Abstract
There are growing concerns about potential delayed, neuropsychiatric consequences (e.g, cognitive decline, mood or anxiety disorders) of sports-related traumatic brain injury (TBI). Autopsy studies of brains from a limited number of former athletes have described characteristic, pathologic changes of chronic traumatic encephalopathy (CTE) leading to questions about the relationship between these pathologic and the neuropsychiatric disturbances seen in former athletes. Research in this area will depend on in vivo methods that characterize molecular changes in the brain, linking CTE and other sports-related pathologies with delayed emergence of neuropsychiatric symptoms. In this pilot project we studied former National Football League (NFL) players using new neuroimaging techniques and clinical measures of cognitive functioning. We hypothesized that former NFL players would show molecular and structural changes in medial temporal and parietal lobe structures as well as specific cognitive deficits, namely those of verbal learning and memory. We observed a significant increase in binding of [11C]DPA-713 to the translocator protein (TSPO), a marker of brain injury and repair, in several brain regions, such as the supramarginal gyrus and right amygdala, in 9 former NFL players compared to 9 age-matched, healthy controls. We also observed significant atrophy of the right hippocampus. Finally, we report that these same former players had varied performance on a test of verbal learning and memory, suggesting that these molecular and pathologic changes may play a role in cognitive decline. These results suggest that localized brain injury and repair, indicated by increased [11C]DPA-713 binding to TSPO, may be linked to history of NFL play. [11C]DPA-713 PET is a promising new tool that can be used in future study design to examine further the relationship between TSPO expression in brain injury and repair, selective regional brain atrophy, and the potential link to deficits in verbal learning and memory after NFL play.
Keywords: Mild traumatic brain injury, Translocator protein, Neuroinflammation, Microglia, Molecular neuroimaging
Introduction
There have been several recent reports of memory deficits, mood disorders, and motor symptoms among former athletes exposed to repetitive, sports-related, traumatic brain injury (TBI) (Guskiewicz et al., 2005, 2007; Hart et al., 2013; McKee et al., 2013; Pearce et al., 2014; Seichepine et al., 2013; Singh et al., 2014; Weir et al., 2009). Coupled with increased public awareness, these studies have fueled scientific investigation into pathologic changes contributing to TBI-related neuro-psychiatric symptoms including cognitive decline or affective changes (e.g., anxiety, mood swings, depression) in the absence of neurologic signs (e.g., slurred speech, parkinsonism). Concurrently, autopsy studies of a limited number of athletes (including football players) who have suffered from TBI have led some researchers to diagnose chronic traumatic encephalopathy (CTE), a putative tauopathy characterized by global brain atrophy with a thinned corpus callosum, enlarged ventricles, and cavum septum pellucidum (Jordan, 2013). The prevalence of CTE among former athletes who have suffered from TBI is unknown, as is the prevalence of other injury-associated brain pathologies, for example those associated with Alzheimer's disease or frontotemporal degeneration. Nevertheless, there are concerns that even mild, repetitive TBI leads to the development of one or more progressive pathologies causing the delayed emergence of these neuropsychiatric disturbances (Collins et al., 1999; Matser et al., 1999; Singh et al., 2014). To best understand these relationships the development of in vivo tools, preferably utilizing brain imaging, is essential.
Regarding CTE, it has been hypothesized that repetitive mild TBI (mTBI) in athletes leads to axonal damage and inflammation, followed by deposition and aggregation of hyperphosphorylated tau protein (ptau) and the formation of neurofibrillary tangles (NFTs) in susceptible areas (McKee et al., 2014; Shively et al., 2012). In stages I and II, foci of tau pathology are limited to the depths of cortical sulci and brainstem areas such as locus coeruleus. By stage III, NFTs have a more widespread distribution (Stein et al., 2014). It is unclear whether the primary pathology of CTE is deposition of p-tau or whether dysregulated inflammation drives protein deposition (Smith et al., 2013). Regional NFT distribution may promote chronic inflammation and neurotoxicity, resulting in underlying changes in local neuron morphology, more diffuse synaptic changes, and possibly changed cholinergic neurotrans-mission (Hellstrom-Lindahl et al., 2000; Rubio et al., 2006). NFT accumulation may also promote aggregation and diminished clearance of other pathologic proteins including amyloid β, TDP-43, and alpha-synuclein, thereby fueling further neurodegeneration, inflammatory response, and associated cognitive decline (Hazrati et al., 2013; McKee et al., 2013; Shively et al., 2012). Although neuroinflammation is not necessarily deleterious and could represent compensatory repair of these other degenerative processes, the ability to image inflammatory brain changes in vivo in former athletes will contribute to putting together the complex puzzle involved.
Former American football players have higher rates of delayed neurological, cognitive or affective impairments, including dementia with aging (Guskiewicz et al., 2005; Lehman et al., 2012; Weir et al., 2009). Those impairments have been attributed to the pathologic effects of repeated TBI, characterized by repetitive biomechanical shearing and inflammation of neuronal axons from rotational, linear, and/or impact decelerations of the head incurred over years of play. A recent study of retired National Football League (NFL) players found cognitive deficits and depression to be more common in this cohort compared to matched healthy controls, and reported specific deficits in naming, word finding, and visual or verbal episodic memory (Hart et al., 2013). Worse performance on verbal learning and memory testing has also been reported in Division I college varsity football and ice hockey athletes when compared to same-level athletes playing noncontact sports (McAllister et al., 2012, 2014). A functional magnetic resonance imaging (fMRI) study of former professional football players has suggested that subtle deficits in learning and memory may be due to functional inefficiencies of brain networks in medial temporal and inferior parietal lobes (Ford et al., 2013).
At present, CTE is diagnosed on the basis of findings seen at autopsy, although recent preliminary brain positron emission tomography (PET) imaging using [18F]FDDNP, a radiopharmaceutical that binds to Alzheimer pathology including p-tau NFTs and amyloid, revealed increased binding in subcortical regions and in the amygdala of five former NFL players in vivo (Small et al., 2013). Novel technology enabling non-invasive, quantitative study of post-traumatic brain changes after TBI will improve the longitudinal study of pathologic responses to trauma, and will add value to prognostic evaluation and therapeutic monitoring. In addition to tau imaging, such technology may also include imaging of TBI-related neuroinflammation by targeting the translocator protein (TSPO).
TSPO is a five-transmembrane protein that spans the outer mitochondrial membrane (Papadopoulos et al., 2006). Although TSPO expression is low in healthy human brain tissue, brain TSPO levels are increased after TBI in animal models, likely due to increased expression by activated microglia in states of neuroinflammation and reactive gliosis. Accordingly, increased expression of TSPO has been used as a marker of brain injury or post-traumatic immune cell response (Papadopoulos and Lecanu, 2009) and has been detected in areas with other co-localized markers of microglial activation using [11C]DPA-713 in animal models of neuroin-flammation (Boutin et al., 2007). Using PET, two previous studies have demonstrated elevated uptake of [11C]R-PK11195, a first-generation radioligand that targets TSPO, in the brains of patients who suffered moderate to severe TBI from several months to 17 years after injury (Folkersma et al., 2011; Ramlackhansingh et al., 2011). However, studies using [11C]R-PK11195 are limited by poor signal-to-noise ratio related to high non-specific binding and poor brain delivery (Chauveau et al., 2008). Analysis of PET imaging data using second generation radio-tracers for TSPO requires correction for rs6971 genotype because the Ala147Thr polymorphism is associated with reduced affinity for the TSPO target (Owen et al., 2012). Recently developed techniques to apply TSPO genotype correction to PET imaging with second generation radiopharmaceuticals not only control for the effect of this effect, but also improve the sensitivity of detecting increased TSPO density in neurodegenerative disease, as demonstrated in study of Alzheimer's disease (Kreisl et al., 2013a, 2013b) and of HIV dementia (Coughlin et al., 2014).
Building on this background, we posited a mechanistic relationship between localized, chronic neuroinflammation, altered regional morphology, and specific cognitive disruption in verbal learning and memory in American football players. Through multimodal, cross-sectional design of this pilot study, we sought to test for these changes in parallel, within a small group of former NFL players. Specifically, we hypothesized increased [11C]DPA-713 binding, consistent with increased TSPO expression, in medial temporal structures and in the supramarginal gyrus (SMG) of the inferior parietal lobe, as these regions are susceptible to NFT deposition and atrophy in well-established CTE. In addition, we hypothesized volume loss and cortical atrophy in these medial temporal and inferior parietal regions. Finally, we hypothesized impaired performance on testing of verbal learning and memory in these same former players. To test these hypotheses, we used [11C]DPA-713 PET to measure binding of this radiopharmaceutical to TSPO in 9 former NFL players and 9 healthy non-football players. Regional binding of [11C]DPA-713 was compared after correction for the effect of rs6971 genotype on binding. Diminished volume and/or cortical thickness in the same regions of interest (ROIs) were evaluated with anatomical MRI data. Finally, we tested the former NFL players for deficits in neurocognitive function, focusing on verbal learning and memory.
Materials and methods
Human subjects
The Johns Hopkins Institutional Review Board approved this study. All subjects provided informed consent prior to participation. Former NFL players were recruited through advertisement at local chapter meetings of the NFL Players Association and through word of mouth among retired NFL players. All participants denied alcohol and illicit substance abuse (confirmed by negative urine toxicology screen). Age comparable, male healthy controls were recruited through local advertising and also were studied (including PET and MRI imaging) after careful clinical interview to ensure health. The healthy control participants were over the age of 55, were medically stable, and denied surgery in the past year. Controls denied current use of prescribed and over-the-counter medications. All healthy participants denied neurologic impairments, psychiatric illness, history of head trauma with loss of consciousness, or a known family history of dementia. All NFL participants were evaluated with the 17-item Hamilton Depression scale (HAMD) (Hamilton, 1960) by a psychiatrist on the study team (J.M.C.).
Clinical assessment and neuropsychological testing in former NFL players
Assessment of each former NFL player was conducted in person by the study neuropsychologist (C.A.M.) and consisted of a battery of tests (Supplemental Table 1) that included the CVLT-II (Delis et al., 2005). The assessment also included a standardized interview with questions about their NFL career and concussion(s), handedness, and a modified version of the Rivermead Post-Concussive Symptom Questionnaire (RPQ) (King et al., 1995). Details about past concussion, as de-fined by the Quality Standards Subcommittee of the American Academy of Neurology (The Quality Standards Subcommittee, 1997) were thus obtained by retrospective report from each former player. In the modi-fied RPQ participants rated the severity of each somatic, cognitive, or affective (i.e. mood, anxiety) symptom on a scale of 0 (absence of the symptom) to 4 (indicating a severe problem) relative to the severity before NFL play. We focused on responses to the 13 symptoms classified as ‘late’ post concussive symptoms in the original RPQ, the RPQ-13, and calculated the sum of the severity scores for these 13 symptoms (total score range 0–52). The California Verbal Learning Test-II (CVLT-II) was administered as a measure of the ability to learn and recall verbally presented words (a 16-item word list) (Delis et al., 2005) as it has been used as the primary outcome measure in other recent studies of athletes involved in contact sports (McAllister et al., 2014). CVLT-II component indices included four outcome measures: the total score for five trials of immediate recall, short-delay free recall, long-delay free recall, and recall discriminability. Raw scores for each component were standardized based on age-adjusted normative data provided in the test's scoring program. Control subjects did not participate in this clinical and neuro-psychological assessment.
DNA extraction and genotyping
Both former NFL players and healthy controls provided a blood sample for DNA extraction (PureGene® Blood Core Kit C, Qiagen, Valencia, CA) and TSPO (rs6971) genotype analysis using the rs6971 TaqMan assay (Applied Biosytems®, Life Technologies, Grand Island, NY). Three different rs6971 genotypes were defined: Ala147/Ala147 (C/C), Thr147/Thr147 (T/T), and Ala147/Thr147 (C/T). [11C]DPA-713 binds to TSPO from individuals of each genotype with a characteristic affinity pattern. The first two groups consisted of homozygotes for high affinity (C/C), and low affinity (T/T) binding phenotype, and the last group consisted of heterozygotes (C/T) with mixed affinity binding phenotype, as previously described (Owen et al., 2012).
In vivo brain imaging
Radiotracer synthesis
[11C]DPA-713 was synthesized as described by (Thominiaux et al., 2006). Radiochemical purity was greater than 95%. It was delivered with high specific radioactivity (244.8 ± 75.2 GBq/μmol) via intravenous bolus injection at the onset of a 90 min dynamic list mode PET acquisition.
Plasma sampling
Measurement of the arterial plasma input function was conducted as previously described (Endres et al., 2009) through collection of 25–35 blood samples (1 mL) over the course of each PET scan and collection of an additional eight serial 4 mL samples for radiolabeled metabolite measurements.
PET acquisition
PET scans were acquired using a High Resolution Research Tomograph scanner (HRRT, Siemens Healthcare, Knoxville,TN), an LSO-based, dedicated brain PET scanner with 2.5 mm reconstructed image resolution. The 90 min list mode data were binned into 30 frames (four 15 s, four 30 s, three 1 min, two 2 min, five 4 min, and twelve 5 min frames). The data were then reconstructed using the iterative ordered subsets expectation maximization (OS-EM) algorithm (with six iterations and 16 subsets), with correction for radioactive decay, dead time, attenuation, scatter and randoms (Rahmim et al., 2004). The attenuation maps were generated from 6 min transmission scans performed with a 137Cs point source prior to the emission scans. The reconstructed image space consisted of cubic voxels, each 1.22 mm3 in size, and spanning dimensions of 31 cm x 31 cm (transaxially) and 25 cm (axially).
MRI acquisition
All subjects underwent brain MRI for anatomic delineation of ROIs on PET images after PET-MRI co-registration (detailed below). MRI T1-weighted images were obtained on a Phillips Achieva 3 T scanner (Andover, MA) with a 32-channel head coil to obtain a 1 × 1 x 1 mm 3D MP-RAGE sequence.
Data analysis and statistics
PET image processing
The software package PMOD (v3.3, PMOD Technologies Ltd, Zurich, Switzerland) was used in initial PET image processing and kinetic analysis. Pre-processing steps included (1) inter-frame motion correction: all 30 frames of PET reconstructed images were rigidly realigned to the 0–30 min mean PET image, which was obtained by averaging frames 1 through 18; and (2) PET-MRI co-registration: the 0–30 min PET mean image and, subsequently, all 30 motion-corrected PET frames, were co-registered to the subject's T1-weighted MRI image using rigid transformations. Based on the T1-weighted MR images, automated cortical reconstruction and volumetric segmentation were performed with the Freesurfer image analysis suite, which is documented and freely available for download online (http://surfer.nmr.mgh.harvard.edu/). The technical details of these procedures are described in prior publications, e.g., (Fischl et al., 2002). From the automated regions generated, we selected 12 ROIs (including hippocampus, amygdala, entorhinal cortex, parahippocampal cortex, supramarginal gyrus (SMG) and temporal pole of right and left hemispheres. These medial temporal lobe structures and the SMG of the inferior parietal lobe were chosen based on hypothesized involvement of these regions in CTE and mTBI-associated cognitive, behavioral and motor deficits. PET time-activity curves (TACs) were then generated for all subjects using the above mentioned ROI definitions.
Calculation of regional total distribution volume (VT)
Based on the TACs obtained, [11C]DPA-713 binding to TSPO was quantified with the use of the metabolite-corrected arterial plasma input function. Following other published studies using second-generation TSPO ligands and the proposed nomenclature for reversibly binding radioligands (Innis et al., 2007), the main outcome measure presented was total distribution volume (VT), defined as the ratio of the concentration of the radioligand in brain tissue to that in plasma at equilibrium. Regional VT is proportional to the receptor density in the defined ROI. VT was obtained using the Logan graphical method with arterial input function for each defined ROI (Logan et al., 1990). The Logan method was chosen due to the relative stability of this measure.
PET data analysis
Differences in VT values between the former NFL players and elderly healthy control (EHC) participants were examined using linear mixed model analysis, assuming a constant residual variance across regions, with proposed full and null models represented as the following:
In these models, index i indicates different regions, index j indicates different cohort or genotype and k indicates different subjects, whereas αi indicates the region specific effect, βi indicates the cohort effect for region i, bk is the random effect for subject k, and ∊ijk represents the error term. Two omnibus tests were conducted, one for cohort effect and the second for genotype effect across all regions tested, generating respective P-values. Region-specific effects and interaction effects were examined with appropriate post-hoc tests, and the threshold for significance was set at P < 0.004, taking into account multiple comparisons for the 12 ROIs using Bonferroni correction (0.05/12 = 0.004). Finally, based on hypothesized regional selectivity of CTE pathology after traumatic injury, we conducted a simplified secondary analysis where these examinations were repeated after the absolute regional VT values were normalized by the total cortical gray matter (GM) VT, defined as VT_GM, obtained from the same subject and scan. This regional outcome measure reflects regional binding relative to global gray matter binding, defined as GMVT = VT/VT_GM, and is assumed to be independent of rs6971 genotype. Using the Student's t test GMVT values for participants of all rs6971 genotypes were compared between NFL players and controls.
MRI data analysis
Freesurfer software was used to obtain the regional volumes for each of right and left hippocampal and amygdala ROIs as well as cortical thickness measures for the right and left hemispheres of the remaining ROIs, including parahippocampal cortices, entorhinal cortices, temporal pole and SMG (http://surfer.nmr.mgh.harvard.edu/). Total cortical gray matter volume and brain parenchymal volume were also measured with this software. Student's t test comparison was used to compare measures of volume/cortical thickness between the former NFL player and healthy control groups. Bonferroni correction for multiple comparisons was employed with significance set to P < 0.0036 (=0.05/14).
Results
Human subject participation
Eleven former NFL players over age 55 (seven Caucasian, four African American) were enrolled in the study. These participants included two linebackers, two running backs, two corner backs, one defensive tackle, one punter, one safety, one defensive end, and one wide receiver. Two of the former players did not participate in the neuroimaging: one subject had pre-existing atrial fibrillation on EKG and the other required surgery that excluded him from further participation. Those two players participated in the clinical assessment. Of the nine remaining former players who completed PET and MRI, one refused clinical and neuropsychological testing, although his PET and MRI data were incorporated in the imaging analyses. Nine controls over the age of 55 years (six Caucasian, three African American) completed imaging (PET and MRI). Nine of the 11 former NFL players and eight of the nine controls were right-handed. Other demographic characteristics for the study participants are in Table 1. All eleven former NFL players were without moderate or severe depression defined as a score >13 on the HAMD, though two players had a HAMD score between 8 and 13, indicating mild depression.
Table 1.
Clinical characteristics for 11 former nfl player participants (NFL)a and 9 elderly healthy controls (EHC).
| Age (years) | Education (years) | BMI | Years since NFL play | Reported number of concussions | RPQ-13b | |
|---|---|---|---|---|---|---|
| NFLa | 66 | 16 | 25.1 | 33 | 3 | 2 |
| 74 | 16 | 35.1 | 42 | 3 | 2 | |
| 72 | 18 | 34.6 | 37 | 5 | 19 | |
| 60 | 16 | 29.8 | 37 | 4 | . | |
| 64 | 16 | 32.7 | 32 | 4 | 32 | |
| 68 | 16 | 29.3 | 35 | 11 | 21 | |
| 67 | 16 | 27.3 | 42 | 0 | 3 | |
| 63 | 20 | 27.0 | 28 | 2 | 20 | |
| 57 | 16 | 36.3 | 24 | 3 | . | |
| 59 | 16 | 32.9 | 32 | 4 | 4 | |
| 63 | 18 | 29.6 | 26 | 40 | 7 | |
| EHC | 62 | 15 | 28.8 | |||
| 56 | 16 | 24.6 | ||||
| 61 | 14 | 34.0 | ||||
| 55 | 16 | 29.4 | ||||
| 56 | 16 | 32.3 | ||||
| 57 | 18 | 23.1 | ||||
| 57 | 16 | 28.0 | ||||
| 55 | 16 | 25.9 | ||||
| 66 | 17 | 22.7 |
BMI = body mass index.
The first 9 of these listed 11 former NFL players participated in [11C]DPA-713 PET.
RPQ-13, Rivermead Post Concussion Symptoms Questionaire, higher scores (0-52) reflect greater severity of late symptoms. Nine of ten players who completed neuropsychiatric assessment completed the RPQ-13.
Clinical and neuropsychological assessment in former NFL players
Historical details about years since last play in the NFL (range 24–42 years), and retrospectively reported number of career concussions (range 0–40 concussions) for the 11 former players are listed in Table 1. The modified RPQ was completed by nine of the ten former NFL players who underwent neuropsychological assessment. Total RPQ-13 scores are listed for each player in Table 1 (severity ratings for each of the 13 symptoms are presented in Supplemental Table 2), with higher scores reflecting greater severity of reported symptoms out of a total possible score of 52. Percentile scores for each of the four CVLT-II components were determined from the standardized scores (based on age-adjusted normative data) and are presented in Table 2.
Table 2.
California Verbal Learning Test, 2nd Edition (CVLT-II) percentile scores for former NFL players (N = 10).a
| Range | Mean (SD) | |
|---|---|---|
| CVLT trials 1 through 5 total | 12-83 | 54 (26) |
| CVLT short delay free recall | 6-93 | 44 (31) |
| CVLT long delay free recall | 16-84 | 46 (24) |
| CVLT recall discriminability | 16-98 | 53 (30) |
One of the 11 former NFL players refused neurocognitive testing.
Genotyping
Assay results of the rs6971 polymorphism identified five EHC participants and five former NFL players with the C/C genotype (Ala/Ala). The remaining four EHC and four former NFL players had the C/T genotype (Ala/Thr). None of the EHC or former NFL player participants had the T/T (Thr/Thr) genotype.
In vivo [11C]DPA-713 brain imaging
There were no significant differences in the [11C]DPA-713 injected, including amount of radioactivity, specific activity and mass, in PET scans across all healthy control and former NFL players (Supplemental Table 3).
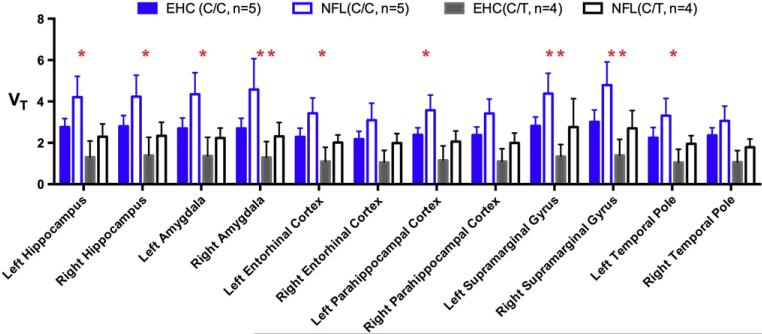
Linear mixed model analysis revealed a significant cohort effect (former NFL player, EHC) on [11C]DPA-713 VT (chi squared = 45.5, df = 13, P = 1.74 e-5). A significant effect of genotype on [11C]DPA-713 VT was also found (chi squared = 44.0, df = 13, P = 3.12 e-5). Post-hoc results of region-specific testing of the respective effect of cohort and of genotype on regional [11C]DPA-713 VT are presented in Table 3. As predicted, individuals of C/C genotype had higher VT values when compared to those with C/T genotype across all 12 ROIs tested (Fig. 1 and Table 3, most P < 0.004); and former NFL players had higher VT values than EHCs in all 12 ROIs, with right amygdala and bilateral SMGs maintaining significance after Bonferroni correction for multiple comparisons (P < 0.004) (Table 3). Representative PET images are depicted in Fig. 2.
Table 3.
Statistical significance (P-values) of the effect of rs6971 genotype and of NFL play on regional [11C]DPA-713 VT using a linear mixed effect model.
| Regions | C/C vs. C/T | EHC vs. NFL |
|---|---|---|
| Left hippocampus | 0.000272** | 0.013996* |
| Right hippocampus | 0.000113** | 0.013028* |
| Left amygdala | 0.000110** | 0.009041* |
| Right amygdala | 0.000038** | 0.002764** |
| Left entorhinal cortex | 0.003471** | 0.037040* |
| Right entorhinal cortex | 0.012038* | 0.064839 |
| Left parahippocampal cortex | 0.002031** | 0.033014* |
| Right parahippocampal cortex | 0.002309** | 0.051094 |
| Left supramarginal gyrus | 0.000501** | 0.002640** |
| Right supramarginal gyrus | 0.000032** | 0.001676** |
| Left temporal pole | 0.004100* | 0.046526* |
| Right temporal pole | 0.003907** | 0.155172 |
Those P-values <0.05 are listed with an asterisk, among which, those that survive testing for multiple comparisons are listed with a double asterisk
P < 0.004.
Fig. 1.
Individuals of C/C genotype demonstrate increased binding of [11C]DPA-713 over those with C/T genotype. When compared with individuals of the same genotype (blue = C/C; black = C/T) the former NFL Players (NFL) had higher binding of [11C]DPA-713 than Elderly Healthy Controls (EHC) across all regions tested. Statistical significance (P -values) of the effect of NFL play on regional [11C]DPA-713 VT were generated using linear mixed model analysis with P < 0.05 indicated with *. Results meeting more stringent threshold of significance (accounting for multiple comparisons) where P < 0.004 are indicated with **. VT = Total distribution volume within the region labeled. Data given as mean ± standard deviation (SD).
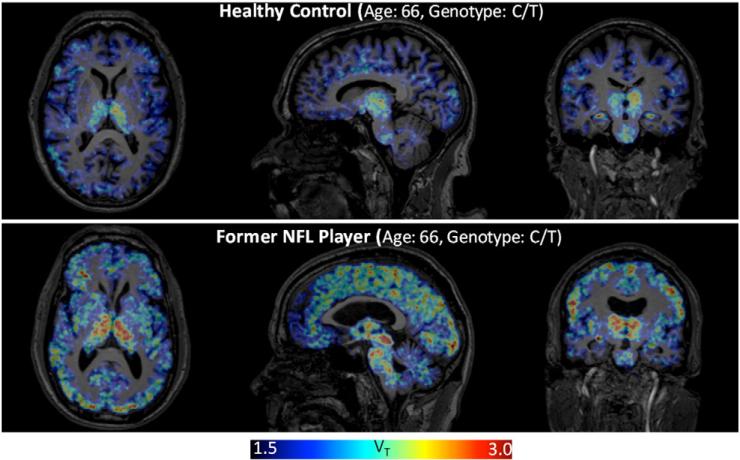
Fig. 2.
Former NFL players demonstrate increased binding of [11C]DPA-713, reported as total distribution volume (VT) across many brain regions compared to binding of [11C]DPA-713 in the brains of elderly healthy controls. Parametric [11C]DPA-713 VT images from one former NFL player and one age- and rs6971 genotype-matched healthy individual are presented for comparison.
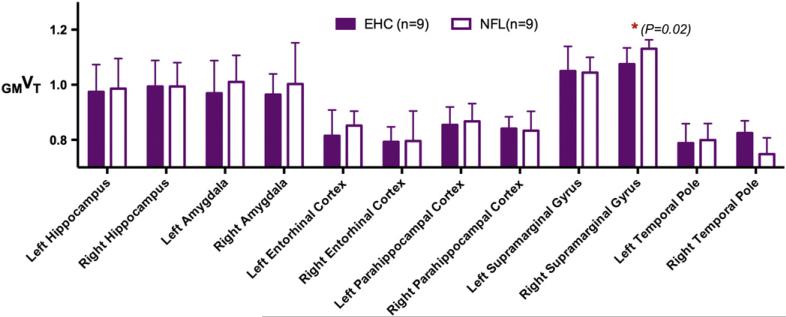
For each individual, regional VT normalized by the overall cortical gray matter VT, defined as GMVT, was generated for each of the 12 ROIs. Results of Student's t-test comparison of regional [11C]DPA-713 GMVT in former NFL players to GMVT of controls revealed little difference in binding between the two groups (Fig. 3) except for in the right SMG, where localized right SMG GMVT trended higher in former NFL players (mean ± SD: 1.13 ± 0.03) compared to that of controls (1.07 ± 0.06) (P = 0.02). This finding did not reach statistical significance, however, after Bonferroni correction for multiple comparisons.
Fig. 3.
Regional VT normalized by the overall cortical gray matter VT, defined as GMVT, was generated for each of the 12 regions of interest. Regional [11C]DPA-713 GMVT in former NFL players (NFL) compared to GMVT of Elderly Healthy Controls (EHC) revealed little difference in binding between the two groups except for in the right supramarginal gyrus, where GMVT was greatly increased in former NFL players (mean ± SD: 1.13 ± 0.03) over that of EHCs (1.07 ± 0.06).
Analysis of volume loss by MRI
Using the MRI data from the cohort of nine former NFL players and nine EHCs who also underwent [11C]DPA-713 PET imaging, volume measurements for right and left hippocampus and amygdala volumes and for cortical thickness of the remaining eight cortical ROIs were computed (Table 4). Total cortical gray matter volume and brain parenchymal volume were also measured. Student's t-test comparison revealed significant atrophy in the right hippocampus of former NFL players after correction for multiple comparisons given the 14 ROIs (12 ROIs from PET analysis plus total gray matter volume and brain parenchymal volume) tested (P < 0.0036).
Table 4.
Structural comparison of regional volume and cortical thickness measurements between the nine former NFL players (NFL) and nine elderly healthy control (EHC) participants who also underwent [11C]DPA-713 PET.
| NFL, mean (SD) | EHC, mean (SD) | P | |
|---|---|---|---|
| Brain region volume (mm3) | |||
| Right hippocampus | 3493.0 (440.4) | 4232.4 (392.0) | 0.002** |
| Left hippocampus | 3526.8 (476.7) | 4174.5 (447.3) | 0.009* |
| Right amygdala | 1799.0 (349.3) | 2111.1 (340.8) | 0.073 |
| Left amygdala | 1556.9 (241.3) | 1832.9 (87.5) | 0.009* |
| Total gray matter | 556,785.8 (34,890.2) | 591,657.7 (41,901.4) | 0.074 |
| Brain parenchymal volume | 1,058,862.9 (55,241.4) | 1,112,091.9 (79,279.2) | 0.120 |
|
Brain region cortical thickness (mm)
| |||
| Right parahippocampal CTX | 2.42 (0.30) | 2.73 (0.40) | 0.07 |
| Left parahippocampal CTX | 2.51 (0.32) | 2.66 (0.38) | 0.41 |
| Right entorhinal CTX | 3.29 (0.38) | 3.36 (0.41) | 0.72 |
| Left entorhinal CTX | 3.13 (0.27) | 3.14(0.31) | 0.95 |
| Right temporal pole | 3.40 (0.31) | 3.66 (0.49) | 0.19 |
| Left temporal pole | 3.45 (0.41) | 3.50 (0.25) | 0.75 |
| Right supramarginal gyrus | 2.22 (0.13) | 2.33 (0.19) | 0.19 |
| Left supramarginal gyrus | 2.27 (0.18) | 2.36 (0.16) | 0.27 |
P < 0.05.
P < 0.0036, indicating a significant difference after correction for multiple testing.
Discussion
In this study of molecular and structural brain changes in former NFL players, we found increased [11C]DPA-713 binding, a putative marker of neuroinflammation, in several regions believed to be susceptible to NFT deposition in CTE and to atrophy in TBI, namely medial temporal lobe and inferior parietal cortical structures. Linear mixed model analysis revealed significantly higher uptake of [11C]DPA-713 VT in 9 of the 12 regions analyzed, with increased binding in right amygdala and bilateral SMGs remaining significant after Bonferroni correction (P < 0.004).
Furthermore, regional brain volume loss was observed in the right hippocampus of former NFL players when compared to controls, and this finding remained significant after Bonferroni correction. While this study is limited by small sample size, our results suggest the utility of [11C]DPA-713 neuroimaging to examine the relationship between TSPO expression, onset of hippocampal atrophy, and related clinical effects.
As predicted, imaging with [11C]DPA-713 demonstrated increased binding in individuals with the C/C genotype over those with the C/T genotype for all ROIs, as previously described for other second generation radiotracers for this target (Kreisl et al., 2013a, 2013b; Mizrahi et al., 2012). Here we compared regional [11C]DPA-713 VT between former NFL players and controls of both C/C and C/T rs6971 genotypes, similar to analyses recently published using [11C]PBR28 (Kreisl et al., 2013a, 2013b). However, as noted by Kreisl et al., other yet-unidentified factors besides rs6971 genotype may also affect binding of second generation TSPO-targeted radiopharmaceuticals, independent of the state of health of the individual undergoing imaging. Those factors may include changes in levels of endogenous ligands that may occupy or alter the binding site of the TSPO receptor or factors that may alter expression of TSPO given its other functional roles in the outer mitochondrial membrane (Drugan, 1996; Gavish et al., 1999). While we acknowledge that work to identify those factors affecting binding is important, we also employed a method of normalizing for the effect of genotype and these other unknown factors, namely, using GMVT (Coughlin et al., 2014). The method of normalizing regional PET-based radiotracer binding to that of whole brain or gray matter has been used for decades as an accepted method to normalize for the effect of global variation in measurement and to improve sensitivity to discern abnormal regional findings, as in studies using [15O]H20 (Fox et al., 1988) or [18F]FDG (Dukart et al., 2010). Employing this gray matter normalization allows use of data from all participants irrespective of their rs6971 SNP genotype, improves test-retest reproducibility of the PET data, and maintains sensitivity of uncovering abnormal regional findings in disease (Coughlin et al., 2014). While the current finding of increased [11C]DPA-713 GMVT in right SMG is limited by the small sample size, this finding suggests that this normalization approach will be useful in application to larger studies of former players of all three genotypes.
While there have been reports of diffuse pathology in the brains of professional players of contact sports, here we focused on six bilateral regions (both cortical and hippocampal) selected a priori based on regions reported to have p-tau deposition in Stage III CTE (Stein et al., 2014). It is possible that our participants have increased expression of TSPO in other areas as is suggested by whole brain images of VT (Fig. 2), which demonstrate increased signal in the thalamus and regions within the brainstem. Indeed, McKee and colleagues have shown dense tau pathology in brainstem structures even in early stages of CTE (McKee et al., 2013). Further studies designed to examine more global distribution of TSPO in the brains of former NFL athletes are needed. Study design using multiple PET radiotracers in the same individuals, such as radiotracers targeting tau (Chien et al., 2014; Small et al., 2013) and TSPO, may also further our understanding of the proposed relationship between football-associated neurotrauma, inflammation, and aggregation of p-tau or other proteins implicated in neurodegenerative pathology.
We acknowledge that PET-based neuroimaging results from study of aged individuals, both in health and disease, may be influenced by regional brain atrophy. Specifically, the regional radiopharmaceutical VT will be diminished in areas where there is less tissue to express the target receptor. Some researchers employ proposed methods of partial volume correction (PVC) to adjust the PET signal for the effect of regional tissue atrophy. However, the risk of employing such PVC corrections is that the regional radiopharmaceutical VT may become falsely elevated, leading to the conclusion that PET-based signal is due to increased binding to the target rather than to overcompensation by the method of analysis. Notably, most PVC methods are applicable only to PET data reconstructed through conventional analytical filtered back-projection, as they are based on assumptions that require shift-invariant Gaussian blurring functions and linear system responses (Erlandsson et al., 2012). Instead, we used an iterative image reconstruction algorithm with resolution recovery in this study, so that our reconstructed PET data have intrinsically higher resolution, reducing partial volume effects. Due to those considerations, while we see significant volume loss in the right hippocampus of former NFL players in this study, we did not use PVC and only report conservative measures of the changes in [11C]DPA-713 binding. Therefore the degree of increased binding of [11C]DPA-713 in regions with volume loss or cortical thinning, particularly in the right hippocampus of former NFL players, may be underreported here.
Perhaps unsurprisingly given symptom overlap with broader conditions, these former players differ widely in symptoms, rated from absent to severe on the modified RPQ-13 (Table 1, Supplementary Table 2). Two of the eleven former players scored between an 8 and 13 on the HAMD, indicating mild depression. We are unable to comment on whether the presence and severity of symptoms are directly linked to play-related head trauma in this small cross-sectional study of players 24–42 years from last NFL play. Through focus on former NFL players, this small pilot was also unable to adequately assess the effects of other factors related to being a professional athlete (including athletes of non-contact sports), history of repetitive versus single head trauma, or the effects of body habitus on reported symptoms. Indeed, there is recent evidence that overnutrition and obesity may independently be a cause and effect of neuroinflammation (Cai, 2013) and be linked to cognitive decline (Miller and Spencer, 2014). These former players also had dissimilar performance on testing of verbal learning and memory: after raw scores were standardized based on age-adjusted normative data, we found variability in CVLT-II short delay recall percentile scores, a measure of the ability to remember verbally presented information after a short delay. Still, some scores were as low as the sixth percentile (Table 2), a finding that is consistent with other published reports of verbal learning and memory deficits in former NFL players (Hart et al., 2013) and Division I college varsity football and ice hockey athletes (McAllister et al., 2012, 2014). Given the functional role of inferior parietal lobe structures (including SMG) in verbal learning and memory pathways, there may be a pathophysiologic link between inflammation in the SMG and onset of even subtle changes in verbal learning and memory. However this study is statistically underpowered to assess correlation between neuropsychological performance and imaging results within players. Longitudinal study using [11C]DPA-713 to measure TSPO in active and former NFL players may help elucidate the role of inflammation in specific brain regions (e.g. SMG) in development of even subtle verbal learning and memory deficits over time.
Conclusion
In this pilot study of former NFL players we observed a significant increase in binding of [11C]DPA-713 to TSPO, a marker of brain injury and repair, in the right amygdala and in the SMG in players with history of sports-related, repetitive TBI. We also observed significant atrophy of the right hippocampus. Finally, we report that these same former players have varied performance on the CVLT-II, suggesting that these molecular and pathologic changes may play a role in cognitive decline. These results suggest that localized brain injury and repair, indicated by increased [11C]DPA-713 binding to TSPO, may be linked to history of NFL play. [11C]DPA-713 PET is a promising new tool that can be used in future study design to further examine the relationship between TSPO expression in brain injury and repair, selective regional brain atrophy, and potential link to deficits in verbal learning and memory after NFL play.
Supplementary Material
Acknowledgments
We are grateful to Alimamy Kargbo for performing PET metabolite analyses, and to the Johns Hopkins PET Center for provision of the radiotracer. This project was funded in part by financial support from the following NIH grants and foundations: NIH 5R21MH082277, NIH 5R01MH092443, NIH R01EB012547, NIEHS ES007062, NIH 5T32EB006351, NIH P50AG005146, the Lupus Foundation for America, NFL Charities and the GE NFL Head Health challenge.
Abbreviations
- CTE
chronic traumatic encephalopathy
- CVLT-II
California Verbal Learning Test, Second Edition
- mTBI
mild traumatic brain injury
- NFL
National Football League
- RPQ
Rivermead Post-Concussive Symptom Questionnaire
- SMG
supramarginal gyrus
Footnotes
Available online on ScienceDirect (www.sciencedirect.com).
Appendix A. Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.nbd.2014.10.019.
References
- Boutin H, et al. 11C-DPA-713: a novel peripheral benzodiazepine receptor PET ligand for in vivo imaging of neuroinflammation. J Nucl Med. 2007;48:573–581. doi: 10.2967/jnumed.106.036764. [DOI] [PubMed] [Google Scholar]
- Cai D. Neuroinflammation and neurodegeneration in overnutrition-induced diseases. Trends Endocrinol Metab. 2013;24:40–47. doi: 10.1016/j.tem.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauveau F, et al. Nuclear imaging of neuroinflammation: a comprehensive review of [11C]PK11195 challengers. Eur J Nucl Med Mol Imaging. 2008;35:2304–2319. doi: 10.1007/s00259-008-0908-9. [DOI] [PubMed] [Google Scholar]
- Chien DT, et al. Early clinical PET imaging results with the novel PHF-tau radioligand [F18]-T808. J Alzheimers Dis. 2014;38:171–184. doi: 10.3233/JAD-130098. [DOI] [PubMed] [Google Scholar]
- Collins MW, et al. Relationship between concussion and neuropsychological performance in college football players. JAMA. 1999;282:964–970. doi: 10.1001/jama.282.10.964. [DOI] [PubMed] [Google Scholar]
- Coughlin JM, et al. Regional brain distribution of translocator protein using [C] DPA-713 PET in individuals infected with HIV. J Neurovirol. 2014;20:219–232. doi: 10.1007/s13365-014-0239-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, et al. Recall discriminability: utility of a new CVLT-II measure in the differential diagnosis of dementia. J Int Neuropsychol Soc. 2005;11:708–715. doi: 10.1017/S1355617705050812. [DOI] [PubMed] [Google Scholar]
- Drugan RC. Peripheral benzodiazepine receptors: molecular pharmacology to possible physiological significance in stress-induced hypertension. Clin Neuropharmacol. 1996;19:475–496. doi: 10.1097/00002826-199619060-00002. [DOI] [PubMed] [Google Scholar]
- Dukart J, et al. Differential effects of global and cerebellar normalization on detection and differentiation of dementia in FDG-PET studies. Neuroimage. 2010;49:1490–1495. doi: 10.1016/j.neuroimage.2009.09.017. [DOI] [PubMed] [Google Scholar]
- Endres CJ, et al. Initial evaluation of 11C-DPA-713, a novel TSPO PET ligand, in humans. J Nucl Med. 2009;50:1276–1282. doi: 10.2967/jnumed.109.062265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlandsson K, et al. A review of partial volume correction techniques for emission tomography and their applications in neurology, cardiology and oncology. Phys Med Biol. 2012;57:R119–R159. doi: 10.1088/0031-9155/57/21/R119. [DOI] [PubMed] [Google Scholar]
- Fischl B, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Folkersma H, et al. Widespread and prolonged increase in (R)-(11)C-PK11195 binding after traumatic brain injury. J Nucl Med. 2011;52:1235–1239. doi: 10.2967/jnumed.110.084061. [DOI] [PubMed] [Google Scholar]
- Ford JH, et al. Episodic memory in former professional football players with a history of concussion: an event-related functional neuroimaging study. J Neurotrauma. 2013;30:1683–1701. doi: 10.1089/neu.2012.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox PT, et al. Enhanced detection of focal brain responses using intersubject averaging and change-distribution analysis of subtracted PET images. J Cereb Blood Flow Metab. 1988;8:642–653. doi: 10.1038/jcbfm.1988.111. [DOI] [PubMed] [Google Scholar]
- Gavish M, et al. Enigma of the peripheral benzodiazepine receptor. Pharmacol Rev. 1999;51:629–650. [PubMed] [Google Scholar]
- Guskiewicz KM, et al. Association between recurrent concussion and late-life cognitive impairment in retired professional football players. Neurosurgery. 2005;57:719–726. doi: 10.1093/neurosurgery/57.4.719. discussion 719–726. [DOI] [PubMed] [Google Scholar]
- Guskiewicz KM, et al. Recurrent concussion and risk of depression in retired professional football players. Med Sci Sports Exerc. 2007;39:903–909. doi: 10.1249/mss.0b013e3180383da5. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart J, Jr., et al. Neuroimaging of cognitive dysfunction and depression in aging retired National Football League players: a cross-sectional study. JAMA Neurol. 2013;70:326–335. doi: 10.1001/2013.jamaneurol.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazrati LN, et al. Absence of chronic traumatic encephalopathy in retired football players with multiple concussions and neurological symptomatology. Front Hum Neurosci. 2013;7:222. doi: 10.3389/fnhum.2013.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellstrom-Lindahl E, et al. Increased levels of tau protein in SH-SY5Y cells after treatment with cholinesterase inhibitors and nicotinic agonists. J Neurochem. 2000;74:777–784. doi: 10.1046/j.1471-4159.2000.740777.x. [DOI] [PubMed] [Google Scholar]
- Innis RB, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27:1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- Jordan BD. The clinical spectrum of sport-related traumatic brain injury. Nat Rev Neurol. 2013;9:222–230. doi: 10.1038/nrneurol.2013.33. [DOI] [PubMed] [Google Scholar]
- King NS, et al. The Rivermead Post Concussion Symptoms Questionnaire: a measure of symptoms commonly experienced after head injury and its reliability. J Neurol. 1995;242:587–592. doi: 10.1007/BF00868811. [DOI] [PubMed] [Google Scholar]
- Kreisl WC, et al. A genetic polymorphism for translocator protein 18 kDa affects both in vitro and in vivo radioligand binding in human brain to this putative biomarker of neuroinflammation. J Cereb Blood Flow Metab. 2013a;33:53–58. doi: 10.1038/jcbfm.2012.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisl WC, et al. In vivo radioligand binding to translocator protein correlates with severity of Alzheimer's disease. Brain. 2013b;136:2228–2238. doi: 10.1093/brain/awt145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman EJ, et al. Neurodegenerative causes of death among retired National Football League players. Neurology. 2012;79:1970–1974. doi: 10.1212/WNL.0b013e31826daf50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan J, et al. Graphical analysis of reversible radioligand binding from time–activity measurements applied to [N-11C-methyl]-(−)-cocaine PET studies in human subjects. J Cereb Blood Flow Metab. 1990;10:740–747. doi: 10.1038/jcbfm.1990.127. [DOI] [PubMed] [Google Scholar]
- Matser EJ, et al. Neuropsychological impairment in amateur soccer players. JAMA. 1999;282:971–973. doi: 10.1001/jama.282.10.971. [DOI] [PubMed] [Google Scholar]
- McAllister TW, et al. Cognitive effects of one season of head impacts in a cohort of collegiate contact sport athletes. Neurology. 2012;78:1777–1784. doi: 10.1212/WNL.0b013e3182582fe7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister TW, et al. Effect of head impacts on diffusivity measures in a cohort of collegiate contact sport athletes. Neurology. 2014;82:63–69. doi: 10.1212/01.wnl.0000438220.16190.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee AC, et al. The spectrum of disease in chronic traumatic encephalopathy. Brain. 2013;136:43–64. doi: 10.1093/brain/aws307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee AC, et al. The neuropathology of sport. Acta Neuropathol. 2014;127:29–51. doi: 10.1007/s00401-013-1230-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AA, Spencer SJ. Obesity and neuroinflammation: a pathway to cognitive impairment. Brain Behav Immun. 2014;42:10–21. doi: 10.1016/j.bbi.2014.04.001. [DOI] [PubMed] [Google Scholar]
- Mizrahi R, et al. Translocator protein (18 kDa) polymorphism (rs6971) explains in-vivo brain binding affinity of the PET radioligand [(18)F]-FEPPA. J Cereb Blood Flow Metab. 2012;32:968–972. doi: 10.1038/jcbfm.2012.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen DR, et al. An 18-kDa translocator protein (TSPO) polymorphism explains differences in binding affinity of the PET radioligand PBR28. J Cereb Blood Flow Metab. 2012;32:1–5. doi: 10.1038/jcbfm.2011.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos V, Lecanu L. Translocator protein (18 kDa) TSPO: an emerging therapeutic target in neurotrauma. Exp Neurol. 2009;219:53–57. doi: 10.1016/j.expneurol.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos V, et al. Translocator protein (18 kDa): new nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol Sci. 2006;27:402–409. doi: 10.1016/j.tips.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Pearce AJ, et al. The long-term effects of sports concussion on retired Australian football players: a study using transcranial magnetic stimulation. J Neurotrauma. 2014;31:1139–1145. doi: 10.1089/neu.2013.3219. [DOI] [PubMed] [Google Scholar]
- Rahmim A, et al. Statistical list-mode image reconstruction for the high resolution research tomograph. Phys Med Biol. 2004;49:4239–4258. doi: 10.1088/0031-9155/49/18/004. [DOI] [PubMed] [Google Scholar]
- Ramlackhansingh AF, et al. Inflammation after trauma: microglial activation and traumatic brain injury. Ann Neurol. 2011;70:374–383. doi: 10.1002/ana.22455. [DOI] [PubMed] [Google Scholar]
- Rubio A, et al. Acetylcholine receptors and tau phosphorylation. Curr Mol Med. 2006;6:423–428. doi: 10.2174/156652406777435444. [DOI] [PubMed] [Google Scholar]
- Seichepine DR, et al. Profile of self-reported problems with executive functioning in college and professional football players. J Neurotrauma. 2013;30:1299–1304. doi: 10.1089/neu.2012.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively S, et al. Dementia resulting from traumatic brain injury: what is the pathology? Arch Neurol. 2012;69:1245–1251. doi: 10.1001/archneurol.2011.3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, et al. Relationship of collegiate football experience and concussion with hippocampal volume and cognitive outcomes. JAMA. 2014;311:1883–1888. doi: 10.1001/jama.2014.3313. [DOI] [PubMed] [Google Scholar]
- Small GW, et al. PET scanning of brain tau in retired national football league players: preliminary findings. Am J Geriatr Psychiatry. 2013;21:138–144. doi: 10.1016/j.jagp.2012.11.019. [DOI] [PubMed] [Google Scholar]
- Smith DH, et al. Chronic neuropathologies of single and repetitive TBI: substrates of dementia? Nat Rev Neurol. 2013;9:211–221. doi: 10.1038/nrneurol.2013.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein TD, et al. Chronic traumatic encephalopathy: a spectrum of neuropathological changes following repetitive brain trauma in athletes and military personnel. Alzheimers Res Ther. 2014;6:4. doi: 10.1186/alzrt234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Quality Standards Subcommittee Practice parameter: the management of concussion in sports (summary statement). Report of the Quality Standards Subcommittee. Neurology. 1997;48:581–585. doi: 10.1212/wnl.48.3.581. [DOI] [PubMed] [Google Scholar]
- Thominiaux C, et al. Improved synthesis of the peripheral benzodiazepine receptor ligand [11C]DPA-713 using [11C]methyl triflate. Appl Radiat Isot. 2006;64:570–573. doi: 10.1016/j.apradiso.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Weir DR, et al. National Football League Player Care Foundation Study of Retired NFL Players. I. f. S. Research. University of Michigan; Ann Arbor: 2009. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.