Abstract
Context:
Regional lymphadenitis is the most common complication of bacille Calmette–Guerin (BCG) vaccination. Most of the BCG lymphadenitis cases are nonsuppurative, but some suppurate and follow abscess formation, rupture, ulceration and cicatrization. Needle aspiration is the rapid, safe and cost-effective method for diagnosis as well as management of suppurative BCG adenitis.
Aims:
The aims of the present study were to assess the clinical and cytological spectrum of BCG lymphadenitis and to evaluate the role of needle aspiration in the management of suppurative BCG lymphadenitis.
Settings and Design:
We have approached every cases of ipsilateral axillary lymphadenopathy having history of BCG vaccination. We designed to aspirate the suppurative axillary lymph nodes and follow-up of nonsuppurative cases.
Subjects and Methods:
30 cases of BCG adenitis were studied during a period of 2 years. 12 cases of suppurative lymphadenitis were approached by needle aspiration and cytologically evaluated, and all the cases were followed-up for 12 weeks after diagnosis. Anti-tubercular drugs were not applied, and surgical excision was reserved for nonhealing lesions.
Statistical Analysis Used:
Data tables.
Results:
Ipsilateral axillary lymph nodes were commonest site and none had constitutional symptoms. Acid-fast bacilli were detected in 11 (91.67%) cases of suppurative BCG lymphadenitis. On follow-up all nonsuppurative adenitis were resolved spontaneously, and 8 suppurative lymphadenitis cases were resolved after 4 weeks of needle aspiration. Four cases needed repeat aspiration among which 3 resolved in 8 weeks, and one needed surgical excision.
Conclusions:
We recommend needle aspiration as a simple, safe, chief and effective modality, which helps in diagnosis as well as in management of suppurative BCG lymphadenitis.
Keywords: Bacille Calmmette–Guerin, lymphadenitis, needle aspiration
INTRODUCTION
The Bacille Calmette–Guerin (BCG) vaccine is a live attenuated vaccine with residual virulence.[1] After the first introduction of BCG vaccine in 1921, it was incorporated in expanded program of immunization in 1974 World Health Organization to decrease the serious complications and mortality of tubercular infection.[2,3] In India, BCG is routinely used to newborns under National Immunization Program and administered intradermal at left deltoid region. Though BCG is widely accepted and safe vaccine, it sometimes causes local adverse reaction such as regional lymphadenitis, local abscess formation, osteomyelitis and rarely disseminated mycobacterial infection.[4,5] BCG induced lymphadenitis is the most common complication of BCG vaccination.[1,2,5] Two forms of BCG lymphadenitis have been noted, and these are (1) Simple, nonsuppurative lymphadenitis and (2) suppurative lymphadenitis.[1,2] Nonsuppurative lymphadenitis is self-resolving and needs only careful follow-up. However, suppurative lymphadenitis often leads to abscess formation, rupture, ulceration followed by cicatrization and sinus formation, if not treated. Few diagnostic and therapeutic modalities like anti-tubercular drugs (ATDs), needle aspiration and surgical intervention have been used as management modalities.[5,6] Very few previous studies have evaluated the efficacy of needle aspiration in diagnosis and management of BCG lymphadenitis. In the present study, we have an aim to evaluate the clinical and cytological features of BCG lymphadenitis and to evaluate the role of needle aspiration in the management of suppurative BCG lymphadenitis.
SUBJECTS AND METHODS
The present study was conducted at the Department of Pathology, in our hospital, from April 2011 to March 2013. A total of 30 case of BCG induced lymphadenitis were evaluated during our study period. Diagnosis of BCG lymphadenitis was done clinically in the pediatrics clinic. The study included the cases of left axillary lymphadenitis (some cases were associated with cervical and supraclavicular lymphadenopathy) with a history of BCG vaccination and without any other identifiable cause of lymphadenitis. Patients were referred to Pathology department for needle aspiration and cytological evaluation. We collected detailed history and documented age of presentation, age of immunization, other signs and symptoms related to immunodeficiency, size and distribution of enlarged lymph nodes and constitutional symptoms. Patients with nonsuppurative lymphadenitis were followed-up without needle aspiration. Cases of generalized lymphadenopathy were not included in our study. Only in the cases of suppurative lymphadenitis, needle aspirations were done with 22–23 G needle attached with 20 cc disposable syringe. Smears were made from aspirated material and stained with Leishman-Giemsa stain and modified Ziehl–Neelsen (ZN) stain. Microscopic evaluation was done for cytomorphology and detection of acid-fast bacilli. Patients were followed-up at 4 weeks, 8 weeks, 12 weeks and 16 weeks after needle aspiration. Re-aspiration was done in the nonresolving cases during follow-up examination. We encountered two cases of ruptured suppurative lymphadenitis at the axillary area, but they were not included in the study group as ulceration and cicatrization had already been developed in those cases.
RESULTS
Total 30 cases of BCG lymphadenitis were evaluated during 2-year study period. All were vaccinated during 1st week of neonatal period. Only 12 cases (40%) out of 30 were suppurative BCG lymphadenitis, which were aspirated for diagnostic as well as therapeutic purpose. Among the 30 cases 17 cases (56.6%) were male and 13 cases (43.3%) were female [Table 1]. Out of 12 suppurative lymphadenitis cases, 7 cases (58.33%) were male and 5 cases (41.67%) were female. Most of the cases were presented at 2–6 months after vaccination (25 cases; 83.33%) [Table 1]. The most common site of involvement in our series was ipsilateral (left) axillary lymph node (all the 30 cases) [Table 2 and Figure 1]. One of nonsuppurative lymphadenitis cases and two of suppurative BCG lymphadenitis cases had ipsilateral cervical lymphadenitis along with axillary lymphadenitis. However on aspiration from cervical lymph nodes, cytology showed reactive hyperplasia only. Two cases of nonsuppurative BCG lymphadenitis and one cases of suppurative lymphadenitis had ipsilateral supraclavicular lymphadenitis also. On aspiration from supraclavicular lymph nodes, cytology showed features of reactive hyperplasia. Only one case of contra lateral cervical lymph node were seen, which were very small (0.5 cm) and cytology showed features of reactive hyperplasia. Largest diameters of all the enlarged lymph nodes were measured. Among 18 ipsilateral axillary nonsuppurative cases, 13 cases (72.22%) were ≤2 cm in largest dimension and 5 cases (27.78%) were >2 cm to ≤4 cm. 8 cases (66.67%) of suppurative lymph nodes were ≤2 cm and 3 cases (25%) were >2 cm to ≤4 cm and only one case (8.33%) was >4 cm in largest dimension [Table 2]. None of the 30 cases had constitutional symptoms like fever, weight loss/malnutrition, poor feeding or any respiratory discomfort. Ulceration at the site of BCG vaccination was noted in 7 cases, 23.33% (3 of nonsupppurative and 4 of suppurative lymphadenitis cases). Needle aspiration was used in 12 suppurative cases. The smears from suppurative lymph nodes aspirate showed predominantly necrosis admixed with mixed inflammatory cells and scattered epithelioid cells. In two cases, ill formed or degenerated granulomas were seen. ZN stained smears showed presence of acid-fast bacilli in 11 cases (91.67% of suppurative and 36.67% of all BCG lymphadenitis) [Figure 2]. On follow-up 15 out of 18 cases nonsuppurative cases (83.33%) and 8 cases out of 12 cases of suppurative lymphadenitis (66.67%) were resolved after 6 weeks. Three (16.67%) out of 18 nonsuppurative lymphadenitis cases undergone suppurative inflammatory changes on follow-up, and they needed needle aspiration. Four of nonresolving suppurative BCG lymphadenitis needed repeat aspiration at 6 weeks. Among these four cases, three cases were resolved after repeat aspiration and in one case sinus formation, cicatrization was developed even after repeat needle aspiration.
Table 1.
Age and sex distribution of BCG lymphadenitis cases
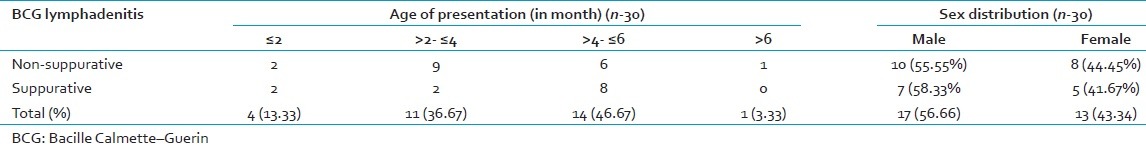
Table 2.
Size and site distribution of BCG lymphadenitis cases
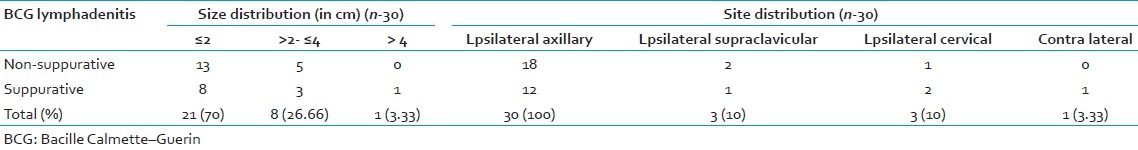
Figure 1.
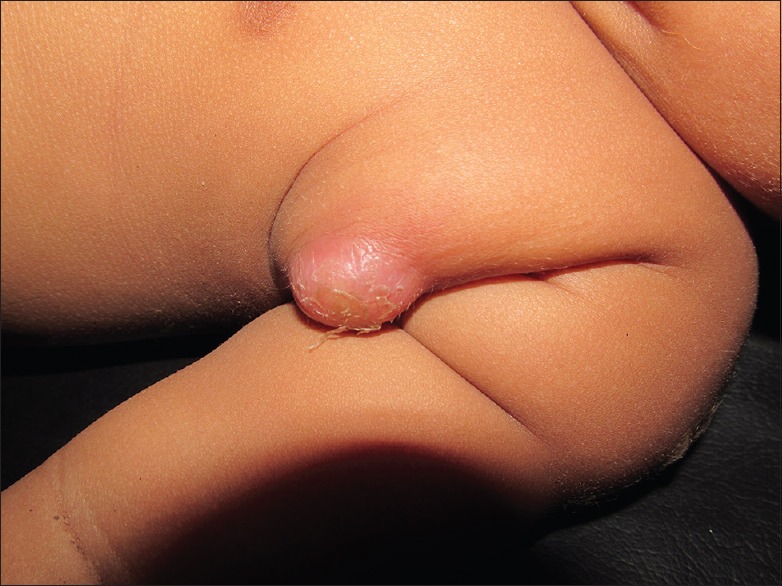
Photograph of suppurative left axillary lympgadenitis in a 7-month-old baby
Figure 2.
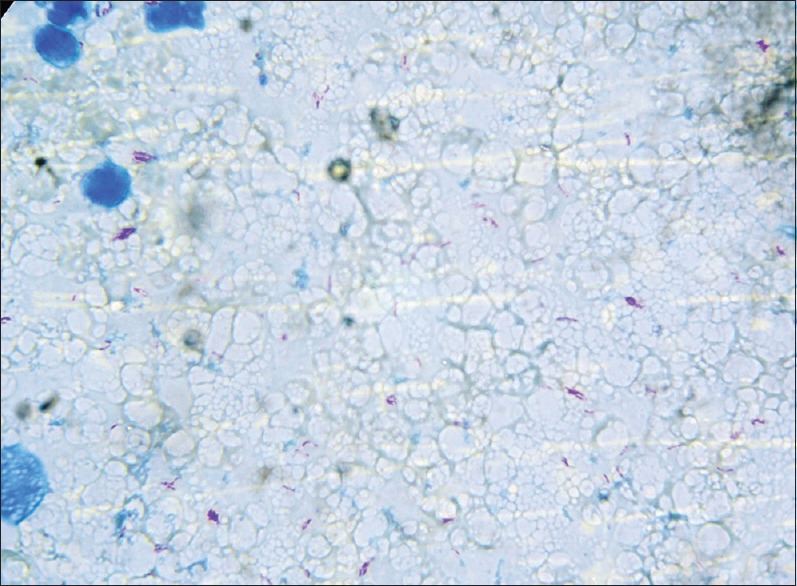
Acid-fast bacilli in aspirates of bacille Calmette–Guerin adenitis (Ziehl–Neelsen, ×400)
DISCUSSION
Bacille Calmette–Guerin vaccination is recommended in expanded program of immunization in India and also in other countries where incidence of tuberculosis is more than 1%. Original BCG vaccine is live attenuated form of Mycobacterium bovis.[1] BCG vaccination is done by intradermal inoculation of 0.05 ml vaccine at left deltoid region (In India). It causes erythematous induration at the site of inoculation, which follows pustule formation after 2–3 weeks; ulceration, drainage and crusting at 4–6 weeks after vaccination. Healing occurs with small residual scar 10–12 weeks after vaccination.[5] BCG vaccination does not prevent primary tuberculous infection or reactivation of latent tuberculosis, but it prevents the complication of tuberculosis (meningitis, disseminated tuberculosis).[6,7] Most of the presently used BCG vaccines are the different strain of M. bovis – Pasteur 1173 P2, Danish 1331, Glaxo 1077 and Tokyo 172 accounting approximately 90% of all used BCG vaccine World Wide.[6,8]
Bacille Calmette–Guerin vaccination has been associated with occasional adverse reactions like prolonged local ulceration, suppuration, lymphadenitis, osteomyelitis and disseminated BCG infection.[7,9]
Reported incidence of BCG vaccine related complications varies from 0.1% to 17% in different studies World Wide.[10] BCG related lymphadenitis is the commonest complication of BCG vaccination.[1,2] It represents as the development of ipsilateral regional lymph node enlargement after BCG vaccination in the absence of any constitutional symptoms, any obvious cause of lymphadenopathy.[2] The factors which affect the frequency and course of BCG lymphadenitis are residual virulence of the vaccine, viability of the product, used dose of the vaccine, age of the newborn and skill of the person who gives vaccination.[2,4] Different previous studies concluded that very early age of the recipient, congenital or acquired immunodeficiency, faulty vaccination technique are the host related factors which predispose to the risk of BCG lymphadenitis.[4,11]
Two forms of BCG lymphadenitis have been noticed (nonsuppurative and suppurative lymphadenitis). Nonsuppurative lymphadenitis regresses spontaneously within few weeks and does not need any therapeutic intervention.[2] Suppurative lymphadenitis is diagnosed with the appearance of fluctuation in the lymph node swelling, erythema and edema of the overlying skin.[12,13] It is seen in (30–80) % of cases of the cases of BCG lymphadenitis.[12] If not treated properly, it is followed by spontaneous rupture, ulceration, sinus formation, cicatrization and healing with scarring; which involve several months.[13]
We approached needle aspiration only in the cases of suppurative BCC lymphadenitis. In our study, we found 56.66% of cases are male child and 43.34% are female child. In other studies by Behjati and Ayatoallahi[1] (53.84% male and 46.16% female) and Abu Zeid and Dahabreh[7] (53% are male and 47% are female) showed similar sex ratio in their study. Bukhari et al. found 69% of cases as male child in their study, quite higher than others.[5] We found that a large percentage of cases (83.33%) presented at 2–6 months of age. Only one case was above 6 months of age. Behjati et al. showed 57.7% cases presented within 4 months and 100% within 6 months of vaccination.[7] In other studies by Bukhari et al. and Nazir and Qazi reported the cases of BCG lymphadenitis occurred mostly within 6 months of vaccination (98% and 92% respectively), which is consistent with our result (96.67% within 6 months of vaccination).[5,14] Ipsilateral axillary lymph node was the commonest site of involvement in our study accounting all the suppurative and nonsuppurative cases (100%). In previous studies, Behjati and Ayatoallahi (92.3%) and Nazir and Qazi (96.61%) showed similar finding.[1,14] In our study, in 70% of cases, size of lymph node were ≤2 cm and another 26.61% were (>2 to ≤4) cm in size. Bukhari et al. found 71.7% cases were within 1–3 cm and 20% cases within 4–6 cm in diameter, consistent with our finding.
Diagnosis of BCG lymphadenitis depends on the history and clinical examination. Cases are diagnosed as ipsilateral enlargement of local lymph node at the site of BCG vaccination in the absence of any constitutional symptoms with other identifiable cause of adenitis.[2,12,15] Size of the lymph node has not been considered in the diagnosis in previous literature. BCG lymphadenitis is often difficult to differentiate from tubercular lymphadenitis. Isolated axillary lymphadenitis of tuberculous etiology is very rare entity.[2] Routine investigations such as blood examination, chest X-ray, Mantoux test have no role in diagnosis.[2,6] Microbiological separation of BCG is confirmatory, but species identification of acid-fast bacilli often need phage typing or genetic analysis which is beyond our scope.[2,6,16]
Few previous studies have proved the efficacy of needle aspiration in the cases of suppurative BCG lymphadenitis, which helps to avoid spontaneous rupture, sinus formation and cicatrization. In our study, 8 cases (66.67%) were resolved after needle aspiration in single sitting and another 3 cases (25%) were resolved after repeat needle aspiration. Hence, efficacy of needle aspiration in our study is 91.67%. Banani and Alborzi showed 95% efficacy of needle aspiration in their study, nearer to our result.[6] But in contrast, in another study in Jordan, Abu Zeid and Dahabreh found that only 50% cases improved with needle aspiration.[7] Banani and Alborzi showed that needle aspiration had significantly higher rate of resolution (95% in cases of needle aspiration vs. 68% in cases of without needle aspiration) with rapidity (average resolution time 6.7 weeks in needle aspiration vs. 11.8 weeks in cases of without needle aspiration).[2,6] They concluded that one aspiration is usually adequate, but repeat aspiration may be necessary for some cases.[2,6] We have experienced similar findings in our study group.
Resolution of suppurative lymph node by simple needle aspiration has been explained as the removal of caseous material containing detrimental substances like large concentration of tuberculin antigens, inflammatory mediators, hydrolytic enzymes and tubercle bacilli. So, the aspiration helps to remove the causes of suppuration in an anoxic and acidic microenvironment.[6]
Poor responses were found in needle aspiration possibly due to inadequate aspiration of suppurative lymph nodes in multiloculated abscess or recollection of necrotic debris due to the severity of delayed hypersensitivity.[6] In cases of recollection, re-aspiration is recommended. Other effective means of treating suppurative BCG lymphadenitis are medical management by ATDs and surgical excision of involved lymph node. Different prior clinical trials showed controversial results in treating with ATDs. Bukhari et al., Abu Zeid and Dahabreh and de Souza et al. reported significant improvement with ATDs.[5,7,17] But in other control trails by Caglayan et al.,[18] Kuyucu et al.,[12] Noah et al.,[19] Close and Nasiiro[20] reported that medical therapy by ATD, have no effect in reducing the risk of suppuration and shortening the duration of healing.[2]
Surgical excision is a curative procedure for suppurative BCG lymphadenitis.[2] But, it should be kept reserved for nonhealing cases or where repeated aspirations fail as in the case of multilocular, matted lymphadenopathy.[2,6] S. Abbas Banani and Alborzi concluded that surgical excision is recommended in nonhealing cases after 3rd attempt of aspiration. Surgical incision and drainage are not recommended in suppurative BCG lymphadenitis because it leads to chronic nonhealing sinus formation, ulceration, and cicatrization.[2,6]
CONCLUSION
Local lymphadenitis is most common complication of BCG vaccination. Nonsuppurative lymphadenitis has simple course and resolves spontaneously where the suppurative adenitis needs treatment. Needle aspiration should be used in all cases of suppurative BCG lymphadenitis as it prevents spontaneous rupture and sinus formation as well as it facilitates rapid regression. Surgical excision is kept reserved for nonhealing cases of multilocular and matted lymph node abscesses which persist even after repeated needle aspiration.
ACKNOWLEDGMENT
Prof. (Dr.) Manaj Kr Chowdhuri.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Behjati B, Ayatoallahi J. Post BCG lymphadenitis in vaccinated infants in Yazd, Iran. Iran J Pediatr. 2008;18:351–6. [Google Scholar]
- 2.Goraya JS, Virdi VS. Bacilli Calmette-Guerin lymphadenitis. Postgrad Med J Lond. 2002;78:327–9. doi: 10.1136/pmj.78.920.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bannon MJ. BCG and tuberculosis. Arch Dis Child. 1999;80:80–3. doi: 10.1136/adc.80.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Milstien JB, Gibson JJ. Quality control of BCG vaccine by WHO: A review of factors that may influence vaccine effectiveness and safety. Bull World Health Organ. 1990;68:93–108. [PMC free article] [PubMed] [Google Scholar]
- 5.Bukhari E, Alzahrani M, Alsubaie S, Alrabiaah A, Alzamil F. Bacillus Calmette-Guerin lymphadenitis: A 6-year experience in two Saudi hospitals. Indian J Pathol Microbiol. 2012;55:202–5. doi: 10.4103/0377-4929.97869. [DOI] [PubMed] [Google Scholar]
- 6.Banani SA, Alborzi A. Needle aspiration for suppurative post-BCG adenitis. Arch Dis Child. 1994;71:446–7. doi: 10.1136/adc.71.5.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abu Zeid AF, Dahabreh MM. Bacille Calmette-Guerin lymphadinitis: A single center experience. J R Med Serv. 2010;17:64–7. [Google Scholar]
- 8.Lussier N, Bourgault AM, Gaudreau C, Turgeon P. A complication of BCG vaccine: A case of localized cutaneous abscess due to Mycobacterium bovis. Can J Infect Dis. 1999;10:257–9. doi: 10.1155/1999/463250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park K. 22nd ed. Jabalpur, India: Banarsidas Bhanot Publishers; 2013. Park's Textbook of Preventive and Social Medicine; pp. 166–84. [Google Scholar]
- 10.Mori T, Yamauchi Y, Shiozawa K. Lymph node swelling due to bacille Calmette-Guérin vaccination with multipuncture method. Tuber Lung Dis. 1996;77:269–73. doi: 10.1016/s0962-8479(96)90012-x. [DOI] [PubMed] [Google Scholar]
- 11.O’Brien KL, Ruff AJ, Louis MA, Desormeaux J, Joseph DJ, McBrien M, et al. Bacillus Calmette-Guérin complications in children born to HIV-1-infected women with a review of the literature. Pediatrics. 1995;95:414–8. [PubMed] [Google Scholar]
- 12.Kuyucu N, Kuyucu S, Ocal B, Teziç T. Comparison of oral erythromycin, local administration of streptomycin and placebo therapy for nonsuppurative Bacillus Calmette-Guérin lymphadenitis. Pediatr Infect Dis J. 1998;17:524–5. doi: 10.1097/00006454-199806000-00020. [DOI] [PubMed] [Google Scholar]
- 13.Caglayan S, Arikan A, Yaprak I, Aksoz K, Kansoy S. Management of suppuration in regional lymph nodes secondary to BCG vaccination. Acta Paediatr Jpn. 1991;33:699–702. doi: 10.1111/j.1442-200x.1991.tb02593.x. [DOI] [PubMed] [Google Scholar]
- 14.Nazir Z, Qazi SH. Bacillus Calmette-Guerin (BCG) lymphadenitis-changing trends and management. J Ayub Med Coll Abbottabad. 2005;17:16–8. [PubMed] [Google Scholar]
- 15.Helmick CG, D’Souza AJ, Goddard N. An outbreak of severe BCG axillary lymphadenitis in Saint Lucia, 1982-83. West Indian Med J. 1986;35:12–7. [PubMed] [Google Scholar]
- 16.Yan JJ, Chen FF, Jin YT, Chang KC, Wu JJ, Wang YW, et al. Differentiation of BCG-induced lymphadenitis from tuberculosis in lymph node biopsy specimens by molecular analyses of pncA and oxyR. J Pathol. 1998;184:96–102. doi: 10.1002/(SICI)1096-9896(199801)184:1<96::AID-PATH989>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 17.de Souza GR, Sant’Anna CC, Lapa e Silva JR, Mano DB, Bethlem NM. Intradermal BCG vaccination complications – Analysis of 51 cases. Tubercle. 1983;64:23–7. doi: 10.1016/0041-3879(83)90046-6. [DOI] [PubMed] [Google Scholar]
- 18.Caglayan S, Yegin O, Kayran K, Timocin N, Kasirga E, Gun M. Is medical therapy effective for regional lymphadenitis following BCG vaccination? Am J Dis Child. 1987;141:1213–4. [PubMed] [Google Scholar]
- 19.Noah PK, Pande D, Johnson B, Ashley D. Evaluation of oral erythromycin and local isoniazid instillation therapy in infants with Bacillus Calmette-Guérin lymphadenitis and abscesses. Pediatr Infect Dis J. 1993;12:136–9. doi: 10.1097/00006454-199302000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Close GC, Nasiiro R. Management of BCG adenitis in infancy. J Trop Pediatr. 1985;31:286. doi: 10.1093/tropej/31.5.286. [DOI] [PubMed] [Google Scholar]


