Abstract
While the primary method for evaluating diabetic retinopathy involves direct and indirect ophthalmoscopy, various imaging modalities are of significant utility in the screening, evaluation, diagnosis, and treatment of different presentations and manifestations of this disease. This manuscript is a review of the important imaging modalities that are used in diabetic retinopathy, including color fundus photography, fluorescein angiography, B-scan ultrasonography, and optical coherence tomography. The article will provide an overview of these different imaging techniques and how they can be most effectively used in current practice.
Keywords: B-scan Ultrasonography, Color Fundus Photography, Diabetic Retinopathy, Fluorescein Angiography, Optical Coherence Tomography, Retinal Imaging
INTRODUCTION
While the primary method for evaluating diabetic retinopathy involves direct and indirect ophthalmoscopy, various imaging modalities are of significant utility in the screening, evaluation, diagnosis, and treatment of the different presentations of this disease. Many imaging techniques can be useful depending on the manifestation of diabetic retinopathy. Important imaging techniques to be familiar with include color fundus photography, fluorescein angiography (FA), B-scan ultrasonography, and optical coherence tomography (OCT). This manuscript is meant to provide an overview of these different imaging techniques and how they relate to the management of diabetic retinopathy.
FUNDUS PHOTOGRAPHY
Color fundus photography is a useful tool in the management of diabetic eye disease. Traditionally, fundus photography has been performed using film, but more recently, digital fundus photography has become widely adopted. Digital images enable easy and immediate review of images, straightforward image magnification, and the ability to easily enhance and manipulate images. Fundus photography is helpful for documentation of the retinopathy as well as counseling the patient and demonstrating to them what their disease looks like. Fundus photography is also useful for monitoring for improvement or progression of diabetic retinopathy over time. There are different types of fundus photography: Standard, wide field, and stereoscopic.
Standard fundus photography
Standard macular fundus photography captures 30° of the posterior pole of the eye, which includes the macula and the optic nerve1 [Figure 1]. Pros of this form of color photography include that it is easy to use and highly available, and can be utilized to assist in documentation. Certain morphologic features such as hard exudates are easy to identify on color photographs. However, standard macular fundus photography cannot confirm clinically significant macular edema (CSME), fine detail is often not apparent, and it can be difficult to obtain good imaging if there is any media opacity.2
Figure 1.
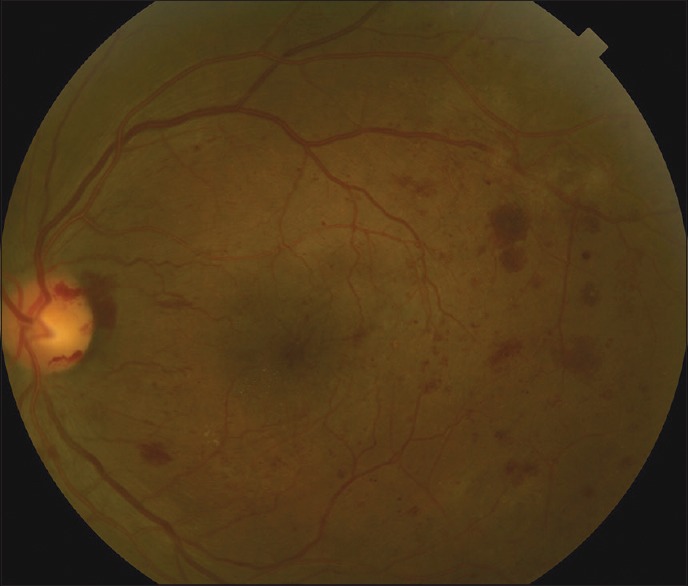
Standard color fundus photo, showing 30° of the posterior pole including the optic nerve and the macula of the left eye of a patient with proliferative diabetic retinopathy. There are diffuse areas of retinal hemorrhages as well as neovascularization along the temporal superior arcade and a small amount of vitreous hemorrhage over the nerve corresponding with neovascularization of the disc
Widefield fundus photography
Recently, widefield fundus photography has been developed, which can image the peripheral retina as well. A standard fundus camera can collect seven fundus fields, which can be combined to create a montage image that shows a 75° field of view [Figure 2]. Newer fundus cameras can capture up to a 200° field of view, even with an undilated pupil, allowing for viewing of over 80% of the total surface retinal area.3 The theoretical advantage of larger fields of view is that they allow for more thorough documentation and detection of peripheral retinal pathology in a minimally invasive fashion. However, widefield imaging does have some limitations, including distortion of images due to the spherical nature of the globe, eyelash artifacts, false color representation of fundus findings, and high equipment costs. Because of these limitations, traditional 30° fundus photography continues to be the standard method to photograph the fundus.
Figure 2.
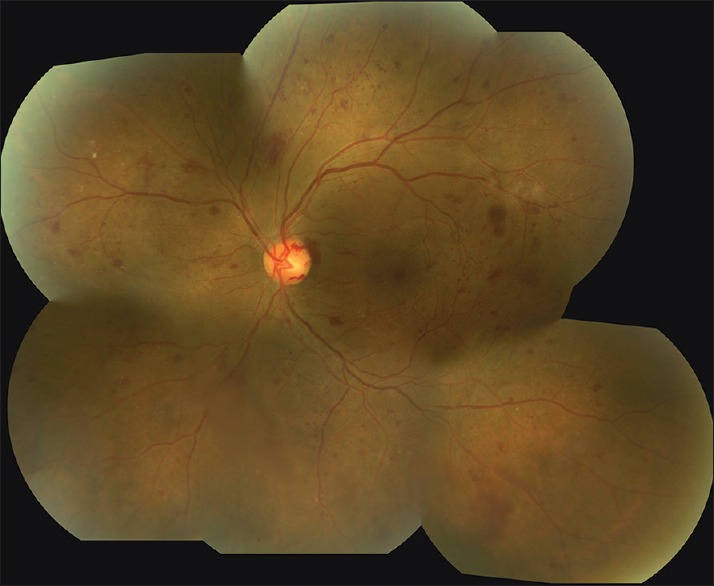
Montage image of the left eye of the same patient seen in Figure 1 using 7-field fundus photography. There is more visualization of the periphery as well as a better appreciation for the extent of the diabetic retinopathy
Stereoscopic fundus photography
With stereoscopic fundus photography, two images are created photographically, one for each eye, and when viewed appropriately, become fused in the brain to form a singular stereoscopic picture.4 This form of photography allows for examination of the patient's pathology in three-dimensions, similar to direct ophthalmoscopy. For distinguishing between IRMA and extraretinal neovascularization elsewhere (NVE), stereoscopic photography may be useful. In clinical practice, however, the utility of stereoscopic photos is debatable and can take more time for the physician to interpret. A comparative study of monoscopic and stereoscopic fundus photography found no difference in the ability of a trained ophthalmologist to evaluate the severity of diabetic retinopathy using Early Treatment of Diabetic Retinopathy Study (ETDRS) criteria.5
Telemedicine
Color fundus photography is also currently being utilized and researched for the purpose of screening of diabetic retinopathy outside of the ophthalmology clinic: This is termed telemedicine. Telemedicine has several potential advantages over needing an ophthalmologist to perform a dilated fundoscopic examination. One advantage is the possibility of increasing compliance with retinopathy screening, as these screenings can be done at a primary care physician's office or a more convenient location for the patient. Other potential advantages include increased efficiency, decreased costs to the patient and society and easier access to screening in areas with poor access to eye care professionals. In addition, some fundus cameras are now able to be used without dilation, allowing screening to be done in a maximally benign fashion.6
There are a number of disadvantages of using color fundus photography for screening, however. Color fundus photography is not a substitute for a comprehensive eye exam, as it cannot be used to look for other ocular diseases such as glaucoma or cataracts. Even widefield photography is not capable of imaging the entire retina. Macular edema and retinal neovascularization cannot always be visualized on fundus photographs, and image interpretation can sometimes be limited due to imaging artifacts or poor image quality. The American Diabetic Association (ADA) states that fundus photography may be used as a screening tool for retinopathy, but is not a substitute for an ophthalmic exam, and in 2014, still recommends an eye care professional evaluate all patients with diabetes.7
A meta-analysis done by the American Academy of Ophthalmology (AAO) showed that single-field fundus photography, when interpreted by trained readers to detect vision-threatening retinopathy, had a sensitivity ranging from 61% to 90% and specificity ranging from 85% to 97% when compared to 7-field fundus photos. The recommendations from the study were that there is the level I evidence that single-field fundus photography can serve as a screening tool for diabetic retinopathy to identify patients for referral to an ophthalmologist.8 As digital fundus photography continues to improve and the volume of patients with this disease increases, color fundus photography will likely serve as an important screening modality. Currently, however, dilated eye examination remains the standard of care.9
FLUORESCEIN ANGIOGRAPHY
Another important imaging technique in the evaluation of nonproliferative and proliferative diabetic retinopathy is FA. The technology was first described in 1961 and was introduced into mainstream ophthalmology by Gass in 1967.10 FA is useful in evaluating diabetic eye disease as it is currently the gold standard for evaluating the retinal vasculature, the part of the retina most affected by diabetes.
Performing fluorescein angiography
To perform FA, sodium fluorescein in a sterile aqueous solution is injected intravenously. The majority of fluorescein in the bloodstream is protein-bound while 20% is unbound. Sodium fluorescein is a fluorescent mineral-based dye; when it is excited by blue light (~480 nm), it then emits, or fluoresces, yellow-green light (~525 nm). FA works using a fundus camera that is outfitted with excitation and barrier filters. White light from the flash is passed through a blue excitation filter and is then absorbed by unbound fluorescein molecules traveling through the arteries and veins in the eye. The molecules then emit light with a longer wavelength in the yellow-green spectrum. The barrier filter in the camera blocks other wavelengths, so the captured image shows only the light emitted by the fluorescein molecules.2,11
Interpreting fluorescein angiography in diabetics
In patients with diabetic retinopathy, imaging photos from FA can show microaneurysms, which manifest as punctate areas of hyperfluorescence. Patchy areas of hypofluorescence can signify ischemia from nonperfused retinal capillaries. An increase in the foveal avascular zone from macular ischemia can be seen using FA, which may help explain vision loss in some diabetic patients. FA can also show abnormal blood vessels in the eye such as intraretinal microvascular abnormalities (IRMA) or retinal neovascularization. Since fluorescein is partially unbound in the blood stream, it can leak out of incompetent blood vessels. The visualization of leakage of fluorescein dye over time is useful in showing the breakdown of the blood-retinal barrier. This is best exemplified in diabetic macular edema, which is visualized as fluorescein leakage over time in the macula. Retinal neovascularization also can cause fluorescein leakage, and FA is a useful test to confirm the diagnosis of neovascularization of the disc and elsewhere in proliferative diabetic retinopathy [Figure 3].
Figure 3.
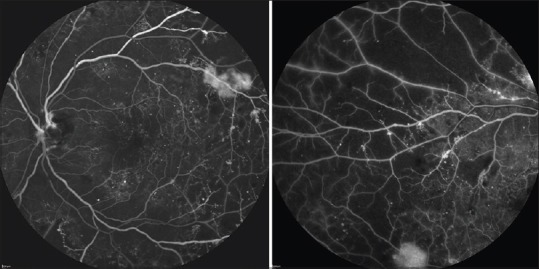
Fluorescein angiography of the same eye seen in Figures 1 and 2. The standard 30° photo on the left shows scattered microaneurysms throughout the macula. There is IRMA present along the superior arcade as well as leakage from an area of neovascularization. A peripheral sweep of the fluorescein angiography in the same patient shows patchy areas of hypofluorscence corresponding with extensive areas of peripheral retinal nonperfusion
Widefield fluorescein angiography
More recently, widefield FA has been developed which has allowed for improved imaging of the peripheral retina [Figure 4]. This technology can be helpful in detecting the peripheral neovascularization, as well as the extent of retinal nonperfusion. Widefield FA may reveal peripheral areas of capillary nonperfusion that are difficult to visualize with standard field FA.12,13 It has been hypothesized that these nonperfused regions may be a source of vascular endothelial growth factor (VEGF), which might contribute to formation of diabetic macular edema.14 VEGF release might theoretically be halted by “targeted” panretinal photocoagulation (PRP) to these areas of nonperfusion, which may then lead to improvement in macular edema.15,16 At this point, however, there has been no conclusive evidence that targeted PRP is clinically useful, and there are no large randomized studies to show whether widefield imaging can help to improve visual outcomes.
Figure 4.

Widefield fluorescein angiography in a patient with proliferative diabetic retinopathy. Note the numerous areas of leakage by the disc and along the arcades corresponding to neovascularization of the disc and elsewhere, respectively. There are extensive areas of nonperfusion in the periphery, and multiple laser scars from panretinal photocoagulation have been targeted to these nonperfused areas
LIMITATIONS OF FLUORESCEIN ANGIOGRAPHY
It is important to note that FA was not part of the ETDRS criteria for determining if a patient has CSME. Rather, CSME is an examination finding diagnosed by fundus biomicroscopy with one of three criteria: Hard exudates within 500 μ of the fovea with associated retinal thickening, retinal thickening within 500 μ of the fovea, or an area of retina thickening over 1500 μ in diameter that is less that 1500 μ from the fovea. Also unclear is the utility of FA for determining treatment parameters for macular edema or determining success of macular edema therapy.17 In addition, visualization of deep retinal and choroidal vessels is limited using FA.
Fluorescein angiography also has a number of potential side effects. The most common complications are transient nausea that occurs in about 2.9% of patients, as well as vomiting in 1.2% of patients.18 There are also various allergic reactions ranging from mild pruritus and urticaria to severe anaphylaxis and death, although the latter are extremely rare.19,20,21 There are no risks or adverse reactions that have been associated with pregnancy, but most physicians avoid performing this test in pregnant patients.22 It is of note that fluorescein dye is not iodine-based, and patients with an iodine allergy can still receive fluorescein dye. The percentage of reactions in patients who had had the previous FA without complication was 1.8%, compared with 48.6% in patients who had had a prior reaction to FA.18
ULTRASONOGRAPHY
Another imaging modality that can be useful in proliferative diabetic retinopathy is B-scan ultrasonography. B-scan ultrasonography creates an image of the eye by using sound waves transmitted at a high frequency from a transducer to the target tissue, which then return to the transducer at varying times and amplitudes. These signals are then interpreted and summed to construct a two-dimensional image of the eye.23 Higher amplitudes correspond to higher densities of tissue that reflect more signal back to the transducer and appear more white in color on the image. Lower amplitudes signify lower-density tissues that do not reflect as much ultrasound signal. B-scan ultrasonography is most useful in patients with vitreous hemorrhage or other media opacity, where the retina cannot be visualized directly on ophthalmic examination. B-scan ultrasonography can demonstrate if a retinal detachment is present and can show other retinal pathology such as a vitreous hemorrhage or posterior vitreous detachment.24 It is not particularly useful for imaging diabetic retinopathy if the media is clear.
OPTICAL COHERENCE TOMOGRAPHY
Optical coherence tomography is an imaging technique capable of evaluating retinal morphology with microscopic resolution.25 Since it became commercially available in 1996, a steady progression of software and hardware improvements to OCT instruments has occurred, and it has now become a mainstay in the management of a variety of retinal diseases, particularly in macular diseases. In diabetics, OCT is most useful to measure and quantify macular edema, and in the era of anti-VEGF therapy, it has become the most important imaging tool in managing patients with diabetic macular edema.
How optical coherence tomography works
Optical coherence tomography works by emitting light and then measuring the resultant time it takes for light to be reflected back from the target tissue; this is analogous to the way sound is used in ultrasonography. By scanning the OCT light source across the macula rapidly, multiple OCT A-scans may be obtained and combined to form a linear image analogous to a B-scan ultrasound image. Because light travels too fast to be detected directly, reflected light must be measured indirectly, using a technique called low coherence interferometry.26
In OCT, the axial resolution is determined by the bandwidth of the light source. Older generations of OCT instruments used light sources capable of 10um axial resolution in the human eye. Recently, wider bandwidth light sources have allowed axial resolutions up to 3 um.27 Transverse resolution is limited to 10–15 μm by natural aberrations in the human eye. In older OCT systems, a reference mirror was continuously moved to detect OCT signal at varying depths within tissue; this was termed time domain OCT. In newer OCT devices, the signal is detected using a stationary reference arm, allowing much faster imaging speeds; this is termed spectral domain OCT.28
Analysis software is critical to the function of OCT devices. Computer algorithms are used to calculate retinal thickness by automatically delineating the inner and outer retinal boundaries. When several OCT images are obtained through the macula, a retinal thickness map can be generated. Previous OCT systems acquired maps by combining data from six linear images. Newer spectral domain OCT systems create macular maps using a much larger amount of OCT data. Automated measurements of retinal thickness can then be compared to normative databases and followed over time, which can be particularly helpful when assessing responses to treatment [Figure 5].
Figure 5.
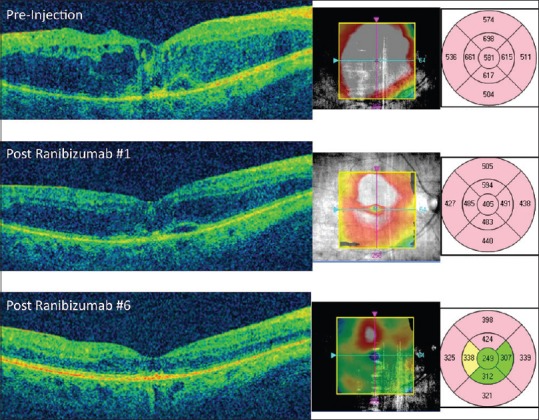
Optical coherence tomography (OCT) of the macula in a patient with diabetic macular edema. On the presentation (top), the patient has severe macular edema with retinal thickening and intraretinal fluid, shown in red on the OCT map to the right. Exact thickness measurements are also shown using a 9-field Early Treatment of Diabetic Retinopathy Study (ETDRS) map. After the first injection of ranibizumab (middle), the retinal edema improves on OCT and the change is quantified by looking at the OCT macular thickness maps, with the central field thickness decreasing from 581 μ to 405 μ on the ETDRS map. After 6 injections, the OCT shows resolution of fovea macular edema with the central field thickness returning to a normal level compared to age-matched controls (turning from red to green on the ETDRS map), showing an excellent overall response to treatment
Advantages of OCT include being noncontact and noninvasive, capable of capturing high-resolution images, quantifying retinal and intraretinal thicknesses, and taking a short amount of time to acquire images. OCT can often be done in a patient who is undilated, although the image quality is often higher in a dilated pupil. Disadvantages include cost of equipment, limitation by media opacity, need for operator skill and training, difficulty capturing images in patients who cannot fixate, and the introduction of imaging artifacts due to automated software errors.
Optical coherence tomography in diabetic macular edema
Currently, OCT is the most important test in evaluation and management of diabetic macular edema. OCT can determine whether diabetic macular edema is center-involving or noncenter-involving, which has become an important distinction in the era of anti-VEGF therapy.29 OCT can also demonstrate a number of microanatomical features in diabetic macular edema. Hard exudates are seen as small hyperreflective lesions typically found in the outer plexiform layer. OCT also can show intraretinal and subretinal fluid, seen as dark “spaces” in or under the retina, respectively [Figure 6]. OCT can demonstrate areas of subclinical macular edema, as well as help confirm the absence of macular thickening.30
Figure 6.
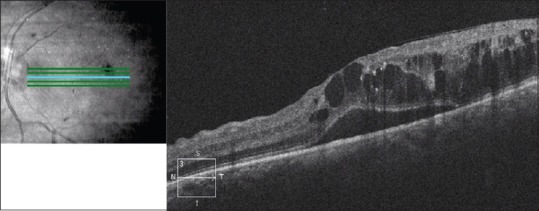
Optical coherence tomography of the macula of the left eye of a patient with diabetic macular edema. There is subretinal fluid as well as cystoid intraretinal fluid present, causing loss of the normal foveal contour. There are areas of increased hyperreflectivity in the outer plexiform layer corresponding to hard exudates
Optical coherence tomography can also demonstrate loss of different layers of the retina, such as the photoreceptors or nerve fiber layer, which can sometimes help to explain visual loss in patients without other macular abnormalities. OCT is also of utility in demonstrating abnormalities of the vitreoretinal interface, such as epiretinal membranes or vitreomacular traction, which may be more amenable to surgical therapy. OCT is also excellent to help gauge the anatomic outcome in patients of various therapies such as laser, intravitreal injections, systemic medications, and surgery.31
Although OCT is extremely helpful in diagnosis of diabetic macular edema, it is not as helpful in the diagnosis of macular ischemia. It is also not clear at what threshold macular edema as seen on OCT should be treated; some patients may have center-involving macular edema on OCT but good visual acuity, and it remains to be seen whether these patients would benefit from treatment. In addition, CSME remains a diagnosis based on slit lamp biomicroscopy, not OCT. It is also not clear whether FA is necessary to determine treatment area for focal laser photocoagulation or if an OCT map may be more useful to guide focal laser treatment.
SUMMARY
In summary, imaging modalities for the management of diabetic retinopathy remain clinically important. Fundus photography can be used to document retinal disease over time, and may be increasingly helpful in screening of diabetic patients for retinopathy. B-scan ultrasonography can be helpful in patients with media opacity, such as vitreous hemorrhage or cataract. FA is helpful to visualize retinal ischemia, as well as leakage from retinal neovascularization and also in macular edema. OCT has become a critical tool in the diagnosis and management of diabetic macular edema. As these technologies have continued to evolve, their importance in the diagnosis and management of diabetic retinopathy has become increasingly evident.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Patrick JS, Tyler ME. 2nd ed. New York: Butterworth-Heinemann Medical; 2001. Fundus photography overview. Ophthalmic Photography: Retinal Photography, Angiography, and Electronic Imaging. [Google Scholar]
- 2.Treatment techniques and clinical guidelines for photocoagulation of diabetic macular edema. Early Treatment Diabetic Retinopathy Study Report Number 2. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1987;94:761–74. doi: 10.1016/s0161-6420(87)33527-4. [DOI] [PubMed] [Google Scholar]
- 3.Witmer MT, Kiss S. The clinical utility of ultra-wide-field imaging. Rev Ophthalmol. 2012. [Last accessed on 2015 Jan 29]. Available from: http://www.reviewofophthalmology.com/content/d/retinal_insider/c/32799 .
- 4.Tyler ME. Stereo fundus photography: Principles and technique. J Ophthalmic Photogr. 1996;18:68–89. [Google Scholar]
- 5.Li HK, Hubbard LD, Danis RP, Esquivel A, Florez-Arango JF, Krupinski EA. Monoscopic versus stereoscopic retinal photography for grading diabetic retinopathy severity. Invest Ophthalmol Vis Sci. 2010;51:3184–92. doi: 10.1167/iovs.09-4886. [DOI] [PubMed] [Google Scholar]
- 6.Li Z, Wu C, Olayiwola JN, Hilaire DS, Huang JJ. Telemedicine-based digital retinal imaging vs standard ophthalmologic evaluation for the assessment of diabetic retinopathy. Conn Med. 2012;76:85–90. [PubMed] [Google Scholar]
- 7.Diabetic Retinopathy. San Francisco, CA: American Academy of Ophthalmology; 2008. [Last accessed on 2015 Jan 29]. American Academy of Ophthalmology Retinal Panel. Preferred Practice Pattern Guidelines. 4th printing 2012. Available from: http://www.aao.org/ppp . [Google Scholar]
- 8.Williams GA, Scott IU, Haller JA, Maguire AM, Marcus D, McDonald HR. Single-field fundus photography for diabetic retinopathy screening: A report by the American academy of ophthalmology. Ophthalmology. 2004;111:1055–62. doi: 10.1016/j.ophtha.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Kim HM, Lowery JC, Kurtz R. Accuracy of digital images for assessing diabetic retinopathy. J Diabetes Sci Technol. 2007;1:531–9. doi: 10.1177/193229680700100411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gass JD, Sever RJ, Sparks D, Goren J. A combined technique of fluorescein funduscopy and angiography of the eye. Arch Ophthalmol. 1967;78:455–61. doi: 10.1001/archopht.1967.00980030457009. [DOI] [PubMed] [Google Scholar]
- 11.Ciardella A, Brown D. Wide field imaging. In: Agarwal A, editor. Fundus Fluorescein and Indocyanine Green Angiography: A Textbook and Atlas. New York: Slack Inc; 2007. pp. 79–83. [Google Scholar]
- 12.Wessel MM, Nair N, Aaker GD, Ehrlich JR, D’Amico DJ, Kiss S. Peripheral retinal ischaemia, as evaluated by ultra-wide field fluorescein angiography, is associated with diabetic macular oedema. Br J Ophthalmol. 2012;96:694–8. doi: 10.1136/bjophthalmol-2011-300774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friberg TR, Gupta A, Yu J, Huang L, Suner I, Puliafito CA, et al. Ultra wide angle fluorescein angiographic imaging: A comparison to conventional digital acquisition systems. Ophthalmic Surg Lasers Imaging. 2008;39:304–11. doi: 10.3928/15428877-20080701-06. [DOI] [PubMed] [Google Scholar]
- 14.Oliver SC, Schwartz SD. Ultra-wide field fluorescein angiography. In: Fernando Arevalo J., editor. Retinal Angiography and Optical Coherence Tomography. New York: Springer Science and Business Media, LLC; 2009. pp. 407–17. [Google Scholar]
- 15.Reddy S, Hu A, Schwartz SD. Ultra wide field fluorescein angiography guided Targeted Retinal Photocoagulation (TRP) Semin Ophthalmol. 2009;24:9–14. doi: 10.1080/08820530802519899. [DOI] [PubMed] [Google Scholar]
- 16.Muqit MM, Marcellino GR, Henson DB, Young LB, Patton N, Charles SJ, et al. Optos-guided pattern scan laser (Pascal)-targeted retinal photocoagulation in proliferative diabetic retinopathy. Acta Ophthalmol. 2013;91:251–8. doi: 10.1111/j.1755-3768.2011.02307.x. [DOI] [PubMed] [Google Scholar]
- 17.Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Early Treatment Diabetic Retinopathy Study research group. Arch Ophthalmol. 1985;103:1796–806. [PubMed] [Google Scholar]
- 18.Kwiterovich KA, Maguire MG, Murphy RP, Schachat AP, Bressler NM, Bressler SB, et al. Frequency of adverse systemic reactions after fluorescein angiography. Results of a prospective study. Ophthalmology. 1991;98:1139–42. doi: 10.1016/s0161-6420(91)32165-1. [DOI] [PubMed] [Google Scholar]
- 19.The diagnosis and management of anaphylaxis. Joint Task Force on Practice Parameters, American Academy of Allergy, Asthma and Immunology, American College of Allergy, Asthma and Immunology, and the Joint Council of Allergy, Asthma and Immunology. J Allergy Clin Immunol. 1998;101:S465–528. [PubMed] [Google Scholar]
- 20.Fineschi V, Monasterolo G, Rosi R, Turillazzi E. Fatal anaphylactic shock during a fluorescein angiography. Forensic Sci Int. 1999;100:137–42. doi: 10.1016/s0379-0738(98)00205-9. [DOI] [PubMed] [Google Scholar]
- 21.Hitosugi M, Omura K, Yokoyama T, Kawato H, Motozawa Y, Nagai T, et al. An autopsy case of fatal anaphylactic shock following fluorescein angiography: A case report. Med Sci Law. 2004;44:264–5. doi: 10.1258/rsmmsl.44.3.264. [DOI] [PubMed] [Google Scholar]
- 22.Berkow JW, Flower RW, Orth DH, Kelley JS. 2nd ed. San Francisco: American Academy of Ophthalmology; 1997. Fluorescein and Indocyanine Green Angiography. [Google Scholar]
- 23.Coleman DJ, Silverman RH, Lizzi FL, Rondeau MJ. 2nd ed. Philadelphia, PA: Lippincott Williams and Wilkins; 2006. Ultrasonography of the Eye and Orbit. [Google Scholar]
- 24.McLeod D, Restori M. Ultrasonic examination in severe diabetic eye disease. Br J Ophthalmol. 1979;63:533–8. doi: 10.1136/bjo.63.8.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, et al. Optical coherence tomography. Science. 1991;254:1178–81. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gabriele ML, Wollstein G, Ishikawa H, Kagemann L, Xu J, Folio LS, et al. Optical coherence tomography: History, current status, and laboratory work. Invest Ophthalmol Vis Sci. 2011;52:2425–36. doi: 10.1167/iovs.10-6312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Drexler W, Morgner U, Ghanta RK, Kärtner FX, Schuman JS, Fujimoto JG. Ultrahigh-resolution ophthalmic optical coherence tomography. Nat Med. 2001;7:502–7. doi: 10.1038/86589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wojtkowski M, Bajraszewski T, Gorczynska I, Targowski P, Kowalczyk A, Wasilewski W, et al. Ophthalmic imaging by spectral optical coherence tomography. Am J Ophthalmol. 2004;138:412–9. doi: 10.1016/j.ajo.2004.04.049. [DOI] [PubMed] [Google Scholar]
- 29.Cunha-Vaz J, Coscas G. Diagnosis of macular edema. Ophthalmologica. 2010;224(Suppl 1):2–7. doi: 10.1159/000315156. [DOI] [PubMed] [Google Scholar]
- 30.Sikorski BL, Malukiewicz G, Stafiej J, Lesiewska-Junk H, Raczynska D. The diagnostic function of OCT in diabetic maculopathy. Mediators Inflamm 2013. 2013 doi: 10.1155/2013/434560. 434560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Diabetic Retinopathy Clinical Research Network Writing Committee. Haller JA, Qin H, Apte RS, Beck RR, Bressler NM, et al. Vitrectomy outcomes in eyes with diabetic macular edema and vitreomacular traction. Ophthalmology. 2010;117:1087–93.e3. doi: 10.1016/j.ophtha.2009.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]


