Abstract
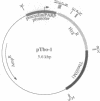
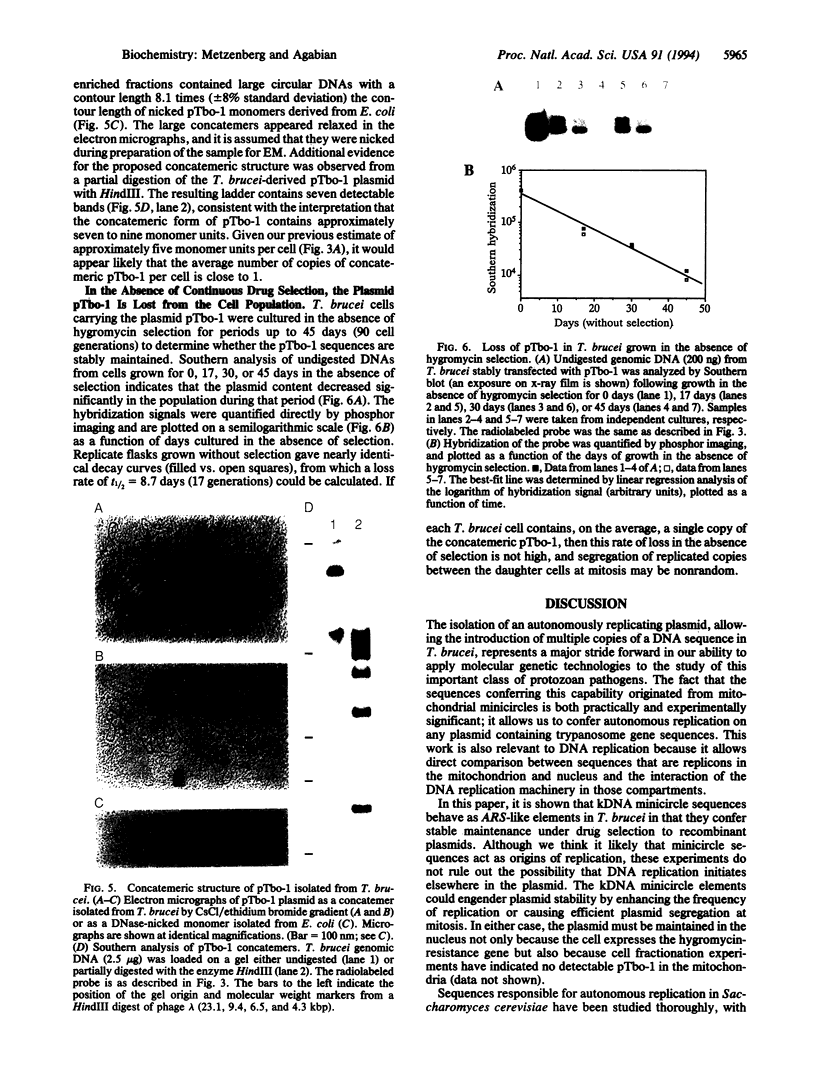
In a search for trypanosome DNA sequences that permit replication and stable maintenance of extrachromosomal elements, a 1-kilobase-pair (kbp) fragment from a mitochondrial kinetoplast DNA (kDNA) minicircle of Trypanosoma brucei was isolated and characterized. The plasmid pTbo-1, carrying the kDNA element, is maintained in T. brucei as a supercoiled concatemer containing approximately seven to nine pTbo-1 monomer units (5.6 kbp each) in a head-to-tail orientation. The concatemer is found in approximately one copy per cell when procyclic trypanosomes are cultured in the presence of 100 micrograms of hygromycin per ml; however, in the absence of continuous hygromycin selection, the plasmid is lost from the population with a t1/2 of approximately 8.7 days (17 cell generations). A second unrelated kDNA minicircle was also able to serve as an autonomously replicating sequence (ARS) element in T. brucei, suggesting that this is a general property of kDNA minicircles. Replication of mitochondrial DNA in the nucleus may be due to either a specific consensus sequence (such as in yeast ARS elements) or nonspecific sequence characteristics (such as the degree of A&T-richness or bent DNA).
Full text
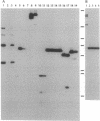
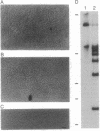
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agabian N. Trans splicing of nuclear pre-mRNAs. Cell. 1990 Jun 29;61(7):1157–1160. doi: 10.1016/0092-8674(90)90674-4. [DOI] [PubMed] [Google Scholar]
- Anderson J. N. Detection, sequence patterns and function of unusual DNA structures. Nucleic Acids Res. 1986 Nov 11;14(21):8513–8533. doi: 10.1093/nar/14.21.8513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellofatto V., Cross G. A. Expression of a bacterial gene in a trypanosomatid protozoan. Science. 1989 Jun 9;244(4909):1167–1169. doi: 10.1126/science.2499047. [DOI] [PubMed] [Google Scholar]
- Blum B., Bakalara N., Simpson L. A model for RNA editing in kinetoplastid mitochondria: "guide" RNA molecules transcribed from maxicircle DNA provide the edited information. Cell. 1990 Jan 26;60(2):189–198. doi: 10.1016/0092-8674(90)90735-w. [DOI] [PubMed] [Google Scholar]
- Broach J. R., Li Y. Y., Feldman J., Jayaram M., Abraham J., Nasmyth K. A., Hicks J. B. Localization and sequence analysis of yeast origins of DNA replication. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):1165–1173. doi: 10.1101/sqb.1983.047.01.132. [DOI] [PubMed] [Google Scholar]
- Buchman A. R., Kimmerly W. J., Rine J., Kornberg R. D. Two DNA-binding factors recognize specific sequences at silencers, upstream activating sequences, autonomously replicating sequences, and telomeres in Saccharomyces cerevisiae. Mol Cell Biol. 1988 Jan;8(1):210–225. doi: 10.1128/mcb.8.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham I. New culture medium for maintenance of tsetse tissues and growth of trypanosomatids. J Protozool. 1977 May;24(2):325–329. doi: 10.1111/j.1550-7408.1977.tb00987.x. [DOI] [PubMed] [Google Scholar]
- Eid J., Sollner-Webb B. Stable integrative transformation of Trypanosoma brucei that occurs exclusively by homologous recombination. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2118–2121. doi: 10.1073/pnas.88.6.2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englund P. T. Free minicircles of kinetoplast DNA in Crithidia fasciculata. J Biol Chem. 1979 Jun 10;254(11):4895–4900. [PubMed] [Google Scholar]
- Feagin J. E., Abraham J. M., Stuart K. Extensive editing of the cytochrome c oxidase III transcript in Trypanosoma brucei. Cell. 1988 May 6;53(3):413–422. doi: 10.1016/0092-8674(88)90161-4. [DOI] [PubMed] [Google Scholar]
- Ferguson M., Torri A. F., Ward D. C., Englund P. T. In situ hybridization to the Crithidia fasciculata kinetoplast reveals two antipodal sites involved in kinetoplast DNA replication. Cell. 1992 Aug 21;70(4):621–629. doi: 10.1016/0092-8674(92)90431-b. [DOI] [PubMed] [Google Scholar]
- Hofmann J. F., Gasser S. M. Identification and purification of a protein that binds the yeast ARS consensus sequence. Cell. 1991 Mar 8;64(5):951–960. doi: 10.1016/0092-8674(91)90319-t. [DOI] [PubMed] [Google Scholar]
- Kapler G. M., Coburn C. M., Beverley S. M. Stable transfection of the human parasite Leishmania major delineates a 30-kilobase region sufficient for extrachromosomal replication and expression. Mol Cell Biol. 1990 Mar;10(3):1084–1094. doi: 10.1128/mcb.10.3.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller B., Delius H. Intervening sequences in chloroplast genomes. Cell. 1984 Mar;36(3):613–622. doi: 10.1016/0092-8674(84)90341-6. [DOI] [PubMed] [Google Scholar]
- Krysan P. J., Smith J. G., Calos M. P. Autonomous replication in human cells of multimers of specific human and bacterial DNA sequences. Mol Cell Biol. 1993 May;13(5):2688–2696. doi: 10.1128/mcb.13.5.2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno K., Kuno S., Matsushima K., Murakami S. Evidence for binding of at least two factors, including T-rich strand-binding factor(s) to the single-stranded ARS1 sequence in Saccharomyces cerevisiae. Mol Gen Genet. 1991 Nov;230(1-2):45–48. doi: 10.1007/BF00290649. [DOI] [PubMed] [Google Scholar]
- Laban A., Tobin J. F., Curotto de Lafaille M. A., Wirth D. F. Stable expression of the bacterial neor gene in Leishmania enriettii. Nature. 1990 Feb 8;343(6258):572–574. doi: 10.1038/343572a0. [DOI] [PubMed] [Google Scholar]
- Milhausen M., Nelson R. G., Parsons M., Newport G., Stuart K., Agabian N. Molecular characterization of initial variants from the IsTat I serodeme of Trypanosoma brucei. Mol Biochem Parasitol. 1983 Nov;9(3):241–254. doi: 10.1016/0166-6851(83)90100-7. [DOI] [PubMed] [Google Scholar]
- Natale D. A., Umek R. M., Kowalski D. Ease of DNA unwinding is a conserved property of yeast replication origins. Nucleic Acids Res. 1993 Feb 11;21(3):555–560. doi: 10.1093/nar/21.3.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterlund M., Luthman H., Nilsson S. V., Magnusson G. Ethidium-bromide-inhibited restriction endonucleases cleave one strand of circular DNA. Gene. 1982 Nov;20(1):121–125. doi: 10.1016/0378-1119(82)90093-2. [DOI] [PubMed] [Google Scholar]
- Patnaik P. K., Kulkarni S. K., Cross G. A. Autonomously replicating single-copy episomes in Trypanosoma brucei show unusual stability. EMBO J. 1993 Jun;12(6):2529–2538. doi: 10.1002/j.1460-2075.1993.tb05908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch C. A., Perez-Morga D., Cozzarelli N. R., Englund P. T. The absence of supercoiling in kinetoplast DNA minicircles. EMBO J. 1993 Feb;12(2):403–411. doi: 10.1002/j.1460-2075.1993.tb05672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray D. S. Conserved sequence blocks in kinetoplast minicircles from diverse species of trypanosomes. Mol Cell Biol. 1989 Mar;9(3):1365–1367. doi: 10.1128/mcb.9.3.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson D. R., Gull K. Basal body movements as a mechanism for mitochondrial genome segregation in the trypanosome cell cycle. Nature. 1991 Aug 22;352(6337):731–733. doi: 10.1038/352731a0. [DOI] [PubMed] [Google Scholar]
- Schmidt A. M., Herterich S. U., Krauss G. A single-stranded DNA binding protein from S. cerevisiae specifically recognizes the T-rich strand of the core sequence of ARS elements and discriminates against mutant sequences. EMBO J. 1991 Apr;10(4):981–985. doi: 10.1002/j.1460-2075.1991.tb08032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman D. R., Janz L., Hug M., Clayton C. Anatomy of the parp gene promoter of Trypanosoma brucei. EMBO J. 1991 Nov;10(11):3379–3386. doi: 10.1002/j.1460-2075.1991.tb04902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson L. Kinetoplast DNA in trypanosomid flagellates. Int Rev Cytol. 1986;99:119–179. doi: 10.1016/s0074-7696(08)61426-6. [DOI] [PubMed] [Google Scholar]
- Teixeira S. M., Schenberg Frascino A. C., Galembeck E. V., de Oliveira Azevedo M., Astolfi Filho S. Isolation of Trypanosoma cruzi DNA fragments which function as ARS elements in Saccharomyces cerevisiae. Gene. 1986;44(1):171–175. doi: 10.1016/0378-1119(86)90059-4. [DOI] [PubMed] [Google Scholar]
- Tzfati Y., Abeliovich H., Kapeller I., Shlomai J. A single-stranded DNA-binding protein from Crithidia fasciculata recognizes the nucleotide sequence at the origin of replication of kinetoplast DNA minicircles. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):6891–6895. doi: 10.1073/pnas.89.15.6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker S. S., Francesconi S. C., Eisenberg S. A DNA replication enhancer in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4665–4669. doi: 10.1073/pnas.87.12.4665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker S. S., Francesconi S. C., Tye B. K., Eisenberg S. The OBF1 protein and its DNA-binding site are important for the function of an autonomously replicating sequence in Saccharomyces cerevisiae. Mol Cell Biol. 1989 Jul;9(7):2914–2921. doi: 10.1128/mcb.9.7.2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White T. C., Fase-Fowler F., van Luenen H., Calafat J., Borst P. The H circles of Leishmania tarentolae are a unique amplifiable system of oligomeric DNAs associated with drug resistance. J Biol Chem. 1988 Nov 15;263(32):16977–16983. [PubMed] [Google Scholar]
- Williams J. S., Eckdahl T. T., Anderson J. N. Bent DNA functions as a replication enhancer in Saccharomyces cerevisiae. Mol Cell Biol. 1988 Jul;8(7):2763–2769. doi: 10.1128/mcb.8.7.2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn K., Blattner F. R. Sequence-induced DNA curvature at the bacteriophage lambda origin of replication. Nature. 1985 Oct 3;317(6036):451–453. doi: 10.1038/317451a0. [DOI] [PubMed] [Google Scholar]
- ten Asbroek A. L., Mol C. A., Kieft R., Borst P. Stable transformation of Trypanosoma brucei. Mol Biochem Parasitol. 1993 May;59(1):133–142. doi: 10.1016/0166-6851(93)90014-o. [DOI] [PubMed] [Google Scholar]