Abstract
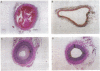
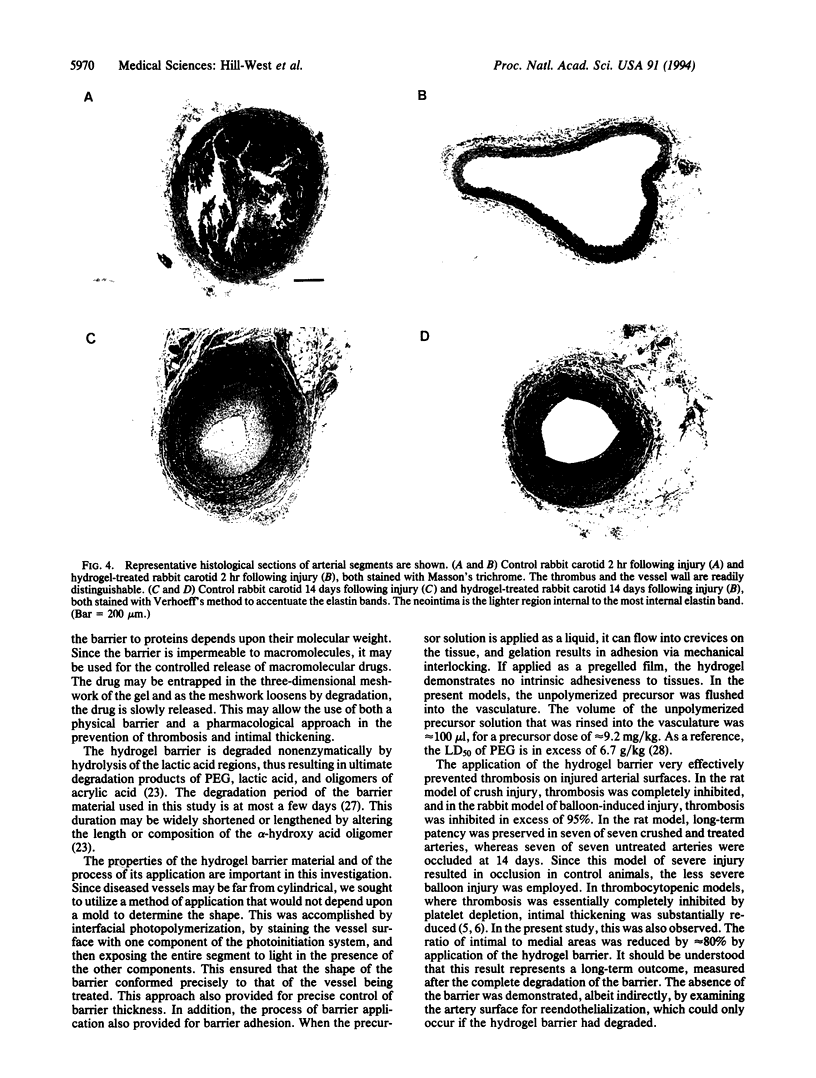
Thin hydrogel barriers formed on the inner surface of injured arteries by interfacial photopolymerization dramatically reduced thrombosis and intimal thickening in rat and rabbit models of vascular injury. This polymerization technique allowed the synthesis of a thin hydrogel barrier that conformed to the vessel wall, directly blocking contact between blood and the damaged vessel. The illumination conditions could be varied to control the thickness of the barrier from 10 microns to > 50 microns. The hydrogel was designed to degrade by nonenzymatic hydrolysis. In rats in which the carotid artery had been severely injured by crushing, treatment with the hydrogel barrier completely eliminated thrombosis (P < 0.01) and preserved long-term patency (P < 0.01). Treatment in a rabbit model of balloon injury inhibited thrombosis (P < 0.02) and reduced long-term intimal thickening by approximately 80% (P < 0.003). These results suggest that blood-borne signals acting in the early phases of healing play an important role in stimulating thickening of the intima.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berk B. C., Taubman M. B., Cragoe E. J., Jr, Fenton J. W., 2nd, Griendling K. K. Thrombin signal transduction mechanisms in rat vascular smooth muscle cells. Calcium and protein kinase C-dependent and -independent pathways. J Biol Chem. 1990 Oct 5;265(28):17334–17340. [PubMed] [Google Scholar]
- Deuel T. F. Polypeptide growth factors: roles in normal and abnormal cell growth. Annu Rev Cell Biol. 1987;3:443–492. doi: 10.1146/annurev.cb.03.110187.002303. [DOI] [PubMed] [Google Scholar]
- Edelman E. R., Nugent M. A., Smith L. T., Karnovsky M. J. Basic fibroblast growth factor enhances the coupling of intimal hyperplasia and proliferation of vasa vasorum in injured rat arteries. J Clin Invest. 1992 Feb;89(2):465–473. doi: 10.1172/JCI115607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferns G. A., Raines E. W., Sprugel K. H., Motani A. S., Reidy M. A., Ross R. Inhibition of neointimal smooth muscle accumulation after angioplasty by an antibody to PDGF. Science. 1991 Sep 6;253(5024):1129–1132. doi: 10.1126/science.1653454. [DOI] [PubMed] [Google Scholar]
- Fingerle J., Johnson R., Clowes A. W., Majesky M. W., Reidy M. A. Role of platelets in smooth muscle cell proliferation and migration after vascular injury in rat carotid artery. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8412–8416. doi: 10.1073/pnas.86.21.8412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. J., Stemerman M. B., Wenz B., Moore S., Gauldie J., Gent M., Tiell M. L., Spaet H. The effect of thrombocytopenia on experimental arteriosclerotic lesion formation in rabbits. Smooth muscle cell proliferation and re-endothelialization. J Clin Invest. 1977 Nov;60(5):1191–1201. doi: 10.1172/JCI108872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster V., Badimon L., Badimon J. J., Chesebro J. H. The pathogenesis of coronary artery disease and the acute coronary syndromes (1). N Engl J Med. 1992 Jan 23;326(4):242–250. doi: 10.1056/NEJM199201233260406. [DOI] [PubMed] [Google Scholar]
- Fuster V., Badimon L., Badimon J. J., Chesebro J. H. The pathogenesis of coronary artery disease and the acute coronary syndromes (2). N Engl J Med. 1992 Jan 30;326(5):310–318. doi: 10.1056/NEJM199201303260506. [DOI] [PubMed] [Google Scholar]
- Graham D. J., Alexander J. J. The effects of thrombin on bovine aortic endothelial and smooth muscle cells. J Vasc Surg. 1990 Feb;11(2):307–313. doi: 10.1067/mva.1990.17098. [DOI] [PubMed] [Google Scholar]
- Groves H. M., Kinlough-Rathbone R. L., Mustard J. F. Development of nonthrombogenicity of injured rabbit aortas despite inhibition of platelet adherence. Arteriosclerosis. 1986 Mar-Apr;6(2):189–195. doi: 10.1161/01.atv.6.2.189. [DOI] [PubMed] [Google Scholar]
- Heras M., Chesebro J. H., Webster M. W., Mruk J. S., Grill D. E., Penny W. J., Bowie E. J., Badimon L., Fuster V. Hirudin, heparin, and placebo during deep arterial injury in the pig. The in vivo role of thrombin in platelet-mediated thrombosis. Circulation. 1990 Oct;82(4):1476–1484. doi: 10.1161/01.cir.82.4.1476. [DOI] [PubMed] [Google Scholar]
- Hill-West J. L., Chowdhury S. M., Sawhney A. S., Pathak C. P., Dunn R. C., Hubbell J. A. Prevention of postoperative adhesions in the rat by in situ photopolymerization of bioresorbable hydrogel barriers. Obstet Gynecol. 1994 Jan;83(1):59–64. [PubMed] [Google Scholar]
- Jawien A., Bowen-Pope D. F., Lindner V., Schwartz S. M., Clowes A. W. Platelet-derived growth factor promotes smooth muscle migration and intimal thickening in a rat model of balloon angioplasty. J Clin Invest. 1992 Feb;89(2):507–511. doi: 10.1172/JCI115613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner V., Lappi D. A., Baird A., Majack R. A., Reidy M. A. Role of basic fibroblast growth factor in vascular lesion formation. Circ Res. 1991 Jan;68(1):106–113. doi: 10.1161/01.res.68.1.106. [DOI] [PubMed] [Google Scholar]
- Lindner V., Reidy M. A. Proliferation of smooth muscle cells after vascular injury is inhibited by an antibody against basic fibroblast growth factor. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3739–3743. doi: 10.1073/pnas.88.9.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M. W., Roubin G. S., Robinson K. A., Black A. J., Hearn J. A., Siegel R. J., King S. B., 3rd Trapidil in preventing restenosis after balloon angioplasty in the atherosclerotic rabbit. Circulation. 1990 Mar;81(3):1089–1093. doi: 10.1161/01.cir.81.3.1089. [DOI] [PubMed] [Google Scholar]
- Muller D. W., Ellis S. G., Topol E. J. Experimental models of coronary artery restenosis. J Am Coll Cardiol. 1992 Feb;19(2):418–432. doi: 10.1016/0735-1097(92)90500-m. [DOI] [PubMed] [Google Scholar]
- Okazaki H., Majesky M. W., Harker L. A., Schwartz S. M. Regulation of platelet-derived growth factor ligand and receptor gene expression by alpha-thrombin in vascular smooth muscle cells. Circ Res. 1992 Dec;71(6):1285–1293. doi: 10.1161/01.res.71.6.1285. [DOI] [PubMed] [Google Scholar]
- Ross R., Raines E. W., Bowen-Pope D. F. The biology of platelet-derived growth factor. Cell. 1986 Jul 18;46(2):155–169. doi: 10.1016/0092-8674(86)90733-6. [DOI] [PubMed] [Google Scholar]
- Sarembock I. J., Gertz S. D., Gimple L. W., Owen R. M., Powers E. R., Roberts W. C. Effectiveness of recombinant desulphatohirudin in reducing restenosis after balloon angioplasty of atherosclerotic femoral arteries in rabbits. Circulation. 1991 Jul;84(1):232–243. doi: 10.1161/01.cir.84.1.232. [DOI] [PubMed] [Google Scholar]
- Schwartz R. S., Holmes D. R., Jr, Topol E. J. The restenosis paradigm revisited: an alternative proposal for cellular mechanisms. J Am Coll Cardiol. 1992 Nov 1;20(5):1284–1293. doi: 10.1016/0735-1097(92)90389-5. [DOI] [PubMed] [Google Scholar]
- Simons M., Edelman E. R., DeKeyser J. L., Langer R., Rosenberg R. D. Antisense c-myb oligonucleotides inhibit intimal arterial smooth muscle cell accumulation in vivo. Nature. 1992 Sep 3;359(6390):67–70. doi: 10.1038/359067a0. [DOI] [PubMed] [Google Scholar]
- Wilcox J. N. Thrombin and other potential mechanisms underlying restenosis. Circulation. 1991 Jul;84(1):432–435. doi: 10.1161/01.cir.84.1.432. [DOI] [PubMed] [Google Scholar]