaf unfolding when the photosynthetic development matched the rate of leaf expansion (Fig. 1-2). After full leaf expansion, the pigments still accumulated and photosynthetic activity increased, but with much weaker rate (Fig. 1-2). Thus, aspen leaves exhibited the phenomenon of “delayed leaf greening” (Miyazawa & Terashima 2001; Miyazawa et al. 2003; Niinemets et al. 2012), although the delay in greening was relatively weak.
Onset of isoprene emission during leaf development
While non-zero photosynthetic electron transport rate was detected already on day 1 (Fig. 2c) and positive net CO2 uptake on day 2 (Fig. 2a), isoprene emission rate became above the limit of detection on day 6, further increasing rapidly to a maximum value on day 20 (Fig. 3a). Thus, our data are in agreement with the past evidence of a time-lag between the onset of photosynthesis and isoprene emission (s. Introduction).
There is evidence that delayed isoprene emission can be explained by deferred induction of isoprene synthase (IspS) activity. Numerous reports have suggested that in developing leaves IspS protein plays a key role in controlling the rate of isoprene synthesis (Silver & Fall 1991; Monson et al. 1992; Kuzma & Fall 1993; Lehning et al. 2001; Wiberley et al. 2005; Vickers et al. 2010; Vickers et al. 2011) and that the delay in isoprene emission is regulated by the transcription of the IspS gene (Mayrhofer et al. 2005; Wiberley et al. 2005). The level of IspS mRNA increases somewhat in advance of IspS protein level, and a certain expression activity maybe already present before the onset of detectable isoprene emission, but IspS activity approaches a maximum simultaneously with leaf maturation (Sharkey et al. 2008; Cinege et al. 2009). Parallel trends in isoprene emission and IspS protein level have been observed in several studies (Mayrhofer et al. 2005; Wiberley et al. 2005; Wiberley et al. 2009; Vickers et al. 2010). In fact, the share of developmental and environmental control on the onset of isoprene emission is also likely determined at the level of IspS. Induction of isoprene emission in developing leaves occurs earlier at higher ambient temperatures (Monson et al. 1994) that enhance the rate of leaf development and pigment accumulation (Niinemets et al. 2012) and isoprene synthase expression (Cinege et al. 2009; Li & Sharkey 2013b; Rosenkranz & Schnitzler 2013).
Importance of expression of IspS is underscored by our data demonstrating increases in the apparent rate constant of isoprene emission (Fig. 3) and apparent νm (Supplemental Material S1, Fig. S1.2) with increasing leaf age. Although both of these characteristics were likely somewhat underestimated due to competition by other DMADP-consuming reactions in young leaves (Fig. 4, see below), both of them were also significantly less in young than in old leaves according to global fitting of data (Fig. 5) and according to alendronate-inhibition experiments (Fig. 6-7), demonstrating that isoprene synthase does exert an important control on developmental changes in isoprene emission rate during leaf development.
Low isoprene emission in young leaves is partly driven by competition by other DMADP consuming processes
When the amount of catalytically active enzyme is increasing, its νm is expected to increase, but its Michaelis-Menten constant (Km) should remain constant as long as the catalytic mechanism is not modified. Thus, the finding of age-dependent increase of isoprene apparent Km (Km,iso,app) derived from postillumination isoprene release data (Supplemental Material S1, Figs. S1.1 and S1.2) is initially puzzling. However, the method applied assumes that during the postillumination decay, chloroplastic DMADP pool size decreases only due to isoprene emission, and no other reactions besides IspS compete for DMADP. In fully mature leaves, where the build-up of the photosynthetic machinery has been completed, this assumption is very likely to be true even if a certain part of DMADP is used for maintenance of essential cellular functions such as synthesis of abscisic acid and for pigment turnover (Barta & Loreto 2006).
In contrast, in young leaves, synthesis of pigments, plastoquinone and isoprenoid-based plant hormones such as gibberellins and cytokinins can potentially importantly compete for chloroplastic DMADP, especially at the level of geranyl diphosphate (GDP) synthase that forms geranyl diphosphate from DMADP and isopentenyl diphosphate (IDP). According to in vitro analyses, GDP synthase has a much higher affinity for DMADP (Km,GDP) than isoprene synthase with values between 8-27 μM (Tholl, Croteau & Gershenzon 2001; Orlova et al. 2009; Wang & Dixon 2009) reported in the literature. Accordingly, consumption of DMADP by GDP formation is expected to importantly alter the isoprene emission vs. apparent DMADP pool size relationships with the magnitude depending on the rate of isoprene synthase and other DMADP consuming reactions (Fig. 4). Analogously, Km values for other prenyltransferases and terpenoid synthases downstream GDP are low, typically between 1-20 μM (Fraser, Schuch & Bramley 2000; Tholl et al. 2001; Tholl et al. 2004). For example, phytoene synthase that is considered as the rate-limiting enzyme in carotenoid synthesis has a Km for geranylgeranyl diphosphate of 5 μM (Fraser et al. 2000), indicating that downstream reactions unlikely limit consumption of DMADP.
We used this vast difference in Km values to derive global values of Km for isoprene and other DMADP-consuming reactions (assumed to be mainly GDP formation for pigment synthesis, Km,pig), and determine the νm values for isoprene (νm,iso) and pigment synthesis (νm,pig) and steady-state DMADP pool sizes for individual leaves (Eqs. 4-5, Figs. 4-5). The in vivo effective Km for prenyltransferases derived in our study by global fitting of the model of chloroplastic DMADP use after switching off the light (Eq. 4-5) was 265 nmol m−2 (20 μM), agreeing with literature observations on GDP affinity towards DMADP in vitro (Tholl et al. 2001; Tholl et al. 2004; Orlova et al. 2009; Chang et al. 2010).
Excellent fits to the data were obtained by globally fitting constant Km,iso and Km,pig values to all leaves (Supplemental Material S2, Fig. S2.1), suggesting that the observed age-dependent changes in Km,iso,app (Supplemental Material S1, Fig. S1.2) reflect consumption of DMADP by other ongoing processes, in particular pigment synthesis. This model analysis further indicated that in mature leaves, the competition from pigment synthesis was minor, and thus, the kinetic method for chloroplastic DMADP pool size estimation provided a realistic estimate of chloroplastic DMADP pool size (Fig. 5a). In fully-mature isoprene-emitting leaves, it has been suggested that the metabolic flux to carotenoids makes up only 1-2% of the MEP pathway flux to isoprene (Sharkey, Chen & Yeh 2001). Our study suggested that the flux to alternative DMADP-consuming reactions may be somewhat larger 5-10% (Fig. 5a), but nevertheless demonstrating that isoprene emission is the dominant sink of the MEP pathway. Differently from mature leaves, in young leaves just starting isoprene emission, DMADP used for isoprene emission constituted a small part of overall DMADP consumption, and in addition to low isoprene synthase activity, isoprene emission rate was significantly constrained by competition from pigment synthesis (Fig. 5c).
This model-based analysis was confirmed by experiments with alendronate inhibitor that specifically inhibits isoprenoid synthesis reactions downstream of DMADP (Bergstrom et al. 2000; Lange et al. 2001; Burke et al. 2004). Significantly higher isoprene emission rate was obtained in young alendronate-inhibited leaves (Fig. 6a), while the effect was minor for mature leaves (Fig. 6b). In addition, apparent Km,iso value was increased in alendronate/inhibited young leaves, while no effect was observed for mature leaves (Fig. 7). Although possible accumulation of DMADP and IDP in alendronate-feeding experiments can result in feedback inhibition of deoxyxylulose 5-phosphate synthase (DXS), the first enzyme in the pathway, the inhibition constants (Ki) for both DMADP and IDP are relatively high (Banerjee et al. 2013), and our data did not suggest changes in the degree of DXS control on pathway flux in response to alendronate feeding (Figs. 6-7). We conclude that the alendronate-inhibition experiments are in broad agreement with the control of isoprene emission by competition from other DMADP-consuming reactions with greater affinity for DMADP.
In our study, no evidence of emission of other volatile isoprenoids such as monoterpenes was observed (Supplemental Material S3). Monoterpene emissions have occasionally been observed from aspen (Hakola, Rinne & Laurila 1998) and young leaves of hybrid poplar (Brilli et al. 2009) and tropical evergreens (Kuhn et al. 2004), and such episodic emission are not currently understood. Nevertheless, monoterpene emissions are typically elicited by biotic stresses (Blande et al. 2007; Toome et al. 2010; Copolovici et al. 2011), suggesting that these emissions in past studies may have reflected biotic stress.
The rate of pigment synthesis in growing leaves
There is surprisingly little quantitative information on the rate of pigment synthesis in growing leaves and on pigment turnover in mature leaves. So far, 14C (Bhaya & Castelfranco 1985; Schulze-Siebert & Schulze 1987; Goericke & Welschmeyer 1993; Beisel et al. 2010; Beisel, Schurr & Matsubara 2011), or 15N (Vavilin, Brune & Vermaas 2005; Vavilin & Vermaas 2007) pulse-chase labeling methods have been employed to estimate the rate of pigment synthesis. However, these methods typically have been conducted in chloroplastic or cellular systems (Bhaya & Castelfranco 1985; Schulze-Siebert & Schulze 1987; Vavilin et al. 2005; Vavilin & Vermaas 2007) with a few exceptions (Beisel et al. 2010; Beisel et al. 2011), and yield relative information that is hard to quantitatively relate to foliage absolute pigment contents in intact leaves. We used three methods to estimate the rate of photosynthetic pigment synthesis during leaf development. Estimation of pigment synthesis rate from the rate of pigment accumulation (Fig. 1b, Fig. 8) yielded estimates between 0.3 nmol m−2 s−1 (DMADP equivalents) in mature leaves to 2.7 nmol m−2 s−1 in young leaves. On the other hand, the rates of alternative DMADP-consuming reactions estimated from fitting of postillumination isoprene decay data and from alendronate-inhibition experiments were 0.6-1.3 nmol m−2 s−1 for mature leaves and 4.6-5.8 nmol m−2 s−1 for young leaves.
Somewhat lower estimates from pigment accumulation data can be explained by several factors. First, the values derived from the kinetic analysis of isoprene emission data provide the rate of whole set of alternative DMADP-consuming reactions, including synthesis of plastoquinone pool and isoprenoid-based phytohormones, In addition, the pigment accumulation-based method does not consider turnover of pigments that is possibly low in developing leaves, but could still contribute to a certain extent to DMADP consumption (Beisel et al. 2010; Beisel et al. 2011). Nevertheless, there is evidence that phytol can be reused in chlorophyll turnover (Vavilin & Vermaas 2007), implying no DMADP consumption. Second, the pigment accumulation-based method assumes a constant rate of pigment synthesis over 12 h light period. However, DMADP pool size varies during the day due to variations in light and temperature (Rosenstiel et al. 2002; Rasulov et al. 2009b; Rasulov et al. 2010; Li et al. 2011), also likely altering the rate of pigment synthesis. In fact, scaling the pigment synthesis rate estimated during the gas-exchange measurements at 30 °C to the average ambient temperature results in very similar pigment synthesis rates in the field and in the lab estimations (data not shown, see Fig. 8b inset for temperature correction for isoprene). This evidence suggests that the average daily pigment synthesis rate possibly underestimated the maximum pigment synthesis rate. Finally, we assumed that one mol DMADP is used for synthesis of one mol pigment during formation of GDP, while the rest of the pigment isoprenoid residues comes from IDP. However, chloroplastic DMADP is in equilibrium with IDP, with the equilibrium depending on the rate of DMADP and IDP consumption and IDP isomerase (IDI) activity (Brüggemann & Schnitzler 2002a). Thus, due to partial conversion of DMADP to IDP, active pigment synthesis may draw down DMADP pool faster than in the case of lower pigment synthesis rate. This would suggest that isoprene-emission based methods overestimate the rate of synthesis of compounds downstream of GDP. Despite these discrepancies between the different methods, all methods provided a similar magnitude of alternative DMADP-consuming reactions, collectively providing conclusive evidence of high rate of alternative DMADP consumption in developing leaves.
Balance between “essential” and “non-essential” isoprenoid synthesis during leaf development
Development of leaf photosynthetic activity in aspen consisted of two different phases: a rapid increase from day 0 to day 6, followed by a slower phase from day 6 to day 20 (Figs. 2-3). The induction of IspS occurred during the second phase when photosynthesis was slowly approaching the maximum. Thus, isoprene emission was induced when the rates of photosynthetic pigments and hormones such as cytokinins (Shani et al. 2010) synthesized via chloroplastic isoprenoid pathway were decelerating. As the long-chain length isoprenoids such as the phytyl residue of chlorophyll and carotenoids mainly rely on IDP, during most active pigment synthesis, DMADP is expected to be just a smaller branch of the pathway, while dominant carbon stream goes through IDP, both synthesized by the hydroxymethylbutenyl diphosphate reductase (HDR). The ratio of these two parallel products of the HDR reaction is strongly shifted towards IDP (Tritsch et al. 2010), but both products, IDP and DMADP, are mutually bound through the IDI reaction (Berthelot et al. 2012) and a significant part of DMADP needed for isoprenoid synthesis is formed from IDP (Tritsch et al. 2010; Berthelot et al. 2012). We suggest that as long as IDP is rapidly consumed (e.g., in developing leaves), DMADP does not accumulate significantly, because the pathway is shifted towards IDP synthesis. As soon as the consumption of IDP and DMADP for production of higher-order isoprenoids slows down during maturation of chloroplasts in maturing leaves, IDP accumulates, inducing accumulation of the parallel product DMADP. It has also been demonstrated that the equilibrium of IDI reaction is shifted towards DMADP (Page et al. 2004; Berthelot et al. 2012; Zhou et al. 2013), implying that as IDP consumption slows down, DMADP gradually becomes the dominant product of the MEP pathway. Implicit in this reasoning is that IDI activity is large enough for adequate conversion rates such that IDI simply operates to meet the downstream metabolic pulls. In fact, IDI activity does vary and in oak Quercus robur, it was correlated with IspS activity (Brüggemann & Schnitzler 2002b), suggesting that changes in IDI activity can constitute an additional control point affecting DMADP pool size.
Clearly a complementary character of isoprene emission and pigment synthesis is evident in our data. Quantification of carbon streams going into isoprene emission and pigment synthesis resulted in comparable estimates, especially if isoprene emission rate was scaled to ambient temperature (Fig. 8). This evidence collectively suggests a balance between the synthesis of “essential” and “non-essential” isoprenoids: as a key MEP pathway sink became weaker with leaf maturation, it was replaced by isoprene emission.
However, there is also evidence that the enzymatic capacity of the whole MEP pathway can simultaneously increase with increasing the activity of IspS. Activation of the transcription of MEP pathway genes DXR and DXS (Mayrhofer et al. 2005; Wiberley et al. 2009), accompanied by increasing catalytic activities of both enzymes (Ghirardo et al. 2010) paralleled the synthesis of IspS protein (Mayrhofer et al. 2005; Wiberley et al. 2009; Ghirardo et al. 2010). Such synchronous expression of IspS, DXR and DXS genes and corresponding enzymatic activities suggests that isoprene emission is cooperatively regulated not only by processes downstream of HDR, but also by enzymes upstream of HDR (Wiberley et al. 2009). Given the high Km value of isoprene synthase, high DMADP pool sizes are needed to maintain high isoprene emission rates.
An order of magnitude lower Km value for prenyltransferases (in particular GDP synthase) than for isoprene synthase has overall important consequences for leaves supporting high DMADP pool, e.g. in mature leaves with high isoprene emission capacity. In such leaves, synthesis of “essential” isoprenoids can be elicited with a significant rate as soon as these isoprenoids are needed such as during repair processes in leaves recovering from photoinhibition, and during photosynthetic apparatus acclimation to modifications in environmental conditions (e.g., Niinemets & Anten 2009). Although the permanent running of the MEP pathway towards isoprene in the constitutively emitting species may seem futile in the first glance, it can play a major role in fast plant responses to stress conditions requiring turning-on the synthetic reactions, and allowing for rapid switch between the synthesis of “non-essential” and “essential” isoprenoids.
Conclusions
In this work, a kinetic gas-exchange approach combined with modeling was applied to gain insight into processes determining the induction and regulation of isoprene emission during foliage development. This approach is unique in that it resolves the chloroplastic DMADP pool that is difficult to estimate by other methods due to high cytosolic DMADP pool (Loreto et al. 2004; Falbel & Sharkey 2005; Rasulov et al. 2009a; Weise et al. 2013). Also, methods based on 13C-labeling can be problematic due to inclusion of “older” not readily labeled carbon in isoprene that can be of cytosolic origin and enter the MEP pathway at the level of pyruvate or originate from chloroplastic starch (e.g., Trowbridge et al. 2012).
Induction of isoprene synthase activity is a crucial event for the onset of isoprene emission, and this was not observed before pigment-synthesizing reactions consuming the bulk of MEP pathway metabolites started to slow down, and substrate became available for isoprene synthesis. Thus, presence of a certain threshold chloroplastic DMADP pool may be the key factor leading to accumulation of functionally active isoprene synthase in chloroplasts. Despite decreasing rates of pigment synthesis, isoprene emission in young leaves was still strongly curbed by competition by other DMADP-consuming reactions, reflecting greater affinity of the prenyltransferases consuming DMADP (mainly GDP synthase). Thus, isoprene emission in growing leaves was co-limited by DMADP and isoprene synthase activity, and the balance of MEP pathway was almost stoichiometrically shifted towards isoprene during leaf maturation as isoprene synthase activity increased and rate of synthesis of isoprenoid components of photosynthetic machinery slowed down. The competition for DMADP by isoprene synthase and prenyltransferases observed in our study supports the hypothesis of a role of isoprene emission, in addition to its antioxidant and membrane stabilizing role (for recent reviews see Fineschi et al. 2013; Possell & Loreto 2013), in maintaining MEP pathway active, thereby allowing for rapid synthesis of “essential isoprenoids” when they are needed, e.g. during repair processes elicited after stress events (e.g. for rapid response of carotenoids and tocopherols to high irradiance stress in aspen, Niinemets et al. 2003; García-Plazaola et al. 2004).
Supplementary Material
Summary statement.
Isoprene emission and prenyltransferase reactions of isoprenoid pigment and hormone synthesis compete for the same precursor, dimethylallyl diphosphate (DMADP). Due to an almost an order of magnitude higher specificity for DMADP of prenyltransferases compared with isoprene synthase, isoprene emission always “loses” when pigment synthesis rate is activated such as in developing leaves. This study demonstrates that differences in enzyme kinetics control the balance between “essential” and “non-essential” isoprenoid synthesis.
Acknowledgements
We thank three reviewers and Tom Sharkey for insightful comments on the MS. This work was supported by grants from the Estonian Ministry of Science and Education (institutional grant IUT-8-3, grant SF0180045s08), the Estonian Science Foundation (grant 9253), and the European Commission through European Regional Fund (the Center of Excellence in Environmental Adaptation) and European Research Council (advanced grant 322603, SIP-VOL+).
References
- Atkinson R, Arey J. Gas-phase tropospheric chemistry of biogenic volatile organic compounds: a review. Atmospheric Environment. 2003;37:197–219. [Google Scholar]
- Banerjee A, Wu Y, Banerjee R, Li Y, Yan H, Sharkey TD. Feedback inhibition of deoxy-D-xylulose 5-phosphate synthase regulates the methyl erythritol 4-phosphate pathway. The Journal of Biological Chemistry. 2013:xx–xx. doi: 10.1074/jbc.M113.464636. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barta C, Loreto F. The relationship between the methyl-erythritol phosphate (MEP) pathway leading to emission of volatile isoprenoids and abscisic acid content in leaves. Plant Physiology. 2006;141:1676–1683. doi: 10.1104/pp.106.083063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beisel KG, Jahnke S, Hofmann D, Köppchen S, Schurr U, Matsubara S. Continuous turnover of carotenes and chlorophyll a in mature leaves of Arabidopsis revealed by 14CO2 pulse-chase labeling. Plant Physiology. 2010;152:2188–2199. doi: 10.1104/pp.109.151647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beisel KG, Schurr U, Matsubara S. Altered turnover of β-carotene and chl a in Arabidopsis leaves treated with lincomycin or norflurazon. Plant and Cell Physiology. 2011;52:1193–1203. doi: 10.1093/pcp/pcr069. [DOI] [PubMed] [Google Scholar]
- Bergstrom JD, Bostedor RG, Masarachia PJ, Reszka AA, Rodan G. Alendronate is a specific, nanomolar inhibitor of farnesyl diphosphate synthase. Archives of Biochemistry and Biophysics. 2000;373:231–241. doi: 10.1006/abbi.1999.1502. [DOI] [PubMed] [Google Scholar]
- Berthelot K, Estevez Y, Deffieux A, Peruch F. Isopentenyl diphosphate isomerase: a checkpoint to isoprenoid biosynthesis. Biochemie. 2012;94:1621–1634. doi: 10.1016/j.biochi.2012.03.021. [DOI] [PubMed] [Google Scholar]
- Bhaya D, Castelfranco PA. Chlorophyll biosynthesis and assembly into chlorophyll-protein complexes in isolated developing chloroplasts. Proceedings of the National Academy of Sciences of the United States of America. 1985;82:5370–5374. doi: 10.1073/pnas.82.16.5370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blande JD, Tiiva P, Oksanen E, Holopainen JK. Emission of herbivore-induced volatile terpenoids from two hybrid aspen (Populus tremula × tremuloides) clones under ambient and elevated ozone concentrations in the field. Global Change Biology. 2007;13:2538–2550. [Google Scholar]
- Brilli F, Ciccioli P, Frattoni M, Prestininzi M, Spanedda AF, Loreto F. Constitutive and herbivore-induced monoterpenes emitted by Populus x euramericana leaves are key volatiles that orient Chrysomela populi beetles. Plant, Cell and Environment. 2009;32:542–552. doi: 10.1111/j.1365-3040.2009.01948.x. [DOI] [PubMed] [Google Scholar]
- Brüggemann N, Schnitzler J-P. Diurnal variation of dimethylallyl diphosphate concentrations in oak (Quercus robur) leaves. Physiologia Plantarum. 2002a;115:190–196. doi: 10.1034/j.1399-3054.2002.1150203.x. [DOI] [PubMed] [Google Scholar]
- Brüggemann N, Schnitzler J-P. Relationship of isopentenyl diphosphate (IDP) isomerase activity to isoprene emission of oak leaves. Tree Physiology. 2002b;22:1011–1018. doi: 10.1093/treephys/22.14.1011. [DOI] [PubMed] [Google Scholar]
- Burke C, Klettke K, Croteau R. Heteromeric geranyl diphosphate synthase from mint: construction of a functional fusion protein and inhibition by bisphosphonate substrate analogs. Archives of Biochemistry and Biophysics. 2004;422:52–60. doi: 10.1016/j.abb.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Cai Z-Q, Slot M, Fan Z-X. Leaf development and photosynthetic properties of three tropical tree species with delayed greening. Photosynthetica. 2005;43:91–98. [Google Scholar]
- Chang T-H, Hsieh F-L, Ko T-P, Teng K-H, Liang P-H, Wang AH-J. Structure of a heterotetrameric geranyl pyrophosphate synthase from mint (Mentha piperita) reveals intersubunit regulation. The Plant Cell. 2010;22:454–467. doi: 10.1105/tpc.109.071738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinege G, Louis S, Hänsch R, Schnitzler J-P. Regulation of isoprene synthase promoter by environmental and internal factors. Plant Molecular Biology. 2009;69:593–604. doi: 10.1007/s11103-008-9441-2. [DOI] [PubMed] [Google Scholar]
- Copolovici L, Kännaste A, Remmel T, Vislap V, Niinemets Ü. Volatile emissions from Alnus glutinosa induced by herbivory are quantitatively related to the extent of damage. Journal of Chemical Ecology. 2011;37:18–28. doi: 10.1007/s10886-010-9897-9. [DOI] [PubMed] [Google Scholar]
- Dickmann DI, Gordon JC. Incorporation of 14C-photosynthate into protein during leaf development in young Populus plants. Plant Physiology. 1975;56:23–27. doi: 10.1104/pp.56.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichelmann H, Oja B, Rasulov B, Padu E, Bichele I, Pettai H, Vapaavuori E, Niinemets Ü, Laisk A. Development of leaf photosynthetic parameters in Betula pendula Roth. leaves: correlations with Photosystem I density. Plant Biology. 2004;6:307–318. doi: 10.1055/s-2004-820874. [DOI] [PubMed] [Google Scholar]
- Falbel TG, Sharkey TD. Determining the DMAPP that is available for isoprene synthesis. Poster 57 - Secondary metabolism, Plant Biology 2005: Saturday, July 16 - Wednesday July 20, 2005 - Seattle, Washington, USA. 2005.
- Fall R, Wildermuth MC. Isoprene synthase: from biochemical mechanism to emission algorithm. Journal of Geophysical Research. 1998;103:25599–25609. [Google Scholar]
- Fineschi S, Loreto F, Staudt M, Peñuelas J. Diversification of volatile isoprenoid emissions from trees: evolutionary and ecological perspectives. In: Niinemets Ü, Monson RK, editors. Biology, controls and models of tree volatile organic compound emissions. Springer; Berlin: 2013. pp. 1–20. [Google Scholar]
- Fletcher RA, McCullagh D. Cytokinin-induced chlorophyll formation in cucumber cotyledons. Planta. 1971;101:88–90. doi: 10.1007/BF00387693. [DOI] [PubMed] [Google Scholar]
- Fraser PD, Schuch W, Bramley PM. Phytoene synthase from tomato (Lycopersicon esculentum) chloroplasts. Partial purification and biochemical properties. Planta. 2000;211:361–369. doi: 10.1007/s004250000293. [DOI] [PubMed] [Google Scholar]
- García-Plazaola JI, Becerril JM, Hernández A, Niinemets Ü, Kollist H. Acclimation of antioxidant pools to the light environment in a natural forest canopy. The New Phytologist. 2004;163:87–97. doi: 10.1111/j.1469-8137.2004.01096.x. [DOI] [PubMed] [Google Scholar]
- Genty B, Briantais J-M, Baker NR. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochimica et Biophysica Acta. 1989;990:87–92. [Google Scholar]
- Ghirardo A, Zimmer I, Brüggemann N, Schnitzler J-P. Analysis of 1-deoxy-D-xylulose 5-phosphate synthase activity in grey poplar leaves using isotope ratio mass spectrometry. Phytochemistry. 2010;71:918–922. doi: 10.1016/j.phytochem.2010.02.016. [DOI] [PubMed] [Google Scholar]
- Goericke R, Welschmeyer NA. The carotenoid-labeling method: measuring specific rates of carotenoid synthesis in natural phytoplankton communities. Marine Ecology - Progress Series. 1993;98:157–172. [Google Scholar]
- Graus M, Schnitzler J-P, Hansel A, Cojocariu C, Rennenberg H, Wisthaler A, Kreuzwieser J. Transient release of oxygenated volatile organic compounds during light-dark transitions in grey poplar leaves. Plant Physiology. 2004;135:1967–1975. doi: 10.1104/pp.104.043240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinspoon J, Bowman WD, Fall R. Delayed onset of isoprene emission in developing velvet bean (Mucuna sp.) leaves. Plant Physiology. 1991;97:170–174. doi: 10.1104/pp.97.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grote R, Monson RK, Niinemets Ü. Leaf-level models of constitutive and stress-driven volatile organic compound emissions. In: Niinemets Ü, Monson RK, editors. Biology, controls and models of tree volatile organic compound emissions. Springer; Berlin: 2013. pp. 315–355. [Google Scholar]
- Guenther A. Upscaling biogenic volatile compound emissions from leaves to landscapes. In: Niinemets Ü, Monson RK, editors. Biology, controls and models of tree volatile organic compound emissions. Springer; Berlin: 2013. pp. 391–414. [Google Scholar]
- Guidolotti G, Calfapietra C, Loreto F. The relationship between isoprene emission, CO2 assimilation and water use efficiency across a range of poplar genotypes. Physiologia Plantarum. 2011;142:297–304. doi: 10.1111/j.1399-3054.2011.01463.x. [DOI] [PubMed] [Google Scholar]
- Hakola H, Rinne J, Laurila T. The hydrocarbon emission rates of tea-leafed willow (Salix phylicifolia), silver birch (Betula pendula) and European aspen (Populus tremula) Atmospheric Environment. 1998;32:1825–1833. [Google Scholar]
- Hansel A, Jordan A, Holzinger R, Prazeller P, Vogel W, Lindinger W. Proton transfer reaction mass spectrometry: on-line trace gas analysis at the ppb level. International Journal of Mass Spectrometry and Ion Processes. 1995:149–150. 609–619. [Google Scholar]
- Harley PC, Litvak ME, Sharkey TD, Monson RK. Isoprene emission from velvet bean leaves. Interactions among nitrogen availability, growth photon flux density, and leaf development. Plant Physiology. 1994;105:279–285. doi: 10.1104/pp.105.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Gil R, Schaedle M. Functional and structural changes in senescing Populus deltoides (Bartr.) chloroplasts. Plant Physiology. 1973;51:245–249. doi: 10.1104/pp.51.2.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang I, Sakakibara H. Cytokinin biosynthesis and perception. Physiologia Plantarum. 2006;126:528–538. [Google Scholar]
- Kakimoto T. Biosynthesis of cytokinins. Journal of Plant Research. 2003;116:233–239. doi: 10.1007/s10265-003-0095-5. [DOI] [PubMed] [Google Scholar]
- Kennedy RA, Johnson DA. Changes in photosynthetic characteristics during leaf development in apple. Photosynthesis Research. 1981;2:213–223. doi: 10.1007/BF00032360. [DOI] [PubMed] [Google Scholar]
- Köksal M, Zimmer I, Schnitzler J-P, Christianson DW. Structure of isoprene synthase illuminates the chemical mechanism of teragram atmospheric carbon emission. Journal of Molecular Biology. 2010;402:363–373. doi: 10.1016/j.jmb.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn U, Rottenberger S, Biesenthal T, Wolf A, Schebeske G, Ciccioli P, Brancaleoni E, Frattoni M, Tavares TM, Kesselmeier J. Seasonal differences in isoprene and light-dependent monoterpene emission by Amazonian tree species. Global Change Biology. 2004;10:663–682. [Google Scholar]
- Kuzma J, Fall R. Leaf isoprene emission rate is dependent on leaf development and the level of isoprene synthase. Plant Physiology. 1993;101:435–440. doi: 10.1104/pp.101.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laisk A, Oja V. Dynamics of leaf photosynthesis: rapid-response measurements and their interpretations. CSIRO Publishing; Canberra: 1998. [Google Scholar]
- Laisk A, Oja V, Rasulov B, Rämma H, Eichelmann H, Kasparova I, Pettai H, Padu E, Vapaavuori E. A computer-operated routine of gas exchange and optical measurements to diagnose photosynthetic apparatus in leaves. Plant, Cell and Environment. 2002;25:923–943. [Google Scholar]
- Lange BM, Ketchum REB, Croteau RB. Isoprenoid biosynthesis. Metabolite profiling of peppermint oil gland secretory cells and application to herbicide target analysis. Plant Physiology. 2001;127:305–314. doi: 10.1104/pp.127.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehning A, Zimmer I, Steinbrecher R, Brüggemann N, Schnitzler JP. Isoprene synthase activity and its relation to isoprene emission in Quercus robur L. leaves. Plant, Cell and Environment. 1999;22:495–504. [Google Scholar]
- Lehning A, Zimmer W, Zimmer I, Schnitzler JP. Modeling of annual variations of oak (Quercus robur L.) isoprene synthase activity to predict isoprene emission rates. Journal of Geophysical Research. 2001;106:3157–3166. [Google Scholar]
- Li Z, Ratliff EA, Sharkey TD. Effect of temperature on postillumination isoprene emission in oak and poplar. Plant Physiology. 2011;155:1037–1046. doi: 10.1104/pp.110.167551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Sharkey TD. Metabolic profiling of the methylerythritol phosphate pathway reveals the source of post-illumination isoprene burst from leaves. Plant, Cell and Environment. 2013a;36:429–437. doi: 10.1111/j.1365-3040.2012.02584.x. [DOI] [PubMed] [Google Scholar]
- Li Z, Sharkey TD. Molecular and pathway controls on biogenic volatile organic compound emissions. In: Niinemets Ü, Monson RK, editors. Biology, controls and models of tree volatile organic compound emissions. Springer; Berlin: 2013b. pp. 119–151. [Google Scholar]
- Lichtenthaler HK. Biosynthesis, accumulation and emission of carotenoids, α-tocopherol, plastoquinone, and isoprene in leaves under high photosynthetic irradiance. Photosynthesis Research. 2007;92:163–179. doi: 10.1007/s11120-007-9204-y. [DOI] [PubMed] [Google Scholar]
- Lichtenthaler HK. Biosynthesis and accumulation of isoprenoid carotenoids and chlorophylls and emission of isoprene by leaf chloroplasts. Bulletin of the Georgian National Academy of Science. 2009;3:81–94. [Google Scholar]
- Lichtenthaler HK, Babani F. Light adaptation and senescence of the photosynthetic apparatus. Changes in pigment composition, chlorophyll fluorescence parameters and photosynthetic activity. In: Papageorgiou GC, Govindjee, editors. Chlorophyll fluorescence: a signature of photosynthesis. Springer; Dordrecht: 2004. pp. 713–736. [Google Scholar]
- Lichtenthaler HK, Buschmann C. Chlorophylls and carotenoids: measurement and characterisation by UV-VIS. In: Wrolstad RE, Acree TE, Decker EA, Penner MH, Reid DS, Schwartz SJ, Shoemaker CF, Smith DM, Sporns P, editors. Current protocols in food analytical chemistry. John Wiley & Sons; Madison: 2001. pp. F4.3.1–F4.3.8. [Google Scholar]
- Lindinger W, Hansel A, Jordan A. On-line monitoring of volatile organic compounds at pptv levels by means of proton-transfer-reaction mass spectrometry (PTR-MS): medical applications, food control and environmental research. International Journal of Mass Spectrometry and Ion Processes. 1998;173:191–241. [Google Scholar]
- Long SP, Postl WF, Bolhár-Nordenkampf HR. Quantum yields for uptake of carbon dioxide in C3 vascular plants of contrasting habitats and taxonomic groupings. Planta. 1993;189:226–234. [Google Scholar]
- Loreto F, Pinelli P, Brancaleoni E, Ciccioli P. 13C labelling reveals chloroplastic and extra-chloroplastic pools of dimethylallyl pyrophosphate and their contribution to isoprene formation. Plant Physiology. 2004;135:1903–1907. doi: 10.1104/pp.104.039537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreto F, Sharkey TD. A gas-exchange study of photosynthesis and isoprene emission in Quercus rubra L. Planta. 1990;182:523–531. doi: 10.1007/BF02341027. [DOI] [PubMed] [Google Scholar]
- Mayrhofer S, Teuber M, Zimmer I, Louis S, Fischbach RJ, Schnitzler J-P. Diurnal and seasonal variation of isoprene biosynthesis-related genes in grey poplar leaves. Plant Physiology. 2005;139:474–484. doi: 10.1104/pp.105.066373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazawa S-I, Livingston NJ, Turpin DH. Stomatal development in new leaves is related to the stomatal conductance of mature leaves in poplar (Populus trichocarpa × P. deltoides) Journal of Experimental Botany. 2006;57:373–380. doi: 10.1093/jxb/eri278. [DOI] [PubMed] [Google Scholar]
- Miyazawa SI, Makino A, Terashima I. Changes in mesophyll anatomy and sink-source relationships during leaf development in Quercus glauca, an evergreen tree showing delayed leaf greening. Plant, Cell and Environment. 2003;26:745–755. [Google Scholar]
- Miyazawa SI, Satomi S, Terashima I. Slow leaf development of evergreen broad-leaved tree species in Japanese warm temperate forests. Annals of Botany. 1998;82:859–869. [Google Scholar]
- Miyazawa SI, Terashima I. Slow development of leaf photosynthesis in an evergreen broad-leaved tree, Castanopsis sieboldii: relationships between leaf anatomical characteristics and photosynthetic rate. Plant, Cell and Environment. 2001;24:279–291. [Google Scholar]
- Monson RK, Grote R, Niinemets Ü, Schnitzler J-P. Tansley review. Modeling the isoprene emission rate from leaves. The New Phytologist. 2012;195:541–559. doi: 10.1111/j.1469-8137.2012.04204.x. [DOI] [PubMed] [Google Scholar]
- Monson RK, Harley PC, Litvak ME, Wildermuth M, Guenther AB, Zimmerman PR, Fall R. Environmental and developmental controls over the seasonal pattern of isoprene emission from aspen leaves. Oecologia. 1994;99:260–270. doi: 10.1007/BF00627738. [DOI] [PubMed] [Google Scholar]
- Monson RK, Jaeger CH, Adams WW, III, Driggers EM, Silver GM, Fall R. Relationships among isoprene emission rate, photosynthesis, and isoprene synthase activity as influenced by temperature. Plant Physiology. 1992;98:1175–1180. doi: 10.1104/pp.98.3.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott KA. Opinion: Stomatal responses to light and CO2 depend on the mesophyll. Plant, Cell and Environment. 2009;32:1479–1486. doi: 10.1111/j.1365-3040.2009.02022.x. [DOI] [PubMed] [Google Scholar]
- Mott KA, Sibbernsen ED, Shope JC. The role of the mesophyll in stomatal responses to light and CO2. Plant, Cell and Environment. 2008;31:1299–1306. doi: 10.1111/j.1365-3040.2008.01845.x. [DOI] [PubMed] [Google Scholar]
- Nakano T, Kimura T, Kaneko I, Nagata N, Matsuyama T, Asami T, Yoshida S. Molecular mechanism of chloroplast development regulated by plant hormone. RIKEN Review. 2001;41:86–87. [Google Scholar]
- Nelissen H, Rymen B, Jikumaru Y, Demuynck K, Van Lijsebettens M, Kamiya Y, Inzés D, Beemster GTS. A local maximum in gibberellin levels regulates maize leaf growth by spatial control of cell division. Current Biology. 2012;22:1183–1187. doi: 10.1016/j.cub.2012.04.065. [DOI] [PubMed] [Google Scholar]
- Niinemets Ü. Whole plant photosynthesis. In: Terrestrial photosynthesis in a changing environment. In: Flexas J, Loreto F, Medrano H, editors. A molecular, physiological and ecological approach. Cambridge University Press; Cambridge: 2012. pp. 399–423. [Google Scholar]
- Niinemets Ü, Anten NPR. Packing the photosynthesis machinery: from leaf to canopy. In: Laisk A, Nedbal L, Govindjee, editors. Photosynthesis in silico: understanding complexity from molecules to ecosystems. Springer Verlag; Berlin: 2009. pp. 363–399. [Google Scholar]
- Niinemets Ü, Arneth A, Kuhn U, Monson RK, Peñuelas J, Staudt M. The emission factor of volatile isoprenoids: stress, acclimation, and developmental responses. Biogeosciences. 2010a;7:2203–2223. [Google Scholar]
- Niinemets Ü, García-Plazaola JI, Tosens T. Photosynthesis during leaf development and ageing. In: Flexas J, Loreto F, Medrano H, editors. Terrestrial photosynthesis in a changing environment. A molecular, physiological and ecological approach. Cambridge University Press; Cambridge: 2012. pp. 353–372. [Google Scholar]
- Niinemets Ü, Kollist H, García-Plazaola JI, Hernández A, Becerril JM. Do the capacity and kinetics for modification of xanthophyll cycle pool size depend on growth irradiance in temperate trees? Plant, Cell and Environment. 2003;26:1787–1801. [Google Scholar]
- Niinemets Ü, Kuhn U, Harley PC, Staudt M, Arneth A, Cescatti A, Ciccioli P, Copolovici L, Geron C, Guenther AB, Kesselmeier J, Lerdau MT, Monson RK, Peñuelas J. Estimations of isoprenoid emission capacity from enclosure studies: measurements, data processing, quality and standardized measurement protocols. Biogeosciences. 2011;8:2209–2246. [Google Scholar]
- Niinemets Ü, Monson RK. Preface. In: Niinemets Ü, Monson RK, editors. Biology, controls and models of tree volatile organic compound emissions. Springer; Berlin: 2013. pp. V–X. [Google Scholar]
- Niinemets Ü, Monson RK, Arneth A, Ciccioli P, Kesselmeier J, Kuhn U, Noe SM, Peñuelas J, Staudt M. The leaf-level emission factor of volatile isoprenoids: caveats, model algorithms, response shapes and scaling. Biogeosciences. 2010b;7:1809–1832. [Google Scholar]
- Orlova I, Nagegowda DA, Kish CM, Gutensohn M, Maeda H, Varbanova M, Fridman E, Yamaguchi S, Hanada A, Kamiya Y, Krichevsky A, Citovsky V, Pichersky E, Dudareva N. The small subunit of snapdragon geranyl diphosphate synthase modifies the chain length specificity of tobacco geranylgeranyl diphosphate synthase in planta. The Plant Cell. 2009;21:4002–4017. doi: 10.1105/tpc.109.071282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne B, Garrett M. Quantum yields for CO2 uptake in some diploid and tetraploid plant species. Plant, Cell and Environment. 1983;6:135–144. [Google Scholar]
- Owen SM, Peñuelas J. Opportunistic emissions of volatile isoprenoids. Trends in Plant Science. 2005;10:420–426. doi: 10.1016/j.tplants.2005.07.010. [DOI] [PubMed] [Google Scholar]
- Page JE, Hause G, Raschke M, Gao W, Schmidt J, Zenk MH, Kutchan TM. Functional analysis of the final steps of the 1-deoxy-D-xylulose 5-phosphate (DXP) pathway to isoprenoids in plants using virus-induced gene silencing. Plant Physiology. 2004;134:1–13. doi: 10.1104/pp.103.038133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike RC, Young PJ. How plants can influence tropospheric chemistry: the role of isoprene emissions from the biosphere. Weather. 2009;64:332–336. [Google Scholar]
- Possell M, Loreto F. The role of volatile organic compounds in plant resistance to abiotic stresses: responses and mechanisms. In: Niinemets Ü, Monson RK, editors. Biology, controls and models of tree volatile organic compound emissions. Springer; Berlin: 2013. pp. 209–235. [Google Scholar]
- Rajabi Memari H., Pazouki L, Niinemets Ü. The biochemistry and molecular biology of volatile messengers in trees. In: Niinemets Ü, Monson RK, editors. Biology, controls and models of tree volatile organic compound emissions. Springer; Berlin: 2013. pp. 47–93. [Google Scholar]
- Ramos-Valdivia AC, Heijden R, Verpoorte R. Isopentenyl diphosphate isomerase: a core enzyme in isoprenoid biosynthesis. A review of its biochemistry and function. Nat. Prod. Rep. 1997;14:591–603. doi: 10.1039/np9971400591. [DOI] [PubMed] [Google Scholar]
- Rasulov B, Copolovici L, Laisk A, Niinemets Ü. Postillumination isoprene emission: in vivo measurements of dimethylallyldiphosphate pool size and isoprene synthase kinetics in aspen leaves. Plant Physiology. 2009a;149:1609–1618. doi: 10.1104/pp.108.133512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasulov B, Hüve K, Bichele I, Laisk A, Niinemets Ü. Temperature response of isoprene emission in vivo reflects a combined effect of substrate limitations and isoprene synthase activity: a kinetic analysis. Plant Physiology. 2010;154:1558–1570. doi: 10.1104/pp.110.162081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasulov B, Hüve K, Laisk A, Niinemets Ü. Induction of a longer-term component of isoprene release in darkened aspen leaves: origin and regulation under different environmental conditions. Plant Physiology. 2011;156:816–831. doi: 10.1104/pp.111.176222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasulov B, Hüve K, Välbe M, Laisk A, Niinemets Ü. Evidence that light, carbon dioxide and oxygen dependencies of leaf isoprene emission are driven by energy status in hybrid aspen. Plant Physiology. 2009b;151:448–460. doi: 10.1104/pp.109.141978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz M, Schnitzler J-P. Genetic engineering of BVOC emissions from trees. In: Niinemets Ü, Monson RK, editors. Biology, controls and models of tree volatile organic compound emissions. Springer; Berlin: 2013. pp. 95–118. [Google Scholar]
- Rosenstiel TN, Ebbets AL, Khatri WC, Fall R, Monson RK. Induction of poplar leaf nitrate reductase: a test of extrachloroplastic control of isoprene emission rate. Plant Biology. 2004;6:12–21. doi: 10.1055/s-2003-44722. [DOI] [PubMed] [Google Scholar]
- Rosenstiel TN, Fisher AJ, Fall R, Monson RK. Differential accumulation of dimethylallyl diphosphate in leaves and needles of isoprene- and methylbutenol-emitting and nonemitting species. Plant Physiology. 2002;129:1276–1284. doi: 10.1104/pp.002717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanadze GA, Dzhaiani GI. Carbohydrate partition within isoprene molecule from photosynthetically assimilated CO2. Fiziologiya Rastenii. 1972;19:1082–1089. [Google Scholar]
- Sanderson MG, Jones CD, Collins WJ, Johnson CE, Derwent RG. Effect of climate change on isoprene emissions and surface ozone levels. Geophysical Research Letters. 2003;30:1936. [Google Scholar]
- Schmülling T, Schafer S, Romanov G. Cytokinins as regulators of gene expression. Physiologia Plantarum. 1997;100:505–519. [Google Scholar]
- Schnitzler J-P, Zimmer I, Bachl A, Arend M, Fromm J, Fischbach R. Biochemical properties of isoprene synthase in poplar (Populus x canescens) Planta. 2005;222:777–786. doi: 10.1007/s00425-005-0022-1. [DOI] [PubMed] [Google Scholar]
- Schnitzler JP, Arenz R, Steinbrecher R, Lehning A. Characterization of an isoprene synthase from leaves of Quercus petraea (Mattuschka) Liebl. Botanica Acta. 1996;109:216–221. [Google Scholar]
- Schreiber U, Bilger W, Neubauer C. Chlorophyll fluorescence as a nonintrusive indicator for rapid assessment of in vivo photosynthesis. In: Schulze E-D, Caldwell MM, editors. Ecophysiology of photosynthesis. Springer; Verlag, Berlin - Heidelberg - New York - London - Paris - Tokyo - Hong Kong - Barcelona - Budapest: 1994. pp. 49–70. [Google Scholar]
- Schulze-Siebert D, Schulze G. ß-carotene synthesis in isolated chloroplasts. Plant Physiology. 1987;84:1233–1237. doi: 10.1104/pp.84.4.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shani E, Ben-Gera H, Shleizer-Burko S, Burko Y, Weiss D, Ori N. Cytokinin regulates compound leaf development in tomato. The Plant Cell. 2010;22:3206–3217. doi: 10.1105/tpc.110.078253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey TD. The future of isoprene research. Bulletin of the Georgian National Academy of Sciences. 2009;3:106–113. [Google Scholar]
- Sharkey TD, Chen XY, Yeh S. Isoprene increases thermotolerance of fosmidomycin-fed leaves. Plant Physiology. 2001;125:2001–2006. doi: 10.1104/pp.125.4.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey TD, Loreto F. Water stress, temperature, and light effects on the capacity for isoprene emission and photosynthesis of kudzu leaves. Oecologia. 1993;95:328–333. doi: 10.1007/BF00320984. [DOI] [PubMed] [Google Scholar]
- Sharkey TD, Loreto F, Delwiche CF. High carbon dioxide and sun/shade effects on isoprene emission from oak and aspen tree leaves. Plant, Cell and Environment. 1991;14:333–338. [Google Scholar]
- Sharkey TD, Singsaas EL, Vanderveer PJ, Geron C. Field measurements of isoprene emission from trees in response to temperature and light. Tree Physiology. 1996;16:649–654. doi: 10.1093/treephys/16.7.649. [DOI] [PubMed] [Google Scholar]
- Sharkey TD, Wiberley AE, Donohue AR. Isoprene emission from plants: why and how. Annals of Botany. 2008;101:5–18. doi: 10.1093/aob/mcm240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp RE, Matthews MA, Boyer JS. Kok effect and the quantum yield of photosynthesis. Light partially inhibits dark respiration. Plant Physiology. 1984;75:95–101. doi: 10.1104/pp.75.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shesták Z, editor. Photosynthesis during leaf development. Dr. W. Junk Publishers; Dordrecht - Boston - Lancaster: 1985. [Google Scholar]
- Silver GM, Fall R. Enzymatic synthesis of isoprene from dimethylallyl diphosphate in aspen leaf extracts. Plant Physiology. 1991;97:1588–1591. doi: 10.1104/pp.97.4.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singsaas EL, Ort DR, DeLucia EH. Variation in measured values of photosynthetic quantum yield in ecophysiological studies. Oecologia. 2001;128:15–23. doi: 10.1007/s004420000624. [DOI] [PubMed] [Google Scholar]
- Skoog A, Armstrong D. Cytokinins. Annual Review of Plant Physiology. 1970;21:359–384. [Google Scholar]
- Stitt M. Rising CO2 levels and their potential significance for carbon flow in photosynthetic cells. Plant, Cell and Environment. 1991;14:741–762. [Google Scholar]
- Sun Z, Copolovici L, Niinemets Ü. Can the capacity for isoprene emissions acclimate to environmental modifications during autumn senescence in temperate deciduous tree species Populus tremula? Journal of Plant Research. 2012a;125:263–274. doi: 10.1007/s10265-011-0429-7. [DOI] [PubMed] [Google Scholar]
- Sun Z, Niinemets Ü, Hüve K, Noe SM, Rasulov B, Copolovici L, Vislap V. Enhanced isoprene emission capacity and altered light responsiveness in aspen grown under elevated atmospheric CO2 concentration. Global Change Biology. 2012b;18:3423–3440. [Google Scholar]
- Suzuki S, Nakamoto H, Ku MSB, Edwards GE. Influence of leaf age on photosynthesis, enzyme activity, and metabolite levels in wheat. Plant Physiology. 1987;84:1244–1248. doi: 10.1104/pp.84.4.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo CM, Matsumura Y, Fukura S, Martin MB, Sanders JM, Sengupta S, Cieslak JA, Loftus TC, Lea CR, Lee H-J, Koohang A, Coates RM, Sagami H, Oldfield E. Inhibition of geranylgeranyl diphosphate synthase by bisphosphonates and diphosphates: a potential route to new bone antiresorption and antiparasitic agents. Journal of Medical Chemistry. 2002;45:2185–2196. doi: 10.1021/jm010412y. [DOI] [PubMed] [Google Scholar]
- Tholl D, Croteau R, Gershenzon J. Partial purification and characterization of the short-chain prenyltransferases, geranyl diphosphate synthase and farnesyl diphosphate synthase, from Abies grandis (grand fir) Archives of Biochemistry and Biophysics. 2001;386:233–242. doi: 10.1006/abbi.2000.2212. [DOI] [PubMed] [Google Scholar]
- Tholl D, Kish CM, Orlova I, Sherman D, Gershenzon J, Pichersky E, Dudareva N. Formation of monoterpenes in Antirrhinum majus and Clarkia breweri flowers involves heterodimeric geranyl diphosphate synthases. The Plant Cell. 2004;16:977–992. doi: 10.1105/tpc.020156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toome M, Randjärv P, Copolovici L, Niinemets Ü, Heinsoo K, Luik A, Noe SM. Leaf rust induced volatile organic compounds signalling in willow during the infection. Planta. 2010;232:235–243. doi: 10.1007/s00425-010-1169-y. [DOI] [PubMed] [Google Scholar]
- Tritsch D, Hemmerlin A, Bach TJ, Rohmer M. Plant isoprenoid biosynthesis via the MEP pathway: in vivo IPP/DMAPP ratio produced by (E)-4-hydroxy-3-methylbut-2-enyl diphosphate reductase in tobacco BY-2 cell cultures. FEBS Letters. 2010;584:129–134. doi: 10.1016/j.febslet.2009.11.010. [DOI] [PubMed] [Google Scholar]
- Trowbridge AM, Asensio D, Eller ASD, Way DA, Wilkinson MJ, Schnitzler J-P, Jackson RB, Monson RK. Contribution of various carbon sources toward isoprene biosynthesis in poplar leaves mediated by altered atmospheric CO2 concentrations. PloS ONE. 2012;7:e32387. doi: 10.1371/journal.pone.0032387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vavilin D, Brune DC, Vermaas W. 15N-labeling to determine chlorophyll synthesis and degradation in Synechocystis sp. PCC 6803 strains lacking one or both photosystems. Biochimica et Biophysica Acta - Bioenergetics. 2005;1708:91–101. doi: 10.1016/j.bbabio.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Vavilin D, Vermaas W. Continuous chlorophyll degradation accompanied by chlorophyllide and phytol reutilization for chlorophyll synthesis in Synechocystis sp. PCC 6803. Biochimica et Biophysica Acta - Bioenergetics. 2007;1767:920–929. doi: 10.1016/j.bbabio.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Vickers CE, Possell M, Hewitt CN, Mullineaux PM. Genetic structure and regulation of isoprene synthase in poplar (Populus spp.) Plant Molecular Biology. 2010;73:547–558. doi: 10.1007/s11103-010-9642-3. [DOI] [PubMed] [Google Scholar]
- Vickers CE, Possell M, Laothawornkitkul J, Ryan AC, Hewitt CN, Mullineaux PM. Isoprene synthesis in plants: lessons from a transgenic tobacco model. Plant, Cell and Environment. 2011;34:1043–1053. doi: 10.1111/j.1365-3040.2011.02303.x. [DOI] [PubMed] [Google Scholar]
- von Caemmerer S, Farquhar GD. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta. 1981;153:376–387. doi: 10.1007/BF00384257. [DOI] [PubMed] [Google Scholar]
- Wang G, Dixon RA. Heterodimeric geranyl(geranyl)diphosphate synthase from hop (Humulus lupulus) and the evolution of monoterpene biosynthesis. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:9914–9919. doi: 10.1073/pnas.0904069106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weise SE, Li Z, Sutter AE, Corrion A, Banerjee A, Sharkey TD. Measuring dimethylallyl diphosphate available for isoprene synthesis. Analytical Biochemistry. 2013;435:27–34. doi: 10.1016/j.ab.2012.11.031. [DOI] [PubMed] [Google Scholar]
- Wiberley AE, Donohue AR, Westphal MM, Sharkey TD. Regulation of isoprene emission from poplar leaves throughout a day. Plant Cell and Environment. 2009;32:939–947. doi: 10.1111/j.1365-3040.2009.01980.x. [DOI] [PubMed] [Google Scholar]
- Wiberley AE, Linskey AR, Falbel TG, Sharkey TD. Development of the capacity for isoprene emission in kudzu. Plant, Cell and Environment. 2005;28:898–905. [Google Scholar]
- Wolfertz M, Sharkey TD, Boland W, Kühnemann F. Rapid regulation of the methylerythritol 4-phosphate pathway during isoprene synthesis. Plant Physiology. 2004;135:1939–1945. doi: 10.1104/pp.104.043737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SD, Greer DH, Laing WA, McManus MT. Changes in photosynthetic efficiency and carotenoid composition in leaves of white clover at different developmental stages. Plant Physiology and Biochemistry. 2003;41:887–893. [Google Scholar]
- Zhou C, Li Z, Wiberley-Bradford AE, Weise SE, Sharkey TD. Isopentenyl diphosphate and dimethylallyl diphosphate ratio measured with recombinant isopentenyl diphosphate isomerase and isoprene synthase. Analytical Biochemistry. 2013;440:130–136. doi: 10.1016/j.ab.2013.05.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.