Abstract
The reasons for aspiration in healthy adults remain unknown. Given that the pharyngeal phase of swallowing is a key component of the safe swallow, it was hypothesized that healthy older adults who aspirate are likely to generate less pharyngeal peak pressures when swallowing. Accordingly, pharyngeal and upper esophageal sphincter pressures were examined as a function of aspiration status (i.e., nonaspirator vs. aspirator), sensor location (upper vs. lower pharynx), liquid type (i.e., water vs. milk), and volume (i.e., 5 vs. 10 ml) in healthy older adults. Manometric measurements were acquired with a 2.1-mm catheter during flexible endoscopic evaluation. Participants (N = 19, mean age = 79.2 years) contributed 28 swallows; during 8 swallows, simultaneous manometric measurements of upper and lower pharyngeal and upper esophageal pressures were obtained. Pharyngeal manometric peak pressure was significantly less for aspirators (mean = 82, SD = 31 mmHg) than for nonaspirators (mean = 112, SD = 20 mmHg), and upper pharyngeal pressures (mean = 85, SD = 32 mmHg) generated less pressure than lower pharyngeal pressures (mean = 116, SD = 38 mmHg). Manometric measurements vary with respect to aspiration status and sensor location. Lower pharyngeal pressures in healthy older adults may predispose them to aspiration.
Keywords: Swallowing, Manometry, Age, Gender, Bolus volume, Deglutition, Deglutition disorders
Numerous factors affect pharyngeal function while healthy adults swallow. For example, dry swallows elicit greater pharyngeal pressures and longer pharyngeal durations than liquid swallows [1–4] or “simpler” pressure patterns [5]. Findings from studies exploring pharyngeal pressure as a function of viscosity have been equivocal. Whereas Perlman et al. [3] found no difference in pressure or timing variables in water versus apple sauce conditions, Dantas et al. [6] found increases in pharyngeal pressure duration and increased upper esophageal sphincter (UES) relaxation with increases in bolus viscosity from liquid barium to paste barium. Likewise, Butler et al. [7] found, in general, increases in lower pharyngeal pressure with increases in viscosity across thin, nectar, honey, and pudding conditions.
Pharyngeal pressure and duration are also affected by bolus volume. Gumbley et al. [8] found no statistically significant differences in pharyngeal peak pressures or duration across 5-, 10-, and 20-ml bolus volumes; however, UES nadir pressures were inversely proportionate to bolus volume. Kahrilas et al. [9] also reported an increase in UES duration with larger boluses. Similarly, Castell et al. [2] found greater pharyngeal contraction and greater time to the peak of the contraction with increases in bolus volume of 5 vs. 20 ml. Butler et al. [7] found increases in lower pharyngeal pressure with increases in volume (5 and 10 ml) and the inverse of that relationship at the UES. Relative to duration, they found that 5-ml boluses elicited longer upper pharyngeal durations than did 10-ml boluses. Ghosh et al. [10] also reported increased mean intrabolus pressure during the UES relaxation interval with increasing bolus volumes. Cook et al. [11] also reported increases in “upstream” pharyngeal intrabolus pressures with increases in bolus volume; velocity of pharyngeal peristalsis did not vary with bolus volume [12]. In contrast, Kern et al. [13] reported that 10-ml barium boluses elicited significantly higher intrabolus pharyngeal pressures than did 5-ml barium boluses. Some differences in findings may be attributed to differences in manometric catheter sizes and/or sensor locations. When utilizing smaller unidirectional-type sensor catheters, significantly higher pressure was evident for the lower versus the upper pharyngeal location [7, 14, 15].
Little is known about the role of pharyngeal pressure in swallowing in healthy older adults. It is reasonable to expect a concomitant decline in swallowing with a general decline in physical or cognitive function that accompanies aging. For example, even in healthy older adults, sarcopenia (loss of muscle mass and strength) could lead to reduced pharyngeal peak pressures when swallowing, causing a greater risk of aspiration. Given that the pharyngeal phase of swallowing is a key component of the safe swallow, decreased pharyngeal strength may be an indicator of pharyngeal pathophysiology in healthy older adults and thus worthy of further investigation.
The recent observation of a strikingly high prevalence of aspiration (i.e., approximately 30% and most episodes being silent) among healthy older adults over 65 years old during flexible endoscopic evaluation of swallowing (FEES) [16, 17] was the impetus for this study. It was subsequently hypothesized that healthy older adults who aspirate may have less pharyngeal strength (i.e., pharyngeal peak pressures with swallowing) than healthy older adults who do not aspirate. Thus, the purpose of this study was to examine pharyngeal pressure during concurrent manometric and FEES assessment as a function of aspiration status (i.e., nonaspirator vs. aspirator), sensor location (i.e., upper vs. lower pharynx), liquid type (i.e., milk vs. water), and bolus volume (i.e., 5 vs. 10 ml). The inclusion of a milk bolus was deemed important since aspiration is observed with milk significantly more frequently than with water (S.G. Butler et al., unpublished). Concurrent assessment of upper and lower pharyngeal pressures was undertaken as pharyngeal pressures vary as a function of manometric sensor location [7, 14, 15].
Methods
Participants
Twenty adults were enrolled in the study; however, one participant underwent only the endoscopic portion of the study. The 19 participants were volunteers over 65 years of age (mean = 79.2 years, range = 69–87); 9 women (mean age = 79.4 years, SD = 5.4) and 10 men (mean age = 79.0 years, SD = 4.7) were included. Participants reported via questionnaire that they had no history of swallowing or voice problems; speech disorders; known otolaryngological, pulmonary, or neurological disease; structural disorders; and were not taking medications known to change swallowing function. Participants were asymptomatic for reflux as determined by the Reflux Symptom Index [18]. All participants were ambulatory and self-reported to be in good health. Participants were recruited by bulletins approved by the Wake Forest University Health Sciences Institutional Review Board. Informed consent was obtained.
Apparatus
A KayPENTAX Swallowing Workstation (KayPENTAX, Inc., Lincoln Park, NJ) with concurrent manometry and FEES was utilized. The manometry catheter was 100 cm long and 2.1 mm in diameter (Model CTS3 + emg, Galtek, Hackensack, NJ) with solid-state unidirectional, posteriorly oriented sensors consistent with proposed catheter standards [19]. Pressures were measured in the upper esophageal sphincter (UES) and at 3 and 5 cm proximally, the approximate levels of inferior constrictor and tongue base, respectively. Digital 12-bit samples were obtained with a sampling frequency of 500 Hz and displayed in a -100- to 250-mmHg display window. The system software generated pressure waveforms as a function of time. Videoendoscopic imaging, as described previously [7, 15, 20], was time-locked with the manometric waveforms and displayed on the same monitor.
Procedure
Catheter calibrations were conducted with a sphygmomanometer calibration kit that has an accuracy of ±0.75 mmHg. A 3.1-mm digital flexible endoscope was lubricated and passed transnasally by a speech-language pathologist (SLP) to obtain a superior view of the hypopharynx allowing for easy, visualized placement of the manometric catheter. Once endoscopic placement was assured, the tip of the manometric catheter was lubricated and passed transnasally by another SLP or a trained nursing assistant through the other nare into the hypopharynx, and through the UES with cued swallows and water swallows as needed. If difficulty was encountered passing the manometric catheter through the opposite nare, then the catheter was passed through the same nare as the endoscope. This is a routine procedure in our clinic and in research studies and is well tolerated whether one or two nares are used.
Using a pull-through technique, the catheter was pulled back until the high-pressure zone of the UES was observed in the waveform of sensor one. Once the high-pressure zone of the UES was identified, the catheter was stabilized in the high-pressure zone for approximately 20 s and UES resting pressure was recorded. The catheter was then pulled back (i.e., moved superiorly) an additional 1–2 cm so that UES relaxation could be measured at the height of the swallow (i.e., to compensate for laryngeal elevation with swallowing), and the characteristic M-wave [21] was seen in the channel for sensor one. A posterior orientation of sensors two and three at the levels of the inferior constrictor and the base of the tongue, respectively, was obtained and assured via endoscopic visualization throughout data collection. The manometric clinician took the hand that was holding the manometric catheter transnasally and braced it on the participant’s nose or cheek to assure that minimal inferior or superior movement of the catheter in the pharynx occurred. However, given the dynamic nature of swallowing, some reorientation of the catheter was needed during data collection and it was performed by again advancing sensor one to the highpressure zone of the UES and then pulling the catheter back (i.e., superiorly) 1 cm as described above.
Determining Aspiration Status
Fourteen conditions were studied to determine aspiration status. Four of the 14 conditions were conducted with the manometric catheter in (i.e., 5- and 10-ml volumes of water and milk) and the remaining ten conditions were conducted with the manometric catheter out (i.e., 5- and 10-ml volumes of water and milk delivered via syringe and 5-, 10-, and 15-ml volumes of water and milk delivered via cup). Conditions were randomized across participants. Two trials of each condition were administered. Thus, each participant contributed 28 swallows for determination of aspiration status. Because of previous reports that aspiration status was not significantly affected by delivery method or catheter condition [17], aspiration status was not stratified as a function of delivery method nor catheter condition.
At the beginning of the study, participants were instructed to swallow the water or milk when they were ready. All boluses were dyed with green food coloring to offer better visualization. Swallowing position was maintained during all bolus administrations. Swallowing position required that the distal end of the endoscope be just above the top of the epiglottis so that the entire base of tongue, the tip of the epiglottis, posterior pharyngeal wall, lateral pharyngeal walls (e.g., lateral channels), and laryngeal vestibule were visualized prior to bolus administration. The endoscope was maintained in swallowing position throughout the study except for the seconds that the scope was advanced to post-swallow position following a bolus presentation. To obtain post-swallow position, the distal end of the scope was advanced lower into the pharynx, past the tip of the epiglottis and into the upper portion of the laryngeal vestibule where the glottis and trachea below could be well visualized. Post-swallow position was held long enough to determine the penetration-aspiration scale (PAS) score [22] and then the scope was pulled back up into swallowing position.
Obtaining Manometric Data
As noted above, simultaneous manometric measures were obtained during FEES in four conditions (i.e., 5- and 10-ml volumes of water and milk). Each participant contributed eight swallows across two trials, yielding a total of 152 swallows for manometric analyses. Pressure measures were acquired at the upper pharynx, lower pharynx, and UES locations. Accordingly, 456 data points were possible for statistical analyses. Four of the 456 possible data points were missing in either trial one or two (i.e., 0.01%) for a particular test condition. There were no instances where both trials of a particular condition were missing. The missing data resulted from misoriented sensors.
Manometric waveforms were analyzed offline. Peak amplitudes of the two pharyngeal sensors and the relaxation nadir of the UES from sensor one were measured in mmHg. UES resting pressure measurements were also acquired for each participant. Five-seconds were selected from the waveform of sensor one when the sensor was located in the highest pressure region of the UES. The average pressure (in mmHg) across the 5 s was used for the UES resting pressure. As trial does not affect measurements of pressure, duration, and onset [7, 15, 20], the two trials for each condition were averaged for each participant. If one trial was missing (n = 4), the trial that was present was used for the averaged/collapsed data set.
Results
Aspiration was observed on 22 (of the 748 total) swallows from seven participants (i.e., those identified as aspirators). Three of the seven aspirators aspirated once; the remaining four aspirators aspirated twice or more. Eighty-six percent (19/22) of the aspiration events were “silent,” eliciting no throat clearing or cough. All aspiration events occurred with milk or water test boluses, with more aspiration events occurring with milk (68%; 15/22 swallows) than water (32%; 7/22 swallows).
Mean pharyngeal peak pressures as a function of aspiration status, sensor location, liquid type, and bolus volume with standard errors are presented in Table 1. A mixed four-factor ANOVA was performed to investigate pharyngeal peak pressures as a function of aspiration status, pharyngeal sensor location, liquid type, and bolus volume. For all analyses, an α level of 0.05 was adopted. Relative treatment effect sizes (i.e., proportion of variance accounted for) and statistical power were indexed by η2 [23], and ϕ [24], respectively, for all analyses. Small, medium, and large effect sizes were considered with values as 0.10, 0.25, and 0.40, respectively [24]. A significant main effect of aspiration status [F(1,17) = 6.33, p = 0.02, η2 = 0.27, ϕ = 0.66 at α = 0.05] and pharyngeal sensor location [F(1,17) = 12.73, p = 0.002, η2 = 0.43, ϕ = 0.92 at α = 0.05] was found. All other main effects and interactions were not statistically significant (p[0.05).
Table 1.
Mean manometric peak pharyngeal pressure (mmHg) as a function of aspiration status, sensor location, liquid type, and bolus size
| Aspiration status | Sensor location | Liquid type | Bolus volume | |||
|---|---|---|---|---|---|---|
| Upper | Lower | Milk | Water | 5 ml | 10 ml | |
| Aspirator (N = 7) | 58 (10) | 105 (14) | 81 (14) | 83 (9) | 82 (11) | 81 (9) |
| Nonaspirator (N = 12) | 101 (7) | 123 (11) | 105 (7) | 118 (10) | 115 (8) | 108 (7) |
Standard errors are presented in parentheses
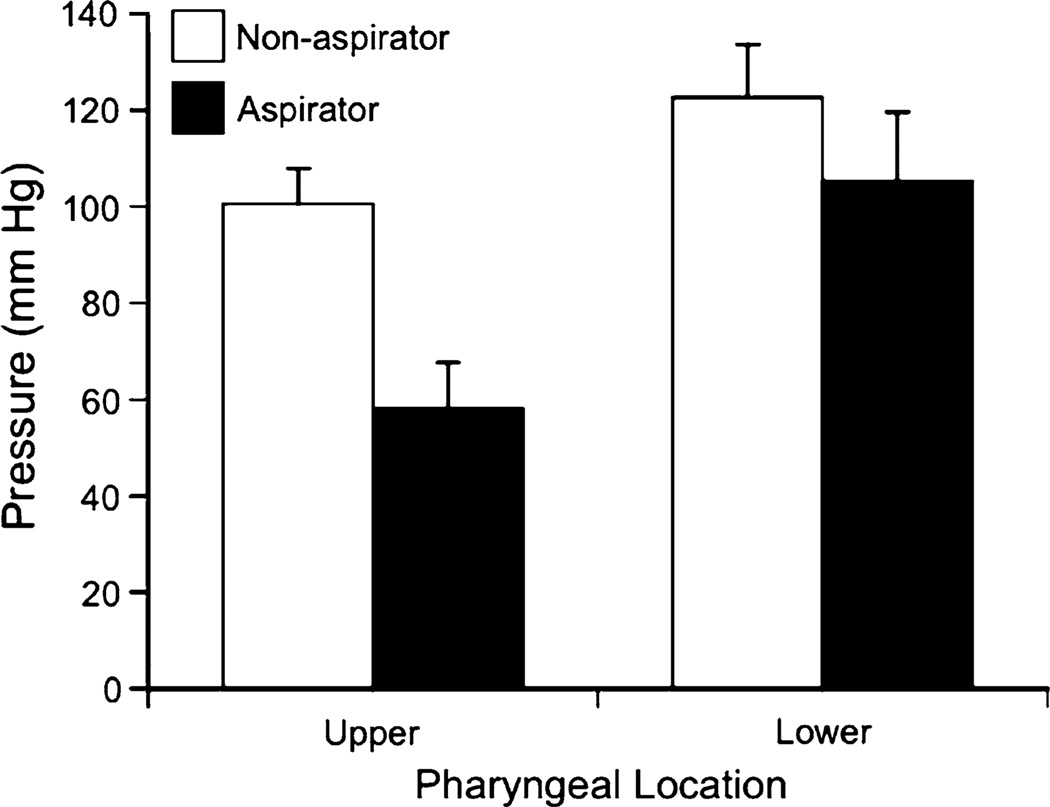
As seen in Fig. 1, aspirators had lower peak pharyngeal pressures than nonaspirators, and lower pharyngeal peak pressure was found at the upper versus lower pharyngeal sensor location in aspirators and nonaspirators. The grand mean pharyngeal peak pressures (with 95% lower- and upper-bound confidence intervals), collapsed across liquid type and bolus volume for the nonaspirating participants’ upper and lower pharyngeal sensor locations, were 100 mmHg (range = 86–116) and 123 mmHg (range = 100–146), respectively. Similarly, for the aspirating participants they were 58 mmHg (range = 37–78) and 105 mmHg (range = 74–136).
Fig. 1.
Mean pharyngeal peak pressures (mmHg) as a function of aspiration status and pharyngeal sensor location. Error bars represent ±1 SEM
A mixed three-factor ANOVA was also performed to investigate UES nadir pressure as a function of aspiration status, liquid type, and bolus volume. No significant main effects or interactions were found (p > 0.05). The grand mean with 95% confidence intervals (with lower and upper bounds) collapsed across aspiration status, liquid type, and bolus volume was 8 mmHg (5 and 12).
An independent t test was performed to investigate UES resting pressure as a function of group (i.e., nonaspirator vs. aspirator). There was no significant group difference (p = 0.25). The grand mean with 95% confidence interval (with lower and upper bounds) collapsed across group was 33 mmHg (23 and 44).
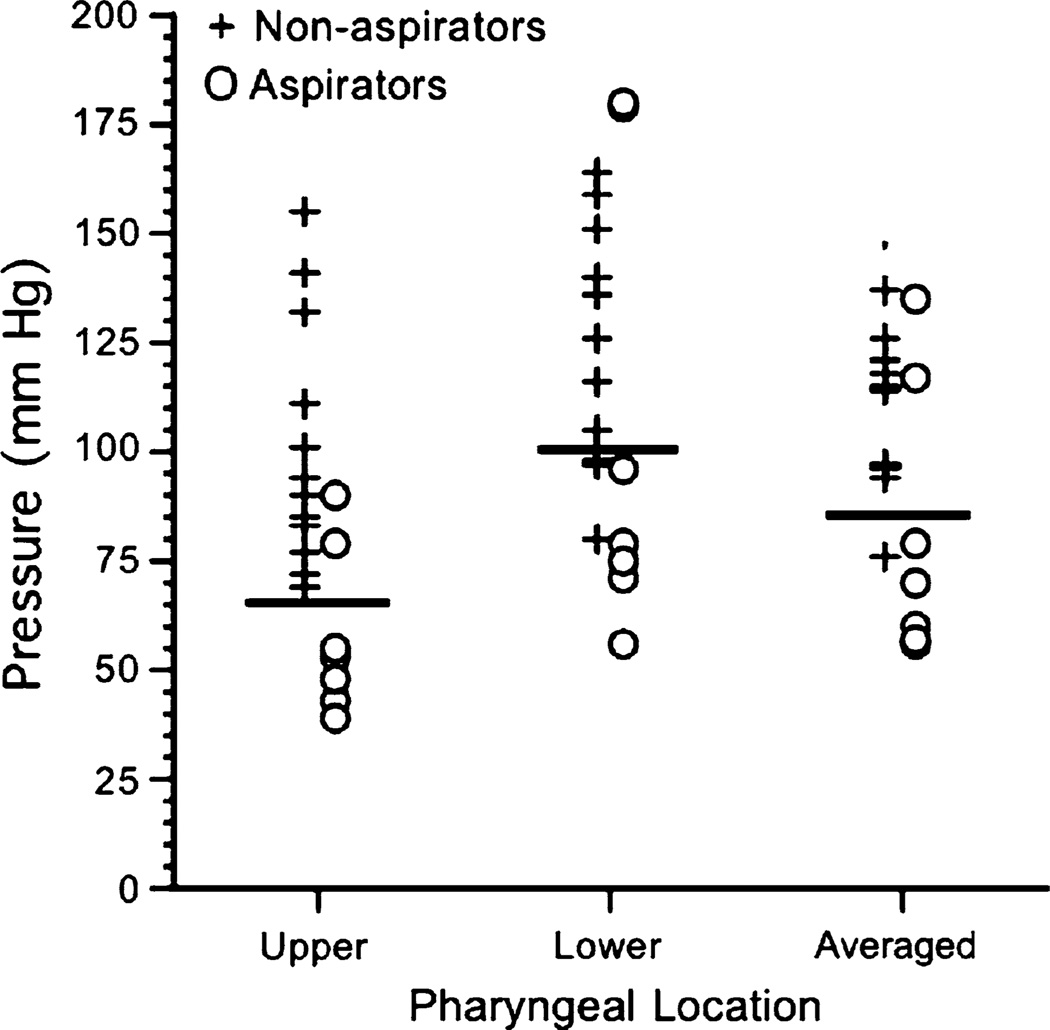
To investigate the association between aspiration status and pharyngeal strength, an examination of pharyngeal peak pressures (in mmHg) across participants was undertaken (Fig. 2). As noted above, aspirators had statistically significant lower peak pharyngeal pressures than nonaspirators. Group separation was drawn at 65, 100, and 85 mmHg for upper, lower, and averaged pharyngeal sensor locations, respectively. Group association as a function of pharyngeal pressure was clear except for two aspirators.
Fig. 2.
Pharyngeal peak pressures (mmHg) for each participant collapsed across liquid type and bolus volume as a function of sensor location and aspiration status. Horizontal bars delineate group separation at 65, 100, and 85 mmHg for upper, lower, and averaged pharyngeal sensor locations
Discussion
Consistent with the hypothesis posed above, healthy older adults who aspirated generated lower pharyngeal peak pressures than healthy older adults who did not aspirate. In concert with previous findings [7, 14, 15], less pharyngeal peak pressure was generated in the upper versus lower pharynx. It was previously reported that 30–40% of healthy adults undergoing FEES aspirated, raising questions about the pathophysiological differences between healthy older adults who aspirate and those who do not [17, S.G. Butler et al., unpublished]. The current results suggest that decreased pharyngeal peak pressure (i.e., throat strength) during swallowing at least partially explains this difference.
While all participants identified as aspirators reported good general health and a negative history of neurological disease, an undiagnosed condition could have existed. Furthermore, since these data are from a cross-sectional design, it is uncertain whether the relative pharyngeal weakness developed prior to the development of aspiration. Longitudinal studies and intervention studies targeting pharyngeal strength will be required to establish the causative role of weakness in the occurrence of aspiration in healthy older adults.
In this study, there was no significant age difference between aspirators (mean = 79.0 years; range = 69–83) and nonaspirators (mean = 79.3 years; range = 69–87). Thus, weakness is not simply a proxy for age. As Fries observed, “variation between healthy persons of the same age is far greater than the variation due to age” [25]. Sarcopenia, the age-related loss of muscle mass and strength, is universally observed in human aging; however, the variability observed between older adults is substantial. Rantanen et al. [26] have commented that “muscle strength may have long-term implications for mortality. Additionally, greater strength itself may provide greater physiological and functional reserve that protects against mortality” (p. 168).
These results are consistent with the idea that pharyngeal peak pressures in swallowing may mirror other physical declines associated with age-related disability. If so, a number of important questions regarding aspiration and pharyngeal strength are suggested by analogy with other studies of muscle weakness in older adults. Does aspiration without known precipitating factors in healthy older adults confer higher risk for morbidity and mortality? If healthy adult aspiration results in negative health consequences, is the aspiration treatable/reversible?
Further investigations into decreased oral, pharyngeal, and laryngeal strength associated with subclinical aspiration in healthy older adults are needed to explore the implications for age-related morbidity (specifically pneumonia). For example, maximum isometric tongue strength is reduced in older versus young adults [27–32]; however, whether reduced isometric tongue strength is associated with aspiration status in healthy older adults is unknown. However, there is some evidence to suggest that patients with dysphagia (but not necessarily aspiration) have lower isometric tongue strength than controls [29]. A laryngeal component possibly contributing to aspiration in healthy older adults outside of reduced pharyngeal strength is presbylarynx (vocal fold atrophy and bowing), whereby laryngeal atrophy results in decreased laryngeal closure. However, again, associations between changes in vocal fold closure patterns and aspiration in healthy older adults remain unknown. Thus, it will be important to identify other variables, aside from pharyngeal peak pressure, related to aspiration (as in the two participants with “normal” pharyngeal peak pressure in Fig. 2) in healthy older adults.
In Fig. 2, putative pharyngeal strength thresholds below which aspiration risk increases were delineated. The actual value of these targets should be replicated by other groups. However, the existence of such thresholds suggests that silent aspiration may be treatable by sufficiently strengthening muscles involved. Thus, if a patient has a mean pharyngeal peak pressure below the thresholds, an oropharyngeal treatment program could be started to reverse his/her aspiration status. Also, it is clear from these data that although pharyngeal strength thresholds may delineate most aspirators from nonaspirators, they do not delineate all aspirators from nonaspirators, since two aspirators had pharyngeal strength similar to that of nonaspirators. In those two patients, other pathophysiological mechanisms— currently unknown but possibly including decreased laryngeal closure or delayed pharyngeal response—are likely responsible for aspiration. Future studies are planned to investigate laryngeal closure, reflux findings, pharyngeal response times, and residue severity as a function of aspiration status.
Lower pharyngeal pressures were significantly higher than upper pharyngeal pressures, which mirrors our previous investigations. It is logical that the sensor closest to the tongue would yield the highest pressures since the posterior tongue drives the bolus into the hypopharynx. However, given that multiple investigations have found that lower pharyngeal peak pressures are higher [7, 14, 15], perhaps greater pressures are needed to safely propel the bolus past the possible entrance of the trachea and efficiently into the esophagus.
While no difference was found in pharyngeal peak pressures with milk relative to water in the present study, milk was associated with significantly more frequent episodes of aspiration in healthy older adults (S.G. Butler et al., unpublished). Thus, although aspirators have lower pharyngeal peak pressure than nonaspirators, either another pathophysiological mechanism is responsible for the greater frequency of aspiration seen with milk or the effect of pharyngeal peak pressures for milk versus water was not detectable in this small sample.
Gumbley et al. [8] previously found no effect of bolus volume on pharyngeal peak pressures in young adults. This is consistent with the present findings in older adults, regardless of aspiration status. However, Gumbley et al. [8] did find that smaller bolus volumes yielded greater UES nadir measurements. This agrees with our previous work that found, in general, that 5-ml boluses elicited greater UES nadir than 10-ml boluses [7], but it differs from our current findings. The current findings may be different since more aspirators participated in this study; the pathophysiology associated with decreased pharyngeal peak pressures may affect lack of bolus accommodation across bolus volumes.
The finding that healthy older adults who aspirated had less pharyngeal strength during swallowing compared to those who did not aspirate is a novel one that may have parallels to other areas of decreased physical strength/ functioning seen in older adults with a high risk for morbidity and mortality. Accordingly, additional mechanistic studies are needed in healthy older adults who aspirate versus those who do not to determine other potential oral, pharyngeal, or laryngeal pathophysiology associated with aspiration status. From the present observations, one could theorize the existence of a pathway by which generalized functional decline may lead to specific age-associated morbidities such as community-acquired pneumonia. Longitudinal studies are needed to determine if aspiration status and/or decreased pharyngeal strength predict negative health consequences.
Acknowledgments
This work was supported by a new investigators research grant from the American Speech Language Hearing Foundation and in part by the Wake Forest School of Medicine Claude D. Pepper Older Americans Independence Center (P30 AG21332). We thank Karen Potvin Klein, MA, ELS (Research Support Core, Wake Forest University Health Sciences) for her editorial contributions to the manuscript.
Footnotes
Presented at the 18th Annual Meeting of the Dysphagia Research Society, San Diego, CA, March 5–7, 2010.
Contributor Information
Susan G. Butler, Email: sbutler@wfubmc.edu, Department of Otolaryngology, Wake Forest University School of Medicine, Medical Center Boulevard, Winston-Salem, NC 27157, USA.
Andrew Stuart, Department of Communication Sciences and Disorders, East Carolina University, Greenville, NC 27858, USA.
Erika Wilhelm, Department of Communication Sciences and Disorders, Appalachian State University, Boone, NC 28608, USA.
Catherine Rees, Department of Otolaryngology, Wake Forest University School of Medicine, Medical Center Boulevard, Winston-Salem, NC 27157, USA.
Jeff Williamson, Department of Otolaryngology, Wake Forest University School of Medicine, Medical Center Boulevard, Winston-Salem, NC 27157, USA.
Stephen Kritchevsky, Department of Otolaryngology, Wake Forest University School of Medicine, Medical Center Boulevard, Winston-Salem, NC 27157, USA.
References
- 1.Witte U, Huckabee ML, Doeltgen SH, Gumbley F, Robb M. The effect of effortful swallow on pharyngeal manometric measurements during saliva and water swallowing in healthy participants. Arch Phys Med Rehabil. 2008;89(5):822–828. doi: 10.1016/j.apmr.2007.08.167. [DOI] [PubMed] [Google Scholar]
- 2.Castell JA, Dalton CB, Castell DO. Pharyngeal and upper esophageal sphincter manometry in humans. Am J Physiol. 1990;258(2 Pt 1):G173–G178. doi: 10.1152/ajpgi.1990.258.2.G173. [DOI] [PubMed] [Google Scholar]
- 3.Perlman AL, Schultz JG, VanDaele DJ. Effects of age, gender, bolus volume, and bolus viscosity on oropharyngeal pressure during swallowing. J Appl Physiol. 1993;75(1):33–37. doi: 10.1152/jappl.1993.75.1.33. [DOI] [PubMed] [Google Scholar]
- 4.Dodds WJ, Hogan WJ, Lydon SB, Stewart ET, Stef JJ, Arndorfer RC. Quantitation of pharyngeal motor function in normal human subjects. J Appl Physiol. 1975;39(4):692–696. doi: 10.1152/jappl.1975.39.4.692. [DOI] [PubMed] [Google Scholar]
- 5.Cerenko D, McConnel FM, Jackson RT. Quantitative assessment of pharyngeal bolus driving forces. Otolaryngol Head Neck Surg. 1989;100(1):57–63. doi: 10.1177/019459988910000109. [DOI] [PubMed] [Google Scholar]
- 6.Dantas RO, Kern MK, Massey BT, Dodds WJ, Kahrilas PJ, Brasseur JG, Cook IJ, Lang IM. Effect of swallowed bolus variables on oral and pharyngeal phases of swallowing. Am J Physiol. 1990;258(5 Pt 1):G675–G681. doi: 10.1152/ajpgi.1990.258.5.G675. [DOI] [PubMed] [Google Scholar]
- 7.Butler SG, Stuart A, Castell D, Russell GB, Koch K, Kemp S. Effects of age, gender, bolus condition, viscosity, and volume on pharyngeal and upper esophageal sphincter pressure and temporal measurements during swallowing. J Speech Lang Hear Res. 2009;52(1):240–253. doi: 10.1044/1092-4388(2008/07-0092). [DOI] [PubMed] [Google Scholar]
- 8.Gumbley F, Huckabee ML, Doeltgen SH, Witte U, Moran C. Effects of bolus volume on pharyngeal contact pressure during normal swallowing. Dysphagia. 2008;23(3):280–285. doi: 10.1007/s00455-007-9137-9. [DOI] [PubMed] [Google Scholar]
- 9.Kahrilas PJ, Logemann JA, Lin S, Ergun GA. Pharyngeal clearance during swallowing: a combined manometric and videofluoroscopic study. Gastroenterology. 1992;103(1):128–136. doi: 10.1016/0016-5085(92)91105-d. [DOI] [PubMed] [Google Scholar]
- 10.Ghosh SK, Pandolfino JE, Zhang Q, Jarosz A, Kahrilas PJ. Deglutitive upper esophageal sphincter relaxation: a study of 75 volunteer subjects using solid-state high-resolution manometry. Am J Physiol Gastrointest Liver Physiol. 2006;291(3):G525–G531. doi: 10.1152/ajpgi.00081.2006. [DOI] [PubMed] [Google Scholar]
- 11.Cook IJ, Dodds WJ, Dantas RO, Massey B, Kern MK, Lang IM, Brasseur JG, Hogan WJ. Opening mechanisms of the human upper esophageal sphincter. Am J Physiol. 1989;257(5 Pt 1):G748–G759. doi: 10.1152/ajpgi.1989.257.5.G748. [DOI] [PubMed] [Google Scholar]
- 12.Jacob P, Kahrilas PJ, Logemann JA, Shah V, Ha T. Upper esophageal sphincter opening and modulation during swallowing. Gastroenterology. 1989;97(6):1469–1478. doi: 10.1016/0016-5085(89)90391-0. [DOI] [PubMed] [Google Scholar]
- 13.Kern M, Bardan E, Arndorfer R, Hofmann C, Ren J, Shaker R. Comparison of upper esophageal sphincter opening in healthy asymptomatic young and elderly volunteers. Ann Otol Rhinol Laryngol. 1999;108(10):982–989. doi: 10.1177/000348949910801010. [DOI] [PubMed] [Google Scholar]
- 14.Olsson R, Nilsson H, Ekberg O. Simultaneous videoradiography and pharyngeal solid state manometry (videomanometry) in 25 nondysphagic volunteers. Dysphagia. 1995;10(1):36–41. doi: 10.1007/BF00261278. [DOI] [PubMed] [Google Scholar]
- 15.Huckabee ML, Butler SG, Barclay M, Jit S. Submental surface electromyographic measurement and pharyngeal pressures during normal and effortful swallowing. Arch Phys Med Rehabil. 2005;86(11):2144–2149. doi: 10.1016/j.apmr.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 16.Butler SG, Stuart A, Kemp S. Flexible endoscopic evaluation of swallowing in healthy young and older adults. Ann Otol Rhinol Laryngol. 2009;118(2):99–106. doi: 10.1177/000348940911800204. [DOI] [PubMed] [Google Scholar]
- 17.Butler SG, Stuart A, Markley L, Rees C. Penetration and aspiration in healthy older adults as assessed during endoscopic evaluation of swallowing. Ann Otol Rhinol Laryngol. 2009;118(3):190–198. doi: 10.1177/000348940911800306. [DOI] [PubMed] [Google Scholar]
- 18.Belafsky PC, Postma GN. Validity and reliability of the reflux symptom index (RSI) J Voice. 2002;16(2):274–277. doi: 10.1016/s0892-1997(02)00097-8. [DOI] [PubMed] [Google Scholar]
- 19.Salassa JR, DeVault KR, McConnel FM. Proposed catheter standards for pharyngeal manofluorography (videomanometry) Dysphagia. 1998;13:105–110. doi: 10.1007/PL00009553. [DOI] [PubMed] [Google Scholar]
- 20.Hiss SG, Huckabee ML. Timing of pharyngeal and upper esophageal sphincter pressures as a function of normal and effortful swallowing in young healthy adults. Dysphagia. 2005;20(2):149–156. doi: 10.1007/s00455-005-0008-y. [DOI] [PubMed] [Google Scholar]
- 21.Castell JA, Castell DO. Recent developments in the manometric assessment of upper esophageal sphincter function and dysfunction. Dig Dis. 1997;15(Suppl 1):28–39. doi: 10.1159/000171619. [DOI] [PubMed] [Google Scholar]
- 22.Rosenbek JC, Robbins JA, Roecker EB, Coyle JL, Wood JL. A penetration-aspiration scale. Dysphagia. 1996;11(2):93–98. doi: 10.1007/BF00417897. [DOI] [PubMed] [Google Scholar]
- 23.Keppel G, Wickens TD. Design and analysis: a researcher’s handbook. 4th ed. Englewood Cliffs, NJ: Prentice Hall; 2004. [Google Scholar]
- 24.Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates; 1989. [Google Scholar]
- 25.Fries JF. Aging, natural death, and the compression of morbidity. N Engl J Med. 1980;303(3):130–135. doi: 10.1056/NEJM198007173030304. [DOI] [PubMed] [Google Scholar]
- 26.Rantanen T, Harris T, Leveille SG, Visser M, Foley D, Masaki K, Guralinik JM. Muscle strength and body mass index as long-term predictors of mortality in initially healthy men. J Gerontol A Biol Sci Med Sci. 2000;55:M168–M173. doi: 10.1093/gerona/55.3.m168. [DOI] [PubMed] [Google Scholar]
- 27.Crow HC, Ship JA. Tongue strength and endurance in different aged individuals. J Gerontol A Biol Sci Med Sci. 1996;51(5):M247–M250. doi: 10.1093/gerona/51a.5.m247. [DOI] [PubMed] [Google Scholar]
- 28.Nicosia MA, Hind JA, Roecker EB, Carnes M, Doyle J, Dengel GA, Robbins J. Age effects on the temporal evolution of isometric and swallowing pressure. J Gerontol A Biol Sci Med Sci. 2000;55(11):M634–M640. doi: 10.1093/gerona/55.11.m634. [DOI] [PubMed] [Google Scholar]
- 29.Stierwalt JA, Youmans SR. Tongue measures in individuals with normal and impaired swallowing. Am J Speech Lang Pathol. 2007;16(2):148–156. doi: 10.1044/1058-0360(2007/019). [DOI] [PubMed] [Google Scholar]
- 30.Robbins J, Levine R, Wood J, Roecker EB, Luschei E. Age effects on lingual pressure generation as a risk factor for dysphagia. J Gerontol A Biol Sci Med Sci. 1995;50(5):M257–M262. doi: 10.1093/gerona/50a.5.m257. [DOI] [PubMed] [Google Scholar]
- 31.Youmans SR, Stierwalt JA. Measures of tongue function related to normal swallowing. Dysphagia. 2006;21(2):102–111. doi: 10.1007/s00455-006-9013-z. [DOI] [PubMed] [Google Scholar]
- 32.Youmans SR, Youmans GL, Stierwalt JA. Differences in tongue strength across age and gender: is there a diminished strength reserve? Dysphagia. 2009;24(1):57–65. doi: 10.1007/s00455-008-9171-2. [DOI] [PubMed] [Google Scholar]