Abstract
Introduction:
Acute spinal cord injury (SCI) is often treated with induced hypertension to enhance spinal cord perfusion. The optimal mean arterial pressure (MAP) likely varies between patients. Arbitrary goals are often set, frequently requiring vasopressors to achieve, with no clear evidence supporting this practice. We hypothesize that increased MAP goals and episodes of relative hypotension do not affect hospital outcome.
Materials and Methods:
All cervical and thoracic SCI patients treated at a level one trauma and regional SCI center over at 2.5-year period were retrospectively reviewed. Lowest and average hourly MAP was recorded for the first 72 h of hospitalization, allowing for quantification of mean MAP and the total number of episodic relative hypotensive events. These data were further compared to daily American spinal injury association motor score (AMS), which was used to determine the severity of SCI and improvement/decline during hospitalization. Patient's data were finally analyzed at theoretic MAP set points.
Results:
One hundred and five patients had complete data during the study period. At higher theoretic MAP set points (85 and 90), increased number of relative hypotensive episodes correlated with lower admission AMS (85 mmHg: <10 episodes, AMS 66.2; >50 episodes, 22.0; P < 0.001) and the need for vasopressors (P < 0.03) but showed no statistical change in AMS by hospital discharge. The need for vasopressors correlated with the number of hypotensive episodes and inversely related to admission AMS at all theoretic MAP goal set points but was not correlated with the change in AMS during the hospitalization.
Conclusions:
The frequency of relative hypotension and the need for vasopressors are progressively related to more severe SCI, as denoted by lower admission AMS. However, episodes of hypotension and the need for vasopressors did not affect the change in AMS during the acute hospitalization, regardless of theoretic MAP goal set-point. Arbitrarily elevated MAP goals may not be efficacious.
Keywords: Hypotension, mean arterial pressure goals, spinal cord injury, spinal cord perfusion
INTRODUCTION
Acute spinal cord injury (SCI) is a potentially devastating occurrence that affects 270,000 people each year in the United States and is expected to increase.[1,2,3] Recovery from SCI is highly variable and felt to be associated with a multitude of factors including the care rendered during the acute hospital setting.
One such intervention is raising the blood pressure to improve blood flow to the penumbra of tissue near the injury site that is at risk of edema and infarction. This risk is accentuated by impaired vascular reactivity and loss of auto-regulation of the injured spine, making it vulnerable to systemic hypotension and impaired functional recovery.[4,5,6,7]
Raising the blood pressure is often accomplished with volume resuscitation and vasopressor support; however, there is no gold standard in terms of optimal perfusion.[8] There have been several published studies on the effect of blood pressure management in acute SCI, the largest of which contained 103 patients, 41 of which required vasopressors.[9,10,11,12,13,14,15,16,17,18,19] Despite this, there only exists Level III evidence suggesting a target mean arterial pressure (MAP) goal of 85-90 mmHg.[9]
The most tangible outcome of improved spinal cord perfusion is ultimate functional neurologic outcome. In this current study, we hope to assess the largest published cohort of acute SCI patients and evaluate functional outcome as it relates to set MAP goals during hospitalization. We hypothesize that increased spinal cord perfusion will not be associated with improved functional outcome by hospital discharge. Spinal cord perfusion will be measured using both MAP and number of episodes of hypotension during the initial hospitalization.
MATERIALS AND METHODS
This single institution study was conducted at a level 1 trauma center and regional SCI center (one of 14 such centers designated by the National Institute on Disability and Rehabilitation Research). With Institutional Review Board approval, a retrospective review of the prospectively-entered trauma database was performed to identify all patients with an acute cervical or thoracic SCI during a 2.5-year period, January 2007 through June 2009.
The database was used to collect standard demographic data. The hospital's electronic medical record was then queried for lowest and average MAP for each hour of the initial 72 h of hospitalization. This allowed for quantification of mean MAP and the total number of episodic relative hypotensive events (maximum 1/h). These relative hypotensive events were subsequently denoted by the creation of theoretic MAP set points (>90, >85, >70, >65 mmHg) for which the data were compared. At each MAP set point, patients were divided into a quartile distribution based on the number of relative hypotensive events. Division into quartiles and not using the absolute number of events was necessary as the number of events differed significantly based on the theoretic MAP goal set point.
Severity of SCI and functional improvement was assessed using the American spinal injury association's (ASIA) motor score (AMS). AMS is more reliable than the more popular ASIA grade because it allows for each limb to be scored separately increasing its sensitivity and has thus been found to be the best indicator for observing the effects of therapeutic interventions.[19,20,21] AMS is calculated by assessing function in 5 key muscle groups per extremity, each muscle group with a maximum score of 5, creating a total body maximum score of 100.[7]
American spinal injury association motor score data were abstracted from the daily progress notes of the physical medicine and rehabilitation (PM&R) physicians. In a similar fashion to mean, MAP and number of relative hypotensive episodes, admission AMS and the change in AMS during the hospitalization was assessed in quartile fashion using the same theoretic MAP goal set points. Finally, the need for vasopressor support during the initial 72 h of hospitalization was also recorded for each patient.
Statistical analysis was performed using SPSS Version 20.0.0 (SPSS, Inc., 2011, Chicago, IL, USA). All data were subjected to descriptive statistical analysis yielding frequency scores for categorical data and mean scores with standard deviations for continuous/interval data. Statistically significant differences were evaluated by conducting inferential statistical analysis using the Chi-square test or Fisher's Exact test for categorical variable comparisons and the Student's t-test or analysis of variance (ANOVA) for continuous/interval data comparisons. Bonferroni correction was used to compare the number of patients in one quartile with the other quartiles while running the ANOVA. A P ≤ 0.05 was considered as statistically significant.
RESULTS
Two hundred and twenty-three patients presented with acute cervical and thoracic SCI during the study period. Figure 1 displays those patients who were excluded from data analysis for missing telemetry data, AMS documentation, or missing charts. Ultimately, 105 patients were used for a complete analysis.
Figure 1.
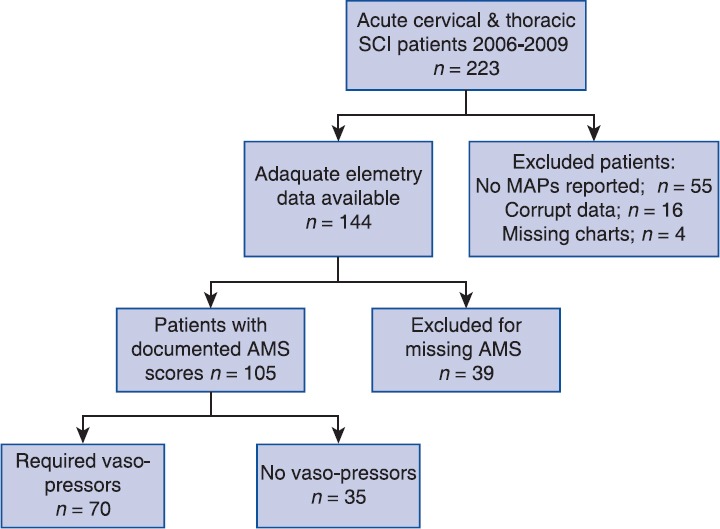
Patient selection and exclusion criteria. SCI: spinal cord injury, MAP: mean arterial pressure, AMS: ASIA motor score
When patient telemetry data were subjected to theoretic MAP set points, the number of episodes below that set point was calculated. Patients were then grouped into quartiles for statistical analysis based on the number of episodes. Table 1 displays the results of the set points 90, 85, 70, and 65 mmHg. At the 90 and 85 mmHg set points, a statistically significantly higher number of relative hypotensive episodes related to lower admission AMS; however, there was no related difference in the change in AMS during the acute hospitalization. This difference was no longer statistically significant at lower theoretic MAP set points (70 and 65 mmHg).
Table 1.
The effect of admission AMS and change in AMS during hospitalization on the number of episodes of hypotension (stratified by quartile); further stratified by theoretic MAP goal set-point
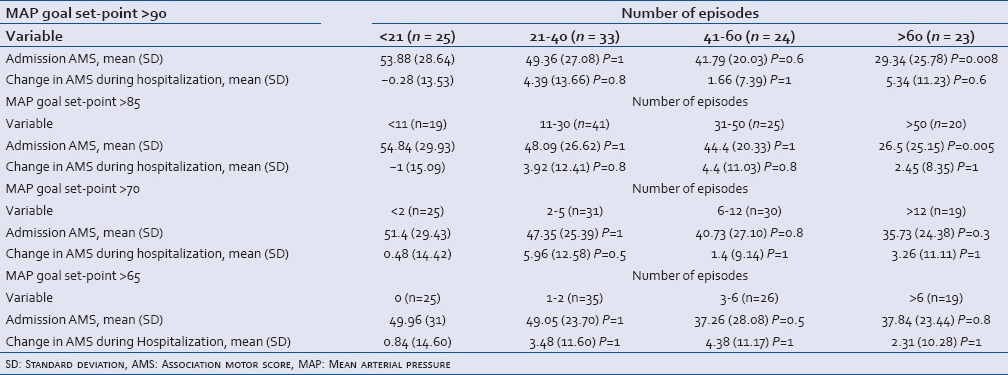
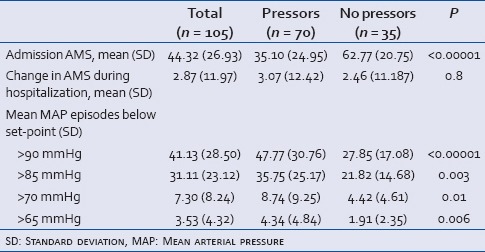
Table 2 displays patient demographics, mechanisms of injury, and injury level. It then further segregates data by improvement or worsening of AMS during the acute hospital admission. Other than injury severity score, no statistically significant differences were found. Table 3 displays the need for vasopressors and correlates that with the AMS at admission change in AMS during hospitalization, and the number of hypotensive episodes at each theoretic MAP goal set point. Results revealed the need for vasopressors correlated with the number of hypotensive episodes and inversely related to admission AMS at all theoretic MAP goal set points, but was not correlated with the change in AMS during the hospitalization.
Table 2.
Patient demographics and clinical features with comparison of those whose AMS improved vs. those whose worsened during the admission
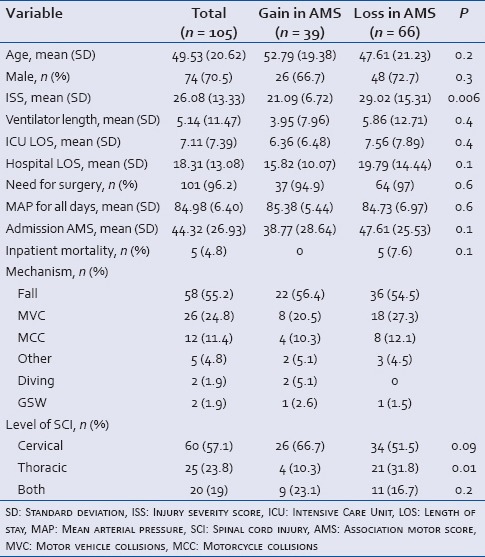
Table 3.
The effect of the admission AMS, change in AMS during hospitalization and the number of hypotensive episodes by theoretic MAP goal set-point versus the need for vasopressors
DISCUSSION
This study revealed significant variances in the hemodynamics of acute SCI patients. Our results showed lower MAPs, more frequent hypotensive episodes, and an increased need for vasopressors occurred in patients with more severe injuries. These factors, however, did not affect the change in AMS during hospitalization. These findings are significant because contemporary clinical practice in SCI still includes driving MAP goals to supra-physiologic levels.
Maintaining supra-physiologic MAP goals is not without consequence. Additional intra-vascular volume and vasopressors are often employed to meet treatment objectives. Whereas in true vasoplegia, where these agents improve outcome despite side-effects, there use to create relative hypertension alters this risk/benefit ratio. Further, over resuscitation with volume can result in fluid overload that has been associated with both cardio-pulmonary complications and surgical complications.[22,23] Thus, deploying these strategies warrants appropriate considerations of the deleterious effects and determining the associated risk/benefit ratio.
Appropriate patient selection is one method of optimizing the risk to benefit ratio. Complete recovery is extremely rare and even more so with complete SCI. Fortunately, approximately 60% of acute SCIs are incomplete, a percentage that has increased in the last 15 years.[3] Focusing therapy on this select population may be an area worthy of future study. Similarly, this study only included cervical and thoracic SCI patients. This select population was chosen secondary to the greater likelihood of autonomic dysregulation from loss of normal sympathetic function. Lumbar SCI patients are theoretically less likely to benefit from induced hypertension.
Having conducted this study at a regional SCI center, the study population is likely biased toward more severe injuries that community practitioners felt were too complex. These more severe injuries may have biased our results but at the same time represent the most at-risk population. The lack of support for increasing MAP goals in our results is despite the fact that not only does our center practice induced hypertension, but it also has all of the collaborative resources of a regional SCI center. This includes the multidisciplinary coordination of care between trauma, orthopedics, neurosurgery, PM&R, amongst others.
An added benefit of regional SCI centers is the close association with rehabilitation facilities. Substantial recovery can take place in the weeks to months following injury. AMS improvements can, therefore, be noted during the acute hospitalization, but also at rehab and beyond. Our dataset was limited to the acute hospitalization, but future studies of more delayed outcomes may yield different findings.
Another consideration for our negative findings is the diversity of our patient population. Perhaps some patients normally require a hypertensive state due to chronic vascular disease and after injury are relatively hypotensive as compared to their baseline. This is well described in hypertensive end-stage renal patients who become transiently relatively hypotensive during dialysis and develop cardiac ischemia.[24] In our study, we did not look at preexisting hypertension but this may be a more select population to study for the benefits of increased MAP goals after SCI.
Another limitation of the current study is the fact that we only looked at the lowest MAP during a 1 h period, but we do not have a record of how long that hypotensive episode lasted. In theory, this limitation should affect both groups equally but this cannot be measured within the limits of our retrospective dataset. Finally, a single institution and retrospective nature of this study are also noted limitations.
CONCLUSION
The frequency of episodes of relative hypotension and the need for vasopressors are progressively related to more severe SCI, as denoted by lower admission AMS. However, episodes of hypotension and the need for vasopressors did not affect the change in AMS during the acute hospitalization, regardless of theoretic MAP goal set-point. Arbitrarily elevated MAP goals may not be efficacious but further prospective study with longer-term follow-up is needed.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Spinal Cord Injury Facts and Figures at a Glance. [Last accessed on 2014 Feb 21]. Available from: https://www.nscisc.uab.edu/PublicDocuments/fact_figures_docs/Facts%202012%20Feb%20Final.pdf .
- 2.Lee BB, Cripps RA, Fitzharris M, Wing PC. The global map for traumatic spinal cord injury epidemiology: Update 2011, global incidence rate. Spinal Cord. 2014;52:110–6. doi: 10.1038/sc.2012.158. [DOI] [PubMed] [Google Scholar]
- 3.Devivo MJ. Epidemiology of traumatic spinal cord injury: Trends and future implications. Spinal Cord. 2012;50:365–72. doi: 10.1038/sc.2011.178. [DOI] [PubMed] [Google Scholar]
- 4.Amar AP, Levy ML. Pathogenesis and pharmacological strategies for mitigating secondary damage in acute spinal cord injury. Neurosurgery. 1999;44:1027–39. doi: 10.1097/00006123-199905000-00052. [DOI] [PubMed] [Google Scholar]
- 5.King BS, Gupta R, Narayan RK. The early assessment and intensive care unit management of patients with severe traumatic brain and spinal cord injuries. Surg Clin North Am. 2000;80:855–70. doi: 10.1016/s0039-6109(05)70100-6. viii. [DOI] [PubMed] [Google Scholar]
- 6.Tator CH. Hemodynamic issues and vascular factors in acute experimental spinal cord injury. J Neurotrauma. 1992;9:139–40. doi: 10.1089/neu.1992.9.139. [DOI] [PubMed] [Google Scholar]
- 7.Kirshblum SC, Burns SP, Biering-Sorensen F, Donovan W, Graves DE, Jha A, et al. International standards for neurological classification of spinal cord injury (revised 2011) J Spinal Cord Med. 2011;34:535–46. doi: 10.1179/204577211X13207446293695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ploumis A, Yadlapalli N, Fehlings MG, Kwon BK, Vaccaro AR. A systematic review of the evidence supporting a role for vasopressor support in acute SCI. Spinal Cord. 2010;48:356–62. doi: 10.1038/sc.2009.150. [DOI] [PubMed] [Google Scholar]
- 9.AANS Guidelines for the Management of ASCI and SCI. [Last accessed on 2014 Feb 21]. Available from: http://www.aans.org/Education%20and%20Meetings/~/media/Files/Education%20and%20Meetingf/Clinical%20Guidelines/TraumaGuidelines.ashx .
- 10.Tator CH, Rowed DW, Schwartz ML, Gertzbein SD, Bharatwal N, Barkin M, et al. Management of acute spinal cord injuries. Can J Surg. 1984;27:289–93, 6. [PubMed] [Google Scholar]
- 11.Zäch GA, Seiler W, Dollfus P. Treatment results of spinal cord injuries in the Swiss Parplegic Centre of Basle. Paraplegia. 1976;14:58–65. doi: 10.1038/sc.1976.9. [DOI] [PubMed] [Google Scholar]
- 12.Lehmann KG, Lane JG, Piepmeier JM, Batsford WP. Cardiovascular abnormalities accompanying acute spinal cord injury in humans: Incidence, time course and severity. J Am Coll Cardiol. 1987;10:46–52. doi: 10.1016/s0735-1097(87)80158-4. [DOI] [PubMed] [Google Scholar]
- 13.Levi L, Wolf A, Belzberg H. Hemodynamic parameters in patients with acute cervical cord trauma: Description, intervention, and prediction of outcome. Neurosurgery. 1993;33:1007–16. [PubMed] [Google Scholar]
- 14.Vale FL, Burns J, Jackson AB, Hadley MN. Combined medical and surgical treatment after acute spinal cord injury: Results of a prospective pilot study to assess the merits of aggressive medical resuscitation and blood pressure management. J Neurosurg. 1997;87:239–46. doi: 10.3171/jns.1997.87.2.0239. [DOI] [PubMed] [Google Scholar]
- 15.Wolf A, Levi L, Mirvis S, Ragheb J, Huhn S, Rigamonti D, et al. Operative management of bilateral facet dislocation. J Neurosurg. 1991;75:883–90. doi: 10.3171/jns.1991.75.6.0883. [DOI] [PubMed] [Google Scholar]
- 16.Levi L, Wolf A, Rigamonti D, Ragheb J, Mirvis S, Robinson WL. Anterior decompression in cervical spine trauma: Does the timing of surgery affect the outcome? Neurosurgery. 1991;29:216–22. [PubMed] [Google Scholar]
- 17.Bilello JF, Davis JW, Cunningham MA, Groom TF, Lemaster D, Sue LP. Cervical spinal cord injury and the need for cardiovascular intervention. Arch Surg. 2003;138:1127–9. doi: 10.1001/archsurg.138.10.1127. [DOI] [PubMed] [Google Scholar]
- 18.Mathias CJ, Frankel HL, Christensen NJ, Spalding JM. Enhanced pressor response to noradrenaline in patients with cervical spinal cord transection. Brain. 1976;99:757–70. doi: 10.1093/brain/99.4.757. [DOI] [PubMed] [Google Scholar]
- 19.Kong CY, Hosseini AM, Belanger LM, Ronco JJ, Paquette SJ, Boyd MC, et al. A prospective evaluation of hemodynamic management in acute spinal cord injury patients. Spinal Cord. 2013;51:466–71. doi: 10.1038/sc.2013.32. [DOI] [PubMed] [Google Scholar]
- 20.Marino RJ, Graves DE. Metric properties of the ASIA motor score: Subscales improve correlation with functional activities. Arch Phys Med Rehabil. 2004;85:1804–10. doi: 10.1016/j.apmr.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 21.Fawcett JW, Curt A, Steeves JD, Coleman WP, Tuszynski MH, Lammertse D, et al. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: Spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials. Spinal Cord. 2007;45:190–205. doi: 10.1038/sj.sc.3102007. [DOI] [PubMed] [Google Scholar]
- 22.Holte K, Sharrock NE, Kehlet H. Pathophysiology and clinical implications of perioperative fluid excess. Br J Anaesth. 2002;89:622–32. doi: 10.1093/bja/aef220. [DOI] [PubMed] [Google Scholar]
- 23.Nessim C, Sidéris L, Turcotte S, Vafiadis P, Lapostole AC, Simard S, et al. The effect of fluid overload in the presence of an epidural on the strength of colonic anastomoses. J Surg Res. 2013;183:567–73. doi: 10.1016/j.jss.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 24.Ritz E, Koch M. Morbidity and mortality due to hypertension in patients with renal failure. Am J Kidney Dis. 1993;21:113–8. doi: 10.1016/0272-6386(93)70102-5. [DOI] [PubMed] [Google Scholar]


