Abstract
This is a summary of current and emerging pharmacologic therapies utilized in the treatment of diabetic retinopathy (DR). Current therapies, such as ranibizumab, bevacizumab, triamcinolone acetonide, and fluocinolone acetonide, inhibit angiogenesis and inflammation and may be used alone or in combination with laser treatment. Emerging therapies aim to reduce oxidative stress or inhibit other signal transduction pathways, including the protein kinase C cascade and aldose reductase pathway. Future therapies may target other molecules crucial to the pathogenesis of DR, including hepatocyte growth factors and matrix metalloproteinase 9. Finally, the emergence of novel mechanisms of medication delivery may also be on the horizon.
Keywords: Corticosteroids, Diabetic Retinopathy, Eye, Intravitreal Injection, Treatment, Vascular Endothelial Growth Factor
INTRODUCTION
Diabetic retinopathy (DR) is a leading cause of visual impairment in the working aged population in industrialized countries. Greater than one-third of patients with type 2 diabetes mellitus have at least one microvascular complication at diagnosis, either retinopathy, neuropathy, and/or nephropathy.1 In addition, approximately one-quarter of all patients develop severe sight-threatening levels of retinopathy.2 By far, the most common complications of diabetes are diabetic macular edema (DME) and proliferative diabetic retinopathy (PDR).
There are two main categories of DR, nonproliferative diabetic retinopathy (NPDR) and proliferative diabetic retinopathy (PDR). DME may be present in both. Moderate vision loss in patients with diabetes is secondary to DME resulting from leakage of retinal vessels while severe vision loss is often secondary to intraocular angiogenesis, characteristic of PDR. Inflammation, ischemia, and vitreoretinal traction are all associated with the development of DR, although the pathogenesis of DR is still not completely understood. Another contributing factor is chronic hyperglycemia, which results in the overproduction of reactive oxygen species (ROS) in the mitochondria, especially in tissues with high oxygen demand, such as the retina. This begins a cascade of events that ultimately manifest as DR. As new molecules/factors in the pathogenesis of DR are elucidated, new therapies are emerging.
The management of DR begins with improved glycemic control. The diabetes control and complications trial (DCCT) reported up to a 76% decrease in the progression of DR in type 1 diabetic patients receiving intensive insulin therapy during a 9 year period3 and a persistent reduction in risk for four additional years, despite hyperglycemia.4 The United Kingdom Prospective Diabetes Study was performed in type 2 diabetics and reported similar findings to DCCT, as well as decreased diabetes-related complications in patients undergoing aggressive blood pressure management.5,6
Other therapies for DR, directly targeting the retina, include panretinal photocoagulation (PRP) and focal laser photocoagulation. The diabetic retinopathy study (DRS)7 reported a 50% reduced risk of severe vision loss in eyes with high-risk PDR treated with PRP. The early treatment of diabetic retinopathy study (ETDRS)8 reported that PRP reduced the risk of severe vision loss to <2% if performed in a timely fashion. In addition, as the results of ETDRS9 showed that moderate vision loss secondary to clinically significant macular edema (CSME) in patients with diabetes could be reduced with focal or grid laser photocoagulation treatment, this has remained a mainstay of treatment since 1985. Laser photocoagulation for DME was defined by ETDRS results where focal laser was indicated for focal lesions located between 500 and 3000 μm from the center of the macula (noncenter-involving).8,10 Grid laser photocoagulation with a milder power than focal laser photocoagulation is indicated for DME within 500 μm from the center of the macula (center-involving DME).8,10 These therapeutic modalities can greatly reduce visual morbidity due to DR although their use can be limited by associated adverse events or side effects. This has led to the search for additional therapies, including pharmacotherapies, for the treatment of DR.
Currently, DR can be managed with a variety of pharmacotherapies, and there are others on the horizon. The purpose of this review was to describe past, current, and emerging pharmacotherapies for the treatment of DR as well as to discuss future directions.
PAST, CURRENT, AND EMERGING PHARMACOTHERAPIES
Potential therapies for DR aim to block angiogenic growth factors or other intracellular signaling pathways, as well as disrupt the inflammatory cascade.
Blockade of angiogenesis and increased retinal vascular permeability
Inhibitors of vascular endothelial growth factor
Vascular endothelial growth factor (VEGF) is a glycoprotein with four isoforms. An increase in retinal VEGF concentrations and retinal neovascularization has been demonstrated in the setting of hypoxia,11 and has been implicated in the pathogenesis of DR. Current medications that inhibit VEGF include pegaptanib (Macugen, Pfizer, New York, NY) – a ribonucleic acid aptamer, ranibizumab (Lucentis, Genentech, Inc., South San Francisco, CA) – a humanized monoclonal antibody fragment, bevacizumab (Avastin, Genentech, Inc.) – a recombinant full-length humanized monoclonal antibody and aflibercept (Eylea, Regeneron, Tarrytown, NY) – a soluble decoy receptor fusion protein [Table 1].12,13,14
Table 1.
Pharmacologic agents, current and emerging, for the treatment of diabetic retinopathy, their drug class and/or mechanism of action and the phase of investigation
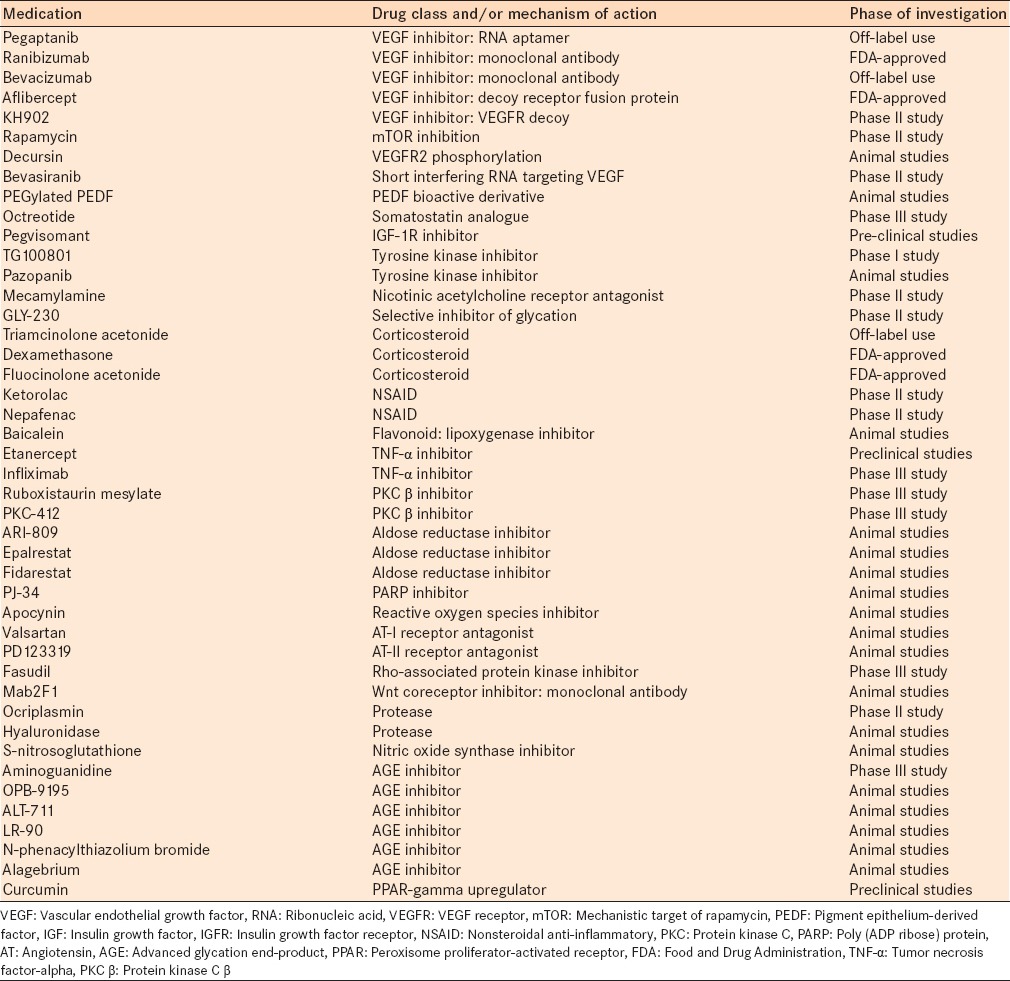
All of the aforementioned anti-VEGF agents are used in the treatment of DR, although pegaptanib has fallen out of favor since the introduction of ranibizumab and bevacizumab. Early studies with pegaptanib showed that there were better visual acuity (VA) outcomes in patients treated with pegaptanib than sham and that there was a decreased need for additional laser therapy. Sultan et al.14 reported a VA gain of ≥10 letters in 36.8% of patients treated with pegaptanib versus 19.7% of untreated patients (P = 0.0047) at 54 weeks.
Soon after the introduction of pegaptanib, intravitreal ranibizumab, and bevacizumab emerged. Studies evaluating the efficacy of intravitreal ranibizumab for the treatment of DME have reported significantly better VA outcomes with treatment compared to controls. In the Diabetic Retinopathy Clinical Research Network (DRCR.net)15 study, performed in patients with decreased VA and center-involving DME, intravitreal ranibizumab with deferred (≥24 weeks) or prompt focal/grid laser photocoagulation was found to be superior to focal/grid laser alone at 1-year. Although grid laser photocoagulation is currently indicated for the treatment of center involving DME, there is a continuing search for additional treatment modalities as laser photocoagulation alters the anatomy of the retina. In addition, results from DRCR.net16 showed that in the short-term, eyes with center-involving DME receiving prompt PRP at the same time as focal/grid laser were more likely to have increased ME and greater VA loss than eyes without central DME receiving prompt PRP without focal/grid laser.
In the RISE and RIDE17 studies, patients treated with ranibizumab 0.3 mg and 0.5 mg had significantly improved VA outcomes with more patients losing <15 letters when compared to sham. In RISE,17 a VA gain of ≥15 letters was reported in 18.1, 44.8, and 39.2% of sham, ranibizumab 0.3 mg and ranibizumab 0.5 mg groups, respectively, at 24 months (P < 0.0001 and P < 0.001, respectively). Similar findings were also reported in RIDE17 with 33.6% and 45.7% of patients in the ranibizumab 0.3 mg and 0.5 mg groups, respectively, compared to 12.3% in the sham group (P < 0.0001 for both) gaining 15 letters. Notably, in RIDE17 the proportion of patients losing <15 letters was not significantly different between the sham-injection and ranibizumab 0.5 mg groups (P = 0.1384). The beneficial effects of ranibizumab on DME were evaluated in the RESTORE study and were sustained long-term.18 The LUCIDATE study,19 reported improved retinal function and structure in patients with DME treated with ranibizumab compared to macular laser therapy. Studies have also demonstrated improved visual outcomes in patients with DR treated with intravitreal bevacizumab compared to sham/observation or laser.20 The BOLT study reported an increase in the mean best-corrected VA (BCVA) in the bevacizumab group and a decrease in mean BCVA in the laser group at 12 months.20
A more recently approved medication for the treatment of DME is aflibercept. Studies have showed superiority of intravitreal aflibercept over laser in functional and anatomic endpoints with BCVA gains from baseline of 12.5, 10.7 and 0.2 letters at 52 weeks in the aflibercept 2 mg every 4 weeks (q4wk), aflibercept 2 mg q8wk and laser groups, respectively (P < 0.0001).21 The DA VA Informatics and Computing Infrastructure study also reported more favorable VA outcomes in patients receiving aflibercept compared to laser (P ≤ 0.0085) at 24 weeks.22 The efficacy of intravitreal anti-VEGF agents for the treatment of PDR has been demonstrated by multiple studies, and the use of these medications has expanded greatly, as often, these medications are used as first-line therapies.11,12
Other inhibitors of angiogenesis
The success of current anti-VEGF therapies has made the way for other anti-VEGF agents. KH902 is a VEGF receptor (VEGFR) decoy, which blocks ocular neovascularization by binding VEGF and placental growth factor (PlGF).23 4 weeks after intravitreal injection of KH902, KH902-treated rats exhibited improved retinal electrophysiological function, less retinal vascular leakage, and decreased levels of VEGFR2, PlGF, and PI3K (all involved angiogenesis), than sham or bevacizumab-treated rats.
Other drugs that ultimately affect the production and/or function of VEGF include rapamycin, decursin, and bevasiranib. Rapamycin (Sirolimus, MAcuSight, Inc., Union City, CA) is a macrolide antibiotic that can lead to upstream blockade of VEGF production. Studies have showed that subconjuctival rapamycin can lead to improvements in VA and foveal thickness in patients with DME,24 although no significant recent successes with this medication have been reported. Decursin, a compound isolated from the root of Angelica gigas Nakai, induces phosphorylation of VEGFR2 and inhibits neovascularization.25 Yang et al.25 found that systemic decursin attenuated glucose-induced proliferation and angiogenesis in animal models of DR. Intravitreal administration of short interfering ribonucleic acids targeting VEGF have been reported to prevent retinal and choroidal neovascularization in mice.26 The RNAi assessment of bevasiranib (OPKO Health Inc., Ontario, Canada) in DME (RACE) trial was a phase II investigation of the preliminary efficacy and safety of bevasiranib and showed a trend toward decreasing macular thickness with a dose-dependent effect.27
Numerous other molecules have also been identified as inhibitors of angiogenesis, including pigment epithelium-derived factor (PEDF), and have become targets for the development of anti-angiogenic medications.28 Pigment epithelium-derived factor has been reported to be the most potent endogenous anti-angiogenic agent28 and studies have shown that intravitreal injection of exogenous PEDF reduces areas of retinal neovascularization and induces apoptosis of vascular endothelial cells.29 Bai et al.30 reported that in vivo and in vitro administration of PEGylated-PEDF (a polyethylene glycol carrier) prevented neovascularization. Liu et al.31 reported that PEDF bioactive derivatives reached the retina via a topical formulation delivered to the surface of the eye and that treatment with these derivatives reduced DR complications in mice.
Previous studies have also evaluated the effects of systemic somatostatin analogs, such as octreotide (Sandostatin, Novartis, East Hanover, NJ) on DR as somatostatin expression is downregulated in DR.32,33,34,35 Grant et al.32 reported a decreased incidence of progression to PDR needing PRP in patients treated with octreotide and Boehm et al.33 reported a decreased incidence of progression to PDR and vitreous hemorrhage requiring vitreoretinal surgery in patients treated with octreotide. In addition, one study reported effective treatment of DME with the use of octreotide.34 More recently, studies have found that topical somatostatin analogs may prevent retinal neurodegeneration in diabetic model animals.35
Insulin growth factor (IGF-1) is also an important regulator of angiogenesis and has been directly implicated in the pathogenesis of DR. Blocking the IGF-1 receptor by pegvisomant, a mutated growth hormone, may have favorable effects on DR, although currently, one study in patients with PDR reported that subcutaneous treatment with pegvisomant did not produce regression of diabetic retinal neovascularization at 24 weeks.36
Another medication studied for the treatment of DR is TG100801 (TargeGen, Inc., San Diego, CA), a topical tyrosine kinase inhibitor. It has been shown to decrease VEGF-mediated retinal leakage, angiogenesis, and inflammation.37 Another tyrosine kinase inhibitor, pazopanib (Votrient, Glaxo Smith Kline, East Durham, NY) blocks VEGFR (VEGFR1, VEGFR2, and VEGFR3) and platelet-derived growth-factor receptor (PDGFR). Pazopanib eye drops were studied in animal models with diabetes and were found to significantly reduce leukostasis and the vitreous-to-plasma protein ratio, therefore alleviating retinal complications in DR.38 Another topical medication, mecamylamine, antagonizes the nicotinic acetylcholine receptor pathway, inhibiting angiogenesis. Thus far, while this medication is well-tolerated, its efficacy in DR is unclear.
Small molecules are also in development for the treatment of DR. GLY-230, an oral medication in phase II trials, is a selective inhibitor of glycation that leads to inhibition of VEGF and PEDF.39 Glycated albumin emerged as a target for therapy in DR as it is increased in diabetes and is toxic to retinal pericytes.40 Studies have reported that inhibiting the formation of glycated albumin mitigates vitreous changes in angiogenic and metabolic factors associated with the DR.39
Blockade of inflammation
A second mechanism for the development of DR is based on increased intraocular inflammation. For this reason, medications such as corticosteroids–which inhibit the production of prostaglandins and leukotrienes, and nonsteroidal anti-inflammatory (NSAID) agents–which inhibit the production of prostaglandins, have been utilized for the treatment of DME. Corticosteroids can be administered intravitreally, subtenon or topically, including periocular injection and eye drops. Miyamoto et al.41 reported that intravitreal triamcinolone acetonide (IVTA) reduces macular edema as soon as 1-h postinjection. 3-year data from DRCR.net42 showed no increased benefit of intravitreal triamcinolone (1.0 mg or 4.0 mg) over focal/grid laser photocoagulation in patients with DME with a VA gain of 5, 0, and 2 letters in laser and triamcinolone 1.0 mg and 4.0 mg. In addition, there was a significantly higher rate of cataract extraction in the triamcinolone groups (P < 0.001 for both groups).42 Boyer et al.43 showed significant improvement in BCVA and reduction in central retinal thickness (CRT) in patients treated with dexamethasone intravitreal implant (Ozurdex, Allergan, Inc., Irvine, CA) at 3 years. Dexamethasone is more potent than triamcinolone and may be associated with higher rates of elevated intraocular pressure. One advantage of the corticosteroids, over other medications, is that they are dosed less frequently, often every 3 months versus monthly for anti-VEGF agents. Corticosteroids can also be used in combination with other therapies, including anti-VEGF agents and/or laser treatment, for greater overall effect. Goto et al.44 reported improvement in BCVA and significant improvement in mean retinal thickness (P = 0.006) in patients treated with corticosteroid eye drops, difluprednate ophthalmic emulsion 0.05%. Currently, in patients unresponsive to laser photocoagulation, corticosteroids show promise in the treatment of DME, especially in pseudophakic patients.
One of the most recent medications to be Food And Drug Administration-approved for the treatment of DME is a fluocinolone acetonide intravitreal implant (Iluvien, Alimera Sciences, Inc., Alpharetta, GA). Studies have shown improvement in BCVA in patients receiving fluocinolone at 36 months, with an improvement of ≥15 letters in 29 (P = 0.018), 28 and 19% of patients in the low-dose (0.2 g/day), high-dose (0.5 μg/day) and sham groups, respectively.45 In addition, patients with DME for 3 or more years, were more likely to achieve a ≥15 letter gain in BCVA compared to patients in the sham group (P < 0.001 and P = 0.002, low-dose and high-dose, respectively).45 Another fluocinolone acetonide implant, Retisert (Bausch and Lomb, Rochester, NY), has also been used for the treatment of DR. The fluocinolone acetonide implant is active for about 36 months and the dexamethasone implant is active for about 3 months.
As prostaglandins and other inflammatory molecules have been implicated in the pathophysiology of DR, they have become targets for potential therapy. Schoenberger et al.46 reported significantly decreased aqueous and vitreous levels of interleukin 8 and decreased vitreous levels of PDGF-AA, both factors implicated in intraocular inflammation, in patients with DR treated with topical ketorolac 0.45%, a topical NSAID. Similarly, other studies have also reported on the use of topical NSAIDs for the treatment of DME. In a small case series of patients with DME treated with nepafenac 0.1% (Nevanac, Alcon, Ft. Worth, TX), average central foveal thickness decreased significantly, from 417 μm to 267 μm after a mean of 178 days.47 Another study found inhibition of diabetes-induced microvascular abnormalities with nepafenac treatment.48 Another medication, baicalein, was found to suppress inflammatory processes and inhibit vascular abnormalities and neuron loss in retinas of diabetic model animals.49
Two medications known to block the inflammatory molecule tumor necrosis factor-α are etanercept (Enbrel, Amgen, Inc., Thousand Oaks, CA and Wyeth, Madison, NJ) and infliximab (Remicade, Centocor, Horsham, PA). One of the effects of etanercept is to reduce intercellular adhesion molecule-1 (ICAM-1) expression, involved in the mediation of leukocyte adhesion to the vasculature and the progression of endothelial cell injury in DR. The effects of etanercept on DR require further evaluation as there are few studies evaluating its efficacy, one of which reported no significant improvement in refractory DME.50 On the other hand, intravitreal infliximab has been shown to lead to functional and anatomic improvement in DME in a small case series.51 Interferon α-2a (IFNα) has anti-inflammatory and anti-proliferative properties. In a case report, one patient with refractory DME was found to have a drastic improvement in BCVA and CRT as a result of treatment with subtenon injection of the natural leukocyte IFNα.52
Blockade of signal transduction pathways
Protein kinase C β inhibition
Protein kinase C β (PKC β) is a serine/threonine kinase that is activated during the signal transduction mechanism initiated by VEGF. Aiello et al.53 reported that selective inhibition of PKC β in vitro prevented VEGF-mediated cell growth and inhibition of PKC α stimulated cell growth. In animal studies, PKC β inhibition alleviated the decline in retinal blood flow associated with DR, helped prevent increased vascular permeability54 and reduced the stimulus for neovascularization.55 Ruboxistaurin (LY333531) mesylate (Arxxant, Eli Lilly/Takeda, Indianapolis, IN) is a selective inhibitor of PKC β1 and β2 isoforms. While some studies have not been able to clearly demonstrate a treatment effect from ruboxistaurin, the Protein Kinase C (PKC) Beta Inhibitor-Diabetic Retinopathy Study (PKC-DRS) and PKC-DRS2 showed significantly reduced occurrence of sustained moderate visual loss by 41% (P = 0.011) and reduced encroachment of CSME to the center of the macula in patients treated with ruboxistaurin compared to placebo.56 In addition, treatment with ruboxistaurin was shown to increase the improvement in VA in patients with moderately severe to very severe NPDR.56 PKC-412 is also an inhibitor of PKC and has been found to prevent the formation of retinal neovascularization in mice when administered orally.57 In a randomized, multicenter, double-masked study, patients treated with oral PKC-412 had a decrease in CRT and improvement in VA (P = 0.007) in the 100 mg/day group.58
Inhibition of other signal transduction pathways
Aldose reductase produces sorbitol during glucose metabolism in patients with diabetes, depriving cells of glutathione and increasing oxidative stress. Studies in diabetic animals have reported decreased prevalence of microaneurysms, basement membrane thickness, VEGF expression, and gliosis in retinas with treatment with aldose reductase inhibitors (ARI).59,60 To date, many of the older ARIs have not been found to be efficacious, significantly beneficial and/or tolerable in patients, including: Sorbinil, tolrestat, lidorestat, and ponalrestat.61 Newer ARIs have shown greater success in animal studies, with greater selectivity for aldose reductase and greater potency. These medications include ARI-809, epalrestat, and fidarestat.60 Hattori et al.61 studied fidarestat in vivo and reported significantly decreased concentrations of sorbitol and fructose in the retinas of diabetic model rats with less leukocyte accumulation (P < 0.01) and suppression of ICAM-1 expression.
Poly (ADP ribose) protein (PARP), a nuclear enzyme, is activated in diabetic retinas and can lead to DNA damage and oxidative stress.62 PJ-34 is a PARP inhibitor that has been found to inhibit the loss of pericytes and the formation of acellular capillaries in diabetic mice.62 Another molecule, apocynin–a ROS inhibitor, has been reported to prevent diabetes-induced upregulation of PARP in the retina.63
The renin-angiotensin system (RAS) has been implicated in the pathogenesis of DR, as studies have reported increased levels of renin, prorenin, and angiopoietin-2 (Ang-2) in the vitreous of patients with DR.64 As RAS upregulates VEGF, ICAM-1, and nuclear factor-kB (NF-kB), inhibition of this system may lead to a decrease in these inflammatory factors. Studies in diabetic rats have shown that treatment with oral valsartan–angiotensin-I (AT-I) receptor antagonist and subcutaneous PD123319–AT-II receptor antagonist, diminishes increased retinal VEGF expression.65 On the other hand, the appropriate blood control in diabetes trial did not show benefit with ACE-inhibitor treatment in patients with DR.66
Fasudil (Asahi Kasei Pharma Corporation, Tokyo, Japan) is a rho-associated protein kinase inhibitor has that been studied for the treatment of DME. Studies report that fasudil protects the vascular endothelium by inhibiting neutrophil adhesion and reducing neutrophil-induced endothelial injury. There are reports of improvement in DME with use of intravitreal fasudil and intravitreal bevacizumab in patients with persistent DME after macular laser photocoagulation and multiple intravitreal injections of bevacizumab.67
Another target for treatment in the signaling pathway for DR is Wnt, a cysteine-rich glycoprotein that contributes to retinal inflammation, vascular leakage, and neovascularization. Mab2F1 is a monoclonal antibody directed against the Wnt coreceptor and studies in DR models have demonstrated that Mab2F1 can reduce retinal vascular leakage and block the overexpression of inflammatory/angiogenic factors.68
Intravitreal anti-VEGF treatment can lead to the upregulation of connective tissue growth factor (CTGF), increasing the risk of fibrosis and tractional retinal detachment. In an effort to diminish the upregulation of CTGF, anti-CTGF agents are emerging. One such medication is a transfection reagent-treated nonviral vector carrying an anti-CTGF short hairpin RNA (shRNA). Hu et al.69 reported ameliorated retinal microvessel ultrastructural damage in patients treated with combination therapy of an anti-VEGF agent and a CTGF shRNA than in patients with monotherapy. The second study, the DEGAS study,70 showed a dose-related tendency toward improvement in BCVA in DME patients treated with the RTP-801 gene.
Disruption of the vitreoretinal interface
It is believed that some complications of DR may be secondary to the interactions between the vitreous and retinal surface. Nasrallah et al. reported a significantly higher rate of a posterior vitreous detachment (PVD) in diabetic patients without macular edema than those with macular edema, speculating that the vitreous may have a role in the development of DME.71 Currently, the only available intravitreal medication for inducing a PVD is ocriplasmin (Jetrea, ThromboGenics, Iselin, NJ) and there are no data for its efficacy in DR. Intravitreal hyaluronidase (Vitrase, ISTA Pharmaceuticals, Irvine, CA) also disrupts the vitreoretinal interface and its use has shown some efficacy in reducing vitreous hemorrhage secondary to PDR.72
Other mechanisms of action and therapies
Current data for other therapies for DR are emerging, but limited. Xu et al.73 developed a gene-based intraocular erythropoietin therapy, AAV2-CMV-hEPO and reported long-term protective effects on diabetic retinas when administered systemically in rats. Another study evaluated the effects of S-nitrosoglutathione eye drops in an experimental animal DR model, reporting mitigated nitrosative stress and slowing of the early structural changes in the retina, with improved retinal function.74 It is thought that diabetic stimuli may trigger reactive nitrogen species generation, leading to inflammation and tissue injury. Therefore, mitigation of this nitrosative stress may prevent and/or treat DR.
Advanced glycation end-product (AGE) inhibitors have recently emerged as possible therapies for DR. The accumulation of AGEs and their interaction with receptors (RAGEs) adversely affects the microvasculature in patients with diabetes. Kaji et al.75 reported significantly inhibited blood-retinal barrier breakdown, increased leukostasis and decreased expression of ICAM-1 in the retinas of mice with AGE inhibition. Aminoguanidine, an inhibitor of AGE formation, used in diabetic dogs, showed diminished pericyte drop-out, reduced progression of vascular occlusion and inhibited abnormal endothelial cell proliferation.76 Multiple AGE inhibitors have been developed and are being evaluated for use in humans with DR, including OPB-9195, ALT-711, LR-90, N-phenacylthiazolium bromide and alagebrium.77
Curcumin, peroxisome proliferator-activated receptor-gamma upregulator, has been studied as a potential treatment and while results show a beneficial effect in inhibiting the pathways responsible for DR, no definitive studies have demonstrated its use as an effective therapy in humans. One study by Steigerwalt et al.78 showed that treatment with Meriva, a lecithin delivery system of curcumin, led to improvement in VA and retinal edema compared to controls (P < 0.025 and P < 0.05, respectively).
Combination therapies
Response to intravitreal monotherapy can vary and may not be adequate, but combination therapy has yielded better results. The RESTORE study showed a greater improvement in mean BCVA in patients treated with both intravitreal ranibizumab and laser than in patients treated with monotherapy.18 Similarly, Gilles et al.79 reported an increased likelihood of a 10-letter improvement in BCVA from baseline in patients treated with IVTA and laser versus only laser at 2 years. On the other hand, the READ-2 study reported no significant difference in visual outcomes in the combination therapy group, although combination treatment provided an improvement in BCVA and a greater decrease in macular edema with fewer injections.80
Future therapies
The future of treatment of DR relies not only on the development of medications targeting molecules crucial to the pathogenesis of DR, but also on the development of novel delivery techniques. In order to maximize the treatment effect and minimize systemic adverse effects, targeted delivery of medication to the retina is ideal.
The administration of pharmacotherapies for DR in a topical form, like eye drops, is ideal as it is not invasive and minimizes associated systemic effects. Data are emerging that medications administered as eye drops are able to reach the retina in therapeutic concentrations. In addition, the use of depot deposits and trans-scleral delivery may allow for sustained effect of medication. Medications or genes might also be able to be delivered to the retina via an adenovirus.
Future targets for the development of therapies for the treatment of DR include hepatocyte growth factor, matrix metalloproteinase 9 (MMP-9), monocyte chemotactic protein-1 (MCP-1), kallikrein, Ang-2, and NF-kB. Hepatocyte growth factor has been found to increase retinal vascular permeability in vivo and is increased in the serum of patients with PDR81 and MMP-9 has been found in increased concentrations in the retina and vitreous of patients with diabetes.82 Inhibition of these two molecules may prevent DR or cause regression of PDR. An increase in MCP-1 in retinal vascular endothelial cells has be associated with hyperglycemia and MCP-1 levels in the vitreous have been reported to be increased in patients with diabetes.83 MCP-1 receptor antagonism is being evaluated in ongoing studies in patients with DME as previous studies have reported a potential role for MCP-1 in altering the blood-retina barrier. Kallikrein activation has been shown to contribute to increased retinal vascular permeability in patients with DME.84 Similarly, Ang-2 has been associated with an increase in retinal vascular permeability.85 The effects of these molecules on vascular permeability make them novel targets in the treatment of DR. Finally, NF-kB inhibition shows promise as treatment with pyrrolidinedithiocarbamate, an NF-kB inhibitor, in an animal model of ischemic retinopathy, resulted in the suppression of ischemia-induced retinal neovascularization.86
Many of these studies have been found effective in animal models, and the next step is to further develop these medications with the eventual goal of administration in humans. Efficacy and safety data for many of the aforementioned medications are sparse since data are limited to case reports and small retrospective or prospective studies. As the exact mechanisms involved in the pathogenesis of DR are elucidated more therapeutic targets will emerge, and the armamentarium of treatment options for DR will expand greatly.
CONCLUSION
While PRP and focal laser photocoagulation are the standard of care for the treatment of high-risk PDR and focal DME, the damaging effects of laser therapy and other potential complications make these treatment modalities less than ideal. On the other hand, PRP is often still necessary for the inducing a more permanent regression of vessels, as the effects of anti-VEGF therapy are transient. Unfortunately, these treatment modalities are associated with significant adverse events and sometimes patients are unresponsive to therapy. For this reason, in the search for novel pharmacotherapies for the treatment of DR, the aim is to deliver optimal visual results with a reasonable safety profile and decreased treatment burden.
The ideal medication for the treatment of DR is fast-acting, long-lasting and above all, safe. As the development of DR is a multifactorial process, involving inflammation and ischemia, future therapies, especially combination therapies, targeting different pathways may lead to more favorable outcomes.
Footnotes
Source of Support: Sophie J. Bakri, was supported by Research to Prevent Blindness, New York, NY.
Conflict of Interest: None declared.
REFERENCES
- 1.Ruigómez A, García Rodríguez LA. Presence of diabetes related complication at the time of NIDDM diagnosis: An important prognostic factor. Eur J Epidemiol. 1998;14:439–45. doi: 10.1023/a:1007484920656. [DOI] [PubMed] [Google Scholar]
- 2.Klein R, Klein BE, Moss SE. Visual impairment in diabetes. Ophthalmology. 1984;91:1–9. [PubMed] [Google Scholar]
- 3.The effect of intensive diabetes treatment on the progression of diabetic retinopathy in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial. Arch Ophthalmol. 1995;113:36–51. doi: 10.1001/archopht.1995.01100010038019. [DOI] [PubMed] [Google Scholar]
- 4.The Diabetes Control and Complications Trial. Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. N Engl J Med. 2000;342:381–9. doi: 10.1056/NEJM200002103420603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837–53. [PubMed] [Google Scholar]
- 6.The United Kingdom Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes (UKPDS 38) Br Med J. 1998;317:703–13. [PMC free article] [PubMed] [Google Scholar]
- 7.The Diabetic Retinopathy Study Research Group. Photocoagulation treatment of proliferative diabetic retinopathy. Clinical application of diabetic retinopathy study (DRS) findings, DRS Report Number 8. Ophthalmology. 1981;88:583–600. [PubMed] [Google Scholar]
- 8.Early photocoagulation for diabetic retinopathy. ETDRS report number 9. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991;98(5 Suppl):766–85. [PubMed] [Google Scholar]
- 9.Early Treatment Diabetic Retinopathy Study Research Group. Photocoagulation for diabetic macular edema: Early Treat-ment Diabetic Retinopathy Study report number 1. Arch Ophthalmol. 1985;103:1796–806. [PubMed] [Google Scholar]
- 10.Techniques for scatter and local photocoagulation treatment of diabetic retinopathy: Early Treatment Diabetic Retinopathy Study Report no. 3. The Early Treatment Diabetic Retinopathy Study Research Group. Int Ophthalmol Clin. 1987;27:254–64. doi: 10.1097/00004397-198702740-00005. [DOI] [PubMed] [Google Scholar]
- 11.Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331:1480–7. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- 12.Heier JS, Brown DM, Chong V, Korobelnik JF, Kaiser PK, Nguyen QD, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119:2537–48. doi: 10.1016/j.ophtha.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 13.CATT Research Group. Martin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL, et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364:1897–908. doi: 10.1056/NEJMoa1102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sultan MB, Zhou D, Loftus J, Dombi T, Ice KS Macugen 1013 Study Group. A phase 2/3, multicenter, randomized, double-masked, 2-year trial of pegaptanib sodium for the treatment of diabetic macular edema. Ophthalmology. 2011;118:1107–18. doi: 10.1016/j.ophtha.2011.02.045. [DOI] [PubMed] [Google Scholar]
- 15.Elman MJ, Aiello LP, Beck RW, Bressler NM, Bressler SB, Edwards AR, et al. Randomized trial evaluating ranibizumab versus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Diabetic Retinopathy Clinical Research Network. Ophthalmology. 2010;117:1064–77. doi: 10.1016/j.ophtha.2010.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brucker AJ, Qin H, Antoszyk AN, Beck RW, Bressler NM, et al. Diabetic Retinopathy Clinical Research Network, Observational study of the development of diabetic macular edema following panretinal (scatter) photocoagulation given in 1 or 4 sittings. Arch Ophthalmol. 2009;127:132–40. doi: 10.1001/archophthalmol.2008.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nguyen QD, Brown DM, Marcus DM, Boyer DS, Patel S, Feiner L, et al. Ranibizumab for diabetic macular edema: Results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119:789–801. doi: 10.1016/j.ophtha.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt-Erfurth U, Lang GE, Holz FG, Schlingemann RO, Lanzetta P, Massin P, et al. Three-year outcomes of individualized ranibizumab treatment in patients with diabetic macular edema: The RESTORE extension study. Ophthalmology. 2014;121:1045–53. doi: 10.1016/j.ophtha.2013.11.041. [DOI] [PubMed] [Google Scholar]
- 19.Comyn O, Sivaprasad S, Peto T, Neveu MM, Holder GE, Xing W, et al. A randomized trial to assess functional and structural effects of ranibizumab versus laser in diabetic macular edema (the LUCIDATE study) Am J Ophthalmol. 2014;157:960–70. doi: 10.1016/j.ajo.2014.02.019. [DOI] [PubMed] [Google Scholar]
- 20.Michaelides M, Kaines A, Hamilton RD, Fraser-Bell S, Rajendram R, Quhill F, et al. A prospective randomized trial of intravitreal bevacizumab or laser therapy in the management of diabetic macular edema (BOLT study) 12-month data: Report 2. Ophthalmology. 2010;117:1078–86.e2. doi: 10.1016/j.ophtha.2010.03.045. [DOI] [PubMed] [Google Scholar]
- 21.Korobelnik JF, Do DV, Schmidt-Erfurth U, Boyer DS, Holz FG, Heier JS, et al. Intravitreal aflibercept for diabetic macular edema. Ophthalmology. 2014;121:2247–54. doi: 10.1016/j.ophtha.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 22.Do DV, Schmidt-Erfurth U, Gonzalez VH, Gordon CM, Tolentino M, Berliner AJ, et al. The DA VINCI Study: Phase 2 primary results of VEGF Trap-Eye in patients with diabetic macular edema. Ophthalmology. 2011;118:1819–26. doi: 10.1016/j.ophtha.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 23.Huang J, Li X, Li M, Li S, Xiao W, Chen X, et al. Effects of intravitreal injection of KH902, a vascular endothelial growth factor receptor decoy, on the retinas of streptozotocin-induced diabetic rats. Diabetes Obes Metab. 2012;14:644–53. doi: 10.1111/j.1463-1326.2012.01584.x. [DOI] [PubMed] [Google Scholar]
- 24.Blumenkranz M. Boston, MA: 2007. Sep 27-30, Sirolimus Demonstrates Favorable Safety Profile and Improvements in Visual Acuity. 40th Annual Meeting of the Retina Society. [Google Scholar]
- 25.Yang Y, Yang K, Li Y, Li X, Sun Q, Meng H, et al. Decursin inhibited proliferation and angiogenesis of endothelial cells to suppress diabetic retinopathy via VEGFR2. Mol Cell Endocrinol. 2013;378:46–52. doi: 10.1016/j.mce.2013.04.021. [DOI] [PubMed] [Google Scholar]
- 26.Shen J, Yang X, Xiao WH, Hackett SF, Sato Y, Campochiaro PA. Vasohibin is up-regulated by VEGF in the retina and suppresses VEGF receptor 2 and retinal neovascularization. FASEB J. 2006;20:723–5. doi: 10.1096/fj.05-5046fje. [DOI] [PubMed] [Google Scholar]
- 27.Prenner JL. Lauderdale, FL: 2007. Oct 5, RACE Study Group. The RACE Study: Bevasiranib for the treatment of diabetic macular edema (abstract 5045). Association for Research in Vision and Ophthalmology. [Google Scholar]
- 28.Dawson DW, Volpert OV, Gillis P, Crawford SE, Xu H, Benedict W, et al. Pigment epithelium-derived factor: A potent inhibitor of angiogenesis. Science. 1999;285:245–8. doi: 10.1126/science.285.5425.245. [DOI] [PubMed] [Google Scholar]
- 29.Gao G, Li Y, Fant J, Crosson CE, Becerra SP, Ma Jx. Difference in ischemic regulation of vascular endothelial growth factor and pigment epithelium – derived factor in brown norway and sprague dawley rats contributing to different susceptibilities to retinal neovascularization. Diabetes. 2002;51:1218–25. doi: 10.2337/diabetes.51.4.1218. [DOI] [PubMed] [Google Scholar]
- 30.Bai YJ, Huang LZ, Xu XL, Du W, Zhou AY, Yu WZ, et al. Polyethylene glycol-modified pigment epithelial-derived factor: New prospects for treatment of retinal neovascularization. J Pharmacol Exp Ther. 2012;342:131–9. doi: 10.1124/jpet.112.192575. [DOI] [PubMed] [Google Scholar]
- 31.Liu Y, Leo LF, McGregor C, Grivitishvili A, Barnstable CJ, Tombran-Tink J. Pigment epithelium-derived factor (PEDF) peptide eye drops reduce inflammation, cell death and vascular leakage in diabetic retinopathy in Ins2(Akita) mice. Mol Med. 2012;18:1387–401. doi: 10.2119/molmed.2012.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grant MB, Mames RN, Fitzgerald C, Hazariwala KM, Cooper-DeHoff R, Caballero S, et al. The efficacy of octreotide in the therapy of severe nonproliferative and early proliferative diabetic retinopathy: A randomized controlled study. Diabetes Care. 2000;23:504–9. doi: 10.2337/diacare.23.4.504. [DOI] [PubMed] [Google Scholar]
- 33.Boehm BO, Lang GK, Jehle PM, Feldman B, Lang GE. Octreotide reduces vitreous hemorrhage and loss of visual acuity risk in patients with high-risk proliferative diabetic retinopathy. Horm Metab Res. 2001;33:300–6. doi: 10.1055/s-2001-15282. [DOI] [PubMed] [Google Scholar]
- 34.Kuijpers RW, Baarsma S, van Hagen PM. Treatment of cystoid macular edema with octreotide. N Engl J Med. 1998;338:624–6. doi: 10.1056/NEJM199802263380917. [DOI] [PubMed] [Google Scholar]
- 35.Hernández C, Simó R. European Consortium for the Early Treatment of Diabetic Retinopathy (EUROCONDOR). Somatostatin replacement: A new strategy for treating diabetic retinopathy. Curr Med Chem. 2013;20:3251–7. doi: 10.2174/09298673113209990024. [DOI] [PubMed] [Google Scholar]
- 36.Growth Hormone Antagonist for Proliferative Diabetic Retinopathy Study Group. the effect of a growth hormone receptor antagonist drug on proliferative diabetic retinopathy. Ophthalmology. 2001;108:2266–72. doi: 10.1016/s0161-6420(01)00853-3. [DOI] [PubMed] [Google Scholar]
- 37.A Phase 1 Safety Study of TG100801 Eye Drops in Healthy Volunteers. [Last accessed on 2014 Sep 17]. Available from: http://www.clinicaltrials.gov/ct2/show/NCT00414999 .
- 38.Thakur A, Scheinman RI, Rao VR, Kompella UB. Pazopanib, a multitargeted tyrosine kinase inhibitor, reduces diabetic retinal vascular leukostasis and leakage. Microvasc Res. 2011;82:346–50. doi: 10.1016/j.mvr.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cohen MP, Hud E, Wu VY, Shearman CW. Amelioration of diabetes-associated abnormalities in the vitreous fluid by an inhibitor of albumin glycation. Invest Ophthalmol Vis Sci. 2008;49:5089–93. doi: 10.1167/iovs.08-1993. [DOI] [PubMed] [Google Scholar]
- 40.Cohen MP, Lautenslager GT, Hud E, Shea E, Wang A, Chen S, et al. Inhibiting albumin glycation attenuates dysregulation of VEGFR-1 and collagen IV subchain production and the development of renal insufficiency. Am J Physiol Renal Physiol. 2007;292:F789–95. doi: 10.1152/ajprenal.00201.2006. [DOI] [PubMed] [Google Scholar]
- 41.Miyamoto N, Iossifov D, Metge F, Behar-Cohen F. Early effects of intravitreal triamcinolone on macular edema: Mechanistic implication. Ophthalmology. 2006;113:2048–53. doi: 10.1016/j.ophtha.2006.05.037. [DOI] [PubMed] [Google Scholar]
- 42.Beck RW, Edwards AR, Aiello LP, Bressler NM, Ferris F, et al., editors. Diabetic Retinopathy Clinical Research Network (DRCR. net). Three-year follow-up of a randomized trial comparing focal/grid photocoagulation and intravitreal triamcinolone for diabetic macular edema. Arch Ophthalmol. 2009;127:245–51. doi: 10.1001/archophthalmol.2008.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boyer DS, Yoon YH, Belfort R, Jr, Bandello F, Maturi RK, Augustin AJ, et al. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology. 2014;121:1904–14. doi: 10.1016/j.ophtha.2014.04.024. [DOI] [PubMed] [Google Scholar]
- 44.Nakano Goto S, Yamamoto T, Kirii E, Abe S, Yamashita H. Treatment of diffuse diabetic macular oedema using steroid eye drops. Acta Ophthalmol. 2012;90:628–32. doi: 10.1111/j.1755-3768.2010.02066.x. [DOI] [PubMed] [Google Scholar]
- 45.Campochiaro PA, Brown DM, Pearson A, Chen S, Boyer D, Ruiz-Moreno J, et al. Sustained delivery fluocinolone acetonide vitreous inserts provide benefit for at least 3 years in patients with diabetic macular edema. Ophthalmology. 2012;119:2125–32. doi: 10.1016/j.ophtha.2012.04.030. [DOI] [PubMed] [Google Scholar]
- 46.Schoenberger SD, Kim SJ, Shah R, Sheng J, Cherney E. Reduction of interleukin 8 and platelet-derived growth factor levels by topical ketorolac, 0.45%, in patients with diabetic retinopathy. JAMA Ophthalmol. 2014;132:32–7. doi: 10.1001/jamaophthalmol.2013.6203. [DOI] [PubMed] [Google Scholar]
- 47.Callanan D, Williams P. Topical nepafenac in the treatment of diabetic macular edema. Retina. 2010;30:459–67. doi: 10.2147/opth.s3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kern TS, Miller CM, Du Y, Zheng L, Mohr S, Ball SL, et al. Topical administration of nepafenac inhibits diabetes-induced retinal microvascular disease and underlying abnormalities of retinal metabolism and physiology. Diabetes. 2007;56:373–9. doi: 10.2337/db05-1621. [DOI] [PubMed] [Google Scholar]
- 49.Yang LP, Sun HL, Wu LM, Guo XJ, Dou HL, Tso MO, et al. Baicalein reduces inflammatory process in a rodent model of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2009;50:2319–27. doi: 10.1167/iovs.08-2642. [DOI] [PubMed] [Google Scholar]
- 50.Tsilimbaris MK, Panagiotoglou TD, Charisis SK, Anastasakis A, Krikonis TS, Christodoulakis E. The use of intravitreal etanercept in diabetic macular oedema. Semin Ophthalmol. 2007;22:75–9. doi: 10.1080/08820530701418243. [DOI] [PubMed] [Google Scholar]
- 51.Sfikakis PP, Markomichelakis N, Theodossiadis GP, Grigoropoulos V, Katsilambros N, Theodossiadis PG. Regression of sight-threatening macular edema in type 2 diabetes following treatment with the anti-tumor necrosis factor monoclonal antibody infliximab. Diabetes Care. 2005;28:445–7. doi: 10.2337/diacare.28.2.445. [DOI] [PubMed] [Google Scholar]
- 52.Cellini M, Balducci N, Strobbe E, Campos EC. Subtenon injection of natural leukocyte interferon α-2a in diabetic macular edema: A case report. BMC Ophthalmol. 2013;13:63. doi: 10.1186/1471-2415-13-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aiello LP, Bursell SE, Clermont A, Duh E, Ishii H, Takagi C, et al. Vascular endothelial growth factor-induced retinal permeability is mediated by protein kinase C in vivo and suppressed by an orally effective beta-isoform-selective inhibitor. Diabetes. 1997;46:1473–80. doi: 10.2337/diab.46.9.1473. [DOI] [PubMed] [Google Scholar]
- 54.Ishii H, Jirousek MR, Koya D, Takagi C, Xia P, Clermont A, et al. Amelioration of vascular dysfunctions in diabetic rats by an oral PKC beta inhibitor. Science. 1996;272:728–31. doi: 10.1126/science.272.5262.728. [DOI] [PubMed] [Google Scholar]
- 55.Danis RP, Bingaman DP, Jirousek M, Yang Y. Inhibition of intraocular neovascularization caused by retinal ischemia in pigs by PKCbeta inhibition with LY333531. Invest Ophthalmol Vis Sci. 1998;39:171–9. [PubMed] [Google Scholar]
- 56.Aiello LP, Vignati L, Sheetz MJ, Zhi X, Girach A, Davis MD, et al. Oral protein kinase c Beta inhibition using ruboxistaurin: efficacy, safety and causes of vision loss among 813 patients (1,392 eyes) with diabetic retinopathy in the Protein Kinase C Beta Inhibitor- Diabetic Retinopathy Study and the Protein Kinase C Beta Inhibitor-Diabetic Retinopathy Study 2. Retina. 2011;31:2084–94. doi: 10.1097/IAE.0b013e3182111669. [DOI] [PubMed] [Google Scholar]
- 57.Seo MS, Kwak N, Ozaki H, Yamada H, Okamoto N, Yamada E, et al. Dramatic inhibition of retinal and choroidal neovascularization by oral administration of a kinase inhibitor. Am J Pathol. 1999;154:1743–53. doi: 10.1016/S0002-9440(10)65430-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Abiko T, Abiko A, Clermont AC, Shoelson B, Horio N, Takahashi J, et al. Characterization of retinal leukostasis and hemodynamics in insulin resistance and diabetes: Role of oxidants and protein kinase-C activation. Diabetes. 2003;52:829–37. doi: 10.2337/diabetes.52.3.829. [DOI] [PubMed] [Google Scholar]
- 59.Sun W, Oates PJ, Coutcher JB, Gerhardinger C, Lorenzi M. A selective aldose reductase inhibitor of a new structural class prevents or reverses early retinal abnormalities in experimental diabetic retinopathy. Diabetes. 2006;55:2757–62. doi: 10.2337/db06-0138. [DOI] [PubMed] [Google Scholar]
- 60.Drel VR, Pacher P, Ali TK, Shin J, Julius U, El-Remessy AB, et al. Aldose reductase inhibitor fidarestat counteracts diabetes-associated cataract formation, retinal oxidative-nitrosative stress, glial activation, and apoptosis. Int J Mol Med. 2008;21:667–76. [PMC free article] [PubMed] [Google Scholar]
- 61.Hattori T, Matsubara A, Taniguchi K, Ogura Y. Aldose reductase inhibitor fidarestat attenuates leukocyte-endothelial interactions in experimental diabetic rat retina in vivo. Curr Eye Res. 2010;35:146–54. doi: 10.3109/02713680903447918. [DOI] [PubMed] [Google Scholar]
- 62.Zheng L, Kern TS. Role of nitric oxide, superoxide, peroxynitrite and PARP in diabetic retinopathy. Front Biosci (Landmark Ed) 2009;14:3974–87. doi: 10.2741/3505. [DOI] [PubMed] [Google Scholar]
- 63.Mohammad G, Siddiquei MM, Abu El-Asrar AM. Poly (ADP-ribose) polymerase mediates diabetes-induced retinal neuropathy. Mediators Inflamm 2013. 2013 doi: 10.1155/2013/510451. 510451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Satofuka S, Ichihara A, Nagai N, Noda K, Ozawa Y, Fukamizu A, et al. (Pro) renin receptor-mediated signal transduction and tissue renin-angiotensin system contribute to diabetes-induced retinal inflammation. Diabetes. 2009;58:1625–33. doi: 10.2337/db08-0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang X, Lassila M, Cooper ME, Cao Z. Retinal expression of vascular endothelial growth factor is mediated by angiotensin type 1 and type 2 receptors. Hypertension. 2004;43:276–81. doi: 10.1161/01.HYP.0000113628.94574.0f. [DOI] [PubMed] [Google Scholar]
- 66.Mohamed Q, Wong TY. Emerging drugs for diabetic retinopathy. Expert Opin Emerg Drugs. 2008;13:675–94. doi: 10.1517/14728210802584035. [DOI] [PubMed] [Google Scholar]
- 67.Ahmadieh H, Nourinia R, Hafezi-Moghadam A. Intravitreal fasudil combined with bevacizumab for persistent diabetic macular edema: A novel treatment. JAMA Ophthalmol. 2013;131:923–4. doi: 10.1001/jamaophthalmol.2013.143. [DOI] [PubMed] [Google Scholar]
- 68.Lee K, Hu Y, Ding L, Chen Y, Takahashi Y, Mott R, et al. Therapeutic potential of a monoclonal antibody blocking the Wnt pathway in diabetic retinopathy. Diabetes. 2012;61:2948–57. doi: 10.2337/db11-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hu B, Zhang Y, Zeng Q, Han Q, Zhang L, Liu M, et al. Intravitreal injection of ranibizumab and CTGF shRNA improves retinal gene expression and microvessel ultrastructure in a rodent model of diabetes. Int J Mol Sci. 2014;15:1606–24. doi: 10.3390/ijms15011606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nguyen QD, Schachar RA, Nduaka CI, Sperling M, Basile AS, Klamerus KJ, et al. Dose-ranging evaluation of intravitreal siRNA PF-04523655 for diabetic macular edema (the DEGAS study) Invest Ophthalmol Vis Sci. 2012;53:7666–74. doi: 10.1167/iovs.12-9961. [DOI] [PubMed] [Google Scholar]
- 71.Nasrallah FP, Jalkh AE, Van Coppenolle F, Kado M, Trempe CL, McMeel JW, et al. The role of the vitreous in diabetic macular edema. Ophthalmology. 1988;95:1335–9. doi: 10.1016/s0161-6420(88)33004-6. [DOI] [PubMed] [Google Scholar]
- 72.Kuppermann BD, Thomas EL, de Smet MD, Grillone LR Vitrase for Vitreous Hemorrhage Study Groups. Safety results of two phase III trials of an intravitreous injection of highly purified ovine hyaluronidase (Vitrase) for the management of vitreous hemorrhage. Am J Ophthalmol. 2005;140:585–97. doi: 10.1016/j.ajo.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 73.Xu H, Zhang L, Gu L, Lu L, Gao G, Li W, et al. Subretinal delivery of AAV2-mediated human erythropoietin gene is protective and safe in experimental diabetic retinopathy. Invest Ophthalmol Vis Sci. 2014;55:1519–30. doi: 10.1167/iovs.13-13155. [DOI] [PubMed] [Google Scholar]
- 74.Rosales MA, Silva KC, Duarte DA, de Oliveira MG, de Souza GF, Catharino RR, et al. S-nitrosoglutathione inhibits inducible nitric oxide synthase upregulation by redox posttranslational modification in experimental diabetic retinopathy. Invest Ophthalmol Vis Sci. 2014;55:2921–32. doi: 10.1167/iovs.13-13762. [DOI] [PubMed] [Google Scholar]
- 75.Kaji Y, Usui T, Ishida S, Yamashiro K, Moore TC, Moore J, et al. Inhibition of diabetic leukostasis and blood-retinal barrier breakdown with a soluble form of a receptor for advanced glycation end products. Invest Ophthalmol Vis Sci. 2007;48:858–65. doi: 10.1167/iovs.06-0495. [DOI] [PubMed] [Google Scholar]
- 76.Kern TS, Engerman RL. Pharmacological inhibition of diabetic retinopathy: Aminoguanidine and aspirin. Diabetes. 2001;50:1636–42. doi: 10.2337/diabetes.50.7.1636. [DOI] [PubMed] [Google Scholar]
- 77.Bhatwadekar A, Glenn JV, Figarola JL, Scott S, Gardiner TA, Rahbar S, et al. A new advanced glycation inhibitor, LR-90, prevents experimental diabetic retinopathy in rats. Br J Ophthalmol. 2008;92:545–7. doi: 10.1136/bjo.2007.127910. [DOI] [PubMed] [Google Scholar]
- 78.Steigerwalt R, Nebbioso M, Appendino G, Belcaro G, Ciammaichella G, Cornelli U, et al. Meriva®, a lecithinized curcumin delivery system, in diabetic microangiopathy and retinopathy. Panminerva Med. 2012;54:11–6. [PubMed] [Google Scholar]
- 79.Gillies MC, McAllister IL, Zhu M, Wong W, Louis D, Arnold JJ, et al. Intravitreal triamcinolone prior to laser treatment of diabetic macular edema: 24-month results of a randomized controlled trial. Ophthalmology. 2011;118:866–72. doi: 10.1016/j.ophtha.2010.09.029. [DOI] [PubMed] [Google Scholar]
- 80.Nguyen QD, Shah SM, Khwaja AA, Channa R, Hatef E, Do DV, et al. Two-year outcomes of the ranibizumab for edema of the mAcula in diabetes (READ-2) study. Ophthalmology. 2010;117:2146–51. doi: 10.1016/j.ophtha.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 81.Nishimura M, Nakano K, Ushiyama M, Nanbu A, Ohtsuka K, Takahashi H, et al. Increased serum concentrations of human hepatocyte growth factor in proliferative diabetic retinopathy. J Clin Endocrinol Metab. 1998;83:195–8. doi: 10.1210/jcem.83.1.4499. [DOI] [PubMed] [Google Scholar]
- 82.Jin M, Kashiwagi K, Iizuka Y, Tanaka Y, Imai M, Tsukahara S. Matrix metalloproteinases in human diabetic and nondiabetic vitreous. Retina. 2001;21:28–33. doi: 10.1097/00006982-200102000-00005. [DOI] [PubMed] [Google Scholar]
- 83.Capeans C, De Rojas MV, Lojo S, Salorio MS. C-C chemokines in the vitreous of patients with proliferative vitreoretinopathy and proliferative diabetic retinopathy. Retina. 1998;18:546–50. [PubMed] [Google Scholar]
- 84.Feener EP. Plasma kallikrein and diabetic macular edema. Curr Diab Rep. 2010;10:270–5. doi: 10.1007/s11892-010-0127-1. [DOI] [PubMed] [Google Scholar]
- 85.Rangasamy S, Srinivasan R, Maestas J, McGuire P, Das A. A potential role of angiopoietin-2 in the alteration of the blood-retinalbarrier in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2011;52:3784–91. doi: 10.1167/iovs.10-6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yoshida A, Yoshida S, Ishibashi T, Kuwano M, Inomata H. Suppression of retinal neovascularization by the NF-kappaB inhibitor pyrrolidine dithiocarbamate in mice. Invest Ophthalmol Vis Sci. 1999;40:1624–9. [PubMed] [Google Scholar]


