Abstract
Background
Factors associated with survival in pemphigus have not yet been thoroughly addressed. Therefore, in the present study, risk factors for overall mortality in a large group of patients with pemphigus vulgaris and foliaceus were investigated.
Methods
A retrospective hospital-based cohort study was carried out, between October 1998 and November 2012, in the Department of Dermatology of the University of Medicine and Pharmacy “Iuliu Hatieganu”, Cluj-Napoca, Romania. The investigated prognostic endpoint was the overall survival of the patients.
Results
A total of 130 patients were studied (108 with pemphigus vulgaris and 22 with pemphigus foliaceus). In pemphigus vulgaris group, univariate analysis found a statistically significant association between the age of onset ≥ 65 years (p < 0.001), presence of coronary heart disease (p = 0.006), presence of cardiac arrhythmia (p = 0.004), level of anti-desmoglein1 autoantibodies ≥ 100 U/mL (p = 0.047) at diagnosis and the survival of the patients. An age-adjusted analysis showed significant results for coronary heart disease. Multivariate analysis identified the age of onset ≥ 65 years and the presence of coronary heart disease at diagnosis as independent risk factors associated with overall mortality. In patients with pemphigus foliaceus, age of onset ≥ 65 years (p = 0.021) was associated with poor survival.
Conclusions
In addition to common prognostic factors, including older age and cardiovascular comorbidities, level of autoantibodies was found to be a disease-specific factor associated with overall mortality in pemphigus vulgaris. The newly identified factors have major implications for the stratification of patients and should greatly facilitate further epidemiological studies in pemphigus. In addition, they provide useful information for the design of personalized therapeutic plans in the clinical setting.
Keywords: Risk factors, Pemphigus, Survival rate, Age factor, Cardiovascular disease, Anti-desmoglein 1 autoantibodies
Background
Pemphigus encompasses a group of autoimmune blistering skin diseases, characterized by autoreactive T and B lymphocytes, targeting antigens of the intercellular junctions of keratinocytes [1]. Two major forms of pemphigus, pemphigus vulgaris (PV; ORPHA704) and pemphigus foliaceus (PF; ORPHA79481), show specific clinical, histological and immunopathological findings [2]. If not treated, pemphigus vulgaris is often a fatal disease. Despite the fact that prognosis of the condition has dramatically improved after the advent of glucocorticoids and immunosuppresive therapy, recent population-based studies documented an increased risk of death in patients with pemphigus [3,4].
While several studies have been undertaken to evaluate the risk factors for disease course, relapses and remissions in patients with pemphigus [5-8], few investigations evaluated the risk factors for overall mortality in patients with PV [9-12] and none for PF as a separate entity.
Previous studies provided fragmented information suggesting that an older age of onset [3,10,11,13], concomitant involvement of the skin and mucosal surface at early disease presentation [9,14,15], delayed initiation of corticosteroid treatment [9] and the therapeutic combination of corticosteroids plus azathioprine, vs corticosteroids alone [11] are possible risk factors for overall mortality in patients with pemphigus. Further putative risk factors, including comorbidities and pathogenetically-relevant immunopathological features have not yet been assessed sistematically. Therefore, in a hospital-based retrospective approach, we aimed to identify possible risk factors for overall mortality in a characterised group of patients with PV and PF.
Methods
Design overview
This retrospective cohort study was performed in the Department of Dermatology at the University of Medicine and Pharmacy “Iuliu Hatieganu”, from Cluj-Napoca, Romania. Patients diagnosed between October 1998 and November 2012 were included. The study was approved by the local Ethic Committee and performed in accordance with the Declaration of Helsinki.
Patients
The study group consisted of hospitalized or ambulatory patients, which were included in clinical files and electronic data bases. Epidemiological, clinical and immunological data were collected from patients' medical records. Death records data were obtained from the National Population Register. Missing data from patients’ files were obtained from the patients themselves by the use of telephone interviews. An informed consent was signed by all patients.
Inclusion criteria for patients with PV were: 1) presence of skin bullae and/or erosions on mucous membranes, 2) intraepidermal acantholysis on histopathological examination of skin and/or mucosa biopsies, 3) intraepidermal IgG and/or C3 deposits by direct immunofluorescence and presence of anti-desmoglein 3 +/− anti-desmoglein 1 autoantibodies, measured by ELISA method. Inclusion criteria for patients with PF were: 1) presence of skin bullae, 2) lack of mucosal lesions, 3) intraepidermal acantholysis on histopathological examination of skin biopsies, 4) intraepidermal IgG and/or C3 deposits by direct immunofluorescence and presence of anti-desmoglein 1 autoantibodies, with lack of anti-desmoglein 3 autoantibodies, measured by enzyme-linked immunosorbent assay (ELISA) method. Patients diagnosed with other subtypes than PV or PF were not included in our study.
The collected epidemiological variables were the age of onset, gender, ethnicity, alcohol consumption and smoking status. The clinical variables were the subtype of disease (PV/PF), subtype of pemphigus vulgaris (cutaneous, mucosal, mucocutaneous), associated comorbidities at diagnosis, value of erythrocyte sedimentation rate (ESR) (mm/h) at diagnosis, initial dose of corticosteroids (mg/kg/day) and follow-up time. The immunopathological features recorded were the direct immunofluorescence microscopy (DIF) findings, titres of anti-desmoglein1 (Dsg1) and anti-desmoglein3 (Dsg3) autoantibodies.
Collection of immunopathological data
DIF was performed in all patients. In order to determine levels of specific anti-Dsg1 and anti-Dsg3 autoantibodies at diagnosis, commercially available ELISA kits were used (MBL laboratories, Nagoya, Japan). The cut-off value for positive levels of anti-desmoglein1 and anti-desmoglein3 autoantibodies was taken as ≥ 20 U/mL. To use standardized conditions, reduce bias and pre-analytical sources of error, sera from newly diagnosed patients were frozen after collection. Subsequently, ELISA analyses were performed at regular intervals.
Endpoints
The overall survival (OS) rate, representing the period (months) from diagnosis to death, was chosen as endpoint. Overall survival was assessed after a follow-up of minimum 12 months.
For the survival analysis, the cut off values used for numeric continuous variables were similar to those found in the literature. Age was assessed both as a continuous and dichotomous variable. Since information on age as a risk factor for overall mortality in pemphigus is largely lacking, in line with the World Health Organisation definition of an “elderly” or “older” person [16] we used the cut-off value of 65 years. A level of ESR > 30 mm/h has been previously described as risk factor for death among patients with bullous pemphigoid [17]. Therefore, we have chosen it as cut-off value.
A previous categorization of levels of anti-desmoglein autoantibodies has been made by Saha et al. [8], who divided the values in three groups, as follows: 0–29 (negative), 30–100 (low and medium levels), >100 (high levels). For the levels of anti-desmoglein 1 and anti-desmoglein 3 autoantibodies, we have taken as cut-off value the level of 100 U/ml, in order to check if there was a survival difference between patients with high levels and patients with negative, low and medium levels, as previously described.
The cut-off value for the corticosteroid dose was 1 mg/kg/day.
Statistical analysis
Association between potential risk factors (epidemiological, clinical and immunopathological variables defined above) was assessed using the log-rank test and the Kaplan Meier curves. The hazard ratios (HR) were computed for all potential risk factors after adjusting for the effect of age as a continuous and dichotomous variable using a Cox proportional hazard model. Subsequently, a multivariate Cox regression analysis was performed, using Akaike information criterion for stepwise backward/forward model selection. Hazard ratios are presented with 95% confidence intervals. The Mann Whitney U test, medians and interquartile range were used for non‐normally distributed variables. For all tests, a two-tailed p-value < 0.05 was considered statistically significant. Statistical analysis was performed using the R software environment for statistical computing and graphics [18].
Results
Out of the 130 patients enrolled in the study, 108 (83.1%) were diagnosed with PV and 22 (16.9%) with PF. The HRs adjusted for age of onset as continuous variable are shown in Table 1 for PV patients and Table 2 for PF patients. Survival differences between groups at 1, 3, 5 and 10 years are shown in Table 3 for patients with PV and Table 4 for patients with PF. Only relevant Kaplan Meier curves have been shown (Figures 1, 2, 3 and 4).
Table 1.
Age of onset in years, adjusted HR analysis for overall survival rates in patients with pemphigus vulgaris
| Characteristic | No (%) | Deaths | HR (95% CI) | p-value |
|---|---|---|---|---|
| No (%) | ||||
| Age of onset ≥ 65 years | 27 (25.0%) | 12 (70.6%) | 9.37 (95%CI 3.3 – 26.65) | <0.001 |
| Age of onset < 65 years | 81 (75.0%) | 5 (29.4%) | ||
| Gender (M) | 46 (42.6%) | 6 (35.3%) | 0.73 (0.26 – 2.09) | 0.561 |
| Gender (F) | 62 (57.4%) | 11 (64.7%) | ||
| Ethnicity (Romanian) | 100 (92.6%) | 16 (94.1%) | 0.57 (0.07 – 4.12) | 0.588 |
| Ethnicity (Roma) | 8 (7.4%) | 1 (5.9%) | ||
| Alcohol consumption (Yes) | 20 (18.5%) | 3 (17.7%) | 1.52 (0.42 – 5.51) | 0.524 |
| Alcohol consumption (No) | 88 (81.5%) | 14 (82.3%) | ||
| Smoking (Yes) | 29 (26.9%) | 2 (11.8%) | 1.36 (0.29 – 6.33) | 0.698 |
| Smoking (No) | 79 (73.1%) | 15 (88.2%) | ||
| Subtype of PV | ||||
| Mucocutaneous | 80 (74.1%) | 14 (82.3%) | 2.60 (0.34 – 19.93) | 0.357 |
| Cutaneous | 13 (12.0%) | 1 (5.9%) | ||
| Mucous | 15 (13.9%) | 2 (11.8%) | 1.72 (0.16 – 18.97) | 0.659 |
| High blood pressure (Yes) | 28 (25.9%) | 6 (35.3%) | 0.95 (0.35 – 2.60) | 0.928 |
| High blood pressure (No) | 80 (74.1%) | 11 (64.7%) | ||
| Coronary heart disease (Yes) | 11 (10.2%) | 5 (29.4%) | 3.04 (1.07 – 8.67) | 0.037 |
| Coronary heart disease (No) | 97 (89.8%) | 12 (70.6%) | ||
| Heart failure (Yes) | 4 (3.7%) | 1 (5.9%) | 0.63 (0.08 – 4.76) | 0.651 |
| Heart failure (No) | 104 (96.3%) | 16 (94.1%) | ||
| Cardiac arrhythmia (Yes) | 5 (4.6%) | 3 (17.7%) | 2.70 (0.75 – 9.59) | 0.125 |
| Cardiac arrhythmia (No) | 103 (95.4%) | 14 (82.3%) | ||
| Diabetes mellitus (Yes) | 8 (7.4%) | 2 (11.8%) | 0.91 (0.21 – 3.99) | 0.898 |
| Diabetes mellitus (No) | 100 (92.6%) | 15 (88.2%) | ||
| Stroke (Yes) | 5 (4.6%) | 1 (5.9%) | 1.08 (0.14 – 8.22) | 0.944 |
| Stroke (No) | 103 (95.4%) | 16 (94.1%) | ||
| Depression (Yes) | 5 (4.6%) | 1 (5.9%) | 2.09 (0.27 – 16.22) | 0.480 |
| Depression (No) | 103 (95.4%) | 16 (94.1%) | ||
| Dental problems (Yes) | 57 (52.8%) | 7 (41.2%) | 0.85 (0.32 – 2.28) | 0.750 |
| Dental problems (No) | 51 (47.2%) | 10 (58.8%) | ||
| ESR ≥ 30 mm/hour | 26 (42.6%) | 9 (75.0%) | 1.51 (0.37 – 6.20) | 0.570 |
| ESR < 30 mm/hour | 35 (57.4%) | 3 (25.0%) | ||
| Steroids ≥ 1 mg/kg/day | 15 (15.0%) | 2 (14.3%) | 0.88 (0.19 – 4.05) | 0.868 |
| Steroids < 1 mg/kg/day | 85 (85.0%) | 12 (85.7%) | ||
| Anti-Dsg1 autoAbs ≥ 100 U/mL | 45 (45.5%) | 11 (64.7%) | 1.98 (0.71 – 5.53) | 0.195 |
| Anti-Dsg1 autoAbs < 100 U/mL | 54 (54.5%) | 6 (35.3%) | ||
| Anti-Dsg3 autoAbs ≥ 100 U/mL | 71 (71.7%) | 11 (64.7%) | 0.60 (0.22 – 1.63) | 0.314 |
| Anti-Dsg3 autoAbs <100 U/mL | 28 (28.3%) | 6 (35.3%) |
Table 2.
Age of onset in years, adjusted HR analysis for overall survival rates in patients with pemphigus foliaceus
| Characteristic | No (%) | Deaths | HR (95% CI) | p-value |
|---|---|---|---|---|
| No (%) | ||||
| Age of onset ≥65 years | 7 (31.8%) | 4 (80.0%) | 13.48 (1.49 – 121.94) | 0.021 |
| Age of onset <65 years | 15 (68.2%) | 1 (20.0%) | ||
| Gender (M) | 4 (18.2%) | 0 (0.0%) | NA | NA |
| Gender (F) | 18 (81.8%) | 5 (100.0%) | ||
| Alcohol (Yes) | 2 (9.1%) | 0 (0.0%) | NA | NA |
| Alcohol (No) | 20 (90.9%) | 5 (100.0%) | ||
| Smoking (Yes) | 9 (40.9%) | 0 (0.0%) | NA | NA |
| Smoking (No) | 13 (59.1%) | 5 (100.0%) | ||
| High blood pressure (Yes) | 6 (27.3%) | 2 (40.0%) | 0.58 (0.09 – 3.80) | 0.570 |
| High blood pressure (No) | 16 (72.7%) | 3 (60.0%) | ||
| Coronary heart disease (Yes) | 3 (13.6%) | 1 (20.0%) | 3.07 (0.26 – 36.36) | 0.375 |
| Coronary heart disease (No) | 19 (86.4%) | 4 (80.0%) | ||
| Diabetes mellitus (Yes) | 2 (9.1%) | 1 (20.0%) | 7.79 (0.54 – 111.18) | 0.130 |
| Diabetes mellitus (No) | 20 (90.9%) | 4 (80.0%) | ||
| Dental problems (Yes) | 12 (54.5%) | 2 (40.0%) | 1.77 (0.24 – 13.24) | 0.579 |
| Dental problems (No) | 10 (45.5%) | 3 (60.0%) | ||
| ESR ≥ 30 mm/hour | 7 (31.8%) | 2 (40.0%) | NA | NA |
| ESR < 30 mm/hour | 5 (22.7%) | 0 (0.0%) | ||
| Steroids ≥ 1mg/kg/day | 4 (18.2%) | 1 (20.0%) | NA | NA |
| Steroids < 1 mg/kg/day | 12 (54.5%) | 2 (40.0%) | ||
| Anti-Dsg1 autoAbs ≥ 100 U/mL | 12 (54.5%) | 4 (80.0%) | 2.59 (0.28 – 24.13) | 0.404 |
| Anti-Dsg1 autoAbs < 100 U/mL | 10 (45.5%) | 1 (20.0%) |
Table 3.
Differences between survival rates at 1, 3, 5 and 10 years, in patients with pemphigus vulgaris
| Characteristic | Survival rate (%) | 3 | 5 | 10 | p-value |
|---|---|---|---|---|---|
| Follow up (years) 1 | |||||
| Age of onset ≥ 65 years | 72.2% | 67.4% | 48.4% | 41.5% | < 0.001 |
| Age of onset < 65 years | 97.3% | 97.3% | 91.4% | 91.4% | |
| Survival difference (%) | 25.1% | 29.9% | 43.0% | 49.9% | |
| Gender (M) | 88.0% | 88.0% | 83.6% | 83.6% | 0.611 |
| Gender (F) | 93.3% | 91.3% | 78.7% | 75.1% | |
| Survival difference (%) | 5.3% | 3.3% | 4.9% | 8.5% | |
| Ethnicity (Romanian) | 90.6% | 89.3% | 81.1% | 79.0% | 0.816 |
| Ethnicity (Roma) | 100.0% | 100.0% | 75.0% | 75.0% | |
| Survival difference (%) | 9.4% | 10.7% | 6.1% | 4.0% | |
| Alcohol consumption (Yes) | 83.3% | 83.3% | 83.3% | 83.3% | 0.997 |
| Alcohol consumption (No) | 92.8% | 91.4% | 80.1% | 77.5% | |
| Survival difference (%) | 9.5% | 8.1% | 3.2% | 5.8% | |
| Smoking (Yes) | 92.1% | 92.1% | 92.1% | 92.1% | 0.299 |
| Smoking (No) | 90.9% | 89.4% | 78.1% | 75.6% | |
| Survival difference (%) | 1.2% | 2.7% | 14.0% | 16.5% | |
| Subtype of PV | |||||
| Mucocutaneous | 90.8% | 89.2% | 76.8% | 76.8% | 0.692 |
| Cutaneous | 92.3% | 92.3% | 92.3% | 92.3% | |
| Mucous | 92.9% | 92.9% | 92.9% | 79.6% | |
| High blood pressure (Yes) | 88.5% | 83.6% | 77.2 % | 70.7 % | 0.303 |
| High blood pressure (No) | 92.0% | 92.0% | 81.8% | 81.8% | |
| Survival difference (%) | 3.5% | 8.4% | 4.6% | 11.1% | |
| Coronary heart disease (Yes) | 70.7% | 70.7% | 58.9% | 44.2% | 0.003 |
| Coronary heart disease (No) | 93.4% | 92.0% | 83.1% | 83.1% | |
| Survival difference (%) | 22.7% | 21.3% | 24.2% | 38.9% | |
| Heart failure (Yes) | NA | 100.0% | 100.0% | 0.0% | 0.697 |
| Heart failure (No) | 90.8% | 89.5% | 79.9% | 79.9% | |
| Survival difference (%) | NA | 10.5% | 20.1% | 79.9% | |
| Cardiac arrhythmia (Yes) | 53.3% | 53.3% | 53.3% | 26.7% | 0.001 |
| Cardiac arrhythmia (No) | 92.8% | 91.5% | 81.7% | 81.7% | |
| Survival difference (%) | 39.5% | 38.2% | 28.4% | 55.0% | |
| Diabetes mellitus (Yes) | 87.5% | 87.5% | 65.6% | 65.6% | |
| Diabetes mellitus (No) | 91.5% | 90.2% | 81.8% | 79.6% | |
| Survival difference (%) | 4.0% | 2.7% | 16.2% | 14.0% | 0.438 |
| Stroke (Yes) | 80.0% | 80.0% | 80.0% | 80.0% | 0.749 |
| Stroke (No) | 91.7% | 90.5% | 80.6% | 78.4% | |
| Survival difference (%) | 11.7% | 10.5% | 0.6% | 1.6% | |
| Depression (Yes) | 80.0% | 80.0% | 80.0 % | 80.0% | 0.629 |
| Depression (No) | 91.7% | 90.4% | 80.8% | 78.7% | |
| Survival difference (%) | 11.7% | 10.4% | 0.8% | 1.3% | |
| Dental problems (Yes) | 90.5% | 88.0% | 84.8% | 84.8% | 0.508 |
| Dental problems (No) | 91.9% | 91.9% | 77.2% | 73.6% | |
| Survival difference (%) | 1.4% | 3.9% | 7.6% | 11.2% | |
| ESR ≥ 30 mm/hour | 84.6% | 80.4% | 66.4% | 60.9% | 0.039 |
| ESR < 30 mm/hour | 93.6% | 93.6% | 88.9% | 88.9% | |
| Survival difference (%) | 9.0% | 13.2% | 22.5% | 28.0% | |
| Steroids ≥ 1 mg/kg/day | 93.3% | 93.3% | 84.9% | 84.8% | 0.726 |
| Steroids < 1 mg/kg/day | 93.6% | 92.0% | 81.0% | 78.1% | |
| Survival difference (%) | 0.3% | 1.3% | 3.9% | 6.7% | |
| Anti-Dsg1 autoAbs ≥ 100 U/mL | 84.2 % | 81.3% | 68.8% | 68.8% | 0.039 |
| Anti-Dsg1 autoAbs < 100 U/mL | 96.1 % | 96.1% | 88.3% | 84.9% | |
| Survival difference (%) | 11.9 % | 14.8% | 19.5 % | 16.1% | |
| Anti-Dsg3 auto Abs ≥ 100 U/mL | 91.3% | 89.5% | 80.4% | 80.4% | 0.506 |
| Anti-Dsg3 auto Abs < 100 U/mL | 89.3% | 89.3% | 78.8% | 71.6% | |
| Survival difference (%) | 2.0% | 0.2% | 1.6% | 8.8% |
Table 4.
Differences between survival rates at 1, 3, 5 and 10 years, in patients with pemphigus foliaceus
| Characteristic |
Survival rate (%)
Follow up (years) 1 |
3 | 5 | 10 | p-value |
|---|---|---|---|---|---|
| Age of onset ≥ 65 years | 68.6% | 34.3% | 34.3% | 34.3% | 0.003 |
| Age of onset < 65 years | NA | 93.3% | 93.3% | 93.3% | |
| Survival difference (%) | NA | 59.0% | 59.0% | 59.0% | |
| Gender (M) | NA | NA | 100.0% | 100.0% | NA |
| Gender (F) | 88.5% | 70.8% | 70.8% | 70.8% | |
| Survival difference (%) | NA | NA | 29.2% | 29.2% | |
| Alcohol (Yes) | NA | NA | NA | 100.0% | NA |
| Alcohol (No) | 89.7% | 73.9% | 73.9% | 73.9% | |
| Survival difference (%) | NA | NA | NA | 26.1% | |
| Smoking (Yes) | NA | 100.0% | 100.0% | 100.0% | NA |
| Smoking (No) | 83.9% | 58.7% | 58.7% | 58.7% | |
| Survival difference (%) | NA | 41.3% | 41.3% | 41.3% | |
| High blood pressure (Yes) | 83.3% | 66.7% | 66.7% | 66.7% | 0.518 |
| High blood pressure (No) | 93.3% | 80.0% | 80.0% | 80.0% | |
| Survival difference (%) | 10.0% | 13.3% | 13.3% | 13.3% | |
| Coronary heart disease (Yes) | 66.7% | 66.7% | 66.7% | 66.7% | 0.545 |
| Coronary heart disease (No) | 94.4% | 77.8% | 77.8% | 77.8% | |
| Survival difference (%) | 27.7% | 11.1% | 11.1% | 11.1% | |
| Diabetes mellitus (Yes) | 50.0% | 50.0% | 50.0 % | 50.0% | 0.197 |
| Diabetes mellitus (No) | 94.7% | 79.0% | 79.0% | 79.0% | |
| Survival difference (%) | 44.7% | 29.0% | 29.0% | 29.0% | |
| Dental problems (Yes) | 82.5% | 82.5% | 82.5% | 82.5% | 0.623 |
| Dental problems (No) | NA | 70.0% | 70.0% | 70.0% | |
| Survival difference (%) | NA | 12.5% | 12.5% | 12.5% | |
| ESR ≥ 30 mm/hour | NA | 71.4% | 71.4% | 71.4% | NA |
| ESR < 30 mm/hour | 100.0% | 100.0% | 100.0% | 100.0% | |
| Survival difference (%) | NA | 28.6% | 28.6% | 28.6% | |
| Steroids ≥ 1 mg/kg/day | NA | 75.0% | 75.0% | 75.0% | 0.695 |
| Steroids < 1 mg/kg/day | 100.0% | 81.8% | 81.8% | 81.8% | |
| Survival difference (%) | NA | 6.8% | 6.8% | 6.8% | |
| Anti-Dsg1 autoAbs ≥ 100 U/mL | 83.3% | 66.7% | 66.7% | 66.7% | 0.313 |
| Anti-Dsg1 autoAbs < 100 U/mL | NA | 87.5% | 87.5% | 87.5% | |
| Survival difference (%) | 83.3% | 20.8% | 20.8% | 20.8% |
Figure 1.
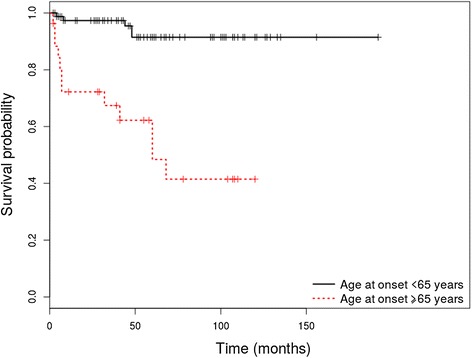
Kaplan-Meier analysis of survival in PV patients with an age of onset ≥ 65 years vs. patients with an age of onset <65 years.
Figure 2.
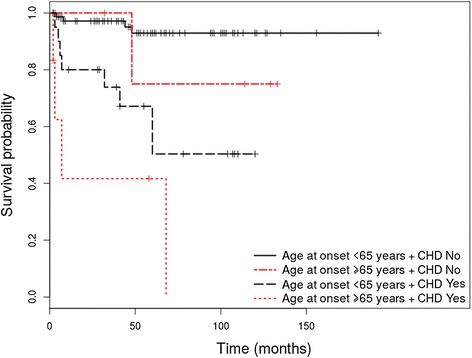
Kaplan-Meier analysis of survival in PV patients with coronary heart disease (CHD) vs. patients without coronary heart disease (CHD) at diagnosis, stratified by age.
Figure 3.
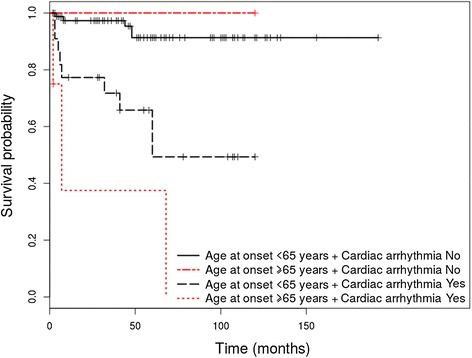
Kaplan-Meier analysis of survival in PV patients with cardiac arrhythmia vs. patients without cardiac arrhythmia at diagnosis, stratified by age.
Figure 4.
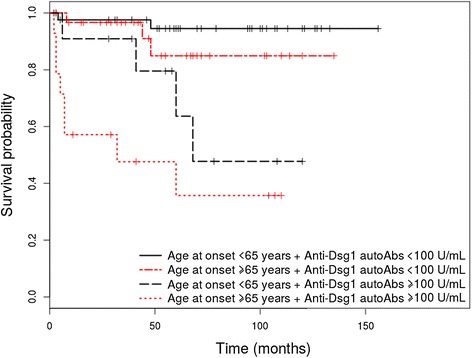
Kaplan-Meier analysis of survival in PV patients with level of anti-Dsg1 autoantibodies ≥ 100 U/mL vs. patients with level of anti-Dsg1 autoantibodies <100 U/mL at diagnosis, stratified by age.
Outcome data
Among patients with PV, a total of 17 died (11 females, 6 males), 9 of them within the first year after diagnosis. The median overall survival among the patients who died was 8 months [2–68]. The median duration of follow up among the patients who were alive was 58 months [2–192]. Among patients with PF, 5 died, 2 of them within the first year after diagnosis. All of them were females. The median overall survival among the patients who died was 17 months [5-36]. The median duration of follow up among the patients who were alive was 68 months [6–126]. In the pemphigus vulgaris group, the cause of death was retrievable in 7 patients, out of which 4 showed an “early death”, within the first year after diagnosis. A number of 5 patients died of “pemphigus-related” causes, out of which 3 within the first year. One patient refused to pursue the medication and died of malnutrition, 6 months after diagnosis. One patient developed an extended form of pemphigus and died of MRSA sepsis, 7 months after diagnosis. Three patients developed an aggressive relapse and died due to the complications induced by corticosteroid therapy (one with gastrointestinal bleeding, one with pulmonary embolism and one with cardiac insufficiency), one at 2 months after diagnosis (gastrointestinal bleeding). Two patients died of ''non-pemphigus related'' causes (one of acute myocardial infarction, 3 months after diagnosis and one of stroke).
In the pemphigus foliaceus group, the cause of death was available in 2 patients. One patient died of ''pemphigus-related causes'', within one year after diagnosis. She developed a relapse and refused to continue the treatment. Additionally, she had a type 2 diabetes mellitus and renal-failure. One patient died of ''non-pemphigus related'' cause (i.e., colorectal carcinoma) 3 years after diagnosis.
The median interval from onset to diagnosis in the pemphigus vulgaris group was 3 months, ranging between 0 and 48 months. The median interval from onset to diagnosis in the pemphigus foliaceus group was 5 months, ranging between 1 and 36 months.
The interval between onset and diagnosis in the PV group of patients who died within the first year after diagnosis varied between 0 and 7 months. Seven of these patients had an age > 65 years and 2 patients had an age < 65 years. In the pemphigus foliaceus group, two patients died within the first year after diagnosis. Both had an age > 65 years. They were diagnosed 1, respectively 2 months after the onset.
Influence of epidemiological characteristics on the overall survival rate
An age of onset ≥ 65 years is associated with a higher risk of overall mortality in patients with pemphigus vulgaris and foliaceus
The median age of onset was 50 years [15–83] in the PV group and 53 [26–87] in the PF group. PV patients with an age of onset ≥ 65 years showed a higher risk for overall mortality, compared to patients with an age of onset < 65 years (HR = 9.37 (95% CI, 3.3-26.65), p < 0.001). Age of onset taken as a continuous variable also represented a risk factor for overall mortality (HR age = 1.12 (95% CI, 1.06 – 1.18), p < 0.001). PV patients with an age of onset ≥ 65 years also demonstrated a lower OS rate at 1, 3, 5 and 10 years (p < 0.001). PF patients with an age of onset ≥ 65 years showed a higher risk for overall mortality, compared to patients with an age of onset < 65 years (HR = 13.48 (95% CI, 1.49 –121.94), p = 0.021). In this subgroup, age taken as a continuous variable also represented a risk factor for overall mortality (HR age = 1.09 (95% CI,1.01 – 1.16), p = 0.025). These patients also demonstrated a lower OS rate at 3, 5 and 10 years (p = 0.003).
Female to male ratio was 1.34:1 among patients with PV and 3:1 among those with PF. The main ethnicity in our studied population was Romanian (92.6% of patients with PV and 100% of patients with PF). The second ethnicity was Roma (7.4% of patients with PV). Gender and ethnicity were not found to be related to overall mortality and did not influence the OS in the PV and PF groups (p > 0.05).
We could not find alcohol intake and smoking status as statistically significant risk factors for overall mortality in patients with PV and PF (p > 0.05). Still, smokers with both PV and PF had a higher OS rate, compared to non-smokers (p > 0.05).
Influence of clinical characteristics on the overall survival rate
Presence of coronary heart disease and cardiac arrhythmia at diagnosis is associated with a higher risk of overall mortality in patients with pemphigus vulgaris
Subtype of pemphigus (PV vs. PF) was not found to be related to overall mortality (p = 0.701). Although patients with PV showed a slightly higher OS survival rate at 1, 3, 5 and 10 years, compared to patients with PF, the results were not statistically significant (p = 0.559).
Of the 108 patients with PV, 80 (74.1%) presented mucocutaneous involvement, 13 (12.0%) presented only cutaneous involvement, while 15 (13.9%) presented only mucous involvement. The subtype of PV (mucocutaneos, cutaneous, mucous) was not found to be related to overall mortality (p > 0.05). Patients with mucocutaneous subtype showed lower OS rates, at 1, 3, 5 and 10 years compared to patients with cutaneous and mucous subtype (p > 0.05).
The evaluated comorbidity profile in patients with PV consisted of the following nosological entities: high blood pressure, coronary heart disease, heart failure, cardiac arrhythmia, diabetes mellitus, stroke, depression and dental problems. None of the patients with PF associated heart failure, cardiac arrhythmia, stroke and depression as comorbidities. Thus, these variables have not been assessed as risk factors in patients with PF. Presence of these comorbidities was assessed at diagnosis, in a cross-sectional manner (presence or absence of the comorbidity at diagnosis). Patients have been diagnosed with coronary heart disease by a cardiologist or general practitioner, before the diagnosis of pemphigus has been made. Coronary heart disease was defined according to the criteria included in the guidelines of the European Society of Cardiology [19].
In the PV group, association of coronary heart disease (HR = 4.27 (95% CI, 1.5- 12.13), p = 0.006) and cardiac arrhythmia (HR = 6.19 (95% CI, 1.77-21.64), p = 0.004) at diagnosis represented statistically significant risk factors for overall mortality in the initial univariate analysis. Adjusting by age, revealed coronary heart disease as a statistically significant risk factor for overall mortality (HR = 3.04 (95% CI, 1.07 – 8.67), p = 0.037 for age adjusted as continuous variable and HR = 2.96 (95% CI,1.03 – 8.54), p = 0.044 for age adjusted as dichotomous variable), while cardiac arrhythmia did not represent a statistically significant risk factor for overall mortality (HR = 2.70 (0.75 – 9.59), p = 0.125, for age adjusted as continuous variable and HR = 2.71 (0.75 – 9.80), p = 0.129, for age adjusted as dichotomous variable). Patients who associated these comorbidities at diagnosis have also shown lower OS rates at 1, 3, 5 and 10 years, compared to patients who did not associate coronary disease or cardiac arrhythmia at diagnosis (p = 0.003 for coronary heart disease and p = 0.001 for cardiac arrhythmia). Although coronary disease was not found to be related to overall mortality in patients with PF (p = 0.375), it has been associated with a decreased OS rate at 1, 3, 5 and 10 years (p = 0.545).
High blood pressure, diabetes mellitus and dental problems were not found to be related to overall mortality in patients with PV and PF (p > 0.05). Patients showing high blood pressure and diabetes mellitus displayed a decreased OS rate at 1, 3, 5 and 10 years, compared to patients with PV or PF who did not associate these comorbidities at diagnosis (p > 0.05).
Presence of heart failure, stroke and depression at diagnosis were not found as additional risk factors for overall mortality in patients with PV. A history of stroke and depression was associated with a decreased OS rate at 1, 3 and 5 years, in patients with PV (p > 0.05).
The median ESR value was 27 mm/h [4–104] in pemphigus vulgaris group and 35 mm/h [4–105] in pemphigus foliaceus group. PV patients with an ESR ≥ 30 mm/h showed a statistically significant decreased OS rate at 1, 3, 5 and 10 years, compared to patients with an ESR < 30 mm/h (p = 0.039). An ESR ≥ 30 mm/h did not represent a risk factor for overall mortality in patients with PV (p > 0.05).
Regarding the administrated treatment in the PV group, 108 patients received corticosteroids. Out of this group, a number of 31 patients received corticosteroids alone. 53 patients received corticosteroids in combination with azathioprine, 9 patients received corticosteroids in combination with cyclophosphamide, while 12 patients received corticosteroids in combination with azathioprine and cyclophosphamide. One patient received corticosteroids in combination with methotrexate. Two patients additionally received intravenous immunoglobulins. One patient received dapsone alone.
Out of a total number of 22 patients with PF, 12 received corticosteroids alone, while 9 received corticosteroids plus azathioprine. One patient received only topical steroids.
The mean initial dose of corticosteroids was 0.7 mg/kg/day [0–2] in patients with PV and 0.6 mg/kg/day [0–1.5] in patients with PF. An initial dose of corticosteroids ≥1 mg/kg was not found to be related to overall mortality in patients with PV or PF (p > 0.05). Also, no differences between survival at 1, 3, 5 and 10 years have been observed between patients with an initial dose of steroids ≥ 1 mg/kg/day and patients with an initial dose of steroids < 1 mg/kg/day (p > 0.05).
Influence of immunopathological characteristics on the overall survival rate
A level of anti-desmoglein 1 autoantibodies ≥100 U/mL at diagnosis associates with a higher risk of overall mortality in patients with pemphigus vulgaris
Values of anti-Dsg1 and anti-Dsg3 autoantibodies were measured in 99 patients with PV and 22 patients with PF. In the PV group, anti-Dsg1 autoantibodies were positive in 57 patients (71.2%) with mucocutaneous type, 8 patients (61.5%) with cutaneous type and 3 patients (20.0%) with mucous type. Anti-Dsg3 autoantibodies were positive in 68 patients (85.0%) with mucocutaneous type, 10 patients (76.9%) with cutaneous type and 13 patients (86.7%) with mucous type.
The median value of anti-Dsg1 autoantibodies was 80 U/mL [0–1000] in PV patients and 182 U/mL [1–12381] in PF patients. The median value of anti-Dsg3 autoantibodies was 175 U/mL [0–8121] in patients with PV.
A level of anti-Dsg1 autoantibodies ≥ 100 U/mL at diagnosis was significantly associated with an increased risk for overall mortality among patients with PV (HR = 2.76 (95% CI, 1.01–7.5), p = 0.047), but not PF (p > 0.05), in the initial univariate analysis. When adjusted by age, the serum levels of anti-Dsg1 autoantibodies did not reach statistical significance as a risk factor for overall mortality (HR = 1.98 (95% CI, 0.71 – 5.53), p = 0.195, for age adjusted as continuous variable and HR = 2.37 (95% CI, 0.87 – 6.46), p = 0.093, for age adjusted as dichotomous variable). Also, patients with PV and a level of anti-Dsg1 autoantibodies ≥ 100 U/mL showed a lower OS rate at 1, 3, 5 and 10 years, compared with patients with a level of anti-Dsg1 autoantibodies <100 U/mL (p = 0.039). Patients with PF and a level of anti-Dsg1 autoantibodies ≥ 100 U/mL have also shown a lower OS rate at 3, 5 and 10 years, compared with patients with a level of anti-Dsg1 autoantibodies < 100 (p > 0.05). In patients with PV we didn't find statistically significant differences with respect to levels of anti-Dsg1 autoantibodies, when the groups were divided by age, coronary heart disease and cardiac arrhythmia. The median and interquartile range for anti-Dsg1 autoantibodies were: age at onset ≥ 65 years 104 (5.33 - 160.6) U/mL versus < 65 years 62.05 (12.65 - 187.25) U/mL, p = 0.972; coronary heart disease present 53.8 (7.7 - 128) versus absent 81 (13.3 - 189), p = 0.580; cardiac arrhythmia present 69.95 (9.1 - 131.35) versus absent 80.25 (13.95 - 188.5), p = 0.444.
An initial serum level of anti-Dsg3 autoantibodies ≥ 100 U/mL could not be identified as risk factor for overall mortality among patients with PV (p = 0.314). We have not observed statistically significant differences between OS rates of patients with anti-Dsg3 autoantibodies ≥ 100 U/mL and anti-Dsg3 autoantibodies < 100 U/mL (p = 0.506).
For the multivariate analysis we started with a model that used all the variables assessed in the univariate analysis, excluding some due either to collinearity issues (i.e., cardiac arrhythmia) or to limited data availability in all patients (e.g., erythrocyte sedimentation rate and dose of steroids), and then we used a stepwise selection procedure to identify important predictors.
For the multivariate analysis, age was assessed both as a continuous and dichotomous variable. The identified independent risk factors for overall mortality in patients with PV using age as a continuous variable were: age of onset (years) (adjusted HR = 1.12 [1.06 - 1.19], p < 0.001) and presence of coronary heart disease at diagnosis (adjusted HR = 3.04 [1.07 - 8.67], p = 0.037). To further explore the relevance of autoantibody levels as risk factor, we manually entered in the previous model the “autoantibody” variable and found the HRs for: anti-Dsg1 autoantibodies ≥ 100 U/mL 2.73 [0.92 – 8.13], p = 0.072; age of onset (years) 1.11 [1.05 – 1.17], p < 0.001; and presence of coronary heart disease at diagnosis 4.21 [1.38 – 12.83], p = 0.011.
The identified independent risk factors for overall mortality among patients with PV using age as a dichotomous variable were: age of onset ≥ 65 years) (adjusted HR = 7.82 [2.71 – 22.59], p < 0.001) and presence of coronary heart disease at diagnosis (adjusted HR = 2.97 [1.03 – 8.58], p = 0.044). Likewise we further explored by manually entering in the model the anti-Dsg1 autoantibodies ≥ 100 U/mL, and found the HR for: anti-Dsg1 autoantibodies ≥ 100 U/mL 3.26 [1.13 – 9.40], p = 0.029; age of onset ≥ 65 years HR = 7.49 [2.61 – 21.54], p < 0.001, and presence of coronary heart disease at diagnosis (adjusted HR = 4.23 [1.40 – 12.84], p = 0.011). In patients with PF, a model containing only the variable age was selected by the stepwise procedure.
Discussion
An age of onset ≥ 65 years, presence of coronary heart disease, cardiac arrhythmia and a level of anti-Dsg1 autoantibodies ≥ 100 U/mL at diagnosis were found to be related to overall mortality in patients with PV. An age of onset ≥ 65 years was found to be related to overall mortality among patients with PF.
A considerable proportion of “early” deaths within the first year after diagnosis has been observed in our group (9 out of 17 patients with PV and 2 out of 5 patients with PF). Based on the causes and age of death in this group of patients described in the Results section and also based on the fact that the diagnosis has been made relatively early after onset, as mentioned above, we could speculate that the important percentage of ''early deaths'' has been due rather to an older age of onset and to pemphigus-related complications, than to a delayed initiation of treatment.
The results of the present study confirmed that general prognostic factors for survival, including older age of onset and cardiovascular disease, are associated with death also in patients with pemphigus. In line with our data, previous studies suggested that an increased age of onset (ranging 60–75 years) represents a risk factor for survival in patients with PV [3,10,11,13].
Regarding the cardiovascular disease, a higher risk of death by this cause in pemphigus population, compared to general population has been previously reported by Huang et al. [3]. Still, the overall survival in pemphigus patients with cardiovascular comorbidities, versus pemphigus patients without cardiovascular comorbidities has not yet been addressed. In addition to the direct cause of death, there are a few possible explanations for the association of cardiac diseases with an increased risk of overall mortality, in patients with pemphigus.
Presence of a chronic inflammatory background may represent a link between pemphigus, cardiovascular comorbidities and a lower overall survival, due to the direct involvement of various cytokines, such as TNF alpha and IL-6. Both molecules have been shown to be involved in the pathogenesis of pemphigus [20,21], as well as atherosclerosis and coronary heart disease [22]. Although we live in the era of TNF-alpha inhibitors, the role of these agents in pemphigus therapy is still controversial, as only a limited efficacy has been proved [23-26]. Therefore, while TNF-alpha inhibitors were shown to reduce development of cardiovascular disease in patients with psoriasis [27], no evidence regarding such an effect is available in pemphigus.
Additionally, an increased inflammatory status in pemphigus could lead to an increased disease activity and, consequently, to a poor outcome. In our cohort, presence of an inflammatory background in PV patients, illustrated by an ESR ≥ 30 mm/h, was shown to be associated with a statistically significant lower overall survival at 1,3,5 and 10 years, compared to patients with an ESR < 30 mm/h (p = 0.039). Rzany et al. demonstrated similar findings among patients with bullous pemphigoid [17]. Still, this association has not been previously reported in patients with pemphigus and could, in our opinion, be explained by the two theories mentioned above, regarding the presence of a subsequently increased disease activity or an increased cardiovascular risk, due to the chronic inflammatory background.
An alternatively, but not most likely explanation would be the cross-reactivity hypothesis. Desmosomes are cell adhesion molecules, known to maintain the integrity of various organs subjected to mechanical stress, such as the skin or the heart [28]. It has been shown that patients from El –Bagre, Colombia, affected by a new variant of endemic pemphigus foliaceus, displayed autoantibodies against multiple cardiac epitopes [29]. Additionally, patients with pemphigus and atypical variants of myocardial infarction developed a high titre of anti-heart autoantibodies, that diminished after corticotherapy [30].
As a paradox, smoking, a very-well known risk factor for cardiovascular comorbidities, was proved to show a limited beneficial effect in pemphigus outcome, due to nicotine, an agonist for the acethylcoline receptors, which are known to play an important role in the pathogenesis of pemphigus [31,32]. In our study, smokers with PV and PF demonstrated a higher OS rate at 1, 3, 5 and 10 years, compared with non-smokers, albeit not reaching statistical significance (p > 0.05).
Smoking, was defined cross-sectionally, as ''smoking at diagnosis''. Our data related to smoking should be interpreted cautiously, because our knowledge on the effects of smoking on the disease severity of pemphigus is limited and is based on few observational retrospective studies and case reports [31,33-37]. Therefore, a causal link between smoking and a clinical improvement in pemphigus cannot be established. Based on the current hypotheses, the actual current smoking exposure, rather than the cumulative long-term nicotine dose, is likely relevant for the pemphigus disease activity. Moreover, patients with severe forms of pemphigus tend to cease smoking, in order to improve their survival. In addition, a dose-dependent effect of nicotine on the disease severity in pemphigus has largely not yet been addressed thorough enough. Therefore, in an initial approach we did not go beyond correlating the smoker/non-smoker status with the survival. Nevertheless, smoking cannot be suggested as therapy in patients with pemphigus. Future studies addressing this issue in-depth in patients and animal models of pemphigus should clarify the role of nicotine in pemphigus and provide a basis for developing therapies using alternative cholinergic agents or similar derivates.
A remarcable finding of the present study is related to the prognostic relevance of the level of pemphigus autoantibodies at the time of diagnosis. Patients with PV and a level of anti-Dsg1 autoantibodies ≥ 100 U/mL showed a lower OS rate at 1, 3, 5 and 10 years, compared with patients with a level of anti-Dsg1 autoantibodies < 100 U/mL (p = 0.039). Thus, a level of anti-Dsg1 autoantibodies ≥100 U/mL at diagnosis represented a risk factor for overall mortality in patients with PV (p = 0.047), in the initial univariate analysis, but not when ajusted by age as continuous or dichotomous variable (p > 0.05). Patients with PF and a level of anti-Dsg1 autoantibodies ≥100 U/mL have also shown a lower OS rate at 3, 5 and 10 years, compared with patients with a level of anti-Dsg1 autoantibodies < 100 (p > 0.05). In multivariate analysis, level of anti-Dsg1 autoantibodies represented an independent risk factor for overall mortality in PV when explored by adding the anti-Dsg1 autoantibodies ≥ 100 U/mL to the initial model adjusted by age as dichotomous (p = 0.029), but not when used as a continuous variable (p > 0.05). In patients with PV we didn't find statistically significant differences with respect to anti-Dsg1 autoantibodies levels when subdivided by age, coronary heart disease and cardiac arrhythmia.
Nevertheless, our novel findings do not exclude that autoantibody levels may represent an independent risk factor for survival. This hypothesis should be further investigated in future studies, using larger patient groups.
While previous studies have mainly focused on assessing the association between the level of autoantibodies to desmogleins and disease activity and their predictive value for relapse in pemphigus [7,38,39], it remains largely unknown whether the levels of Dsg1- and Dsg3-specific autoantibodies also represent a risk factor for overall mortality in patients with PV. One can only speculate that an increased titre of anti-Dsg1 autoantibodies, which are present in the mucocutaneous subtype of PV, associate with a more severe disease and, subsequently, reduced overall survival. Indeed, it has been suggested that, when co-occuring with Dsg3-specific antibodies, high levels of autoantibodies against Dsg1 associate with cutaneous involvement, which may result in an increased risk of cutaneous infections [40], in addition to difficulties in alimentation and subsequent malnutrition, due to painful mucosal erosions [41].
Along the same lines, a correlation between increased levels of anti-Dsg1 autoantibodies and a higher disease activity has previously been reported in patients with pemphigus [38,39]. Titres of anti-Dsg1 autoantibodies were associated with disease severity, in the cutaneous and especially in the mucocutaneous form of PV [38]. Regarding the association between the level of anti-Dsg3 autoantibodies and disease severity, the results are controversial. While some studies have associated a higher level of anti-Dsg3 autoantibodies with a higher disease [7,8], a recent study found no association between levels of anti-Dsg3 autoantibodies and disease severity in none of the PV subtypes (mucous, cutaneous and mucocutaneous) [38].
In agreement with this hypothesis, patients with mucous and cutaneous PV subtype respond better to therapy, while patients with mucocutaneous involvement develop more severe forms of disease [42]. In our study, we showed that levels of anti-Dsg 1 autoantibodies provide a prognostic information. Patients with mucocutaneous involvement, which represented the majority of PV cohort (74.1%), have also demonstrated a lower overall survival rate (p > 0.05), compared with patients with mucous and cutaneous involvement. As described above, the severity of the mucocutaneous type in PV is associated with an increased titre of anti-Dsg1 autoantibodies. Our results suggest that an increased titre of anti-Dsg1 autoantibodies in patients with this PV subtype lead to an increase in disease severity, with a higher resistance to therapy and a lower overall survival rate.
A limitation of our study is related to a low number of observations for several subgroup analyses, which hamper detecting small differences between the subgroups (e.g., cutaneous/mucous/mucocutaneous involvement or ethnicity).
Conclusions
In conclusion, in addition to common prognostic factors, including older age and cardiovascular comorbidities, our study signals the level of anti-desmoglein 1 autoantibodies as a candidate prediction factor for overall survival that should be reinforced in further studies.
While this is the first detailed study of risk factors for overall mortality among patients with pemphigus from Romania, the findings of this study are likely to be replicated in pemphigus patients from other geographic areas or of other genetic background. The newly identified factors have major implications for the stratification of patients and design of personalized therapeutic plans in the clinical setting. In addition, they should greatly facilitate further epidemiological studies in pemphigus.
Acknowledgments
The article processing charge was funded by the German Research Foundation (DFG) and the Albert Ludwigs University Freiburg in the funding programme Open Access Publishing.
This work was supported by grants from the Deutsche Forschungsgemeinschaft SI-1281/5-1 (CS) and from “Iuliu Hatieganu” University of Medicine and Pharmacy, internal grant 1491/10/28.01.2014 (SD). Dr. Daniel-Corneliu Leucuta is a fellow of POSDRU grant no. 159/1.5/S/138776 grant with title: “Model colaborativ institutional pentru translatarea cercetarii stiintifice biomedicale in practica clinica – TRANSCENT”.
The authors acknowledge Mrs Ofelia Vladau and Mrs Garofita Gadalean for their assistance in performance of DIF and ELISA and Dr Maysoon Mustafa, Dr Oana Virtic and Dr Anca Sindrilaru for critical revision of the manuscript.
Abbreviations
- DIF
Direct immunofluorescence microscopy
- Dsg1
Desmoglein 1
- Dsg3
Desmoglein 3
- ELISA
Enzyme-linked immunosorbent assay
- ESR
Erythrocyte sedimentation rate
- PV
Pemphigus vulgaris
- PF
Pemphigus foliaceus
- OS
Overall survival rate
- HR
Hazard ratio
Footnotes
Adrian Baican and Roxana Chiorean contributed equally to this work.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
AB, RC and CS conceived of the study, made the design, coordinated the study and drafted the manuscript. RC also collected the data. DCL performed the statistical analysis. DCL and RC performed interpretation of the results. DC performed DIF and ELISA, with interpretation of the results. CB and SD contributed to collection of the data, interpretation of the results and critical revision of the manuscript. All authors read and approved the final manuscript.
Contributor Information
Adrian Baican, Email: adrianbaican@yahoo.com.
Roxana Chiorean, Email: roxana.chiorean@uniklinik-freiburg.de.
Daniel Corneliu Leucuta, Email: dleucuta@umfcluj.ro.
Corina Baican, Email: cibaican@yahoo.com.
Sorina Danescu, Email: sorinadanescu@yahoo.com.
Dorina Ciuce, Email: dciuce@yahoo.co.uk.
Cassian Sitaru, Email: cassian@mail.sitaru.eu.
References
- 1.Otten JV, Hashimoto T, Hertl M, Payne AS, Sitaru C. Molecular diagnosis in autoimmune skin blistering conditions. Curr Mol Med. 2014;14:69–95. doi: 10.2174/15665240113136660079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hashimoto T. Recent advances in the study of the pathophysiology of pemphigus. Arch Dermatol Res. 2003;295(Suppl 1):S2–11. doi: 10.1007/s00403-002-0366-3. [DOI] [PubMed] [Google Scholar]
- 3.Huang Y-H, Kuo C-F, Chen Y-H, Yang Y-W. Incidence, mortality, and causes of death of patients with pemphigus in Taiwan: a nationwide population-based study. J Invest Dermatol. 2012;132:92–7. doi: 10.1038/jid.2011.249. [DOI] [PubMed] [Google Scholar]
- 4.Langan SM, Smeeth L, Hubbard R, Fleming KM, Smith CJP, West J. Bullous pemphigoid and pemphigus vulgaris–incidence and mortality in the UK: population based cohort study. BMJ. 2008;337:a180. doi: 10.1136/bmj.a180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kavusi S, Daneshpazhooh M, Farahani F, Abedini R, Lajevardi V, Chams-Davatchi C. Outcome of pemphigus vulgaris. J Eur Acad Dermatol Venereol. 2008;22:580–4. doi: 10.1111/j.1468-3083.2007.02537.x. [DOI] [PubMed] [Google Scholar]
- 6.Mimouni D, Bar H, Gdalevich M, Katzenelson V, David M. Pemphigus, analysis of 155 patients. J Eur Acad Dermatol Venereol. 2010;24:947–52. doi: 10.1111/j.1468-3083.2010.03584.x. [DOI] [PubMed] [Google Scholar]
- 7.Herrero-González JE, Iranzo P, Benítez D, Lozano F, Herrero C, Mascaró JM. Correlation of immunological profile with phenotype and disease outcome in pemphigus. Acta Derm Venereol. 2010;90:401–5. doi: 10.2340/00015555-0868. [DOI] [PubMed] [Google Scholar]
- 8.Saha M, Bhogal B, Black MM, Cooper D, Vaughan RW, Groves RW. Prognostic factors in pemphigus vulgaris and pemphigus foliaceus. Br J Dermatol. 2014;170:116–22. doi: 10.1111/bjd.12630. [DOI] [PubMed] [Google Scholar]
- 9.Seidenbaum M, David M, Sandbank M. The course and prognosis of pemphigus. A review of 115 patients. Int J Dermatol. 1988;27:580–4. doi: 10.1111/j.1365-4362.1988.tb02409.x. [DOI] [PubMed] [Google Scholar]
- 10.Savin JA. Some factors affecting prognosis in pemphigus vulgaris and pemphigoid. Br J Dermatol. 1981;104:415–20. doi: 10.1111/j.1365-2133.1981.tb15311.x. [DOI] [PubMed] [Google Scholar]
- 11.Shamsadini S, Fekri AR, Esfandiarpoor I, Saryazdi S, Rahnama Z, Zandi S, et al. Determination of survival and hazard functions for pemphigus patients in Kerman, a southern province of Iran. Int J Dermatol. 2006;45:668–71. doi: 10.1111/j.1365-4632.2005.02614.x. [DOI] [PubMed] [Google Scholar]
- 12.Carson PJ, Hameed A, Ahmed AR. Influence of treatment on the clinical course of pemphigus vulgaris. J Am Acad Dermatol. 1996;34:645–52. doi: 10.1016/S0190-9622(96)80066-1. [DOI] [PubMed] [Google Scholar]
- 13.Baibergenova AT, Weinstock MA, Shear NH. Mortality from acquired bullous diseases of skin in Canadian adults 2000–2007. Int J Dermatol. 2012;51:1325–8. doi: 10.1111/j.1365-4632.2011.05227.x. [DOI] [PubMed] [Google Scholar]
- 14.Herbst A, Bystryn JC. Patterns of remission in pemphigus vulgaris. J Am Acad Dermatol. 2000;42:422–7. doi: 10.1016/S0190-9622(00)90213-5. [DOI] [PubMed] [Google Scholar]
- 15.Chams-Davatchi C, Valikhani M, Daneshpazhooh M, Esmaili N, Balighi K, Hallaji Z, et al. Pemphigus: analysis of 1209 cases. Int J Dermatol. 2005;44:470–6. doi: 10.1111/j.1365-4632.2004.02501.x. [DOI] [PubMed] [Google Scholar]
- 16.WHO Definition of an older or elderly person. http://www.who.int/healthinfo/survey/ageingdefnolder/en/. Accessed 20 July 2014.
- 17.Rzany B, Partscht K, Jung M, Kippes W, Mecking D, Baima B, et al. Risk factors for lethal outcome in patients with bullous pemphigoid: low serum albumin level, high dosage of glucocorticosteroids, and old age. Arch Dermatol. 2002;138:903–8. doi: 10.1001/archderm.138.7.903. [DOI] [PubMed] [Google Scholar]
- 18.R Development Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. 2010. http://www.R-project.org. Accessed 19 July 2014.
- 19.ESC Guidelines. http://www.escardio.org. Accessed 22 July 2014.
- 20.Feliciani C, Toto P, Amerio P, Pour SM, Coscione G, Shivji G, et al. In vitro and in vivo expression of interleukin-1 alpha and tumor necrosis factor-alpha mRNA in pemphigus vulgaris: interleukin-1 alpha and tumor necrosis factor-alpha are involved in acantholysis. J Invest Dermatol. 2000;114:71–7. doi: 10.1046/j.1523-1747.2000.00835.x. [DOI] [PubMed] [Google Scholar]
- 21.Mortazavi H, Babaeijandaghi F, Akbarzadeh M, Rezaei N, Amirzargar AA, Daneshpazhooh M, et al. The influence of systemic therapy on the serum levels of IL-6 and IL-8 in pemphigus vulgaris. J Eur Acad Dermatol Venereol. 2013;27:387–90. doi: 10.1111/j.1468-3083.2011.04319.x. [DOI] [PubMed] [Google Scholar]
- 22.Biswas S, Ghoshal PK, Mandal N. Synergistic effect of anti and pro-inflammatory cytokine genes and their promoter polymorphism with ST-elevation of myocardial infarction. Gene. 2014;544:145–51. doi: 10.1016/j.gene.2014.04.065. [DOI] [PubMed] [Google Scholar]
- 23.Hall RP, Fairley J, Woodley D, Werth VP, Hannah D, Streilein RD, et al. A multi-centered randomized trial of the treatment of pemphigus vulgaris patients with infliximab and prednisone compared to prednisone alone. Br J Dermatol. 2014 doi: 10.1111/bjd.13350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.García-Rabasco A, Alsina-Gibert M, Pau-Charles I, Iranzo P. Infliximab therapy failure in two patients with pemphigus vulgaris. J Am Acad Dermatol. 2012;67:e196–197. doi: 10.1016/j.jaad.2011.05.029. [DOI] [PubMed] [Google Scholar]
- 25.Shetty A, Marcum CB, Glass LF, Carter JD. Successful treatment of pemphigus vulgaris with etanercept in four patients. J Drugs Dermatol. 2009;8:940–3. [PubMed] [Google Scholar]
- 26.Fiorentino DF, Garcia MS, Rehmus W, Kimball AB. A pilot study of etanercept treatment for pemphigus vulgaris. Arch Dermatol. 2011;147:117–8. doi: 10.1001/archdermatol.2010.409. [DOI] [PubMed] [Google Scholar]
- 27.Wu JJ, Poon K-YT, Channual JC, Shen AY-J. Association between tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. Arch Dermatol. 2012;148:1244–50. doi: 10.1001/archdermatol.2012.2502. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt A, Koch PJ. Desmosomes: just cell adhesion or is there more? Cell Adhes Migr. 2007;1:28–32. doi: 10.4161/cam.1.1.4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abreu-Velez AM, Howard MS, Jiao Z, Gao W, Yi H, Grossniklaus HE, et al. Cardiac autoantibodies from patients affected by a new variant of endemic pemphigus foliaceus in Colombia. South America J Clin Immunol. 2011;31:985–97. doi: 10.1007/s10875-011-9574-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chorzelski T, Kuch J. Atypical myocardial infarction in a case of pemphigus in the light of immunofluorescence studies. Clin Exp Immunol. 1969;4:691–5. [PMC free article] [PubMed] [Google Scholar]
- 31.Brenner S, Tur E, Shapiro J, Ruocco V, D’Avino M, Ruocco E, et al. Pemphigus vulgaris: environmental factors. Occupational, behavioral, medical, and qualitative food frequency questionnaire. Int J Dermatol. 2001;40:562–9. doi: 10.1046/j.1365-4362.2001.01266.x. [DOI] [PubMed] [Google Scholar]
- 32.Kalantari-Dehaghi M, Anhalt GJ, Camilleri MJ, Chernyavsky AI, Chun S, Felgner PL, et al. Pemphigus vulgaris autoantibody profiling by proteomic technique. Plos One. 2013;8 doi: 10.1371/journal.pone.0057587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Valikhani M, Kavusi S, Chams-Davatchi C, Hallaji Z, Esmaili N, Ghandi N, et al. Impact of smoking on pemphigus. Int J Dermatol. 2008;47:567–70. doi: 10.1111/j.1365-4632.2008.03645.x. [DOI] [PubMed] [Google Scholar]
- 34.Valikhani M, Kavusi S, Chams-Davatchi C, Daneshpazhooh M, Barzegari M, Ghiasi M, et al. Pemphigus and associated environmental factors: a case–control study. Clin Exp Dermatol. 2007;32:256–60. doi: 10.1111/j.1365-2230.2007.02390.x. [DOI] [PubMed] [Google Scholar]
- 35.Mehta JN, Martin AG. A case of pemphigus vulgaris improved by cigarette smoking. Arch Dermatol. 2000;136:15–7. doi: 10.1001/archderm.136.1.15. [DOI] [PubMed] [Google Scholar]
- 36.Grando SA, Dahl MV. Nicotine and pemphigus. Arch Dermatol. 2000;136:1269. doi: 10.1001/archderm.136.10.1269. [DOI] [PubMed] [Google Scholar]
- 37.Wohl Y, Brenner S. Pemphigus in Israel-an epidemiologic analysis of cases in search of risk factors. Isr Med Assoc J IMAJ. 2003;5:410–2. [PubMed] [Google Scholar]
- 38.Patsatsi A, Kyriakou A, Giannakou A, Pavlitou-Tsiontsi A, Lambropoulos A, Sotiriadis D. Clinical significance of anti-desmoglein-1 and −3 circulating autoantibodies in Pemphigus Patients Measured by Area Index and Intensity Score. Acta Derm Venereol. 2014;94:203–6. doi: 10.2340/00015555-1666. [DOI] [PubMed] [Google Scholar]
- 39.Abasq C, Mouquet H, Gilbert D, Tron F, Grassi V, Musette P, et al. ELISA testing of anti-desmoglein 1 and 3 antibodies in the management of pemphigus. Arch Dermatol. 2009;145:529. doi: 10.1001/archdermatol.2009.9. [DOI] [PubMed] [Google Scholar]
- 40.Leshem YA, Gdalevich M, Ziv M, David M, Hodak E, Mimouni D. Opportunistic infections in patients with pemphigus. J Am Acad Dermatol. 2014;71:284–92. doi: 10.1016/j.jaad.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 41.Daneshpazhooh M, Chams-Davatchi C, Khamesipour A, Mansoori P, Taheri A, Firooz A, et al. Desmoglein 1 and 3 enzyme-linked immunosorbent assay in Iranian patients with pemphigus vulgaris: correlation with phenotype, severity, and disease activity. J Eur Acad Dermatol Venereol Jeadv. 2007;21:1319–24. doi: 10.1111/j.1468-3083.2007.02254.x. [DOI] [PubMed] [Google Scholar]
- 42.Cozzani E, Di Zenzo G, Riva S, Calabresi V, Sera F, Drosera M, et al. Are clinical phenotype and autoantibody profile always concordant in pemphigus? A study in a cohort of pemphigus patients. Eur J Dermatol. 2013;23:40–8. doi: 10.1684/ejd.2012.1903. [DOI] [PubMed] [Google Scholar]


