Abstract
Purpose:
To evaluate changes in corneal topography and biomechanical properties after collagen cross-linking (CXL) for progressive keratoconus.
Patients and Methods:
Collagen cross-linking was performed on 97 eyes. We assessed uncorrected visual acuity (UCVA) and best corrected visual acuity (BCVA). Corneal topography indices were evaluated using placido disc topography, scanning slit anterior topography (Orbscan II), and rotating Scheimpflug topography (Pentacam). Specular microscopy and corneal biomechanics were evaluated.
Results:
A 1-year-follow-up results revealed that UCVA improved from 0.31 to 0.45 and BCVA changed from 0.78 to 0.84 (P < 0.001). The mean of average keratometry value decreased from 49.62 to 47.95 D (P < 0.001). Astigmatism decreased from 4.84 to 4.24 D (P < 0.001). Apex corneal thickness decreased from 458.11 to 444.46 μm. Corneal volume decreased from 56.66 to 55.97 mm3 (P < 0.001). Posterior best fit sphere increased from 55.50 to 46.03 mm (P = 0.025). Posterior elevation increased from 99.2 to 112.22 μm (P < 0.001). Average progressive index increased from 2.26 to 2.56 (P < 0.001). A nonsignificant decrease was observed in mean endothelial count from 2996 to 2928 cell/mm2 (P = 0.190). Endothelial coefficient of variation (CV) increased nonsignificantly from 18.26 to 20.29 (P = 0.112). Corneal hysteresis changed from 8.18 to 8.36 (P = 0.552) and corneal resistance factor increased from 6.98 to 7.21 (P = 0.202), so these changes were not significant.
Conclusion:
Visual acuity and K values improved after CXL. In spite of the nonsignificant increase in endothelial cell count and increase in the CV, CLX seems to be a safe treatment for keratoconus. Further studies with larger sample sizes and longer follow-up periods are recommended.
Keywords: Biomechanics, Collagen Crosslinking, Corneal, Keratoconus, Specular Microscopy, Topography
INTRODUCTION
Keratoconus is a degenerative noninflammatory corneal disorder.1 It leads to decreased vision by distorting the anterior corneal surface, and inducing apical thinning, high irregular astigmatism, and central scarring of the cornea.1 Several modalities such as hard contact lens, intracorneal stromal ring implantation, and penetrating keratoplasty are used to treat keratoconus.2 All these techniques only correct the refractive error of the cornea with no effect on the progression of keratoconus.2 The only treatment that is believed to have the ability to stop or decrease the progression of keratoconus is collagen cross-linking (CXL).3 CXL changes the biomechanical, thermomechanical, and morphological properties of the cornea.4 It increases stiffness and rigidity of the anterior corneal stroma,5,6 and enhances corneal resistance to proteolytic enzymes by inducing photochemical crosslinking and covalent bindings between individual collagen fibers.3,7 CXL halts the progression of keratoconus with a failure rate of approximately 3% and a complication rate of ≤ 1%.8
Since CXL alters the corneal shape and structure, it would be helpful to assess resulting changes in quantitative descriptors of the cornea, which can affect the clinical outcome of this procedure.9 Previous studies have shown that CXL improves visual acuity, average keratometry values, and definable measures of corneal topography regularity.9 We aimed to evaluate the effect of CXL on corneal parameters, including pachymetry, corneal biomechanics, specular microscopy, and corneal topography, using Scheimpflug imaging (Pentacam, Oculus, Inc., Wetzler, Germany), and slit beam scanning (Orbscan II, Bausch and Lomb, Rochester, New York, USA) during a follow-up period of 1-year.
PATIENTS AND METHODS
In this prospective study, we enrolled 57 patients of white ethnicity with progressive keratoconus, with an age range of 13-30 years. The current project was conducted according to the principles of the declaration of Helsinki, revised in October 2008.10 Study was reviewed by the Ethics Committee of Shiraz University of Medical Sciences, Shiraz, Southern Iran. All the patients gave their written informed consent before participating in the study.
Patients who were <30 years of age with confirmed progressive keratoconus who had a clear cornea without opacity, and central corneal thickness (CCT) >400 μm were included. Patients who had central corneal opacities, CCT <400 μm, history of ocular surgery, herpetic keratitis, severe dry eyes, and connective tissue disease, and those who were not cooperative enough for a 1-year follow-up were excluded from our study. CXL is performed for patients with bacterial and fungal keratitis in our center, but we did not include their data for this study, because we assumed that infectious keratitis may be a confounding factor. We also excluded pregnant patients, because hormonal changes during pregnancy may have a severe impact on the progression of keratoconus. Therefore, we do not perform cross-linking during or directly after pregnancy, and we wait until the corneal curvature has been stabilized. Furthermore, legally we do not have the permission to use UV light for pregnant patients in our center because of its side effects.
Keratoconus progression was proven by central keratometry and pachymetry reports or subjective loss of vision (i.e., increased central keratometry more than 1.0 diopter [D] in 6 months, decreased CCT more than 5% in 6 months, and loss of more than 2 lines of corrected distance visual acuity best corrected visual acuity [BCVA] in 1-year). Patients who needed to change their hard contact lens more than 1 time during 2 years were also considered to have progressive keratoconus.
Follow-up evaluations
Patients were visited preoperatively, in the early postoperative period (until epithelial healing), and 6 and 12 months after CXL. At every follow-up visit, excluding the early.
Postoperative days, a standard examination was performed for all the patients. Ophthalmic examinations included determining uncorrected visual acuity (UCVA) and BCVA, slit lamp examination, dilated funduscopy, subjective and cycloplegic refractions, placido disc corneal topography (Tomey Corp, Nagoya, Japan), scanning-slit anterior topography (Orbscan II, Bausch and Lomb, Rochester, New York, USA), rotating Scheimpflug topography (Pentacam, Oculus, Inc., Wetzler, Germany), corneal biomechanics evaluation by ocular response Analyzer (ORA, Reichert Ophthalmic Instruments, Buffalo, NY, USA), and specular microscopy (SP2000: Topcon Corporation, Tokyo, Japan).
Uncorrected visual acuity and BCVA were determined using the Snellen acuity chart. The data were analyzed using the decimal format. Spheroequivalent (SE), apical keratometry (AK), apical gradient curvature (AGC), and average corneal power in 4 mm zone were evaluated by placidoo disc corneal topography. Scheimpflug topography (Pentacam) was used to evaluate anterior and posterior keratometry, apex and thinnest pachymetry, anterior chamber depth (ACD), corneal volume, average progression index (regarding corneal thickness change from centre to periphery), and corneal asphericity (Q value). Simulated keratometry (Sim K), corneal astigmatism, and anterior and posterior elevation and best fit sphere (BFS) were evaluated by scanning-slit anterior topography (Orbscan II). We assessed endothelial cell mean area, density, and polymegantism (coefficient of variation [CV]) using specular microscopy. We also gathered data on corneal biomechanics such as corneal hysteresis (CH) and corneal resistance factor (CRF) by ORA.
Collagen cross-linking procedure
Central 9.0 mm epithelium was removed by mechanical debridement. Riboflavin 0.1% in 20% dextran was administered topically every 2 min for 30 min. Slit lamp examination was used to confirm riboflavin absorption throughout the corneal stroma and anterior chamber. Then the cornea was exposed to UVA light (365 nm) for 30 min with an optical system (UV-X; Peschke Meditrade, GmbH, Huenenberg, Switzerland) at an irradiance of 3.0 mW/cm2 and 5 cm distance from the cornea. During UVA exposure, isotonic riboflavin was administered every 2 min. Postoperatively, chloramphenicol and betamethasone 0.1% eye drops were administered from day one, and a soft contact lens bandage was placed on the cornea. The contact lens was removed 3 days later, when the epithelial defect was healed. Antibiotic and corticosteroid drops were continued four times a day for 1 and 2 weeks, respectively.
Statistical analysis
Values are expressed as mean ± standard deviation in the tables. Statistical analyses were performed using SPSS software, (version 18, SPSS Inc., Chicago, IL). Data obtained from preoperative and postoperative visits were compared using paired t-test. P <0.05 was considered to be statistically significant.
RESULTS
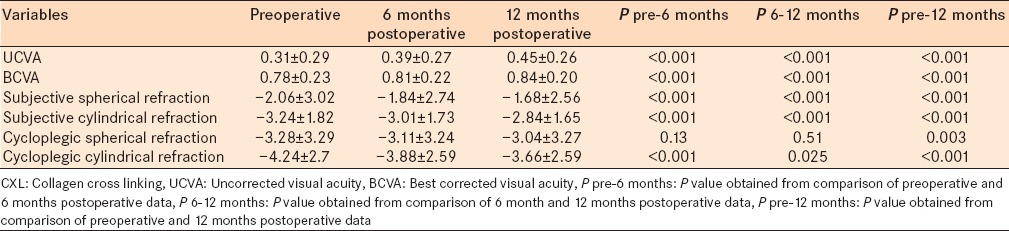
The study comprised 97 eyes with progressive keratoconus. Patients had postoperative visits 6 and 12 months after the CXL procedure. Results from data analysis are shown in the tables. Our results show that the mean UCVA and BCVA improved significantly 6 and 12 months after CXL operation (P < 0.001). The mean subjective spherical and cylindrical refraction values decreased significantly 6 and 12 months postoperatively (P < 0.001). Spherical cycloplegic refraction did not change significantly 6 months after CXL; however, it showed a significant decrease after 1-year compared with the preoperative values. Cylindrical cycloplegic refraction had a significant decrease 6 and 12 months after CXL [Table 1].
Table 1.
Comparison of UCVA, BCVA, subjective spherical, subjective cylindrical, cycloplegic spherical, and cycloplegic cylindrical refractions before and after CXL procedure
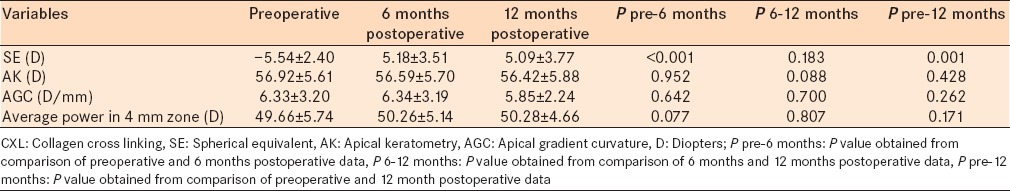
Results obtained from placido disc topography are shown in Table 2. The mean corneal SE reduced significantly 6 and 12 months after CXL compared with preoperative data (P < 0.001). AK, AGC, and average corneal power in the 4 mm zone did not change significantly 6 and 12 months after CXL.
Table 2.
Topographic data using placido disc technique before and after CXL procedure
Tables 3 and 4 show the results obtained from Scheimpflug topography (Pentacam). Anterior surface keratometry in steep and flat axes decreased significantly after 6 and 12 months (P < 0.001). Posterior surface keratometry in steep, flat, and mean axes increased 6 months after CXL (P = 0.018, P < 0.001, and P = 0.002, respectively). However, in the 6 months to 12 months follow-up, posterior surface keratometric values did not change significantly. Ks, Kf, and Km of the back surface were significantly higher after 12 months compared with preoperative data [P = 0.041, P = 0.001, and P < 0.001, respectively, Table 3].
Table 3.
Anterior and posterior surface keratometry in steep and flat axes showed significant corneal changes after CXL procedure
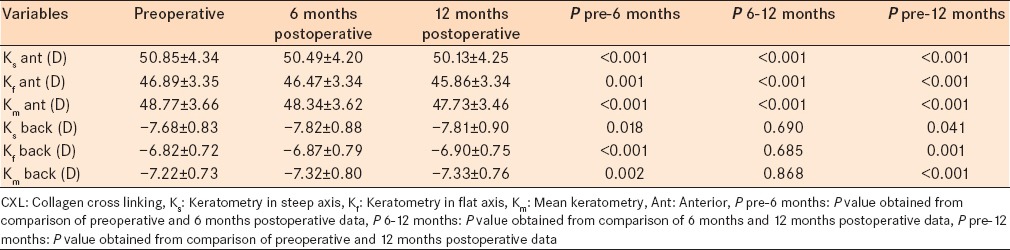
Table 4.
Data obtained from Scheimpflug topography (Pentacam) before and after CXL procedure
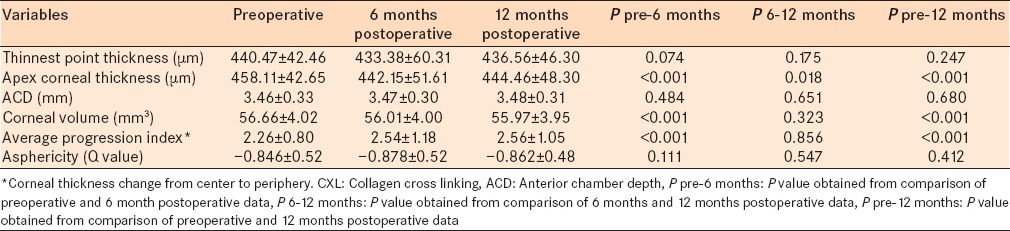
Corneal thickness at the thinnest point did not change significantly over the 6 months and 1-year follow-ups. However, apex corneal thickness decreased significantly 6 and 12 months after CXL (P < 0.001). CXL did not cause statistically significant corneal changes with respect to ACD and asphericity (Q value). Corneal volume decreased and average progression index increased significantly 6 months after CXL compared with preoperative values (P < 0.001); however, they did not change significantly in 6 months to 1-year follow-up. Comparing preoperative and 1-year follow-up data showed significant decrease in corneal volume and significant decrease in the average progression index [P < 0.001, Table 4]. Figure 1 demonstrates changes of corneal parameters in Scheimpflug topography over the 6 months follow-up.
Figure 1.
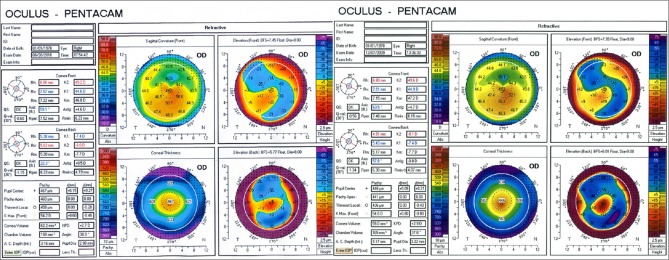
Scheimpflug topography from a patient with progressive keratoconus shows that anterior surface keratometry in steep and flat axes decreased over 6-month follow-up. Posterior surface keratometry remained stable in flat and mean axis and showed minimal increased in steep axis
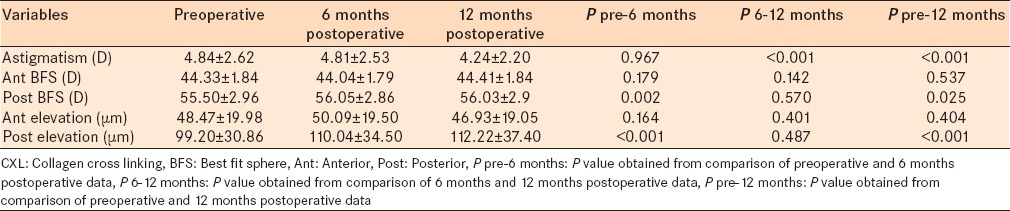
The results obtained from scanning-slit anterior topography (Orbscan II) are shown in Tables 5 and 6. Sim K in steep and flat axes decreased significantly 6 and 12 months after CXL operation [P < 0.001, Table 5]. Sim K decreased by ≥ 2 D in 57 (57%) eyes during the study period. Corneal astigmatism did not change 6 months after CXL; however, it reduced significantly in the 6 months to 1-year follow-up (P < 0.001). We found no significant difference regarding anterior BFS between preoperative and postoperative values. However, posterior BFS increased significantly 6 months and 1-year postoperatively compared with preoperative data (P = 0.002 and P = 0.025, respectively). Anterior elevation did not change postoperatively compared to baseline values. Posterior elevation increased significantly 6 months and 1-year after CXL compared with the baseline [P < 0.001, Table 6].
Table 5.
CXL caused significant decrease in Sim K of cornea
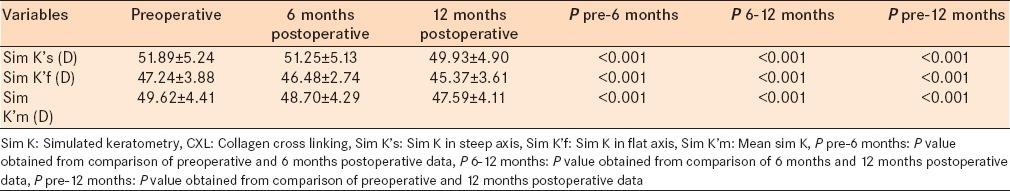
Table 6.
Corneal astigmatism reduced significantly 12 months after CXL procedure, CXL did not change anterior BFS and elevation, but had significant effect on posterior BFS and elevation
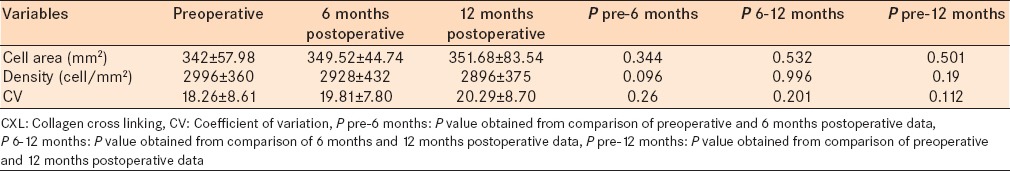
We found that the CXL procedure did not induce any significant changes on endothelial cell size, density, and polymegantism [Table 7]. Endothelial mean cell area changed from 342 ± 57.98 mm2 to 349.52 ± 44.74 mm2 (P = 0.344) and 351.68 ± 83.54 (P = 0.501) mm2 after 6 and 12 months, respectively. The mean endothelial cell density decreased from 2996 ± 360 cell/mm2 to 2928 ± 432 cell/mm2 (P = 0.096) and 2896 ± 375 cell/mm2 (P = 0.190) in the 6 and 12 months follow-up periods, respectively. CV increased from 18.26 ± 8.61 to 19.81 7.80 (P = 0.260) and 20.29 ± 8.70 (P = 0.112) after 6 and 12 months, respectively.
Table 7.
CXL caused no significant changes in specular microscopy over 12 months follow-up
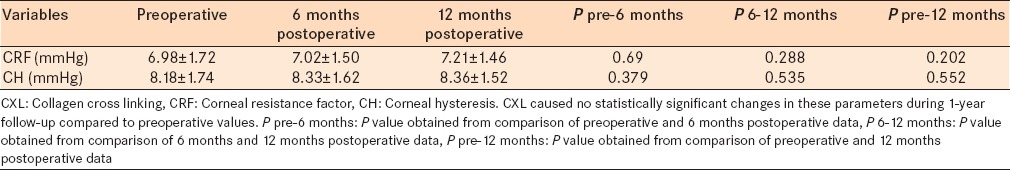
Collagen cross linking caused no statistically significant changes in corneal biomechanical parameters including CRF and CH after 1-year compared with baseline values [Table 8]. CRF changed from 6.98 ± 1.72 mm Hg to 7.02 ± 1.50 mm Hg (P = 0.690) and 7.21 ± 1.46 mm Hg (P = 0.202) after 6 and 12 months, respectively. CH increased from 8.18 ± 1.74 mm Hg to 8.33 ± 1.62 mm Hg (P = 0.379) and 8.36 ± 1.52 mm Hg (P = 0.552) after 6 and 12 months, respectively.
Table 8.
Analyzed data regarding biomechanics of cornea such as CRF and CH after CXL procedure
DISCUSSION
Collagen cross linking is a treatment for stopping the progression of keratoconus, with numerous economical and psychosocial benefits.11 Several studies have shown that visual acuity improves after CXL in patients with keratoconus.3,12,13,14,15,16 The mechanism by which CLX improves or alters vision is not known completely. It might be due to decrease in refractive error, corneal steepness and astigmatism, and also because of improvement in definable topographic indices.4,9 In a study carried out by Wollensak, refractive error decreased 1.14 D after CXL procedure.17 In that study, CXL caused 1.14 line visual acuity improvements over 5 years of follow-up.17 Derakhshan et al. reported 0.55 D decrease in SE over a 6 months follow-up.4 Our results showed that UCVA and BCVA of the patients improved 6 months after CXL, and continued to improve during the 1-year follow-up. This was parallel with improvement in subjective and cycloplegic spherical and cylindrical refractions; furthermore, SE decreased 6 months after CXL and remained stable till 1-year follow-up.
Several studies have reported that maximum keratometry (Kmax), a key topographic indicator of CXL success, decreased significantly after this procedure (up to 2.01 D).2 Furthermore, Saffarian et al. reported 0.44 D decrease of Sim K over a 1-year follow-up period.18 We observed that keratometric values in the steepest and flattest axes, and also mean keratometry in both anterior and posterior surface decreased postoperatively. Mean Sim K and Sim K in flattest and steepest axes also decreased significantly. All these data confirmed that corneal steepness decreased after CXL procedure. However, AK, average corneal power in 4 mm zone, and AGC did not change significantly. ACD also remained stable in spite of corneal changes toward flattening, which may be due to the effect of CXL only on the superficial parts of the cornea. Corneal astigmatism did not change after 6 months, but showed a significant reduction in 6 months to 1-year follow-up. Reduced corneal steepening and astigmatism may contribute to improved visual acuity in postoperative follow-ups in our patients.
It is believed that the cornea assumes a more regular shape after CXL.9 Previous studies have shown CXL causes significant improvement in all of the corneal aberrations such as total coma and spherical aberration.12,19 Unfortunately, we did not perform wave front analysis. However, we evaluated corneal asphericity as an indicator of corneal regularity. Asphericity remained stable and did not progress after CXL procedure.
In a previous study done by Greenstein et al., they showed that corneal apex thinned until 3 months after CXL, then returned to baseline values after 3-6 months post CXL, and was similar to preoperative data in the 1-year follow-up.20 In this study, we observed significant decrease of apex corneal thickness during the 1-year follow-up. The thickness of the thinnest point did not change significantly after 6 and 12 months, which was consistent with a study by Vinciguerra et al.12 They found no significant changes in the pachymetry of the thinnest corneal point.13 However, other researchers found that thinnest point thickness remained slightly reduced 12 months after CXL.20
Collagen fiber compression, changes in corneal hydration and edema, keratocyte apoptosis and changes in glycosaminoglycan's may play an important role in defining corneal thickness.19 Our results show that change in corneal apex thickness was paralleled with increase in the average progression index after 6 months. This index remained stable from 6 months to 1-year. The possible mechanism explaining this change is that CXL affect central cornea more than periphery; so more decrease in CCT compared with periphery induces increased average progression index. To the best of our knowledge, no studies have assessed the average progression index changes after CXL procedure.
It is believed that the first sign of ectasia is alteration in posterior corneal shape.21 In our study, anterior BFS and elevation did not change significantly after CXL, however, posterior BFS and elevation increased 6 months postoperatively, and remained stable during the 12 months follow-up. Increase in posterior elevation can reduce corneal dioptric power and myopia in patients, which may have a role in improved postoperative visual acuity. However, Grewal et al. reported that corneal surface remained stable, and anterior and posterior elevation did not change after CXL.22 In another study, 8 (80%) and 6 (60%) of the 10 eyes, that had underwent CXL to treat keratoconus, showed decrease in the anterior and posterior elevation, respectively at the end of the 12 months follow-up.23 The controversy in these results may partially be explained by the difference in the device used for analyzing corneal elevation.
The data of two mentioned studies were obtained from Scheimpflug topography, while we analyzed our results using scanning slit topography (Orbscan II). The posterior surface data has the potential for artifact with the orbscan device; the increase in posterior elevation can actually represent artifact of edge detection algorithm following high reflectance stromal signal into the corneal stroma and departing from actual posterior surface.
Grewal et al. have shown that CXL does not induce significant edema.22 In their study, corneal thickness and volume remained stable over the 1-year follow-up.22 Another study reported decreased total corneal volume in after 1-year.19 Our data were also indicative of decrease in corneal volume over 6 months, which remained stable in 6 months to 1-year follow-up. The possible etiology of reduced corneal volume may be collagen shrinkage that occurs after CXL procedure.
Persistent corneal edema and possible endothelial cell damage have been reported in a few cases after CXL.24,25,26 Doors et al. evaluated the effect of CXL on the endothelium; they found no significant changes in endothelial cell density.27 We evaluated endothelial cell area, density, and CV. CXL did not induce significant changes in these endothelial parameters, and we concluded that CXL does not affect endothelial cells adversely. Vinciguerra et al. mentioned the lack of endothelial cell loss as an important safety consideration in assessing this new procedure.19
Although corneal stiffening of increases after CXL,28,29 we observed that biomechanical parameters such as CRF and CH showed no change after this procedure. Several previous studies have shown that there are no significant differences in CRF and CH after CXL in keratoconus in 6-month and 1-year follow-up periods.30,31,32,33,34 Tan and Mehta concluded that ORA measures hysteresis, rather than elastic properties of cornea; therefore, it cannot show changes of corneal stiffening.30
CONCLUSION
Collagen cross linking is an effective technique to stabilize or improve visual acuity, refraction, and keratometric values. Cornea assumes a more regular shape as a result of CXL procedure without inducing adverse effect on corneal endothelium and biomechanical parameters such as CRF and CH. We did not meet any problem for measuring corneal parameters. One of the shortcomings of our study was short follow-up. Furthermore, we did not perform wave front analysis. We recommend longer follow-up periods and evaluation of more parameters after this effective and safe procedure.
Footnotes
Source of Support: Poostchi Ophthalmology Research Center, Shiraz University of Medical Sciences, Shiraz, Iran
Conflict of Interest: None declared.
REFERENCES
- 1.Kennedy RH, Bourne WM, Dyer JA. A 48-year clinical and epidemiologic study of keratoconus. Am J Ophthalmol. 1986;101:267–73. doi: 10.1016/0002-9394(86)90817-2. [DOI] [PubMed] [Google Scholar]
- 2.Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-a-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003;135:620–7. doi: 10.1016/s0002-9394(02)02220-1. [DOI] [PubMed] [Google Scholar]
- 3.Asri D, Touboul D, Fournié P, Malet F, Garra C, Gallois A, et al. Corneal collagen crosslinking in progressive keratoconus: Multicenter results from the French National Reference Center for Keratoconus. J Cataract Refract Surg. 2011;37:2137–43. doi: 10.1016/j.jcrs.2011.08.026. [DOI] [PubMed] [Google Scholar]
- 4.Derakhshan A, Shandiz JH, Ahadi M, Daneshvar R, Esmaily H. Short-term outcomes of collagen crosslinking for early keratoconus. J Ophthalmic Vis Res. 2011;6:155–9. [PMC free article] [PubMed] [Google Scholar]
- 5.Wollensak G, Spoerl E, Seiler T. Stress-strain measurements of human and porcine corneas after riboflavin-ultraviolet-A-induced cross-linking. J Cataract Refract Surg. 2003;29:1780–5. doi: 10.1016/s0886-3350(03)00407-3. [DOI] [PubMed] [Google Scholar]
- 6.Kohlhaas M, Spoerl E, Schilde T, Unger G, Wittig C, Pillunat LE. Biomechanical evidence of the distribution of cross-links in corneas treated with riboflavin and ultraviolet a light. J Cataract Refract Surg. 2006;32:279–83. doi: 10.1016/j.jcrs.2005.12.092. [DOI] [PubMed] [Google Scholar]
- 7.Spoerl E, Wollensak G, Seiler T. Increased resistance of crosslinked cornea against enzymatic digestion. Curr Eye Res. 2004;29:35–40. doi: 10.1080/02713680490513182. [DOI] [PubMed] [Google Scholar]
- 8.Koller T, Mrochen M, Seiler T. Complication and failure rates after corneal crosslinking. J Cataract Refract Surg. 2009;35:1358–62. doi: 10.1016/j.jcrs.2009.03.035. [DOI] [PubMed] [Google Scholar]
- 9.Greenstein SA, Fry KL, Hersh PS. Corneal topography indices after corneal collagen crosslinking for keratoconus and corneal ectasia: One-year results. J Cataract Refract Surg. 2011;37:1282–90. doi: 10.1016/j.jcrs.2011.01.029. [DOI] [PubMed] [Google Scholar]
- 10.World Medical Association Declaration of Helsinki-Ethical Principles for Medical Research Involving Human Subjects. 2008. [Last accessed on on 64th WMA General Assembly, Fortaleza, Brazil, October 2013]. Available from: http://www.wma.net/en/30publications/10policies/b317c.pdf .
- 11.Raiskup-Wolf F, Hoyer A, Spoerl E, Pillunat LE. Collagen crosslinking with riboflavin and ultraviolet - A light in keratoconus: long-term results. J Cataract Refract Surg. 2008;34:796–801. doi: 10.1016/j.jcrs.2007.12.039. [DOI] [PubMed] [Google Scholar]
- 12.Vinciguerra P, Albè E, Trazza S, Rosetta P, Vinciguerra R, Seiler T, et al. Refractive, topographic, tomographic, and aberrometric analysis of keratoconic eyes undergoing corneal cross-linking. Ophthalmology. 2009;116:369–78. doi: 10.1016/j.ophtha.2008.09.048. [DOI] [PubMed] [Google Scholar]
- 13.Caporossi A, Mazzotta C, Baiocchi S, Caporossi T. Long-term results of riboflavin ultraviolet a corneal collagen cross-linking for keratoconus in Italy: The Siena eye cross study. Am J Ophthalmol. 2010;149:585–93. doi: 10.1016/j.ajo.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 14.Wittig-Silva C, Whiting M, Lamoureux E, Lindsay RG, Sullivan LJ, Snibson GR. A randomized controlled trial of corneal collagen cross-linking in progressive keratoconus: Preliminary results. J Refract Surg. 2008;24:S720–5. doi: 10.3928/1081597X-20080901-15. [DOI] [PubMed] [Google Scholar]
- 15.Coskunseven E, Jankov MR, 2nd, Hafezi F. Contralateral eye study of corneal collagen cross-linking with riboflavin and UVA irradiation in patients with keratoconus. J Refract Surg. 2009;25:371–6. doi: 10.3928/1081597X-20090401-02. [DOI] [PubMed] [Google Scholar]
- 16.Agrawal VB. Corneal collagen cross-linking with riboflavin and ultraviolet-a light for keratoconus: Results in Indian eyes. Indian J Ophthalmol. 2009;57:111–4. doi: 10.4103/0301-4738.44515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wollensak G. Crosslinking treatment of progressive keratoconus: New hope. Curr Opin Ophthalmol. 2006;17:356–60. doi: 10.1097/01.icu.0000233954.86723.25. [DOI] [PubMed] [Google Scholar]
- 18.Saffarian L, Khakshoor H, Zarei-Ghanavati M, Esmaily H. Corneal Crosslinking for Keratoconus in Iranian Patients: Outcomes at 1 year following treatment. Middle East Afr J Ophthalmol. 2010;17:365–8. doi: 10.4103/0974-9233.71600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vinciguerra P, Albè E, Trazza S, Seiler T, Epstein D. Intraoperative and postoperative effects of corneal collagen cross-linking on progressive keratoconus. Arch Ophthalmol. 2009;127:1258–65. doi: 10.1001/archophthalmol.2009.205. [DOI] [PubMed] [Google Scholar]
- 20.Greenstein SA, Shah VP, Fry KL, Hersh PS. Corneal thickness changes after corneal collagen crosslinking for keratoconus and corneal ectasia: One-year results. J Cataract Refract Surg. 2011;37:691–700. doi: 10.1016/j.jcrs.2010.10.052. [DOI] [PubMed] [Google Scholar]
- 21.Labiris G, Giarmoukakis A, Sideroudi H, Bougatsou P, Lazaridis I, Kozobolis VP. Variability in Scheimpflug image-derived posterior elevation measurements in keratoconus and collagen-crosslinked corneas. J Cataract Refract Surg. 2012;38:1616–25. doi: 10.1016/j.jcrs.2012.04.039. [DOI] [PubMed] [Google Scholar]
- 22.Grewal DS, Brar GS, Jain R, Sood V, Singla M, Grewal SP. Corneal collagen crosslinking using riboflavin and ultraviolet-A light for keratoconus: One-year analysis using Scheimpflug imaging. J Cataract Refract Surg. 2009;35:425–32. doi: 10.1016/j.jcrs.2008.11.046. [DOI] [PubMed] [Google Scholar]
- 23.Henriquez MA, Izquierdo L, Jr, Bernilla C, Zakrzewski PA, Mannis M. Riboflavin/Ultraviolet a corneal collagen cross-linking for the treatment of keratoconus: Visual outcomes and Scheimpflug analysis. Cornea. 2011;30:281–6. doi: 10.1097/ICO.0b013e3181eeaea1. [DOI] [PubMed] [Google Scholar]
- 24.Gokhale NS. Corneal endothelial damage after collagen cross-linking treatment. Cornea. 2011;30:1495–8. doi: 10.1097/ICO.0b013e31820687f7. [DOI] [PubMed] [Google Scholar]
- 25.Bagga B, Pahuja S, Murthy S, Sangwan VS. Endothelial failure after collagen cross-linking with riboflavin and UV-A: Case report with literature review. Cornea. 2012;31:1197–200. doi: 10.1097/ICO.0b013e31823cbeb1. [DOI] [PubMed] [Google Scholar]
- 26.Sharma A, Nottage JM, Mirchia K, Sharma R, Mohan K, Nirankari VS. Persistent corneal edema after collagen cross-linking for keratoconus. Am J Ophthalmol. 2012;154:922–926.e1. doi: 10.1016/j.ajo.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 27.Doors M, Tahzib NG, Eggink FA, Berendschot TT, Webers CA, Nuijts RM. Use of anterior segment optical coherence tomography to study corneal changes after collagen cross-linking. Am J Ophthalmol. 2009;148:844–51.e2. doi: 10.1016/j.ajo.2009.06.031. [DOI] [PubMed] [Google Scholar]
- 28.Spoerl E, Huhle M, Seiler T. Induction of cross-links in corneal tissue. Exp Eye Res. 1998;66:97–103. doi: 10.1006/exer.1997.0410. [DOI] [PubMed] [Google Scholar]
- 29.Wollensak G, Spörl E, Seiler T. Treatment of keratoconus by collagen cross linking. Ophthalmologe. 2003;100:44–9. doi: 10.1007/s00347-002-0700-3. [DOI] [PubMed] [Google Scholar]
- 30.Tan P, Mehta JS. Collagen crosslinking for keratoconus. J Ophthalmic Vis Res. 2011;6:153–4. [PMC free article] [PubMed] [Google Scholar]
- 31.Goldich Y, Barkana Y, Morad Y, Hartstein M, Avni I, Zadok D. Can we measure corneal biomechanical changes after collagen cross-linking in eyes with keratoconus?- A pilot study. Cornea. 2009;28:498–502. doi: 10.1097/ICO.0b013e318190734d. [DOI] [PubMed] [Google Scholar]
- 32.Gkika M, Labiris G, Giarmoukakis A, Koutsogianni A, Kozobolis V. Evaluation of corneal hysteresis and corneal resistance factor after corneal cross-linking for keratoconus. Graefes Arch Clin Exp Ophthalmol. 2012;250:565–73. doi: 10.1007/s00417-011-1897-0. [DOI] [PubMed] [Google Scholar]
- 33.Greenstein SA, Fry KL, Hersh PS. In vivo biomechanical changes after corneal collagen cross-linking for keratoconus and corneal ectasia: 1-year analysis of a randomized, controlled, clinical trial. Cornea. 2012;31:21–5. doi: 10.1097/ICO.0b013e31821eea66. [DOI] [PubMed] [Google Scholar]
- 34.Vinciguerra P, Albè E, Mahmoud AM, Trazza S, Hafezi F, Roberts CJ. Intra- and postoperative variation in ocular response analyzer parameters in keratoconic eyes after corneal cross-linking. J Refract Surg. 2010;26:669–76. doi: 10.3928/1081597X-20100331-01. [DOI] [PubMed] [Google Scholar]


