Abstract
Purpose:
The purpose was to evaluate the features of circumscribed choroidal hemangioma using spectral-domain enhanced depth imaging optical coherence tomography (EDI-OCT).
Design:
Retrospective observational case series.
Participants:
Ten patients with newly diagnosed circumscribed choroidal hemangioma.
Methods:
Spectral-domain EDI-OCT was performed with a Heidelberg Spectralis HRA + OCT (Heidelberg Engineering, Heidelberg, Germany).
Main Outcome Measures:
Tumor thickness and EDI-OCT features.
Results:
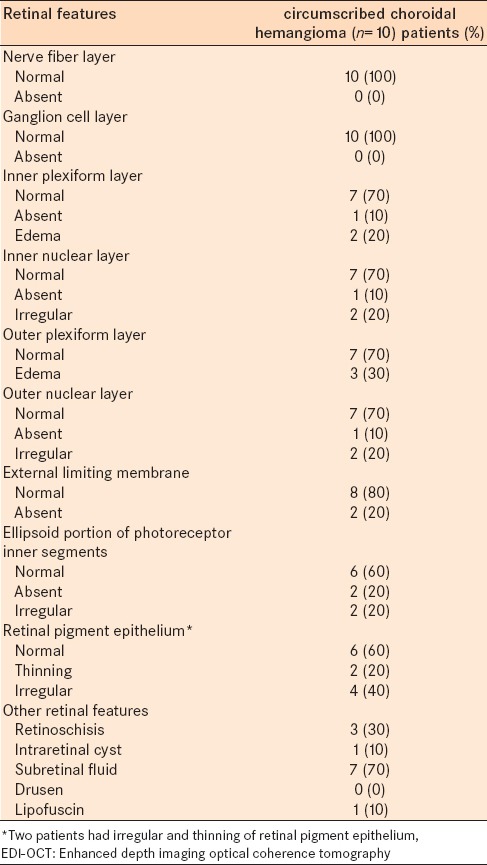
The mean tumor diameter for all eyes was 5.4 mm and mean tumor thickness was 1187 μm by EDI-OCT compared to 2400 μm by ultrasonography. EDI-OCT imaged all tumors as smooth with a gently sloping anterior contour, gradual choroidal expansion, expansion of medium and large size choroidal vessels without compression of choriocapillaris, and intact Bruch's membrane (n = 10, 100%). The height of the medium and large choroidal vessels within the tumor compared to normal medium and large vessels was comparatively increased by a mean of 265% (medium vessels) and 576% (large vessels). Outer retinal abnormalities included subretinal fluid (n = 7, 70%), lipofuscin deposition (n = 1, 10%), irregularity and thinning of retinal pigment epithelium and absence or irregularity of the ellipsoid layer (n = 4, 40%), absent external limiting membrane (n = 2, 20%), and disruption of the outer nuclear layer and outer plexiform layer (n = 3, 30%). The inner retinal abnormalities included irregularity of inner nuclear layer and structural loss or edema of inner plexiform layer (n = 3, 30%). The ganglion cell layer and nerve fiber layer were intact (n = 10, 100%).
Conclusion:
EDI-OCT of circumscribed choroidal hemangiomas depicts a smooth, gently sloping choroidal mass with expansion of medium and large size choroidal vessels without compression of the choriocapillaris. Structural abnormalities of outer and inner retinal layers were noted.
Keywords: Choroid, Enhanced Depth Imaging, Hemangioma, Optical Coherence Tomography, Ultrasonography
INTRODUCTION
Circumscribed choroidal hemangioma is a benign vascular tumor, classically orange in color and located in the para-macular region of the eye. This tumor can lead to visual loss from hyperopic shift with subsequent amblyopia, or by producing macular abnormalities such as subretinal fluid, cystoid edema, retinoschisis, and retinal pigment epithelial (RPE) alterations.1,2,3 The diagnosis of choroidal hemangioma is established with fundus examination and confirmed by diagnostic testing. Diagnostic testing includes ultrasonography demonstrating acoustic density, fluorescein angiography showing early and persistent hyperfluoresence, and indocyanine green angiography revealing early hyperfluoresence with late “washout” hypofluoresence. These features help to differentiate this tumor from simulators such as choroidal nevus, melanoma, metastasis, and lymphoma.
Enhanced depth imaging optical coherence tomography (EDI-OCT) allows for high-resolution imaging of the ocular fundus with approximate axial resolution of 3–4 microns.4,5 This technology provides detailed imaging of the architecture of the retina and choroid.4 Recent publications have described choroidal tumor imaging with EDI-OCT, particularly of choroidal nevus and small choroidal melanoma.6,7,8 In this study, we analyze the EDI-OCT findings in 10 eyes with circumscribed choroidal hemangioma, with a specific focus on the intrinsic qualities of the tumor and its effect on the overlying retina.
METHODS
This retrospective study evaluated the imaging features of newly diagnosed, untreated circumscribed choroidal hemangioma using EDI-OCT. All patients diagnosed with circumscribed choroidal hemangioma and imaged with EDI-OCT over a 1-year period, between August 1, 2010 and August 1, 2011, at the Ocular Oncology Service, Wills Eye Hospital, Philadelphia, PA, USA were included. The clinical and imaging features of the circumscribed choroidal hemangioma were evaluated. This study was approved by the Institutional Review Board.
After pupil dilation, spectral-domain EDI-OCT was performed with the Heidelberg Spectralis HRA + OCT (Heidelberg Engineering, Heidelberg, Germany) and acquisition and analysis software version 5.3.3.0 with automated EDI. The axial resolution was 3.5 μm with a scan speed of 40,000 A-scans/s. The images were captured using custom scan acquisition protocol of up to 13 raster lines of 9 mm scan length with 1536 A-scans/line. Real-time eye tracking using TruTrack Active Eye Tracking was employed, and automatic real-time image averaging was set at 100 images. EDI-OCT was performed using a similar technique described by Spaide et al.4
The patient demographic data included age, race, and sex. The clinical tumor data included quadrantic location of the tumor epicenter, tumor basal diameter (mm), distance of tumor margin to foveola and optic disc (mm), color (orange, amelanotic, melanotic), related subretinal fluid, orange pigment (lipofuscin), drusen (absent, present), fibrous metaplasia of retinal pigment epithelium (absent, present), intraretinal edema, and RPE alterations. The tumor thickness was measured by ultrasonography (mm) and EDI-OCT (um).
The EDI-OCT features included optical qualities of the hemangioma, tumor contour, and status of surrounding tissues including choriocapillaris, medium and large size choroidal vessels, overlying RPE/Bruch's membrane and the retina. The height of medium and large choroidal vessels was measured in the area of normal choroid that was immediately adjacent on both the medial and lateral side of the tumor and in the apical tumor area by measuring the interval distance between the vertical margins of the visible vascular cross section. The medium vessels were defined as those in the middle third of the choroid, defined by an anteroposterior line drawn perpendicular to the underlying sclera. The large vessels were those in the outer third of the choroid. Individual vascular measurements were made anteroposteriorly from endothelial wall to the endothelial wall in the normal immediately adjacent choroid and compared to the similar layer within the apical center of the hemangioma. The retinal layers were evaluated for abnormalities from outer to the inner retina. The presence of subretinal fluid, subretinal lipofuscin deposition, subretinal drusen, and intraretinal edema was noted. A parabolic curve was placed on the anterior (curve 1) and posterior tumor margin (curve 2), with interval distance measured as tumor thickness.
RESULTS
Over this 1-year period, there were 30 patients with circumscribed choroidal hemangioma evaluated at the Ocular Oncology Service at Wills Eye Hospital. Of these, 10 patients met the inclusion criteria with excellent image quality, and 20 were excluded due to previous treatment or poor image quality. The demographic and clinical features of the hemangioma are listed in Table 1. The mean patient age was 50 years (median, 50 years; range 26–63 years). All patients were Caucasian. Seven patients (70%) were females. The hemangioma was located in the post-equatorial region in all cases (n = 10, 100%) with a mean distance to the optic disc of 1.8 mm (median, 1; range 0–6 mm) and mean distance to the foveola of 1.3 mm (median, 1; range 0–3 mm). Associated subretinal fluid was noted in 7 (70%) cases.
Table 1.
EDI-OCT of circumscribed choroidal hemangioma in 10 consecutive patients: Demographic and clinical features
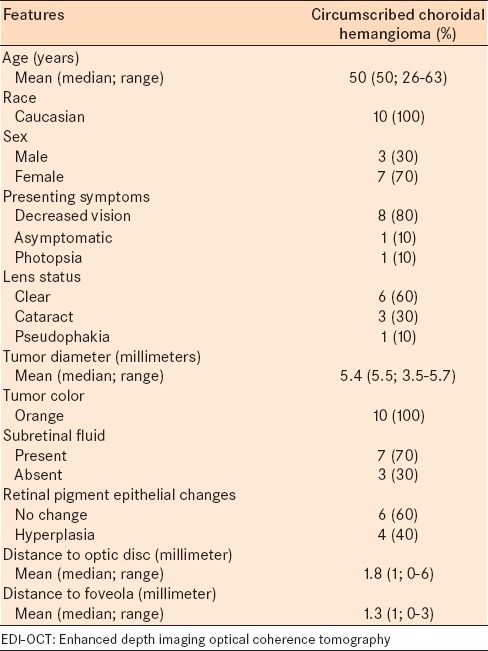
The mean tumor diameter of the hemangioma was 5.4 mm (median, 5.5; range 3.5–5.7 mm) [Table 1], and mean thickness was 2400 μm (median, 2350 μm; range 1900–3100 μm) by ultrasonography [Table 2], and 1187 μm (median, 1069 μm; range, 981 to 1627 μm) by EDI-OCT [Table 3]. The mean difference between tumor thickness measured by ultrasound versus EDI-OCT was 1213 um (50.5% reduction).
Table 2.
EDI-OCT of circumscribed choroidal hemangioma in 10 consecutive cases: Ultrasonographic features
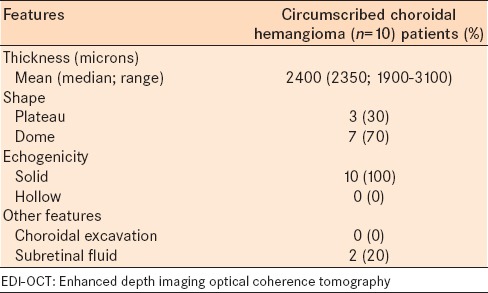
Table 3.
EDI-OCT of circumscribed choroidal hemangioma in 10 consecutive cases: EDI-OCT tumor features
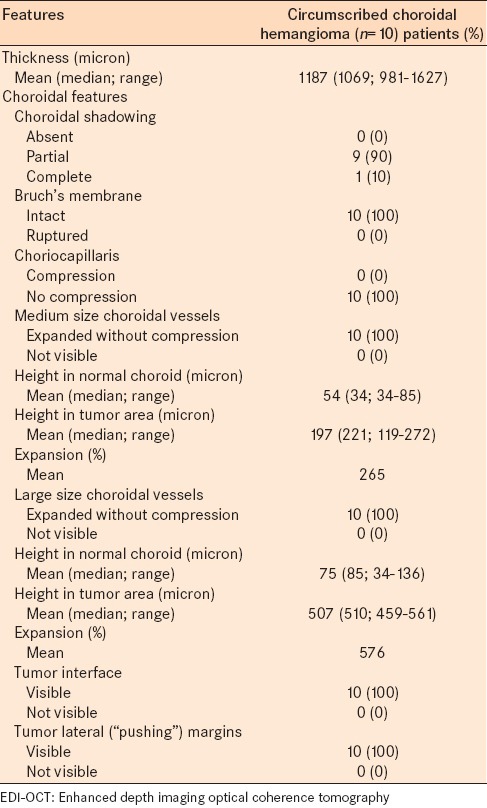
On EDI-OCT, the hemangioma was imaged as smooth with a gently sloping anterior contour (n = 10, 100%), visible tumor interface (n = 10, 100%), gradual choroidal expansion without compression of the choriocapillaris [Figure 1] (n = 10, 100%), and expansion of medium and large size choroidal vessels without compression (n = 10, 100%). The mean height of medium choroidal vessels within the tumor was 197 μm (median, 221 μm; range, 119–272 μm) compared to medium vessels in immediately adjacent normal choroid measuring 54 μm (median, 34 μm; range, 34–85 μm). The mean height of large choroidal vessels within the tumor was 507 μm (median, 510 μm; range, 459–561 μm) compared to large vessels in the immediately adjacent normal choroid measuring 75 μm (median, 85 μm; range, 34–136 μm). Comparatively, medium vessels within the hemangioma were expanded by a mean of 265% compared to those in the normal choroid, and large vessels expanded by a mean of 576% compared to normal.
Figure 1.
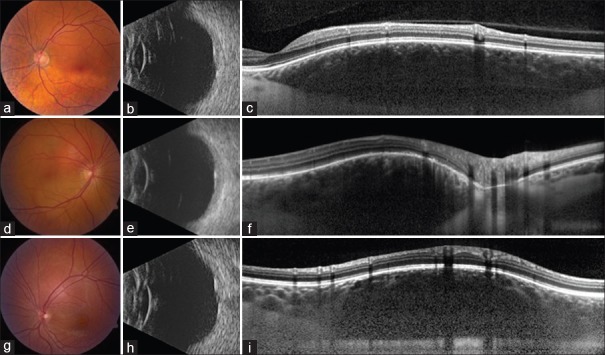
A 52-year-old male with circumscribed choroidal hemangioma in the superonasal macula measuring 7 mm in diameter. (a) Dome-shaped and acoustically dense on B-scan ultrasonography with thickness of 2.2 mm. (b) Enhanced depth imaging optical coherence tomography showed gradual vertical expansion of the choroid without choriocapillaris compression and with partial deep optical shadowing obscuring details. (c) Note the uniform tissue interfaces within the choroid representing the expanded vascular channels. A 46-year-old female with circumscribed choroidal hemangioma in the superonasal macula measuring 4 mm in diameter. (d) Dome-shaped and acoustically dense on B-scan ultrasonography with thickness of 2.3 mm. (e) Enhanced depth imaging optical coherence tomography showed gradual vertical expansion of the choroid in the area of tumor with no compression of choriocapillaris. Note the uniform dilated vascular tissue interfaces within the choroid. (f) A 26-year-old female with circumscribed choroidal hemangioma superotemporal to the optic disc measuring 3.5 mm in diameter. (g) Dome-shaped, acoustically dense on B-scan ultrasonography with thickness of 2.1 mm. (h) Enhanced depth imaging optical coherence tomography showed gradual vertical expansion of the choroid in the area of tumor with no compression of the choriocapillaris. (i) The expanded medium and large choroidal vessels are more clearly noted in the periphery of the mass as central shadowing reduces resolution
Other features included optical shadowing manifesting as partial in 9 (90%) cases or complete in 1 (10%) case. Outer and inner retinal features are listed in Table 4.
Table 4.
EDI-OCT of circumscribed choroidal hemangioma in 10 consecutive cases: EDI-OCT retinal features
DISCUSSION
Circumscribed choroidal hemangioma is a benign vascular tumor that appears clinically as a round or oval orange-red mass. This tumor can remain hidden underlying subretinal fluid, masquerading as central serous chorioretinopathy.1 In other instances, this tumor can simulate amelanotic choroidal melanoma or metastasis.1,2,3 Shields et al.1 reviewed 200 consecutive cases of circumscribed choroidal hemangioma and found that 38% of cases were initially misinterpreted as choroidal melanoma or metastasis before referral to an ocular oncology center.1
Ancillary testing with ultrasonography, fluorescein angiography, and indocyanine green angiography are helpful to establish the diagnosis of circumscribed choroidal hemangioma. In 198 cases of choroidal hemangioma, ultrasonography revealed high internal reflectivity on A-scan and acoustic solidity on B-scan.1 In 195 cases, fluorescein angiography revealed hyperfluorescence in prearterial or arterial phase, with staining and leakage in the late phase.1 In 49 cases, indocyanine green angiography revealed bright hyperfluorescence by 1 min, moderate hyperfluorescnence in the mid-study at 8 min, and moderate hypofluorescence at 20 min with late washout phenomenon, leaving a central hypofluorescent region surrounded by a rim of hyperfluorescence.1,9 In 27 cases, fundus autofluoresence revealed bright hyperautofluorescence overlying the tumor in cases with fresh hemangioma contrasted to dark hypoautofluorescence over those following therapy or those with chronic retinal pigment epithelium hyperplasia and atrophy.10
Time domain OCT has shown poor image resolution of circumscribed choroidal hemangioma as the mass appears optically hyperreflective on its anterior surface but with little information deep within the tumor.11 Time domain OCT can be beneficial for information on the status of the overlying retina, particularly if there is visual loss from subretinal fluid, retinal edema, photoreceptor loss, induced hyperopia, and foveal tilt from the elevated mass.12 Soucek and Cilhelkova evaluated time domain OCT in nine patients with circumscribed choroidal hemangioma following photodynamic therapy and concluded that OCT was useful for evaluation of subretinal fluid resorption, but details of the tumor were limited by technical drawbacks.13
Spectral domain EDI-OCT provides improved visualization of choroidal details. Torres et al. studied the EDI-OCT features of circumscribed choroidal hemangioma in three patients and described the appearance as an elevation of the retina/choroid complex with low to medium reflectivity and a homogenous signal with large intrinsic spaces, presumed to represent the vascular spaces.6 The details of choriocapillaris and inner sclera could be obtained in one patient.6 In our study of 10 patients, we found that most tumors were ideal for imaging due to the perimacular location and ultrasonographic thickness of 3 mm or less. Compared to ultrasonography, tumor thickness measurement by EDI-OCT was 51% less than ultrasonography, likely related to higher resolution of details allowing for precise placement of the measurement cursors on EDI-OCT. Measurement by ultrasound could be artificially increased by inclusion of the overlying retina and inner sclera, particularly when resolution is poor.
The most important features of choroidal hemangioma on EDI-OCT were the gradual dome-shaped anterior tumor contour with expansion of the medium and large choroidal vessels without compression of the choriocapillaris. Other choroidal tumors such as nevus and small choroidal melanoma show similar smooth anterior contours, but different internal features, demonstrating compression of the choriocapillaris and lack of expansile choroidal vessels within the tumor.7,8 The anterior tumor surface differs with choroidal tumors such as metastasis that are “lumpy bumpy,”14 lymphoma with “seasick” surface,15 and sclerochoroidal calcification “rocky and rolling” surface.16 In addition, those tumors show anterior compression of the choroid vessels with deep replacement by solid tumor. Shah et al. reviewed the EDI-OCT features of 104 choroidal nevi and found that 94% had compression of overlying choriocapillaris.7 Shields et al. analyzed 37 small choroidal melanomas with EDI-OCT and found that 100% had compression of choriocapillaris.8 Al-Dahmash et al. performed EDI-OCT in 14 choroidal metastases and found that 93% had anterior choriocapillaris compression.14 These features serve to differentiate choroidal hemangioma from other choroidal tumors. The preservation of internal vascular structures is further highlighted by fluorescein angiography and indocyanine green angiography.
Histopathological analysis of 28 cases of choroidal hemangioma by Jones and Cleasby revealed dilated blood channels of variable size in the region of large and medium-sized choroidal vessels separated by delicate connective tissue.17 In 45 cases of circumscribed choroidal hemangioma reviewed clinicopathologically, Witschel and Font observed large, thin-walled, blood-filled vascular channels and capillary-type vessels, separated by loose, edematous connective tissue, and 75% showed well-demarcated “pushing” peripheral margins with separation from the uninvolved choroid by a layer of compressed melanocytes and choroidal lamellae.18 The choriocapillaris and Bruch's membrane were well-preserved in most instances.18 Using EDI-OCT, we found remarkably similar observations. In particular, we noted the smooth anterior tumor interface, expansion of medium and large size choroidal vessels to approximately 265% (medium vessels) and 576% (large vessels) of the normal size, and preservation of choroiocapillaris. In 100% (n = 10) of cases, we were able to clearly delineate the “pushing” tumor margin, seen as a lenticular-shaped edge. One major drawback of EDI-OCT for choroidal hemangioma and other intraocular tumors remains the optical shadowing, found to some degree in nearly every case. This can be partial or complete shadowing and causes reduction in image resolution of deeper tissue.
In summary, EDI-OCT is a noninvasive imaging technique that provides important information on circumscribed choroidal hemangioma, particularly the smooth, gradually sloping anterior tumor interface, gradual vertical expansion of the choroid particularly of the medium and large size choroidal vessels without choriocapillaris compression. These features can assist in differentiating choroidal hemangioma from other tumors.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Shields CL, Honavar SG, Shields JA, Cater J, Demirci H. Circumscribed choroidal hemangioma: Clinical manifestations and factors predictive of visual outcome in 200 consecutive cases. Ophthalmology. 2001;108:2237–48. doi: 10.1016/s0161-6420(01)00812-0. [DOI] [PubMed] [Google Scholar]
- 2.Shields JA, Shields CL. 2nd ed. Philadelphia: Lippincott Williams and Wilkins; 2008. Intraocular Tumors. An Atlas and Textbook; pp. 230–45. [Google Scholar]
- 3.Mashayekhi A, Shields CL. Circumscribed choroidal hemangioma. Curr Opin Ophthalmol. 2003;14:142–9. doi: 10.1097/00055735-200306000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Spaide RF, Koizumi H, Pozzoni MC. Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol. 2008;146:496–500. doi: 10.1016/j.ajo.2008.05.032. [DOI] [PubMed] [Google Scholar]
- 5.Kiernan DF, Mieler WF, Hariprasad SM. Spectral-domain optical coherence tomography: A comparison of modern high-resolution retinal imaging systems. Am J Ophthalmol. 2010;149:18–31. doi: 10.1016/j.ajo.2009.08.037. [DOI] [PubMed] [Google Scholar]
- 6.Torres VL, Brugnoni N, Kaiser PK, Singh AD. Optical coherence tomography enhanced depth imaging of choroidal tumors. Am J Ophthalmol. 2011;151:586–593.e2. doi: 10.1016/j.ajo.2010.09.028. [DOI] [PubMed] [Google Scholar]
- 7.Shah SU, Kaliki S, Shields CL, Ferenczy SR, Harmon SA, Shields JA. Enhanced depth imaging optical coherence tomography of choroidal nevus in 104 cases. Ophthalmology. 2012;119:1066–72. doi: 10.1016/j.ophtha.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Shields CL, Kaliki S, Rojanaporn D, Ferenczy SR, Shields JA. Enhanced depth imaging optical coherence tomography of small choroidal melanoma: Comparison with choroidal nevus. Arch Ophthalmol. 2012;130:850–6. doi: 10.1001/archophthalmol.2012.1135. [DOI] [PubMed] [Google Scholar]
- 9.Arevalo JF, Shields CL, Shields JA, Hykin PG, De Potter P. Circumscribed choroidal hemangioma: Characteristic features with indocyanine green videoangiography. Ophthalmology. 2000;107:344–50. doi: 10.1016/s0161-6420(99)00051-2. [DOI] [PubMed] [Google Scholar]
- 10.Ramasubramanian A, Shields CL, Harmon SA, Shields JA. Autofluorescence of choroidal hemangioma in 34 consecutive eyes. Retina. 2010;30:16–22. doi: 10.1097/IAE.0b013e3181bceedb. [DOI] [PubMed] [Google Scholar]
- 11.Say EA, Shah SU, Ferenczy S, Shields CL. Optical coherence tomography of retinal and choroidal tumors. J Ophthalmol 2012. 2012 doi: 10.1155/2012/385058. 385058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shields CL, Materin MA, Shields JA. Review of optical coherence tomography for intraocular tumors. Curr Opin Ophthalmol. 2005;16:141–54. doi: 10.1097/01.icu.0000158258.01681.40. [DOI] [PubMed] [Google Scholar]
- 13.Soucek P, Cihelková I. Evaluation of subretinal fluid absorption by optical coherence tomography in circumscribed choroidal hemangioma after photodynamic therapy with Verteporfin. Neuro Endocrinol Lett. 2004;25:109–14. [PubMed] [Google Scholar]
- 14.Al-Dahmash SA, Shields CL, Kaliki S, Johnson T, Shields JA. Enhanced depth imaging optical coherence tomography of choroidal metastasis in 14 eyes. Retina. 2014;34:1588–93. doi: 10.1097/IAE.0000000000000131. [DOI] [PubMed] [Google Scholar]
- 15.Arias JD, Kumar N, Fulco EA, Spaide RF, Yannuzzi LA, Shields JA, et al. Seasick choroid: A finding on enhanced depth imaging spectral domain optical coherence tomography (EDI-OCT) of choroidal lymphoma. Retin Cases Brief Rep. 2013;7:19–22. doi: 10.1097/ICB.0b013e3182733d6b. [DOI] [PubMed] [Google Scholar]
- 16.Fung AT, Arias JD, Shields CL, Shields JA. Sclerochoroidal calcification is primarily a scleral condition based on enhanced depth imaging optical coherence tomography. JAMA Ophthalmol. 2013;131:960–3. doi: 10.1001/jamaophthalmol.2013.67. [DOI] [PubMed] [Google Scholar]
- 17.Jones IS, Cleasby GW. Hemangioma of the choroid: A clinicopathologic analysis. Am J Ophthalmol. 1959;48:612–28. doi: 10.1016/0002-9394(59)90452-0. [DOI] [PubMed] [Google Scholar]
- 18.Witschel H, Font RL. Hemangioma of the choroid. A clinicopathologic study of 71 cases and a review of the literature. Surv Ophthalmol. 1976;20:415–31. doi: 10.1016/0039-6257(76)90067-9. [DOI] [PubMed] [Google Scholar]


