Abstract
Laser photocoagulation has been the mainstay of diabetic retinopathy treatment since its development in mid-20th century. With the advent of antivascular endothelial growth factor therapy, the role of laser therapy appeared to be diminished, however many advances in laser technology have been developed since. This review will describe recent advances in laser treatment of diabetic retinopathy including pattern scan laser, short-pulse duration and a reduced fluence laser, and navigated laser system for proliferative diabetic retinopathy and macular edema.
Keywords: Diabetic Retinopathy, Laser Therapy, Navigated Laser, Pattern Scanning Laser, Subthreshold Diode Micropulse Laser
INTRODUCTION
Diabetic retinopathy is one of the leading causes of blindness in the world; attributing to 1% of the global blindness.1,2 About one-third of patients who have had diabetes for more than 20 years will develop diabetic macula edema (DME).3 Moreover, a significant number of patients who are affected by the diabetic retinopathy are still in working age, which increases the burden of illness on patients and on society.4 Proliferative diabetic retinopathy (PDR) may result in vitreous hemorrhage and tractional retinal detachment, which may cause further visual impairment. Several treatments have been developed for diabetic retinopathy including intraocular steroids, antivascular endothelial growth factor (VEGF) therapy and laser photocoagulation therapy. This paper will briefly review the history and the principle of laser therapy, and outline recent developments in laser treatment for diabetic retinopathy.
HISTORY
The word laser is from an acronym LASER (Light Amplification by Stimulated Emission of Radiation). In 1917, Einstein developed the concepts of laser,5 but Townes and Schawlow constructed a practical device using optical light.6 Meryer-Schwickerath in the late 1940's described retinal photocoagulation using solar coagulation and with the xenon arc photocoagulator in the later years, but the photocoagulator was still too difficult to be used in clinical practice.7 In 1960, Theodor Maiman created the first working laser with a ruby crystal medium: Opening the door for laser treatments in ophthalmic conditions.8 The argon blue-green laser was introduced by L’Esperance in 1968, and krypton laser in 1972.9,10 Subsequently yellow, green and diode lasers were developed and in use since then.11,12
PRINCIPLES OF LASER
Basic elements of lasers include an active medium, a cavity, and an excitation source. The active medium in the laser cavity is stimulated by the excitation source to create a laser beam that has a discrete wavelength (monochromaticity), can be focused to a very small size (spatial coherence), and lack divergence of the light waves (collimation).13 Of the three main laser-tissue interactions: Photochemical interactions, photothermal interactions, photomechanical interactions, laser therapy in diabetic retinopathy involves photothermal interaction in which melanin in the retinal pigment epithelium (RPE) and choroid absorbs the laser energy to produce photocoagulation of the varying retinal layers.14 The extent of the tissue damage in relation with the pulse duration has been assessed via Arrhenius model, in which longer duration is predicted to cause more spread of damage on retinal layers.15
LASER TREATMENT IN DIABETIC RETINOPATHY
The first laser to be used to treat early diabetic neovascular retinopathy was ruby laser photocoagulation by Beetham et al. in 1969.16 More conventional argon laser photocoagulation of diabetic retinopathy was then described by Zweng et al. in 1971.17 Subsequently, two pivotal large, prospective, multicenter, randomized studies – Diabetes Retinopathy Study (DRS) and Early Treatment Diabetes Retinopathy Study (ETDRS) – developed guidelines for laser treatments of diabetic retinopathy.18,19,20 In DRS, panretinal photocoagulation (PRP) was shown to reduce risk of severe visual loss by 60% in 2 years especially in patients with PDR and the following high-risk characteristics: Any neovascularization of disc (NVD) associated with vitreous hemorrhage, moderate to severe NVD (1/4 to 1/3 of the disc, standard photograph 10A), and neovascularization of the retina elsewhere (NVE, 1/2 disc area) with vitreous hemorrhage.18 Later, ETDRS suggested that patients with severe non-PDR might also benefit from scatter photocoagulation as well.19 Although PRP is often used to successfully reduce the risk of severe vision loss from PDR, significant visual side effects are associated with the treatment, such as reduced vision, diminished field of vision, and reduced color and contrast sensitivity.21
The exact mechanism by which PRP aids in regression of neovascularization is not yet clear. It appears to be combined effects of multiple elements including: Facilitation of transports of oxygen and nutrients into the retina from choroid, transport of metabolic waste out of the retina, reduction of retinal metabolic load, and reduced sequestration of proangiogenic cytokines in the photoreceptors, resulting in net reduction of VEGF expression.14,22 Other cytokines such as heat shock protein and transforming growth factor-β2 have been implicated in inflammatory response in retinal photocoagulation as well.23,24
The utility of focal laser therapy was studied in ETDRS, and the laser application in patients with clinically significant macula edema (CSME) was shown to reduce the incidence of moderate vision loss by 50% at the 3 years mark.20 Moreover, focal-direct and grid treatments was shown to be more effective and has fewer side effects than 1 or 4 mg doses of intravitreal triamcinolone in Diabetic Retinopathy Clinical Research Network (DRCR.net) in 2 and 3 years follow-up.25,26 In 2009, Scott et al. reviewed the patients with noncenter-involved (NCI) CSME from ETDRS study, and found that focal/grid laser therapy may be useful for NCI-CSME and associated with relatively stable visual acuity, retinal thickness measurements, and decreased fluorescein leakage at 1-year.27
The exact mechanism for the focal laser treatment is also not clear but presumed to involve stimulation of RPE, closure of leaking microaneurysm, and induction of endothelial cell proliferation.28 Some suggested increased oxygenation lead to constriction of arteriole, resulting in decreased edema.29 Alteration in the biochemical environment in RPE through changes in various cytokines and growth factors also appears to play a role in reducing macular edema.30,31,32,33 This may support the grid treatment alone can effectively reduce macular edema indirectly.34,35,36,37,38
TECHNIQUES FOR LASER TREATMENTS OF DIABETIC RETINOPATHY
In ETDRS, the focal-direct laser treatment of leaking microaneurysm used spot size of 50–100 um in duration of 50–100 ms of sufficient power to induce whitening of the microaneurysm located between 500 and 3000 um from the center of macula. As for focal-grid laser treatment of diffuse edema, spot size of 50–200 um was used in duration of 50–100 ms of sufficient power to induce gray-white light burn 500 um away from the center of macula and the disc.20 Since then, DRCR.net compared modified ETDRS style laser photocoagulation to mild macular grid (MMG) laser photocoagulation for DME (modified Early Treatment Diabetic Retinopathy Study [mETDRS] vs. MMG trial).39 In mETDRS technique, both direct and grid focal laser treatment is applied. All leaking microaneurysms in areas of retinal thickening 500–3000 um from the center of the macula, but not within 500 um of the disc are treated with burn size of 50 um and duration of 50–100 ms. Although the change in microaneurysm color is not required, at least a mild gray-white burn should be seen below the microaneurysms. Subsequent grid laser burns are applied in areas of retinal thickening 500–3,000 um from the center of macula (except temporally 3500 um from the center of macula) with the laser setting of 50 um spot size and duration of 50–100 ms at 2 burn widths apart with barely visible (light gray) intensity. In MMG technique, 200–300 total burns of 50 um size and 50–100 ms duration with barely visible (light gray) intensity are applied to entire area (including unthickened retina) of macula (500–3000 um from center of macula superiorly, nasally and inferiorly and 500–3500 um temporally from the center of macula, and no burns within 500 um of the disc). mETDRS uses lighter burn than what was specified in the ETDRS to reduce possible adverse effects of central scotomas and decreased color vision. MMG has a theoretical advantage over mETDRS in that widespread application may lead to improved oxygenation, development of healthier RPE, and normalize the diabetic cellular changes in the retina.39 At 12 months, however DRCR.net found mETDRS more effective in reducing optical coherence tomography (OCT)-measured retinal thickening when compared to MMG although there was no significant difference in visual acuity outcome. mETDRS technique continues to be a gold standard of laser therapy in treating DME.
A standard full scatter PRP involves spot size of 500 um, 100 ms duration with power sufficient to cause moderate burns in numbers of 1200–1600 that are half burn width apart, more than 2 disc diameter from the center of macula to equator and 500 um from the disc.19 Laser specification for the pattern scan laser is discussed in the section below.
RECENT DEVELOPMENTS IN LASER TREATMENTS IN DIABETIC RETINOPATHY
Pattern scanning laser
Blumenkranz et al. described semiautomated patterned scanning laser in their seminal paper in 2006.40 Pattern scanning laser (PASCAL) allows rapid application of multiple laser spots in an array with shorter pulse duration of 10–30 ms. They reported multiple advantages of PASCAL over conventional laser therapy that includes: Shorter treatment duration, increased safety, uniform and precise spot placement, accurate “subthreshold” grid-pattern placement, and reduced pain and visual field defect.40
In PASCAL, shorter pulse duration was chosen as a new standard in an array of multiple burns in order to provide speed, better spatial localization (avoid ocular movements from affecting the laser placement), and reduced collateral damage by providing more precise control of the depth of the impact.15,41 In result, PASCAL is an ideal laser method to place accurate “subthreshold” (subvisible) focal-grid laser in DME in contrast to conventional laser therapy.42,43 Another benefit of short-pulse duration and semiautomated laser spot placement is decrease in treatment time. Blumenkranz et al. reported that, with 6 × 6 or 7 × 7 array, the total treatment time may be reduced by several folds, in which a single treatment session may be reduced from approximately 25 min to mere 3–5 min using PASCAL.40
In addition, short-pulse duration decreases the width and the axial extent of the retinal burns to RPE and outer retinal layer.41 This treatment avoids collateral damage to the choroid, which may be associated with pain. Several papers studied pain response in patients treated with PASCAL. Nagpal et al. studied PASCAL versus solid-state green laser and found decreased treatment time and pain in treatment with pattern scan laser.44 Two groups looked at pain responses of 20 ms and 100 ms pulse duration, and found 20 ms pulse duration was associated with significantly lower levels of anxiety, headache, pain, and photophobia.45,46
The efficacy of PASCAL laser, however, appears to be diminished compared to conventional laser therapy when the same number of laser spots were delivered.47 Chappelow et al. studied the efficacy of PASCAL in treating high-risk PDR and found that PASCAL is less effective in controlling neovascularization within 6 months of the initial treatment when equal number of spots were applied compared to the traditional argon laser. Using total treatment area, Palanker et al. calculated that one needs to place 1932 burns with PASCAL to achieve the same total area of 1000 standard burns.48
Common PASCAL specification for PRP in PDR includes burn size of 200 um with duration of 20 ms at 2 disc diameter away from the center of macula and 500 um from disc. Typical array can be anywhere from 3 × 3 to 7 × 7, however with increased array size, it is often more difficult to achieve precise laser burn location secondary to eye movements and defocusing of the laser burns in periphery.
Short-pulse duration and a reduced fluence laser
The use of diode laser (810 nm) for macular disease in patients was first described by Friberg and Karatza in 1997.49 The use of diode laser (810 nm) for modified grid photocoagulation were soon compared with traditional argon laser (514 nm), and was shown to be equally effective for diffuse DME.50
Recently, a new technology was developed by modifying diode laser in an effort to reduce inadvertent loss of the visual field from collateral damage of laser therapy. Subthreshold diode micropulse laser photocoagulation (SDM) utilizes diode laser with micropulse technique and lower fluence to achieve subthreshold burns in order to limit the laser burns to RPE.49 Whereas conventional laser therapy utilizes continuous wave of energy delivery, micropulse mode divides a single energy delivery of laser burn with cycles of 100 μs on time and 50 μs off down time until the full duration of laser spot (100–300 ms) is delivered. Histopathological studies have indicated that micropulse 810 nm diode laser affects only RPE without damaging outer retina.51 Subsequently, several papers were published on the SDM in DME, and found it to be equally effective as conventional argon or threshold diode laser.52,53,54 Also there has been a report of using SDM to deliver effective PRP while minimizing retinal damage as well as one describing safety of SDM in transfoveal therapy of CSME.55,56,57
Figueira et al. conducted a prospective randomized controlled trial comparing SDM and conventional green laser for CSME in 2009.58 They reported similar efficacy of SDM in comparison to conventional therapy while minimizing laser scarring in fundus. In 2011, Lavinsky et al. carried out a randomized clinical trial comparing mETDRS versus normal or high-density SDM laser therapy and found that high-density SDM performed superiorly than mETDRS photocoagulation technique, in terms of anatomic and functional measurement.59
End point management in subvisible retinal laser therapy
The advantages of treatment of DME with subthreshold (subvisible) approach include the absence of scotomata, scarring, and improved preservation of color and contrast sensitivity.60 However, due to inability to visualize the laser mark of subvisible therapy, it is often difficult to achieve uniform laser treatment regimen across different studies. In order to achieve more reproducible subvisible laser therapy, Lavinsky et al. created a titration algorithm, Endpoint Mangement (EpM).61 EpM uses PASCAL streamline 577 laser to create a barely visible lesion via titration of power at 20 ms pulse duration, which is defined as a 100% nominal energy level. The energy can be subsequently titrated down for subthreshold therapy. They reported that 120–170% burn energy level corresponded to moderate and light burns at RPE, photoreceptors and inner retina at the highest settings. 50–75% burns corresponded to subvisible laser burn at RPE and photoreceptors detectable with fluorescein angiography and OCT. 30–50% energy level burn correspond to RPE damage that were invisible in vivo multimodal imaging, but were best visualized by scanning electron microscopy.
Navigated laser
Navigated laser (NAVILAS) is a fundus imaging and laser treatment device developed by Neubauer et al.62,63 (OD-OS GmbH, Teltow, Germany) The device utilizes retina navigation via computerized image capture and tracking assistance with high precision and reproducibility of <60 – 110 um.62 Combined with simultaneous fluorescein angiography, the device allows the surgeon to preplan the areas with automatic aim preposition. They report more uniform laser burns with less pain and shorter treatment duration in comparison to the conventional pattern laser. Moreover, it appears that the rate of retreatment for DME is reduced with NAVILAS when compared to the conventional mETDRS focal laser technique.63 It is possible to achieve up to 92% hit rate of microaneurysm via NAVILAS as opposed to 72% in conventional laser focal coagulation.64 Given the above technical advantage, it appears focal laser therapy using NAVILAS will have more impact in future to improve visual acuity and reduce the burden of anti-VEGF injection numbers in patients.65,66 Combined therapy of anti-VEGF therapy and focal/grid NAVILAS therapy is discussed more in below section.
Expanding on the navigated focal laser treatment, Chhablani et al. studied laser spot quality between the conventional slit lamp PASCAL and the NAVILAS for PRP in high-risk PDR patients in 2014.67 They report more uniform laser burns with less pain and shorter treatment duration with NAVILAS when compared to conventional pattern laser.
Role of laser therapy in diabetic retinopathy
With the advent of anti-VEGF therapy, large prospective randomized trials were devised in order to study the efficacy of a new therapy over the conventional laser therapy. In 2010, a prospective randomized trial of intravitreal bevacizumab or laser therapy (BOLT) in the DME management (BOLT study) showed that, with bevacizumab therapy, patients are 5.1 times more likely to gain 10 or greater ETDRS letters over 12 months.68 One of the concern with anti-VEGF therapy in diabetic patients is worsening macular ischemia from antiangiogenesis property of anti-VEGF, however in BOLT study, there was no evidence of worsening macular ischemia on fundus fluorescein angiography at 4-month period in both groups.69 The authors concluded that there are evidences to support the bevacizumab therapy in center-involving CSME without advanced macular ischemia. The RESTORE study in 2011 studied ranibizumab monotherapy and ranibizumab combined with laser therapy over conventional laser therapy in patients with decreased vision from DME.70 The study found equal superiority of ranibizumab monotherapy and ranibizumab combined with laser therapy over standard laser therapy.
In addition to anti-VEGF therapy, the role of intravitreal steroids has been studied in two large trials among others. Soheilian et al. studied the role of bevacizumab alone or combined with triamcinolone versus macular photocoagulation in 150 eyes with DME.71 They found significant superiority of bevacizumab over macular photocoagulation in terms of visual acuity and central macular thickness at 6 months, however the magnitude of the superiority diminished at long term follow-up in 2 years with continued retreatments at 3 months intervals.72 Elman et al. (DRCR.net) studied the role of ranibizumab or triamcinolone combined with prompt or delayed focal/grid laser therapy in comparison to focal/grid laser monotherapy in 854 study eyes with DME in terms of visual acuity improvement and macular thickness reduction at 12 months.73 The authors found that ranibizumab with prompt or deferred laser therapy resulted in greater visual acuity improvement compared to laser monotherapy. Intravitreal steroid therapy achieved a similar macular thickness improvement as in ranibizumab therapy. In pseudophakic subgroup, steroid therapy also achieved comparable visual acuity improvement as in ranibizumab therapy. They observed three eyes (0.8%) with injection-related endophthalmitis and frequent intraocular pressure elevation and development of cataract in intravitreal steroid group. At 2 years follow-up, the authors reported similar result as the original report.74 The median numbers of injections between the 1- and 2 years visits were 2 and 3, while the median numbers of visits were 7 and 10.
Although there have been clear advantages of anti-VEGF therapy in managing DME, there still may be a role for combination therapy of anti-VEGF and laser photocoagulation. Ranibizumab for edema of the mAcula in diabetes-2 study was one of the earliest studies that studied ranibizumab monotherapy, laser monotherapy, and ranibizumab plus laser therapy.75,76 At 2 years follow-up, the authors found decreased number of injections (4.9 injections in combination arm and 9.3 in the ranibizumab monotherapy) and reduced residual edema in the combination arm. However, the above benefit of laser therapy has not been seen in other large randomized control trials.70,73,74 Elman et al. (DRCR.net) found similar median number of injections at 12 months in ranibizumab plus prompt or delayed laser therapy (8 and 9 respectively).73,74 Similarly, RESTORE study found no efficacy difference between the ranibizumab and ranibizumab combined with laser treatment groups.68 After 3 monthly injections, the mean numbers of injections between two treatment arms were also similar (4.1 and 3.8 respectively).
One of the interesting result from RESTORE study is that subgroup analysis of patients who presented with central retinal thickness (CRT) <300 um (Stratus OCT, Carl Zeiss) had similar outcome with laser or anti-VEGF monotherapy.70 Two separate studies by Kernt et al. thus looked into the effect of combination therapy on patients who presented with DME treated with monthly ranibizumab injections with or without focal/grid NAVILAS when CRT was reduced to 440 um or less on Heidelberg Spectralis.65,66 (300 um on Stratus OCT is equivalent to 440 um on Heidelberg Spectralis.77) Both pilot studies found better visual acuity and reduced number of injections to half as much as the control group. The cost-effectiveness of laser therapy in treating DME, however, is not completely clear.78 It is possible to speculate a continued role of focal laser therapy in the treatment of DME as a successful adjunctive therapy as mentioned above.
CONCLUSION
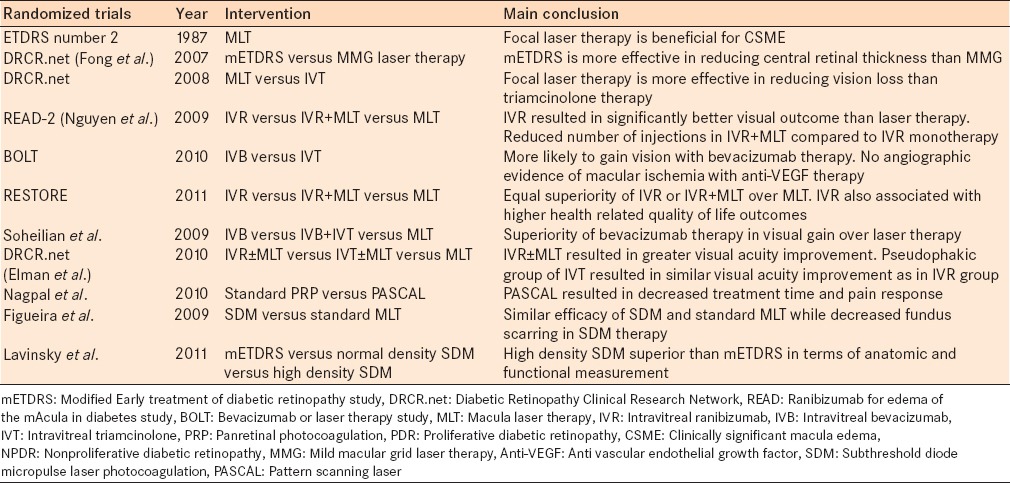
Since the development of laser therapy for diabetic retinopathy in 1969, laser retinal photocoagulation has played a fundamental role in the treatment of the disease. Through randomized control trials, the benefit of PRP and focal/grid macular laser therapy have been clearly demonstrated [Table 1]. Developments in new laser therapy technology continued with PASCAL, making treatment of diabetic retinopathy quicker and more comfortable for patients. New pilot studies have suggested the benefit of subthreshold diode laser and NAVILAS for DME. Randomized control trials of combined NAVILAS and anti-VEGF therapy are still yet to come. Laser therapy has a long term track record in the treatment of diabetic retinopathy.
Table 1.
Major randomized controlled trials of laser therapy in diabetic macula edema
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Geneva: World Health Organization; 2012. World Health Organization. Global Data on Visual Impairments 2010. [Google Scholar]
- 2.Scanlon PH, Aldington SJ, Stratton IM. Epidemiological issues in diabetic retinopathy. Middle East Afr J Ophthalmol. 2013;20:293–300. doi: 10.4103/0974-9233.120007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klein R, Knudtson MD, Lee KE, Gangnon R, Klein BE. The Wisconsin epidemiologic study of diabetic retinopathy XXIII: The twenty-five-year incidence of macular edema in persons with type 1 diabetes. Ophthalmology. 2009;116:497–503. doi: 10.1016/j.ophtha.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen E, Looman M, Laouri M, Gallagher M, Van Nuys K, Lakdawalla D, et al. Burden of illness of diabetic macular edema: Literature review. Curr Med Res Opin. 2010;26:1587–97. doi: 10.1185/03007995.2010.482503. [DOI] [PubMed] [Google Scholar]
- 5.Einstein A. Zur quantentheorie der strahlung. Physiol Zool. 1917;18:121–8. [Google Scholar]
- 6.Schawlow A, Townes C. Infrared and optical masers. Phys Rev. 1958;112:1940–9. [Google Scholar]
- 7.Meyer-Schwickerath G. Photo-coagulation of the ocular fundus and the retina. Ann Ocul (Paris) 1956;189:533–48. [PubMed] [Google Scholar]
- 8.Maiman T. Stimulated optical radiation in ruby. Nature. 1960;187:493–4. [Google Scholar]
- 9.L’Esperance FA., Jr An opthalmic argon laser photocoagulation system: Design, construction, and laboratory investigations. Trans Am Ophthalmol Soc. 1968;66:827–904. [PMC free article] [PubMed] [Google Scholar]
- 10.L’Esperance FA., Jr Clinical photocoagulation with the krypton laser. Arch Ophthalmol. 1972;87:693–700. doi: 10.1001/archopht.1972.01000020695016. [DOI] [PubMed] [Google Scholar]
- 11.Castillejos-Rios D, Devenyi R, Moffat K, Yu E. Dye yellow vs. argon green laser in panretinal photocoagulation for proliferative diabetic retinopathy: A comparison of minimum power requirements. Can J Ophthalmol. 1992;27:243–4. [PubMed] [Google Scholar]
- 12.Krauss JM, Puliafito CA. Lasers in ophthalmology. Lasers Surg Med. 1995;17:102–59. doi: 10.1002/lsm.1900170203. [DOI] [PubMed] [Google Scholar]
- 13.Amirikia A, Puliafito CA. Lasers for vitreoretinal disease. In: Regillo CD, Brown GC, Flynn HW Jr, editors. Vitreoretinal Disease: The Essentials. New York: Thieme Medical Publishers Inc; 1999. pp. 609–29. [Google Scholar]
- 14.Blumenkranz MS. The evolution of laser therapy in ophthalmology: A perspective on the interactions between photons, patients, physicians, and physicists: The LXX Edward Jackson Memorial Lecture. Am J Ophthalmol. 2014;158:12–25.e1. doi: 10.1016/j.ajo.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 15.Sramek C, Paulus Y, Nomoto H, Huie P, Brown J, Palanker D. Dynamics of retinal photocoagulation and rupture. J Biomed Opt. 2009;14:034007. doi: 10.1117/1.3130282. [DOI] [PubMed] [Google Scholar]
- 16.Beetham WP, Aiello LM, Balodimos MC, Koncz L. Ruby-laser photocoagulation of early diabetic neovascular retinopathy: Preliminary report of a long-term controlled study. Trans Am Ophthalmol Soc. 1969;67:39–67. [PMC free article] [PubMed] [Google Scholar]
- 17.Zweng HC, Little HL, Peabody RR. Argon laser photocoagulation of diabetic retinopathy. Arch Ophthalmol. 1971;86:395–400. doi: 10.1001/archopht.1971.01000010397006. [DOI] [PubMed] [Google Scholar]
- 18.Photocoagulation treatment of proliferative diabetic retinopathy: The second report of diabetic retinopathy study findings. Ophthalmology. 1978;85:82–106. doi: 10.1016/s0161-6420(78)35693-1. [DOI] [PubMed] [Google Scholar]
- 19.Early photocoagulation for diabetic retinopathy. ETDRS report number 9. Early Treatment Diabetic Retinopathy Study research group. Ophthalmology. 1991;98(Suppl 5):766–85. [PubMed] [Google Scholar]
- 20.Treatment techniques and clinical guidelines for photocoagulation of diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 2. Early Treatment Diabetic Retinopathy Study research group. Ophthalmology. 1987;94:761–74. doi: 10.1016/s0161-6420(87)33527-4. [DOI] [PubMed] [Google Scholar]
- 21.Fong DS, Girach A, Boney A. Visual side effects of successful scatter laser photocoagulation surgery for proliferative diabetic retinopathy: A literature review. Retina. 2007;27:816–24. doi: 10.1097/IAE.0b013e318042d32c. [DOI] [PubMed] [Google Scholar]
- 22.Foulds WS, Kaur C, Luu CD, Kek WK. A role for photoreceptors in retinal oedema and angiogenesis: An additional explanation for laser treatment? Eye (Lond) 2010;24:918–26. doi: 10.1038/eye.2009.173. [DOI] [PubMed] [Google Scholar]
- 23.Matsumoto M, Yoshimura N, Honda Y. Increased production of transforming growth factor-beta 2 from cultured human retinal pigment epithelial cells by photocoagulation. Invest Ophthalmol Vis Sci. 1994;35:4245–52. [PubMed] [Google Scholar]
- 24.Sramek C, Mackanos M, Spitler R, Leung LS, Nomoto H, Contag CH, et al. Non-damaging retinal phototherapy: Dynamic range of heat shock protein expression. Invest Ophthalmol Vis Sci. 2011;52:1780–7. doi: 10.1167/iovs.10-5917. [DOI] [PubMed] [Google Scholar]
- 25.Diabetic Retinopathy Clinical Research Network. A randomized trial comparing intravitreal triamcinolone acetonide and focal/grid photocoagulation for diabetic macular edema. Ophthalmology. 2008;115:1447–9.e1. doi: 10.1016/j.ophtha.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diabetic Retinopathy Clinical Research Network (DRCR. net) Beck RW, Edwards AR, Aiello LP, Bressler NM, Ferris F, et al. Three-year follow-up of a randomized trial comparing focal/grid photocoagulation and intravitreal triamcinolone for diabetic macular edema. Arch Ophthalmol. 2009;127:245–51. doi: 10.1001/archophthalmol.2008.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scott IU, Danis RP, Bressler SB, Bressler NM, Browning DJ, Qin H, et al. Effect of focal/grid photocoagulation on visual acuity and retinal thickening in eyes with non-center-involved diabetic macular edema. Retina. 2009;29:613–7. doi: 10.1097/IAE.0b013e3181a2c07a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim JW, Ai E. Diabetic retinopathy. In: Regillo CD, Brown GC, Flynn HW Jr, editors. Vitreoretinal Disease: The Essentials. New York: Thieme Medical Publishers Inc; 1999. pp. 133–59. [Google Scholar]
- 29.Arnarsson A, Stefánsson E. Laser treatment and the mechanism of edema reduction in branch retinal vein occlusion. Invest Ophthalmol Vis Sci. 2000;41:877–9. [PubMed] [Google Scholar]
- 30.Ogata N, Ando A, Uyama M, Matsumura M. Expression of cytokines and transcription factors in photocoagulated human retinal pigment epithelial cells. Graefes Arch Clin Exp Ophthalmol. 2001;239:87–95. doi: 10.1007/s004170000235. [DOI] [PubMed] [Google Scholar]
- 31.Ogata N, Tombran-Tink J, Jo N, Mrazek D, Matsumura M. Upregulation of pigment epithelium-derived factor after laser photocoagulation. Am J Ophthalmol. 2001;132:427–9. doi: 10.1016/s0002-9394(01)01021-2. [DOI] [PubMed] [Google Scholar]
- 32.Spranger J, Hammes HP, Preissner KT, Schatz H, Pfeiffer AF. Release of the angiogenesis inhibitor angiostatin in patients with proliferative diabetic retinopathy: Association with retinal photocoagulation. Diabetologia. 2000;43:1404–7. doi: 10.1007/s001250051546. [DOI] [PubMed] [Google Scholar]
- 33.Xiao M, McLeod D, Cranley J, Williams G, Boulton M. Growth factor staining patterns in the pig retina following retinal laser photocoagulation. Br J Ophthalmol. 1999;83:728–36. doi: 10.1136/bjo.83.6.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akduman L, Olk RJ. Subthreshold (invisible) modified grid diode laser photocoagulation in diffuse diabetic macular edema (DDME) Ophthalmic Surg Lasers. 1999;30:706–14. [PubMed] [Google Scholar]
- 35.Lee CM, Olk RJ. Modified grid laser photocoagulation for diffuse diabetic macular edema. Long-term visual results. Ophthalmology. 1991;98:1594–602. doi: 10.1016/s0161-6420(91)32082-7. [DOI] [PubMed] [Google Scholar]
- 36.Olk RJ. Modified grid argon (blue-green) laser photocoagulation for diffuse diabetic macular edema. Ophthalmology. 1986;93:938–50. doi: 10.1016/s0161-6420(86)33638-8. [DOI] [PubMed] [Google Scholar]
- 37.Olk RJ. Argon green (514 nm) versus krypton red (647 nm) modified grid laser photocoagulation for diffuse diabetic macular edema. Ophthalmology. 1990;97:1101–12. doi: 10.1016/s0161-6420(90)32449-1. [DOI] [PubMed] [Google Scholar]
- 38.Striph GG, Hart WM, Jr, Olk RJ. Modified grid laser photocoagulation for diabetic macular edema. The effect on the central visual field. Ophthalmology. 1988;95:1673–9. doi: 10.1016/s0161-6420(88)32957-x. [DOI] [PubMed] [Google Scholar]
- 39.Fong DS, Strauber SF, Aiello LP, Beck RW, Callanan DG, et al. Writing Committee for the Diabetic Retinopathy Clinical Research Network. Comparison of the modified Early Treatment Diabetic Retinopathy Study and mild macular grid laser photocoagulation strategies for diabetic macular edema. Arch Ophthalmol. 2007;125:469–80. doi: 10.1001/archopht.125.4.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blumenkranz MS, Yellachich D, Andersen DE, Wiltberger MW, Mordaunt D, Marcellino GR, et al. Semiautomated patterned scanning laser for retinal photocoagulation. Retina. 2006;26:370–6. doi: 10.1097/00006982-200603000-00024. [DOI] [PubMed] [Google Scholar]
- 41.Jain A, Blumenkranz MS, Paulus Y, Wiltberger MW, Andersen DE, Huie P, et al. Effect of pulse duration on size and character of the lesion in retinal photocoagulation. Arch Ophthalmol. 2008;126:78–85. doi: 10.1001/archophthalmol.2007.29. [DOI] [PubMed] [Google Scholar]
- 42.Muqit MM, Gray JC, Marcellino GR, Henson DB, Young LB, Patton N, et al. In vivo laser-tissue interactions and healing responses from 20- vs 100-millisecond pulse Pascal photocoagulation burns. Arch Ophthalmol. 2010;128:448–55. doi: 10.1001/archophthalmol.2010.36. [DOI] [PubMed] [Google Scholar]
- 43.Muqit MM, Gray JC, Marcellino GR, Henson DB, Young LB, Patton N, et al. Barely visible 10-millisecond pascal laser photocoagulation for diabetic macular edema: Observations of clinical effect and burn localization. Am J Ophthalmol. 2010;149:979–986.e2. doi: 10.1016/j.ajo.2010.01.032. [DOI] [PubMed] [Google Scholar]
- 44.Nagpal M, Marlecha S, Nagpal K. Comparison of laser photocoagulation for diabetic retinopathy using 532-nm standard laser versus multispot pattern scan laser. Retina. 2010;30:452–8. doi: 10.1097/IAE.0b013e3181c70127. [DOI] [PubMed] [Google Scholar]
- 45.Al-Hussainy S, Dodson PM, Gibson JM. Pain response and follow-up of patients undergoing panretinal laser photocoagulation with reduced exposure times. Eye (Lond) 2008;22:96–9. doi: 10.1038/sj.eye.6703026. [DOI] [PubMed] [Google Scholar]
- 46.Muqit MM, Marcellino GR, Gray JC, McLauchlan R, Henson DB, Young LB, et al. Pain responses of Pascal 20 ms multi-spot and 100 ms single-spot panretinal photocoagulation: Manchester Pascal Study, MAPASS report 2. Br J Ophthalmol. 2010;94:1493–8. doi: 10.1136/bjo.2009.176677. [DOI] [PubMed] [Google Scholar]
- 47.Chappelow AV, Tan K, Waheed NK, Kaiser PK. Panretinal photocoagulation for proliferative diabetic retinopathy: Pattern scan laser versus argon laser. Am J Ophthalmol. 2012;153:137–42.e2. doi: 10.1016/j.ajo.2011.05.035. [DOI] [PubMed] [Google Scholar]
- 48.Palanker D, Lavinsky D, Blumenkranz MS, Marcellino G. The impact of pulse duration and burn grade on size of retinal photocoagulation lesion: Implications for pattern density. Retina. 2011;31:1664–9. doi: 10.1097/IAE.0b013e3182115679. [DOI] [PubMed] [Google Scholar]
- 49.Friberg TR, Karatza EC. The treatment of macular disease using a micropulsed and continuous wave 810-nm diode laser. Ophthalmology. 1997;104:2030–8. doi: 10.1016/s0161-6420(97)30061-x. [DOI] [PubMed] [Google Scholar]
- 50.Akduman L, Olk RJ. Diode laser (810 nm) versus argon green (514 nm) modified grid photocoagulation for diffuse diabetic macular edema. Ophthalmology. 1997;104:1433–41. doi: 10.1016/s0161-6420(97)30119-5. [DOI] [PubMed] [Google Scholar]
- 51.Rutledge BK, Wallow IH, Poulsen GL. Sub-pigment epithelial membranes after photocoagulation for diabetic macular edema. Arch Ophthalmol. 1993;111:608–13. doi: 10.1001/archopht.1993.01090050042025. [DOI] [PubMed] [Google Scholar]
- 52.Laursen ML, Moeller F, Sander B, Sjoelie AK. Subthreshold micropulse diode laser treatment in diabetic macular oedema. Br J Ophthalmol. 2004;88:1173–9. doi: 10.1136/bjo.2003.040949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Luttrull JK, Musch DC, Mainster MA. Subthreshold diode micropulse photocoagulation for the treatment of clinically significant diabetic macular oedema. Br J Ophthalmol. 2005;89:74–80. doi: 10.1136/bjo.2004.051540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kumar V, Ghosh B, Mehta DK, Goel N. Functional outcome of subthreshold versus threshold diode laser photocoagulation in diabetic macular oedema. Eye (Lond) 2010;24:1459–65. doi: 10.1038/eye.2010.53. [DOI] [PubMed] [Google Scholar]
- 55.Luttrull JK, Spink CJ. Serial optical coherence tomography of subthreshold diode laser micropulse photocoagulation for diabetic macular edema. Ophthalmic Surg Lasers Imaging. 2006;37:370–7. doi: 10.3928/15428877-20060901-03. [DOI] [PubMed] [Google Scholar]
- 56.Luttrull JK, Musch DC, Spink CA. Subthreshold diode micropulse panretinal photocoagulation for proliferative diabetic retinopathy. Eye (Lond) 2008;22:607–12. doi: 10.1038/sj.eye.6702725. [DOI] [PubMed] [Google Scholar]
- 57.Luttrull JK, Sinclair SH. Safety of transfoveal subthreshold diode micropulse laser for fovea-involving diabetic macular edema in eyes with good visual acuity. Retina. 2014;34:2010–20. doi: 10.1097/IAE.0000000000000177. [DOI] [PubMed] [Google Scholar]
- 58.Figueira J, Khan J, Nunes S, Sivaprasad S, Rosa A, de Abreu JF, et al. Prospective randomised controlled trial comparing sub-threshold micropulse diode laser photocoagulation and conventional green laser for clinically significant diabetic macular oedema. Br J Ophthalmol. 2009;93:1341–4. doi: 10.1136/bjo.2008.146712. [DOI] [PubMed] [Google Scholar]
- 59.Lavinsky D, Cardillo JA, Melo LA, Jr, Dare A, Farah ME, Belfort R., Jr Randomized clinical trial evaluating mETDRS versus normal or high-density micropulse photocoagulation for diabetic macular edema. Invest Ophthalmol Vis Sci. 2011;52:4314–23. doi: 10.1167/iovs.10-6828. [DOI] [PubMed] [Google Scholar]
- 60.Sivaprasad S, Elagouz M, McHugh D, Shona O, Dorin G. Micropulsed diode laser therapy: Evolution and clinical applications. Surv Ophthalmol. 2010;55:516–30. doi: 10.1016/j.survophthal.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 61.Lavinsky D, Sramek C, Wang J, Huie P, Dalal R, Mandel Y, et al. Subvisible retinal laser therapy: Titration algorithm and tissue response. Retina. 2014;34:87–97. doi: 10.1097/IAE.0b013e3182993edc. [DOI] [PubMed] [Google Scholar]
- 62.Kernt M, Cheuteu R, Vounotrypidis E, Haritoglou C, Kampik A, Ulbig MW, et al. Focal and panretinal photocoagulation with a navigated laser (NAVILAS®) Acta Ophthalmol. 2011;89:e662–4. doi: 10.1111/j.1755-3768.2010.02017.x. [DOI] [PubMed] [Google Scholar]
- 63.Neubauer AS, Langer J, Liegl R, Haritoglou C, Wolf A, Kozak I, et al. Navigated macular laser decreases retreatment rate for diabetic macular edema: A comparison with conventional macular laser. Clin Ophthalmol. 2013;7:121–8. doi: 10.2147/OPTH.S38559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kozak I, Oster SF, Cortes MA, Dowell D, Hartmann K, Kim JS, et al. Clinical evaluation and treatment accuracy in diabetic macular edema using navigated laser photocoagulator NAVILAS. Ophthalmology. 2011;118:1119–24. doi: 10.1016/j.ophtha.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 65.Kernt M, Ulbig M, Haritoglou C. Seattle, Washington: The Association for Research in Vision and Ophthalmology; 2013. Combination of ranibizumab and navigated retinal photocoagulation vs ranibizumab mono-therapy for diabetic macular oedema: Twelve month results. [Google Scholar]
- 66.Barteselli G, Kozak I, El-Emam S, Chhablani J, Cortes MA, Freeman WR. 12-month results of the standardised combination therapy for diabetic macular oedema: Intravitreal bevacizumab and navigated retinal photocoagulation. Br J Ophthalmol. 2014;98:1036–41. doi: 10.1136/bjophthalmol-2013-304488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chhablani J, Mathai A, Rani P, Gupta V, Arevalo JF, Kozak I. Comparison of conventional pattern and novel navigated panretinal photocoagulation in proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci. 2014;55:3432–8. doi: 10.1167/iovs.14-13936. [DOI] [PubMed] [Google Scholar]
- 68.Michaelides M, Kaines A, Hamilton RD, Fraser-Bell S, Rajendram R, Quhill F, et al. A prospective randomized trial of intravitreal bevacizumab or laser therapy in the management of diabetic macular edema (BOLT study) 12-month data: Report 2. Ophthalmology. 2010;117:1078–1086.e2. doi: 10.1016/j.ophtha.2010.03.045. [DOI] [PubMed] [Google Scholar]
- 69.Michaelides M, Fraser-Bell S, Hamilton R, Kaines A, Egan C, Bunce C, et al. Macular perfusion determined by fundus fluorescein angiography at the 4-month time point in a prospective randomized trial of intravitreal bevacizumab or laser therapy in the management of diabetic macular edema (Bolt Study): Report 1. Retina. 2010;30:781–6. doi: 10.1097/iae.0b013e3181d2f145. [DOI] [PubMed] [Google Scholar]
- 70.Mitchell P, Bandello F, Schmidt-Erfurth U, Lang GE, Massin P, Schlingemann RO, et al. The RESTORE study: Ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology. 2011;118:615–25. doi: 10.1016/j.ophtha.2011.01.031. [DOI] [PubMed] [Google Scholar]
- 71.Soheilian M, Ramezani A, Obudi A, Bijanzadeh B, Salehipour M, Yaseri M, et al. Randomized trial of intravitreal bevacizumab alone or combined with triamcinolone versus macular photocoagulation in diabetic macular edema. Ophthalmology. 2009;116:1142–50. doi: 10.1016/j.ophtha.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 72.Soheilian M, Garfami KH, Ramezani A, Yaseri M, Peyman GA. Two-year results of a randomized trial of intravitreal bevacizumab alone or combined with triamcinolone versus laser in diabetic macular edema. Retina. 2012;32:314–21. doi: 10.1097/IAE.0b013e31822f55de. [DOI] [PubMed] [Google Scholar]
- 73.Diabetic Retinopathy Clinical Research Network. Elman MJ, Aiello LP, Beck RW, Bressler NM, Bressler SB, et al. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117:1064–1077.e5. doi: 10.1016/j.ophtha.2010.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Elman MJ, Bressler NM, Qin H, Beck RW, Ferris FL, 3rd, Friedman SM, et al. Expanded 2-year follow-up of ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2011;118:609–14. doi: 10.1016/j.ophtha.2010.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nguyen QD, Shah SM, Heier JS, Do DV, Lim J, Boyer D, et al. Primary end point (six months) results of the ranibizumab for edema of the mAcula in diabetes (READ-2) study. Ophthalmology. 2009;116:2175–81.e1. doi: 10.1016/j.ophtha.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 76.Nguyen QD, Shah SM, Khwaja AA, Channa R, Hatef E, Do DV, et al. Two-year outcomes of the ranibizumab for edema of the mAcula in diabetes (READ-2) study. Ophthalmology. 2010;117:2146–51. doi: 10.1016/j.ophtha.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 77.Giani A, Cigada M, Choudhry N, Deiro AP, Oldani M, Pellegrini M, et al. Reproducibility of retinal thickness measurements on normal and pathologic eyes by different optical coherence tomography instruments. Am J Ophthalmol. 2010;150:815–24. doi: 10.1016/j.ajo.2010.06.025. [DOI] [PubMed] [Google Scholar]
- 78.Mitchell P, Annemans L, Gallagher M, Hasan R, Thomas S, Gairy K, et al. Cost-effectiveness of ranibizumab in treatment of diabetic macular oedema (DME) causing visual impairment: Evidence from the RESTORE trial. Br J Ophthalmol. 2012;96:688–93. doi: 10.1136/bjophthalmol-2011-300726. [DOI] [PMC free article] [PubMed] [Google Scholar]


