Cell based therapy is at the forefront of clinical investigation for cardiovascular disease, backed by over a decade of rigorous pre-clinical study of cell biology, mechanism(s) of action, immunology, and phenotypic efficacy1. After early proof-of-concept and safety clinical trials2–5, the field is entering the next phase of clinical evaluation to delineate clinical efficacy. However, several questions still need to be addressed, namely the optimal cell delivery method, cell dosage range, and cell characteristics. Importantly, translating cell therapy into standard clinical practice requires the ability to readily administer a safe and efficacious product at the optimal dosage. An opportunity that greatly enhances the ability to develop such a product is the use of allogeneic therapy, which offers an efficient way to achieve both immediate availability of product and the appropriate number of cells.
The paradigm of allogeneic therapy
Allogeneic therapy is clearly a disruptive concept in biology. Standard immunologic dogma holds that any foreign tissue will elicit an immune reaction6. This concept is clearly evident in solid organ and hematopoietic transplantation, in which aggressive immunosuppression is the norm to protect allografts from rejection6. As the field of cell-based therapy evolves, it has become evident that various cell types – mesenchymal stem cells (MSCs) being the prototype – have sufficient ability to evade7 and/or suppress8 the immune system to the extent that they may be used as allografts without requiring concomitant immunosuppression.
Preclinical Studies
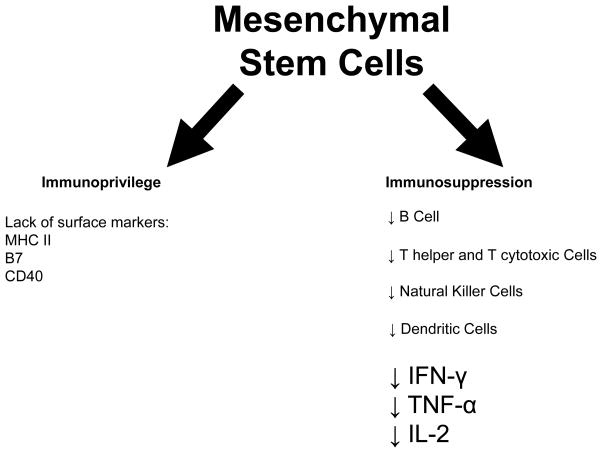
More than ten years ago it was suggested that MSCs act as immune system modulators9. Bartholomew et al.10 performed a mixed lymphocyte reaction in which baboon MSCs were co-cultured with stimulated allogeneic peripheral blood leukocytes and demonstrated that the MSCs suppressed leukocyte proliferation. Extensive studies have subsequently revealed that MSCs evade the immune system via multiple mechanisms, which include having moderate levels of HLA class I expression, lacking expression of HLA class II, B7 and CD40 ligand11 (the underpinning of being immunoprivileged), and secreting paracrine factors12 and exosomes13 (which underlie, among diverse activities, immunosuppressive actions). With regard to the latter, MSCs suppress proliferation of both T helper and cytotoxic T cells8, decrease the production of the pro-inflammatory cytokines IFN-γ, TNF-α and IL-214, inhibit the activation of natural killer cells15, arrest B-cell maturation, and block maturation of dendritic cells, resulting in reduced expression of antigens and costimulatory molecules necessary to activate T-cells (Figure 1)16. These data formed the basis for the pre-clinical and, subsequently, clinical deployment of allogeneic MSC therapy. Indeed, our group demonstrated in porcine models of myocardial infarction (MI)17 and chronic ischemic cardiomyopathy18 that delivery of allogeneic MSCs is effective in improving cardiac structure and function, through engraftment, cell-to-cell interactions, and trilineage transdifferentiation, and does not elicit detrimental immune reactions.
Figure 1.
Mesenchymal stem cells are considered immunoprivileged due to their lack of expression of Major Histocompatibility Class II proteins and costimulatory molecules B7 and CD40 ligand and exert immunosuppressive actions through inhibition of innate and adaptive immunity.
It is important to note, however, that some studies are emerging that raise cautionary notes. Huang et al.19 in a rodent study reported that allogeneic MSCs may lose their immunoprivileged status during differentiation, but this result has not been confirmed in other studies. As previously mentioned, we have demonstrated persistence of differentiated cells, 3 months following intramyocardial delivery in a model of chronic ischemic cardiomyopathy in the pig18. Some have argued that MSCs are not fully immunoprivileged but rather immune-evading7. The difference seems to rely on concentration. In in vitro studies where MSCs were in high concentrations, the immunomodulatory capabilities prevailed7.
Clinical Testing of Allogeneic Stem Cells
Clinical trials have shown that allogeneic bone marrow-derived MSCs may be safely administered to humans without eliciting clinically relevant immune reactions3, 4, 20. In the first clinical trial of allogeneic MSC therapy for acute MI4, intravenous infusion of MSCs did not produce an immune reaction and led to improved outcomes with regard to cardiac arrhythmias, pulmonary function, left ventricular function, and symptomatic global assessment. Subsequent to this acute MI trial, we performed a randomized clinical trial in patients with chronic ischemic cardiomyopathy, POSEIDON3, which had as its main goal the comparison of transendocardial injection of autologous and allogeneic bone marrow-derived MSCs. Although it was not powered to show efficacy as a primary outcome, the trial reported similar safety profiles between the two sources. MSC therapy improved indices of physical functional capacity and quality of life (6-minute walk test and the Minnesota Living with Heart Failure Questionnaire score, respectively) and reduced scar tissue and left-ventricular sphericity index, markers of ventricular remodeling. Importantly, only two patients receiving allogeneic MSCs developed sensitization as measured by the panel reactive antigen (PRA). One exhibited low-level antibodies to antigen specificities not expressed by the donor MSCs and the other showed low-level donor-specific HLA class I antibodies3. Flow cytometric cross-match with serum from the second patient to fresh donor T cells showed a weak positive reaction, indicating low titer, de novo allogeneic sensitization with class I donor antigens. Neither incident developed clinical significance. Similarly, in another clinical trial20 where allogeneic mesenchymal precursor cells (MPCs) were delivered to patients with left ventricular assist devices, donor-specific HLA sensitization developed after randomization in two MPC and three control patients, all of which were resolved by 1 year. The sensitization in control patients was attributed to transfusions received after randomization.
Other related cell types may have similar immunologic properties. Regarding cardiac-derived stem cells, there are encouraging results from employing autologous ckit+ cardiac stem cells (CSCs) in patients with chronic ischemic cardiomyopathy21. Moreover, it has been reported that human CSCs may have immunomodulatory capacity in vitro22, resembling the properties described for MSCs. However, no clinical trial employing allogeneic ckit+ CSCs has been reported yet. The currently ongoing trial ALLogeneic heart STem cells to Achieve myocardial Regeneration (ALLSTAR; NCT01458405) is testing the safety and efficacy of cardiospheres23 without the use of immunosuppression, based on favorable preclinical data24, 25. Of note, emerging evidence shows that cardiospheres have many properties of MSCs, as they express classic markers including CD105, CD90, and CD7326–28, and as such may be cardiac specific stromal or mesenchymal cells. The finding that these cardiac derived CD105+ cells may be used as an allograft suggests that allogeneic cell therapy may be broadly applicable.
Meta-Analysis Of Allogeneic Cell Therapy
As there are early accumulating data regarding allogeneic cell therapy, there is significant value in performing meta-analyses of preclinical data. In this issue of Circulation Research, Jansen of Lorkeers et al.29 present a comprehensive meta-analysis of large animal studies, investigating the effects of stem cell therapy in ischemic heart disease. After the application of strict selection criteria, 82 of 595 publications were included in the meta-analysis. Importantly and expectedly, MSCs were the cell type used in the large majority of the analyzed studies. Three important messages from this meta-analysis are: a) autologous and allogeneic cell therapy exhibit similar effects, b) cell therapy provides an overall significant improvement in left ventricular ejection fraction (LVEF) and decrease in end diastolic volume (EDV), and importantly c) cell therapy appears to be safe. This study makes a critical statement supporting the findings of the two clinical trials in which allogeneic MSCs or MPCs have been used, and furthermore continues to support the fact that large animal studies are highly representative of translation into humans.
As the authors aptly point out, the major issue for the success of allogeneic cell therapy is the lack of an immune response by the recipient to the transplanted cells. With the exception of one study, the MSC studies included in the meta-analysis did not use immunosuppression. Nevertheless, no serious immune reactions were reported in any of the studies and there was no impact on the safety and efficacy profile. In fact, in non-cardiac studies the effect of immunosuppression with cyclosporine and/or methylprednisolone has been mixed, ranging from a positive effect in the treatment of stroke30 to negatively affecting the efficacy of allogeneic MSCs in spinal cord injury31.
The application of allogeneic cell therapy
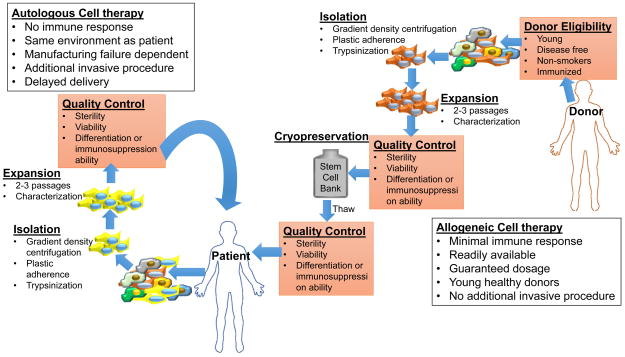
Regarding cell preparation and timely delivery, allogenicity solves logistical issues of a successful stem cell therapy. Allogeneic MSCs are subject to multiple quality controls (Figure 2), from the screening of young healthy donors to pre- and post-cryopreservation control of viability and sterility. When an autologous source is used, the cell product derives from a patient that is usually older and with multiple comorbidities and the cell product is manufacturing dependent. The allogeneic product can be expanded in quantities that may be unattainable for the autologous source, undergo cryopreservation, and be readily available for delivery. In comparison, the manufacture of the autologous product may require up to 4–6 weeks to reach sufficient numbers for administration, and the ability of the patient’s cells to expand is unpredictable.
Figure 2. Comparison of Autologous and Allogeneic stem cell therapy.
Allogeneic stem cell therapy offers advantages over the autologous counterpart. The stem cells are derived from young healthy donors, eliminating any co-morbidities associated with disease states. Allogeneic cells grown and kept in stem cell banks so that they are available for immediate delivery.
In non-cardiac clinical trials, allogeneic sources of stem cells and more specifically MSCs have been widely tested7, 32. Le Blanc et al.33, reporting on the outcomes for 55 patients receiving intravenous infusions of fresh or cryopreserved allogeneic MSCs for steroid-resistant graft versus-host disease, noted no acute side effects. In fact, a growing number of published Phase I and Phase II clinical studies34, 35 of allogeneic MSCs administrated for diverse medical conditions have consistently described a lack of acute complications from doses as high as 5×106 cells/kg. Based upon this background, numerous trials for diverse indications are emerging.
Future issues for consideration
While our immunologic understanding, animal data, and empiric clinical trial data support the use of allogeneic therapy, several issues need to be considered as the field evolves. For instance, tolerance to repeat MSC dosing has not been convincingly demonstrated. And if that is the case, could a different donor be used to avoid anamnestic reaction? Should there be any pre-screening of candidates and appropriate immunophenotypic matching as is the norm with blood banks? The preclinical evidence and empiric safety from clinical trials thus far do not support immunomatching, although strict immunologic monitoring in early stage trials is highly recommended to further define whether immune reactions to allogeneic cell therapy are meaningful and clinically relevant.
In light of these highly encouraging results and intriguing pending questions, larger randomized clinical trials are warranted to investigate whether the immunoprivilege of and immune modulation by MSCs/MPCs observed in preclinical trials in large animals and phase 1 clinical trials translates to larger patient populations. Rigorous and long term monitoring of the immunological profiles of the enrolled patients will provide the evidence needed for stem cell therapy to be transformed into an off-the-shelf treatment option for heart failure and many other diseases.
Acknowledgments
Sources of Funding
Dr. Hare is supported by National Institutes of Health Grants R01HL110737, R01HL084275, R01HL094849, R01HL107110, and UM1HL113460 and the Starr Foundation.
Footnotes
Disclosures
Dr. Hare discloses a relationship with Vestion that includes equity, board membership, and consulting.
References
- 1.Karantalis V, Balkan W, Schulman IH, Hatzistergos KE, Hare JM. Cell-based therapy for prevention and reversal of myocardial remodeling. Am J Physiol Heart Circ Physiol. 2012;303:H256–270. doi: 10.1152/ajpheart.00221.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heldman AW, DiFede DL, Fishman JE, et al. Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: The tac-hft randomized trial. JAMA. 2014;311:62–73. doi: 10.1001/jama.2013.282909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hare JM, Fishman JE, Gerstenblith G, et al. Comparison of allogeneic vs autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: The poseidon randomized trial. JAMA. 2012;308:2369–2379. doi: 10.1001/jama.2012.25321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP, Gerstenblith G, DeMaria AN, Denktas AE, Gammon RS, Hermiller JB, Jr, Reisman MA, Schaer GL, Sherman W. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54:2277–2286. doi: 10.1016/j.jacc.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Houtgraaf JH, den Dekker WK, van Dalen BM, Springeling T, de Jong R, van Geuns RJ, Geleijnse ML, Fernandez-Aviles F, Zijlsta F, Serruys PW, Duckers HJ. First experience in humans using adipose tissue-derived regenerative cells in the treatment of patients with st-segment elevation myocardial infarction. J Am Coll Cardiol. 2012;59:539–540. doi: 10.1016/j.jacc.2011.09.065. [DOI] [PubMed] [Google Scholar]
- 6.Chinen J, Buckley RH. Transplantation immunology: Solid organ and bone marrow. J Allergy Clin Immunol. 2010;125:S324–335. doi: 10.1016/j.jaci.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ankrum JA, Ong JF, Karp JM. Mesenchymal stem cells: Immune evasive, not immune privileged. Nat Biotechnol. 2014;32:252–260. doi: 10.1038/nbt.2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van den Akker F, de Jager SC, Sluijter JP. Mesenchymal stem cell therapy for cardiac inflammation: Immunomodulatory properties and the influence of toll-like receptors. Mediators Inflamm. 2013;2013:181020. doi: 10.1155/2013/181020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tse WT, Pendleton JD, Beyer WM, Egalka MC, Guinan EC. Suppression of allogeneic t-cell proliferation by human marrow stromal cells: Implications in transplantation. Transplantation. 2003;75:389–397. doi: 10.1097/01.TP.0000045055.63901.A9. [DOI] [PubMed] [Google Scholar]
- 10.Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, Hardy W, Devine S, Ucker D, Deans R, Moseley A, Hoffman R. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30:42–48. doi: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]
- 11.Majumdar MK, Keane-Moore M, Buyaner D, Hardy WB, Moorman MA, McIntosh KR, Mosca JD. Characterization and functionality of cell surface molecules on human mesenchymal stem cells. J Biomed Sci. 2003;10:228–241. doi: 10.1007/BF02256058. [DOI] [PubMed] [Google Scholar]
- 12.Murphy MB, Moncivais K, Caplan AI. Mesenchymal stem cells: Environmentally responsive therapeutics for regenerative medicine. Exp Mol Med. 2013;45:e54. doi: 10.1038/emm.2013.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blazquez R, Sanchez-Margallo FM, de la Rosa O, Dalemans W, Alvarez V, Tarazona R, Casado JG. Immunomodulatory potential of human adipose mesenchymal stem cells derived exosomes on stimulated t cells. Front Immunol. 2014;5:556. doi: 10.3389/fimmu.2014.00556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mandi Y, Vecsei L. The kynurenine system and immunoregulation. J Neural Transm. 2012;119:197–209. doi: 10.1007/s00702-011-0681-y. [DOI] [PubMed] [Google Scholar]
- 15.Spaggiari GM, Capobianco A, Abdelrazik H, Becchetti F, Mingari MC, Moretta L. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: Role of indoleamine 2,3-dioxygenase and prostaglandin e2. Blood. 2008;111:1327–1333. doi: 10.1182/blood-2007-02-074997. [DOI] [PubMed] [Google Scholar]
- 16.Zhang W, Ge W, Li C, You S, Liao L, Han Q, Deng W, Zhao RC. Effects of mesenchymal stem cells on differentiation, maturation, and function of human monocyte-derived dendritic cells. Stem Cells Dev. 2004;13:263–271. doi: 10.1089/154732804323099190. [DOI] [PubMed] [Google Scholar]
- 17.Amado LC, Saliaris AP, Schuleri KH, et al. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc Natl Acad Sci U S A. 2005;102:11474–11479. doi: 10.1073/pnas.0504388102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quevedo HC, Hatzistergos KE, Oskouei BN, Feigenbaum GS, Rodriguez JE, Valdes D, Pattany PM, Zambrano JP, Hu Q, McNiece I, Heldman AW, Hare JM. Allogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacity. Proc Natl Acad Sci U S A. 2009;106:14022–14027. doi: 10.1073/pnas.0903201106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang XP, Sun Z, Miyagi Y, McDonald Kinkaid H, Zhang L, Weisel RD, Li RK. Differentiation of allogeneic mesenchymal stem cells induces immunogenicity and limits their long-term benefits for myocardial repair. Circulation. 2010;122:2419–2429. doi: 10.1161/CIRCULATIONAHA.110.955971. [DOI] [PubMed] [Google Scholar]
- 20.Ascheim DD, Gelijns AC, Goldstein D, et al. Mesenchymal precursor cells as adjunctive therapy in recipients of contemporary left ventricular assist devices. Circulation. 2014;129:2287–2296. doi: 10.1161/CIRCULATIONAHA.113.007412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bolli R, Chugh AR, D’Amario D, et al. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): Initial results of a randomised phase 1 trial. Lancet. 2011;378:1847–1857. doi: 10.1016/S0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Lauden L, Boukouaci W, Borlado LR, Lopez IP, Sepulveda P, Tamouza R, Charron D, Al-Daccak R. Allogenicity of human cardiac stem/progenitor cells orchestrated by programmed death ligand 1. Circ Res. 2013;112:451–464. doi: 10.1161/CIRCRESAHA.112.276501. [DOI] [PubMed] [Google Scholar]
- 23.Smith RR, Barile L, Cho HC, Leppo MK, Hare JM, Messina E, Giacomello A, Abraham MR, Marban E. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- 24.Tseliou E, Pollan S, Malliaras K, Terrovitis J, Sun B, Galang G, Marban L, Luthringer D, Marban E. Allogeneic cardiospheres safely boost cardiac function and attenuate adverse remodeling after myocardial infarction in immunologically mismatched rat strains. J Am Coll Cardiol. 2013;61:1108–1119. doi: 10.1016/j.jacc.2012.10.052. [DOI] [PubMed] [Google Scholar]
- 25.Malliaras K, Li TS, Luthringer D, Terrovitis J, Cheng K, Chakravarty T, Galang G, Zhang Y, Schoenhoff F, Van Eyk J, Marban L, Marban E. Safety and efficacy of allogeneic cell therapy in infarcted rats transplanted with mismatched cardiosphere-derived cells. Circulation. 2012;125:100–112. doi: 10.1161/CIRCULATIONAHA.111.042598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tateishi K, Ashihara E, Honsho S, Takehara N, Nomura T, Takahashi T, Ueyama T, Yamagishi M, Yaku H, Matsubara H, Oh H. Human cardiac stem cells exhibit mesenchymal features and are maintained through akt/gsk-3beta signaling. Biochem Biophys Res Commun. 2007;352:635–641. doi: 10.1016/j.bbrc.2006.11.096. [DOI] [PubMed] [Google Scholar]
- 27.Pouly J, Bruneval P, Mandet C, Proksch S, Peyrard S, Amrein C, Bousseaux V, Guillemain R, Deloche A, Fabiani JN, Menasche P. Cardiac stem cells in the real world. J Thorac Cardiovasc Surg. 2008;135:673–678. doi: 10.1016/j.jtcvs.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 28.Mishra R, Vijayan K, Colletti EJ, Harrington DA, Matthiesen TS, Simpson D, Goh SK, Walker BL, Almeida-Porada G, Wang D, Backer CL, Dudley SC, Jr, Wold LE, Kaushal S. Characterization and functionality of cardiac progenitor cells in congenital heart patients. Circulation. 2011;123:364–373. doi: 10.1161/CIRCULATIONAHA.110.971622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jansen Of Lorkeers SJ, Eding JE, Vesterinen HM, van der Spoel TI, Sena ES, Duckers HJ, Doevendans PA, Macleod MR, Chamuleau SA. Similar effect of autologous and allogeneic cell therapy for ischemic heart disease: Systematic review and meta-analysis of large animal studies. Circ Res. 2014 doi: 10.1161/CIRCRESAHA.116.304872. [DOI] [PubMed] [Google Scholar]
- 30.Lees JS, Sena ES, Egan KJ, Antonic A, Koblar SA, Howells DW, Macleod MR. Stem cell-based therapy for experimental stroke: A systematic review and meta-analysis. Int J Stroke. 2012;7:582–588. doi: 10.1111/j.1747-4949.2012.00797.x. [DOI] [PubMed] [Google Scholar]
- 31.Antonic A, Sena ES, Lees JS, Wills TE, Skeers P, Batchelor PE, Macleod MR, Howells DW. Stem cell transplantation in traumatic spinal cord injury: A systematic review and meta-analysis of animal studies. PLoS Biol. 2013;11:e1001738. doi: 10.1371/journal.pbio.1001738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tyndall A, Houssiau FA. Mesenchymal stem cells in the treatment of autoimmune diseases. Ann Rheum Dis. 2010;69:1413–1414. doi: 10.1136/ard.2010.132639. [DOI] [PubMed] [Google Scholar]
- 33.Le Blanc K, Frassoni F, Ball L, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: A phase ii study. Lancet. 2008;371:1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 34.Vaes B, Van’t Hof W, Deans R, Pinxteren J. Application of multistem((r)) allogeneic cells for immunomodulatory therapy: Clinical progress and pre-clinical challenges in prophylaxis for graft versus host disease. Front Immunol. 2012;3:345. doi: 10.3389/fimmu.2012.00345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Christopeit M, Schendel M, Foll J, Muller LP, Keysser G, Behre G. Marked improvement of severe progressive systemic sclerosis after transplantation of mesenchymal stem cells from an allogeneic haploidentical-related donor mediated by ligation of cd137l. Leukemia. 2008;22:1062–1064. doi: 10.1038/sj.leu.2404996. [DOI] [PubMed] [Google Scholar]