SUMMARY
Severe fever with thrombocytopenia syndrome virus (SFTSV) has been prevalent for some time in China and it was first identified in 2010. However, the seroprevalence of SFTSV in the general population in southeastern China and risk factors associated with the infection are currently unclear. Blood samples were collected from seven counties across Zhejiang province and tested for the presence of SFTSV-specific IgG antibodies by ELISA. A total of 1380 blood samples were collected of which 5·51% were seropositive for SFTSV with seroprevalence varying significantly between sites. Seroprevalence of SFTSV in people who were family members of the patient, lived in the same village as the patient, or lived in a different village than the patient varied significantly. There was significant difference in seroprevalence between participants who bred domestic animals and participants who did not. Domestic animals are probably potential reservoir hosts and contact with domestic animals may be a transmission route of SFTSV.
Key words: Seroprevalence, severe fever with thrombocytopenia syndrome, risk factor
INTRODUCTION
Severe fever with thrombocytopenia syndrome (SFTS) is an emerging infectious disease discovered in China in 2010, which is caused by a novel bunyavirus, SFTS virus (SFTSV). The genome of SFTSV contains three segments of negative or ambisense polarity, designated L, M and S segments. The major clinical symptoms and laboratory abnormalities of SFTS are fever, thrombocytopenia, leukopenia, and elevated serum hepatic enzymes, and SFTS patients usually die due to multiple organ failure [1]. The clinical symptoms, however, are less specific and need to be differentiated from various infectious disease, in particular from haemorrhagic fever with renal syndrome (HFRS) caused by hantavirus and human anaplasmosis [2, 3].
In 2011–2012, 2047 cases of SFTS and 129 deaths were reported in over 206 counties of eastern and central China [4]. Cases of SFTS were also identified in Zhejiang province and a total of 65 cases were reported according to the information system for disease control and prevention in recent years [5–7]. However, to date, there have been no efforts to explore the seroprevalence of SFTSV in this region, nor to identify risk factors associated with the infection. In this study, we investigated the prevalence of SFTSV in general human populations for the first time and analysed risk factors for SFTS which will highlight the way for successful control and prevention of this emerging infectious disease.
METHODS
Blood samples
Zhejiang province is located in southeastern of China and is adjacent to Jiangsu and Anhui where SFTS is endemic. The investigated sites including Pujiang, Liandu, Xiangshan, Yiwu, Anji, Haining and Xianju were randomly chosen based on their geographical location and environment (Fig. 1) in Zhejiang province. Blood samples were collected from the seven locations from January to December 2013 and samples of blood serum were prepared after collection. The aims of our study were explained to all participants upon enrolment, and their consent was obtained prior to inclusion in this study. All enrolled participants provided information upon inclusion in the study with regard to their age, gender, place of residence, whether they bred domestic animals, whether they had contact with wildlife, whether they had any outdoor activities in the previous 2 weeks, and whether ticks were present in their environment.
Fig. 1.
Geographical locations of the seven counties where blood samples were collected.
Serological testing
All serum samples were transported to Zhejiang CDC and stored at −80°C prior to use. Serum samples were tested for the presence of SFTSV-specific IgG antibodies using ELISA kits provided by the National Institute for Viral Disease Control and Prevention as described previously [8]. ELISA results were confirmed by immunofluorescence assay (IFA) as appropriate After the samples were diluted 1:10, 1:20, 1:40, and 1:80 in phosphate-buffered saline (PBS)-Tween buffer, the IFA was performed. Positive and negative controls were also used. Immunofluorescence was observed using an epifluorescence microscope. According to the guidelines, a titre of 1:20 was considered indicative of an infection.
Data analysis
Logistic regression analysis, χ2 test or Fisher's exact test were used to compare SFTSV seroprevalence between sites, gender, age groups, place of residence, whether participants bred domestic animals, whether participants had contact with wildlife, whether participants had outdoor activities in the previous 2 weeks, and whether ticks existed in their environment. The difference was considered statistically significant when P < 0·05. Statistical analysis was performed using SPSS software v. 17.0 (SPSS Inc., USA).
The dependent variable in the logistic regression was assigned as the serological status and the independent variables were site, gender, age group, place of residence, breeding domestic animals, contact with wildlife, outdoor activities in the previous 2 weeks, and presence of ticks in their environment (Table 1). The method of logistic regression used was forward-conditional. The stepwise probability was set to 0·05 for entry and 0·10 for removal. The classification cut-off was 0·5 and the maximum number of iterations was 20. Omnibus tests of model coefficients were also conducted.
Table 1.
Assignment of variables in logistic regression analysis
| Variable | Assignment |
|---|---|
| Site | Pujiang = 1, Lishui = 2, Xiangshan = 3, Yiwu = 4, Anji = 5, Haining = 6, Xianju = 7 |
| Gender | Male = 0, female = 1 |
| Age group (years) | >1 = 1, >40 = 2, >55 = 3, >70 = 4 |
| Place of residence | Family member of patient = 1, living in the same village as the patient = 2, living in a different village than the patient = 3 |
| Breeding domestic animals | No = 0, yes = 1 |
| Contact with wildlife | No = 0, yes = 1 |
| Outdoor activities in previous 2 weeks | No = 0, yes = 1 |
| Ticks present in the environment | No = 0, yes = 1 |
| Serological status | Negative = 0, positive = 1 |
Ethical statement
Experimental research reported in this study has been performed with the approval of the Ethics Committee of Zhejiang Provincial Centre for Disease Control and Prevention (Zhejiang CDC). Human research was conducted in compliance with the Helsinki Declaration.
RESULTS
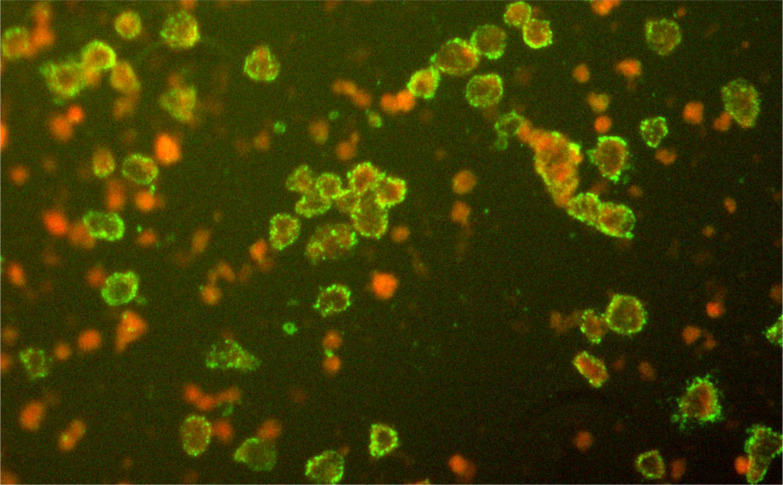
Blood samples were collected from 1380 people living in seven locations across Zhejiang province (Table 2). Overall, 5·51% (76/1380) of blood samples were seropositive for SFTSV and seroprevalence varied significantly between sites within Zhejiang province (1·50–10·57%, χ2 = 29·607, P = 0·000). All 76 ELISA-positives were confirmed by IFA (Fig. 2). Seroprevalence of SFTSV was found to be similar in males and females (6·02% and 5·07%, respectively; χ2 = 0·592, P = 0·441 > 0·05). Furthermore, participants were divided into four age groups (>1, >40, >55, >70 years) and the seroprevalences were 7·29% (21/288), 3·10% (10/323), 3·98% (18/452), 8·52% (27/317), respectively. Of note, seroprevalence in the >70 years and >1 year age groups was significantly higher than in the >40 and >55 years age groups.
Table 2.
Seroprevalence of SFTSV in blood samples from Zhejiang province, China
| Site | Total (n) | Seropositive (n) | Prevalence (%) | χ2 | P |
|---|---|---|---|---|---|
| Pujiang | 54 | 4 | 7·41 | 29·607 | 0·000 |
| Liandu | 220 | 18 | 8·18 | ||
| Xiangshan | 265 | 28 | 10·57 | ||
| Yiwu | 200 | 7 | 3·50 | ||
| Anji | 241 | 5 | 2·07 | ||
| Haining | 200 | 11 | 5·50 | ||
| Xianju | 200 | 3 | 1·50 | ||
| Total | 1380 | 76 | 5·51 |
SFTSV, Severe fever with thrombocytopenia syndrome virus.
Fig. 2.
Positive results of severe fever with thrombocytopenia syndrome virus in serum samples from Zhejiang province by immunofluorescence assay.
Seroprevalence of SFTSV in people who were family members of the patient, lived in the same village as the patient, or lived in a different village than the patient was 18·18%, 8·96% and 4·20%, respectively (χ2 = 14·662, P = 0·001<0·05). Seroprevalence of people who were family members of the patient, or lived in the same village as the patient were significantly higher than that of people who lived in a different village. Moreover, there was significant difference of seroprevalence between participants who bred domestic animals and participants who did not (7·91% and 4·68%, respectively; χ2 = 5·281, P = 0·022<0·05). However, contact with wildlife (0% and 5·63%), outdoor activities in the previous 2 weeks (7·43% and 4·98%) and ticks in the environment (6·69% and 5·24%) were all insignificant factors in SFTSV antibody expression based on χ2 test or Fisher's exact test (Table 3).
Table 3.
Risk factors for seroprevalence of severe fever with thrombocytopenia syndrome virus in Zhejiang province, China
| Characteristics | Seronegative (n = 1304) | Seropositive (n = 76) | χ2 | P |
|---|---|---|---|---|
| Male | 593 | 38 | 0·592 | 0·441 |
| Female | 711 | 38 | ||
| Age group (years) | ||||
| >1 | 267 | 21 | 12·91 | 0·005 |
| >40 | 313 | 10 | ||
| >55 | 434 | 18 | ||
| >70 | 290 | 27 | ||
| Family member of patient | 9 | 2 | 14·662 | 0·001 |
| Live in the same village as the patient | 315 | 31 | ||
| Live in a different village than the patient | 980 | 43 | ||
| Breeding domestic animals | ||||
| Yes | 326 | 28 | 5·281 | 0·022 |
| No | 978 | 48 | ||
| Contact with wildlife | ||||
| Yes | 31 | 0 | 0·411* | |
| No | 1273 | 76 | ||
| Outdoor activities in previous 2 weeks | ||||
| Yes | 274 | 22 | 2·684 | 0·101 |
| No | 1030 | 54 | ||
| Ticks present in the environment | ||||
| Yes | 237 | 17 | 0·841 | 0·359 |
| No | 1067 | 59 |
SFTSV, Severe fever with thrombocytopenia syndrome virus.
Fisher's exact test.
According to results of logistic analysis, the χ2 value in omnibus tests of model coefficients was determined as 20·507 (P < 0·05). Furthermore, the overall correct percentage was found to be 94·5%. Variables in the equation below included site and place of residence and the Wald values were determined to be 7·742 (P = 0·005) and 4·037 (P = 0·045). The equation was:
DISCUSSION
In our study, the seroprevalence of SFTSV was found to be 5·51% in the general population, a percentage similar to the recently reported percentage (6·37%) recorded in Hubei province in China [9], but much higher than the percentage reported in Shandong province (0·84%) [10], and Jiangsu (0·94) [11]. This discrepancy may be attributed to the season of sample collection, age and sex of the subjects, previous exposure or low level of infection, and/or detection method utilized in individual studies. SFTSV antibodies were also found in Liandu and Haining where no cases were confirmed. These results suggest that subclinical SFTSV infections or a relatively mild form of SFTS illness may occur in humans. Although the seroprevalence of SFTSV varied between sites within the province, the fact that populations with SFTSV antibodies were detected across all seven study sites of Zhejiang province indicates that the general population in this region is at risk of exposure to SFTSV. Furthermore, seroprevalence in the >70 years and >1 year age groups was significantly higher. This may suggest poor immunity, but it is also possible that anti-SFTSV antibodies are long-lived, leading to accumulated antibodies in the elderly and that higher ratios in infants are due to maternal antibodies. Anti-hantavirus antibodies can last a lifetime in individuals who have been infected with hantavirus. Further studies, e.g. to study the duration of anti-SFTSV antibodies and correlation of antibody status between infants and their mothers, should be conducted to explore the reasons.
A previously published study reported that SFTSV RNA was detected in acute serum samples which were collected in 2006 indicating that SFTSV has been prevalent for some time in China [12]. However, it was first discovered in 2010 in China and the transmission cycle of SFTSV is not well understood currently. SFTSV is believed to be transmitted by ticks because the virus has been detected in Haemaphysalis longicornis ticks [1]. However, the disease can also be transmitted from person to person through contact with an infected patient's blood or mucous [12–15]. Here, we found that SFTSV seroprevalence in people who were family members of the patient was the highest and seroprevalence of populations who lived in the same village as the patient was significantly higher than that of populations from a different village than the patient. The reasons may be that populations in the first two groups have more chance of exposure to risk factors for SFTS. However, we should not exclude person-to-person transmission between patient and family members.
Interestingly, breeding domestic animals including dogs, cattle, goats, and chickens was a significant determinant of seroprevalence in our study. The data indicate that these domestic animals may be potential reservoir hosts of SFTSV which is consistent with the results of other studies. Niu et al. reported that SFTSV-specific antibodies were detected in sheep (328/472, 69·5%), cattle (509/842, 60·5%), dogs (136/359, 37·9%), pigs (26/839, 3·1%), and chickens (250/527, 47·4%) [16]. Another study in Shandong province showed 111/134 (83%) goats were seropositive for SFTSV [10], and a serosurvey of domesticated animals conducted in Jiangsu province found SFTSV antibody-positive rates of 57% in goats, 32% in cattle, 6% in dogs, 5% in pigs, and 1% in chickens but no antibodies in geese and mice [11]. Additionally, the data also suggest that populations might be infected with SFTSV via contact with secretions although this may not be the major transmission route.
Contact with wildlife and outdoor activities in the previous 2 weeks were insignificant factors for seroprevalence according to χ2 test. The reasons may be that few people have the opportunity for contact with wildlife or that wildlife is probably not a reservoir of SFTSV. Outdoor activities are not risk factors, suggesting that populations can be infected with SFTSV at home and domestic animals are probably reservoirs of SFTSV. The fact that ticks in the environment is also an insignificant factor for seroprevalence is disappointing. This result may be related to bias in the investigation as ticks are very small and many people do not recognize them. However, these data also inform us that other transmission routes may exist besides tick bites.
In summary, our study confirmed that SFTSV antibodies are widespread across Zhejiang province although patients were not identified in many regions. Populations who are family members of the patient, live in the same village as the patient, or breed domestic animals are more likely to have SFTSV antibodies than others. Furthermore, our data also inform that domestic animals are probably potential reservoir hosts and contact with patients or domestic animals may be transmission routes of SFTSV. More studies are needed to elucidate the SFTSV transmission model in nature and risk factors for human infection.
ACKNOWLEDGEMENTS
We thank National Institute for Viral Disease Control and Prevention for providing ELISA kits. We also thank the physicians and staff at Pujiang, Lishui, Xiangshan, Yiwu, Anji, Haining, and Xianju Centres for Disease Control and Prevention for their support and assistance with this investigation.
This research was supported by a grant from Zhejiang Province Major Science and Technology Programme (grant no. 2012C13016-2), the Project of the State Scientific & Technological Development of the 12th Five Year Plan (grant no. 2012ZX10004219) and the Medical Research Programme of Zhejiang Province (grant nos. 2012KYA045, 2014RCA002).
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Yu XJ, et al. Fever with thrombocytopenia associated with a novel bunyavirus in China. New England Journal of Medicine 2011; 364: 1523–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang YZ, et al. Hantavirus infections in humans and animals, China. Emerging Infectious Diseases 2010; 16: 1195–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang L, et al. Nosocomial transmission of human granulocytic anaplasmosis in China. Journal of the American Medicine Association 2008; 300: 2263–2270. [DOI] [PubMed] [Google Scholar]
- 4.Ding F, et al. Epidemiologic features of severe fever with thrombocytopenia syndrome in China, 2011–2012. Clinical Infectious Diseases 2013; 56: 1682–1683. [DOI] [PubMed] [Google Scholar]
- 5.Li SB, et al. Sporadic case infected by severe fever with thrombocytopenia syndrome bunyavirus in a non-epidemic region of China. BioScience Trends 2011; 5: 273–276. [DOI] [PubMed] [Google Scholar]
- 6.Zhang L, et al. Severe fever with thrombocytopenia syndrome Bunyavirus (SFTSV) infections in Zhejiang Province, China. International Journal of Infectious Diseases 2012; 17: e137–e138. [DOI] [PubMed] [Google Scholar]
- 7.Chai CL, et al. Analysis on clinical and epidemiological characteristics of severe fever with thrombocytopenia syndrome in Zhejiang Province. China Preventive Medicine 2012; 13: 904–907. [DOI] [PubMed] [Google Scholar]
- 8.Jiao Y, et al. Preparation and evaluation of recombinant severe fever with thrombocytopenia syndrome virus nucleocapsid protein for detection of total antibodies in human and animal sera by double-antigen sandwich enzyme-linked immunosorbent assay. Journal of Clinical Microbiology 2012; 50: 372–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhan JB, et al. Analysis on antibody levels against severe fever with thrombocytopenia syndrome bunyavirus among healthy population in Hubei province. Chinese Journal of Health Laboratory Technology 2013; 23: 992–993. [Google Scholar]
- 10.Zhao L, et al. Severe fever with thrombocytopenia syndrome virus, Shandong Province, China. Emerging Infectious Diseases 2012; 18: 963–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang WS, et al. Seroepidemiology of severe fever with thrombocytopenia syndrome Bunyavirus in Jiangsu province. Diseases Surveillance 2011; 26: 676–678. [Google Scholar]
- 12.Liu Y, et al. Person-to-person transmission of severe fever with thrombocytopenia syndrome virus. Vector Borne and Zoonotic Diseases 2012; 12: 156–160. [DOI] [PubMed] [Google Scholar]
- 13.Gai ZT, et al. Person to person transmission of severe fever with thrombocytopenia syndrome Bunyavirus through blood contact. Clinical Infectious Diseases 2012; 54: 249–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bao CJ, et al. A family cluster of infections by a newly recognized Bunyavirus in eastern China, 2007: further evidence of person-to-person transmission. Clinical Infectious Diseases 2011; 53: 1208–1214. [DOI] [PubMed] [Google Scholar]
- 15.Chen H, et al. A cluster of cases of human-to-human transmission caused by severe fever with thrombocytopenia syndrome Bunyavirus. International Journal of Infectious Diseases 2013; 17: e206–e208. [DOI] [PubMed] [Google Scholar]
- 16.Niu GY, et al. Severe fever with thrombocytopenia syndrome virus among domesticated animals, China. Emerging Infectious Diseases 2013; 19: 756–763. [DOI] [PMC free article] [PubMed] [Google Scholar]