Abstract
BACKGROUND AND OBJECTIVES:
Heart rate (HR) is frequently used by clinicians in the hospital to assess a patient’s severity of illness and make treatment decisions. We sought to develop percentiles that characterize the relationship of expected HR by age and body temperature in hospitalized children and to compare these percentiles with published references in both primary care and emergency department (ED) settings.
METHODS:
Vital sign data were extracted from electronic health records of inpatients <18 years of age at 2 large freestanding children’s hospitals from July 2011 to June 2012. We selected up to 10 HR-temperature measurement pairs from each admission. Measurements from 60% of patients were used to derive the percentile curves, with the remainder used for validation. We compared our upper percentiles with published references in primary care and ED settings.
RESULTS:
We used 60 863 observations to derive the percentiles. Overall, an increase in body temperature of 1°C was associated with an increase of ∼10 beats per minute in HR, although there were variations across age and temperature ranges. For infants and young children, our upper percentiles were lower than in primary care and ED settings. For school-age children, our upper percentiles were higher.
CONCLUSIONS:
We characterized expected HR by age and body temperature in hospitalized children. These percentiles differed from references in primary care and ED settings. Additional research is needed to evaluate the performance of these percentiles for the identification of children who would benefit from further evaluation or intervention for tachycardia.
Keywords: vital signs, heart rate, temperature, hospital rapid response team, hospital medicine, sepsis
What’s Known on This Subject:
Heart rate (HR) increases with increasing body temperature. Previous studies have characterized the relationship among HR, age, and temperature for patients in primary care and emergency department settings but not in hospitalized children.
What This Study Adds:
Our data demonstrate an overall increase in HR by ∼10 beats/minute for each 1°C increase in body temperature. Expected heart rates for hospitalized children differ from those for primary care and emergency department patients at the same age and temperature.
Heart rate (HR) is frequently used by clinicians in the hospital to aid in determining a patient’s severity of illness and in making treatment decisions. HR is an important component of many pediatric care guidelines and clinical tools including early warning scores and medical emergency team calling criteria,1–8 criteria for the diagnosis of systemic inflammatory response syndrome and sepsis,9 emergency department (ED) triage scores,10,11 and Pediatric Advanced Life Support guidelines.12 Although it is well established that HR increases with increasing body temperature, there is little guidance available to clinicians regarding how to account for this relationship, if at all, when using tools and guidelines that include HR cut points.
The relationship between HR and body temperature has been characterized in 2 nonhospitalized populations: pediatric primary care patients age 3 months to 10 years with suspected acute infection13 and in 2 cohorts of children presenting to the ED.14,15 In this study, we sought to characterize the relationship of HR and body temperature in hospitalized children, which has not been done previously. Our first objective was to derive reference values for HR according to both age and body temperature that could be used by clinicians at the bedside and to facilitate future research aimed at guiding medical decision-making in acutely ill children. Our second objective was to compare our reference values to HR, age, and temperature references developed in primary care and the ED.
Methods
Data Collection
Vital sign data were extracted from electronic health records of all inpatients <18 years of age at the Children’s Hospital of Philadelphia (CHOP) and Cincinnati Children’s Hospital Medical Center (CCHMC) from July 1, 2011, to June 30, 2012. Vital signs obtained while subjects were in any intensive care or cardiac unit were excluded. General medical and surgical patients, as well as patients of all noncardiac subspecialties were included.
The vital sign observations had been previously entered into the electronic health records at each hospital (Epic Systems, Verona, WI) by nurses or nursing assistants in the course of clinical care at a frequency prescribed by the medical team. Over the study period for oral, axillary, and rectal temperatures CHOP used the Welch Allyn SureTemp Plus 692 thermometer Welch Allyn, Skaneateles Falls, NY) and CCHMC used the Alaris Turbo Temp thermometer (CareFusion, San Diego, CA). In practice, heart rate measurement was done using a mix of electronic (via pulse oximetry or electrocardiographic leads) and manual (via auscultation or pulse palpation) methods. The method of assessment is not documented in the chart and thus could not be included in the analysis.
Data Preparation
Only observations with simultaneous recording of both HR and temperature were included. The primary analysis was restricted to observations with a temperature taken either orally or rectally (referred to as “core” from this point forward). Other temperature measurement methods have variable degrees of error when compared with core temperatures, and we were concerned that including these observations could lead to less accurate and precise percentiles.16,17 The most common method of temperature measurement for young children in our sample was axillary, a method that, on average, underestimates body temperature.16
For each observation we determined HR z scores by age (HRZage) using a reference for expected HR in hospitalized children previously developed by our team.18 We excluded observations with extreme values for temperature (<35° or >40.5°C), HR (<30 or >240 beats per minute), or HRZage (<–5, >5, or not calculable) because we suspected that these values were likely to represent data entry errors or rare clinical conditions. A small proportion (0.1%) of temperature measurements were >40°C, and only 91 observations had a core temperature between 40.5°C and 41°C. Reference values are presented only up to 40°C because of the small sample of observations above this range.
We divided data at the patient level into derivation (60%) and validation (40%) data sets after using bootstrapped samples to determine the minimum sample size that would produce stable estimates of outer percentiles. Within each dataset we selected 10 observations from each admission for evaluation, to avoid undue influence by patients with long admissions. We selected observations with more extreme temperatures first to ensure adequate numbers of these observations for analysis. For each admission, we selected up to 3 observations with a body temperature in each of the following ranges, in order, until a maximum of 10 observations were selected for a given admission: 40 to 40.5, 39.5 to 39.9, 39.0 to 39.4, 38.5 to 38.9, 38.0 to 38.4, 35.0 to 35.4, 35.5 to 35.9, and 36 to 37.4. Within each temperature range for each admission, selection of observations was random.
Percentile Curve Development
Analysis was done using Stata 13.1 (Statacorp, College Station, TX) and R 3.0.2 (R Foundation for Statistical Computing, Vienna, Austria). Percentile curves were created by using the Box Cox Power Exponential distribution in the GAMLSS (generalized additive models for location scale and shape) package in R.19,20 After preliminary analyses confirmed a similar relationship between HRZage and temperature across ages, we created a single set of age-nonspecific percentile curves using temperature as the independent variable and HRZage as the dependent variable. Model fit was evaluated using diagnostics available in the GAMLSS package, including QQ plots and worm plots. The age-nonspecific percentile curves were used to create 9 age-specific curves describing expected HR by temperature. This method allowed for increased stability of the outer percentiles at extreme temperatures compared with creating individual age-based percentile curves.
Validation
Using the newly created age-nonspecific reference, we calculated HR z scores and percentiles for age and temperature (HRZagetemp and HRPagetemp) for observations in the validation data set. We determined the overall proportion of observations <5th and >95th percentile. To evaluate the fit of our model across subgroups, we stratified observations into 18 groups based on our 9 age groups and 2 body temperature groups (<38°C and ≥38°C). In each of the 18 groups, we compared the observed proportions <5th or >95th percentile to the expected proportion (5%) and evaluated the statistical significance of differences using the binomial test with α = .0014 (.05 corrected with the Sidak method for 36 comparisons).
Evaluation of Non–Core Temperatures
We evaluated the difference in expected HR for observations with core and noncore body temperatures using linear regression, controlling for recorded body temperature and age. We expected the HR for observations with noncore body temperatures to be higher because of the potential for axillary temperatures, the most common form of noncore temperature measurement, to underestimate true body temperature.16
Analyses by Hospital
We compared the overall proportion of observations <5th and >95th percentile by hospital, evaluating statistical significance of the differences between hospitals with χ2 tests. To explore the extent to which differences in temperature distributions between the hospitals were driven by the thermometer used, we then evaluated the temperature distribution in data obtained from CCHMC from July 1, 2012, to June 30, 2013, when CCHMC was in the process of transitioning to the same Welch Allyn thermometer used at CHOP. We used linear and logistic regression to compare differences in temperature between units at CCHMC that switched to the new thermometer earlier or later during the time period of the secondary data set. To further evaluate differences in the relationship between HR, age, and body temperature by hospital, we created separate percentile curves using only data from (1) CHOP, (2) the CCHMC primary data set in 2011–2012, and (3) the CCHMC secondary data set from 2012–2013, and compared each of these to the percentiles from the primary analysis.
Comparison With Primary Care and ED Patients
We compared the 90th percentiles from our primary analysis with the Thompson primary care percentiles at the medians of the age groups used in that reference (7 months, and 1.5, 3.5, and 7.5 years) at a temperature of 39.5°C.13 We also compared the 95th percentiles for our reference with the Davies ED reference at 39.5°C for the above ages as well as at 12 and 15 years.14 The Thompson reference did not include published values for the 95th percentile, and the Davies reference did not include the 90th percentile. The Hanna et al reference included means and confidence intervals but no percentiles for comparison.15
Ethics
Because deidentified data were used, the study was deemed to be not human subjects research and exempt from review by the institutional review boards of both CHOP and CCHMC.
Results
The extracted data contained 684 749 observations with a HR and body temperature simultaneously recorded. Of these, 596 (0.1%) were excluded because of a temperature, HR, or HRZage that was out of range. There were 16 273 patients with 21 997 admissions from CHOP and 7572 patients with 10 345 admissions from CCHMC (Tables 1 and 2).
TABLE 1.
Subject Demographics
| Characteristic | n (%) |
|---|---|
| Age | |
| 0 to <6 mo | 3086 (13) |
| 6 to <12 mo | 1437 (6) |
| 1 to <3 y | 4133 (17) |
| 3 to <5 y | 2739 (11) |
| 5 to <7 y | 2053 (9) |
| 7 to <9 y | 1775 (7) |
| 9 to <11 y | 1692 (7) |
| 11 to <14 y | 2747 (12) |
| 14 to 18 y | 4183 (18) |
| Race | |
| American Indian/Alaska Native | 14 (0.06) |
| African American or Black | 7363 (31) |
| Asian | 527 (2) |
| Multiracial | 354 (1) |
| Native Hawaiian/Pacific Islander | 15 (0.06) |
| White | 13 294 (56) |
| Other | 2218 (9) |
| Unknown/Refused | 60 (0.3) |
| Gender | |
| Male | 12 870 (54) |
| Female | 10 975 (46) |
| Total | 23 845 |
TABLE 2.
Number of Observations by Age, Temperature (°C), and Temperature Source
| Age | 35–35.9 | 36–36.9 | 37–37.9 | 38–38.9 | 39–39.9 | 40–40.5 | Total |
|---|---|---|---|---|---|---|---|
| 0 to <6 mo | |||||||
| C | 165 | 991 | 2247 | 903 | 296 | 26 | 4628 |
| NC | 7560 | 42 378 | 14 386 | 683 | 158 | 19 | 65 184 |
| 6 to <12 mo | |||||||
| C | 55 | 358 | 629 | 412 | 165 | 27 | 1646 |
| NC | 9852 | 32 308 | 8486 | 893 | 208 | 36 | 51 783 |
| 1 to <3 y | |||||||
| C | 122 | 671 | 989 | 523 | 199 | 30 | 2534 |
| NC | 20 799 | 65 741 | 21 928 | 3289 | 1034 | 186 | 112 977 |
| 3 to <5 y | |||||||
| C | 249 | 3382 | 2833 | 450 | 149 | 20 | 7083 |
| NC | 15 141 | 39 532 | 14 018 | 2053 | 668 | 130 | 71 542 |
| 5 to <7 y | |||||||
| C | 617 | 8211 | 6404 | 960 | 305 | 22 | 16 519 |
| NC | 8954 | 27 027 | 8636 | 1137 | 372 | 60 | 46 186 |
| 7 to <9 y | |||||||
| C | 566 | 10 543 | 7319 | 871 | 311 | 20 | 19 630 |
| NC | 4343 | 14 829 | 5210 | 598 | 156 | 32 | 25 168 |
| 9 to <11 y | |||||||
| C | 1032 | 14 551 | 8665 | 930 | 345 | 28 | 25 551 |
| NC | 3572 | 11 140 | 4001 | 469 | 113 | 33 | 19 328 |
| 11 to <14 y | |||||||
| C | 2261 | 33 581 | 15 912 | 1688 | 561 | 28 | 54 031 |
| NC | 4496 | 15 271 | 6157 | 686 | 249 | 59 | 26 918 |
| 14–18 y | |||||||
| C | 5566 | 68 068 | 26 437 | 2315 | 660 | 16 | 103 062 |
| NC | 5099 | 16 963 | 7152 | 863 | 267 | 39 | 30 383 |
| Total | 90 449 | 405 545 | 161 409 | 19 723 | 6216 | 811 | 684 153 |
C, core temperature; NC, non–core temperature.
Percentile Curve Development
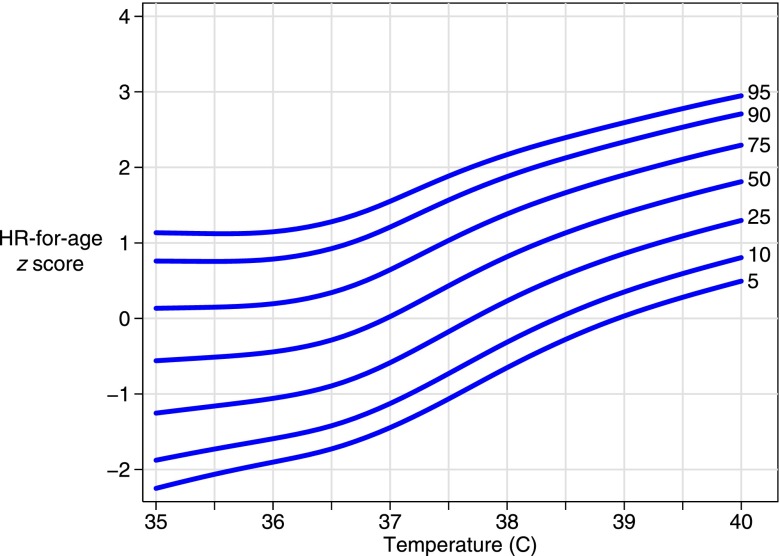
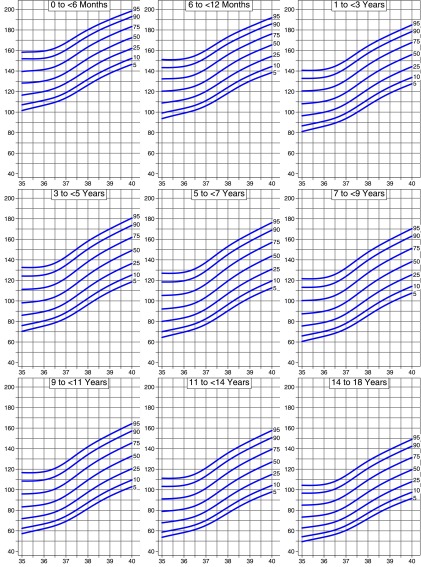
The derivation data set included 60 863 observations; 8.6% of these had a temperature ≥38°C. Age-nonspecific percentile curves for HRZage by temperature were created (Fig 1). We used the age-nonspecific curves to develop HR by temperature curves for each of 9 age groups (Fig 2). Tables to calculate HRZagetemp are available upon request from the authors.
FIGURE 1.
HR-for-age z score percentiles by temperature (age-nonspecific curves).
FIGURE 2.
Heart HR-for-temperature percentiles by age group (age-specific curves).
Validation
In the validation data set (n = 40 096), the proportion of vital signs <5th percentile was 4.9%, and the proportion >95th percentile was 5.3%. In the subgroup analysis, the proportion <5th ranged from 2.1% to 8.4%, and the proportion >95th percentile ranged from 3.5% to 10.4%. The proportion <5th or >95th was statistically different from the expected value of 5% for 4 of 36 comparisons, 2 of which were in infants aged <6 months old (Supplemental Table 4). The group with the most deviation from the expected fit was febrile infants aged <6 months old (n = 374), with 2.1% <5th percentile (P = .008) and 10.4% of febrile infants >95th percentile (P < .001).
Evaluation of Non–Core Temperatures
Controlling for body temperature and age, linear regression showed that the expected HR for observations with a non–core body temperature was 3 beats per minute higher than for observations with a core body temperature.
Analyses by Hospital
The HR distributions were similar for both hospitals (mean HR: CHOP 109, CCHMC 107; mean HRZage CHOP: 0.10, CCHMC: 0.03). The distributions of core temperature differed more substantially (mean temperature: CHOP 36.9°C, CCHMC 36.4°C), with a large difference in the proportion of observations with a core body temperature <36°C (CHOP 0.2%, CCHMC 11.6%; Fig 3). The core temperature distribution of the secondary CCHMC dataset from 2012 to 2013 was intermediate between the CHOP and primary CCHMC data sets (mean temperature: 36.8°C; 8.3% <36°C). Five CCHMC units that switched to the new thermometer earlier in the time period of the secondary data set had a larger increase in mean body temperature and a larger decrease in the proportion of temperatures <36°C, compared with 3 units that switched later. The proportion of observations with a core temperature ≥38°C was similar for both hospitals (CHOP 4.7%, CCHMC 2011–2012 6.0%, CCHMC 2012–2013 5.0%).
FIGURE 3.
Histogram of core temperatures by hospital.
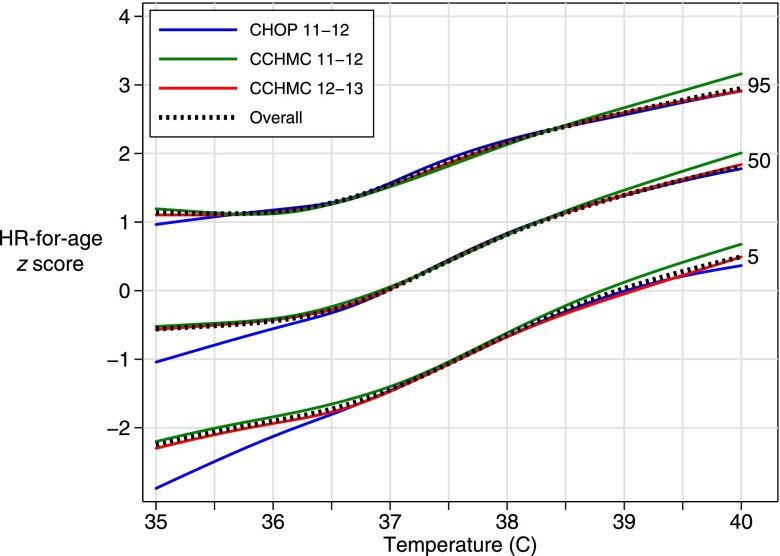
There were small differences by hospital in the proportion of observations <5th percentile (CHOP 5.3%, CCHMC 4.2%, P < .001) and >95th percentile (CHOP 5.5%, CCHMC 5.0%, P = .04). The percentile curves created from individual hospital data sets were similar to the primary analysis percentiles at temperatures from 36° to 40°C (Fig 4).
FIGURE 4.
Comparison of age-nonspecific curves in primary analysis with curves created using each hospital data set separately.
Comparison With Primary Care and ED Patients
Our 90th and 95th percentiles were lower than primary care and ED percentiles at 7 months, 1.5 years, and 3.5 years. Our percentiles were higher than those for nonhospitalized children at 7.5 and 12 years (Table 3).
TABLE 3.
Comparison of Our Percentiles for Hospitalized Children With Existing Percentile Curves From Primary Care and ED Settings
| Hospital 90th | Primary Care 90th | Hospital 95th | ED 95th | |
|---|---|---|---|---|
| 7 mo | 183 | 204 | 189 | 202 |
| 1.5 y | 176 | 191 | 182 | 194 |
| 3.5 y | 169 | 174 | 176 | 177 |
| 7.5 y | 159 | 155 | 167 | 153 |
| 12 y | 147 | N/A | 154 | 145 |
| 15 y | 140 | N/A | 146 | 147 |
N/A, not available.
Discussion
We created percentiles that characterize expected HR in hospitalized children by age and body temperature. As in prior studies, we found that expected HR increases with increasing body temperature across the full range of body temperatures, not only in febrile children.13–15 Our findings support the general relationship described in the heuristics used by some clinicians to expect an increase in HR of ∼10 beats per minute for each increase in body temperature of 1°C, but also allow clinicians to be much more precise regarding the degree to which the HR for their patients deviates from the expected HR for hospitalized patients with the same age and body temperature. For example, the median HR for a 6- to 12-month infant differs by 15 beats per minute at a temperature of 37° vs 38°C, whereas in a 14- to 18-year old, the median HR differs by only 8 beats per minute between 36° vs 37°C.
We restricted our analysis to observations that included a core body temperature measurement, which we defined as either oral or rectal. Our evaluation of HR in children with core and non–core temperature measurements supports our concern that using non–core temperatures would likely have resulted in a small (average 3 beats per minute) overestimation of expected HR. We recognize that in many clinical situations non–core body temperature measurements are necessary or preferred. We recommend using these percentiles without adjustment regardless of the method of body temperature measurement because the difference in expected HR for core and non–core measurements is small and the amount of error in body temperature using non–core methods is variable.
There were some consistent differences between our percentiles and 2 references for nonhospitalized patients in the primary care and ED triage settings.13,14 At the same temperature, young nonhospitalized children, particularly those under 2 years of age, had higher HR than found in our hospitalized subjects. Older nonhospitalized children had similar or somewhat lower HRs compared with our subjects. The difference in HR for young children may reflect differences in patient characteristics between the nonhospitalized and hospitalized populations and possibly the use of non–core body temperature measurements in nonhospitalized patients compared with the core temperatures included in our percentiles. Some of the difference may reflect the emotional stress of young children who are ill and being assessed by strangers, and the effect of this stress on HR. Young inpatients may be somewhat less upset every time vital signs are obtained, and inpatient staff generally have somewhat more flexibility to obtain vital signs when patients are more calm or even asleep. Some of the difference in young children could also be related to measurement. In Thompson et al,13 HR was measured using pulse oximetry, which may be more accurate for high HRs. Measurement is unlikely to account for all of the differences at young ages given that Davies et al14 also used a mix of HR measurement methods.
The differences in expected HR for age and temperature will be important to consider when designing or implementing tools that include HR cut points in inpatient, ED, or primary care settings. For example, tools that can be used to screen for severe sepsis are desired for both inpatients and ED patients. A severe sepsis screening tool that uses the same temperature-adjusted HR cut points in both patient populations would likely identify a higher proportion of infants and toddlers as test-positive in the ED compared with inpatients and a higher proportion of school-age children as test-positive in inpatients compared with ED patients. Because of these differences in the HR distributions between settings, it is possible that different HR cut points are needed in different settings to achieve optimal test performance.
Of note, we found differences in the body temperature distribution between the 2 hospitals from which we obtained data. This was unexpected and seems more likely attributable to differences in temperature measurement rather than differences in body temperature regulation or clinical conditions between the patients at the 2 hospitals. This explanation is supported by our analysis of the temperature distribution at CCHMC during the year it transitioned to a different thermometer. Sensitivity analyses showed small differences by hospital in the relationship among HR, age, and body temperature.
The strengths of our analysis include the large sample size and the validation across subgroups in a separate sample. Our ability to create an initial set of age-nonspecific percentile curves improved the stability of outer percentiles. It also allows researchers to precisely determine HRZagetemp regardless of age, compared with age-stratified curves that may perform less accurately at the extremes of age groups.
There are several limitations to our retrospective analysis. First, the data come from 2 similar hospitals, and the percentile curves have not yet been validated in a population of children from other hospitals. The percentiles generally fit the validation data set well across age and body temperature combinations, but in febrile infants there was some deviation from expected fit that should be evaluated further in other populations. Second, the data included relatively few observations with extremely high temperatures. Finally, our data did not include variables describing other patient-level factors associated with HR such as pain at the time of vital sign observation or medications such as inhaled bronchodilators and β-blockers that influence HR.
The proper application of our percentiles in clinical care is informed by 2 studies that have evaluated the performance of temperature-adjusted HR for the identification of children with serious illness. Cruz et al demonstrated that an age- and temperature-adjusted tachycardia alert identified most subjects with septic shock, with a sensitivity of 81% and a positive predictive value of 3.7%.21 However, when Brent et al compared temperature-adjusted HR-for-age percentiles to HR-for-age percentiles for the identification of serious bacterial illness, they found that HR-for-age was the stronger predictor of serious bacterial illness.22 Further evaluation of our temperature-adjusted HR percentiles will be necessary to determine the test characteristics of various percentile cut points for the early identification of children with serious illness and to determine whether they perform better than non–temperature-adjusted HR cut points. A lower percentile threshold (eg, 90th vs 95th percentile) for temperature-adjusted HR cut points may be needed when sensitivity is valued over specificity such as in a sepsis screen.
Conclusions
We created reference percentile curves for use in hospitalized children that characterize the age-dependent expected increases in HR as body temperature increases. Our sensitivity analyses indicate that these percentiles are appropriate for use regardless of the method of temperature measurement. As in studies of primary care and ED populations, we identified an increase of ∼10 beats per minute in HR for each increase of 1°C. However, our percentiles for hospitalized patients differ from those developed using data from primary care and ED settings. Further research is needed to validate these curves in a distinct population of hospitalized children and to evaluate their performance in identifying children who require further evaluation or intervention for sepsis or other serious conditions.
Supplementary Material
Acknowledgments
We thank William Nieczpiel, Senior Data Architect at CHOP, and Dhiraj Deshmukh, Parth Divekar, and the research data warehouse team at CCHMC for their assistance in obtaining the data.
Footnotes
Dr Daymont conceptualized and designed the study, performed the statistical analysis, participated in the analysis and interpretation of data, and drafted the manuscript; Drs Bonafide and Brady conceptualized and designed the study, acquired data, participated in the analysis and interpretation of data, and critically revised the manuscript for important content; and all authors approved the final manuscript as submitted.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Dr Daymont received support from the Children’s Hospital Research Institute of Manitoba. Dr Bonafide is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award K23HL116427. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Akre M, Finkelstein M, Erickson M, Liu M, Vanderbilt L, Billman G. Sensitivity of the pediatric early warning score to identify patient deterioration. Pediatrics. 2010;125(4). Available at: www.pediatrics.org/cgi/content/full/125/4/e763 10.1542/peds.2009-0338 [DOI] [PubMed] [Google Scholar]
- 2.Brilli RJ, Gibson R, Luria JW, et al. Implementation of a medical emergency team in a large pediatric teaching hospital prevents respiratory and cardiopulmonary arrests outside the intensive care unit. Pediatr Crit Care Med. 2007;8(3):236–246, quiz 247 10.1097/01.PCC.0000262947.72442.EA [DOI] [PubMed] [Google Scholar]
- 3.DeVita MA, Smith GB, Adam SK, et al. “Identifying the Hospitalised Patient in Crisis”—a consensus conference on the afferent limb of rapid response systems. Resuscitation. 2010;81(4):375–382 10.1016/j.resuscitation.2009.12.008 [DOI] [PubMed] [Google Scholar]
- 4.Egdell P, Finlay L, Pedley DK. The PAWS score: validation of an early warning scoring system for the initial assessment of children in the emergency department. Emerg Med J. 2008;25(11):745–749 10.1136/emj.2007.054965 [DOI] [PubMed] [Google Scholar]
- 5.Jones DA, DeVita MA, Bellomo R. Rapid-response teams. N Engl J Med. 2011;365(2):139–146 10.1056/NEJMra0910926 [DOI] [PubMed] [Google Scholar]
- 6.Monaghan A. Detecting and managing deterioration in children. Paediatr Nurs. 2005;17(1):32–35 [DOI] [PubMed] [Google Scholar]
- 7.Parshuram CS, Hutchison J, Middaugh K. Development and initial validation of the Bedside Paediatric Early Warning System score. Crit Care. 2009;13(4):R135 10.1186/cc7998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tibballs J, Kinney S. Reduction of hospital mortality and of preventable cardiac arrest and death on introduction of a pediatric medical emergency team. Pediatr Crit Care Med. 2009;10(3):306–312 10.1097/PCC.0b013e318198b02c [DOI] [PubMed] [Google Scholar]
- 9.Goldstein B, Giroir B, Randolph A, International Consensus Conference on Pediatric Sepsis . International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6(1):2–8 10.1097/01.PCC.0000149131.72248.E6 [DOI] [PubMed] [Google Scholar]
- 10.Warren DW, Jarvis A, LeBlanc L, Gravel J, CTAS National Working Group. Canadian Association of Emergency Physicians. National Emergency Nurses Affiliation. Association des Médecins d’Urgence du Québec. Canadian Paediatric Society. Society of Rural Physicians of Canada . Revisions to the Canadian Triage and Acuity Scale paediatric guidelines (PaedCTAS). CJEM. 2008;10(3):224–243 [PubMed] [Google Scholar]
- 11.Gilboy N, Tanabe P, Travers D, Rosenau AM. Emergency Severity Index (ESI): A Triage Tool for Emergency Department Care, Version 4. Rockville, MD: Agency for Healthcare Research and Quality; 2011 [Google Scholar]
- 12.Chameides L, Samson RA, Schexnayder SM, Fran M, eds. Pediatric Advanced Life Support. Provider Manual. Dallas, TX: American Heart Association; 2011 [Google Scholar]
- 13.Thompson M, Harnden A, Perera R, et al. Deriving temperature and age appropriate heart rate centiles for children with acute infections. Arch Dis Child. 2009;94(5):361–365 10.1136/adc.2008.145011 [DOI] [PubMed] [Google Scholar]
- 14.Davies P, Maconochie I. The relationship between body temperature, heart rate and respiratory rate in children. Emerg Med J. 2009;26(9):641–643 10.1136/emj.2008.061598 [DOI] [PubMed] [Google Scholar]
- 15.Hanna CM, Greenes DS. How much tachycardia in infants can be attributed to fever? Ann Emerg Med. 2004;43(6):699–705 10.1016/S0196064403010539 [DOI] [PubMed] [Google Scholar]
- 16.Craig JV, Lancaster GA, Williamson PR, Smyth RL. Temperature measured at the axilla compared with rectum in children and young people: systematic review. BMJ. 2000;320(7243):1174–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allegaert K, Casteels K, van Gorp I, Bogaert G. Tympanic, infrared skin, and temporal artery scan thermometers compared with rectal measurement in children: a real-life assessment. Curr Ther Res Clin Exp. 2014;76:34–38 10.1016/j.curtheres.2013.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonafide CP, Brady PW, Keren R, Conway PH, Marsolo K, Daymont C. Development of heart and respiratory rate percentile curves for hospitalized children. Pediatrics. 2013;131(4). Available at: www.pediatrics.org/cgi/content/full/131/4/e1150 10.1542/peds.2012-2443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borghi E, de Onis M, Garza C, et al. WHO Multicentre Growth Reference Study Group . Construction of the World Health Organization child growth standards: selection of methods for attained growth curves. Stat Med. 2006;25(2):247–265 10.1002/sim.2227 [DOI] [PubMed] [Google Scholar]
- 20.Stasinopoulos M, Rigby R, Akantziliotou C. Instructions on How to Use the Gamlss Package in R.; 2008. Available at: www.gamlss.com. Accessed April 28, 2014
- 21.Cruz AT, Williams EA, Graf JM, et al. Test characteristics of an automated age- and temperature-adjusted tachycardia alert in pediatric septic shock. Pediatr Emerg Care. 2012;28(9):889–894 10.1097/PEC.0b013e318267a78a [DOI] [PubMed] [Google Scholar]
- 22.Brent AJ, Lakhanpaul M, Ninis N, Levin M, MacFaul R, Thompson M. Evaluation of temperature-pulse centile charts in identifying serious bacterial illness: observational cohort study. Arch Dis Child. 2011;96(4):368–373 10.1136/adc.2010.183129 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.