Abstract
Background:
Bloodstream infection (BSI) and bacteremias due to Enterococcus spp. are increasing worldwide with the current need to understand its causes among hospitalized trauma patients. Hence, the study was conducted.
Methodology:
A 3-year retrospective laboratory cum clinical based study was performed at a level I trauma center in India. Patients with health care associated enterococcal bacteremia were identified using the hospital database, their episodes of BSI/bacteremia calculated and their clinical records and treatment were noted.
Results:
A total of 104 nonrepetitive Enterococcus spp. was isolated of which Enterococcus faecium was the most common (52%). High-level resistance to gentamicin high-level aminoglycoside resistance was seen in all the Enterococcus spp. causing bacteremia, whereas a low resistance to vancomycin and teichoplanin was observed. Overall mortality was more in patients infected with vancomycin-resistant Enterococcus (5/11, 46%) compared to those with vancomycin sensitive Enterococcus (9/93, 10%); though no significant association of mortality with Enterococcus spp. bacteremia (P > 0.05) was seen. The rate of bacteremia due to Enterococcus spp. was 25.4 episodes/1,000 admissions (104/4,094) during the study period.
Conclusion:
Enterococcal bacteremia is much prevalent in trauma care facilities. Here, a microbiologist can act as a sentinel and help in preventing such infections.
Keywords: Bacteremia, drug resistance, Enterococcus sp., trauma, vancomycin-resistant Enterococcus
INTRODUCTION
Infections due to Gram positive bacteria are increasing worldwide. Of these, enterococci have become one of the most common causes of nosocomial infections[1,2] including bacteremia and blood stream infections (BSIs).[3] Enterococcus species are the fourth leading cause of bacteremia[4] and globally account for approximately 10% of all bacteremias.[5,6] Vancomycin resistance is also a big problem in Enterococcus sp. more so in developed countries like USA[7] and European[8,9] countries, though not a matter of much concern in most Asian countries due to low prevalence.[10] In countries like India, the prevalence of Gram-positive bacterial infection is much lower in hospitalized patients than Gram-negative bacteria.[11] Moreover, data regarding bacteremia due to Enterococcus spp. and also its resistance pattern in developing countries, especially in trauma patients are scarce.
This study was, therefore conducted retrospectively with the following aims: (i) To determine the prevalence of enterococcal bacteremia, (ii) to look into the factors associated with mortality and length of hospital stay (LOS) with enterococcal (vancomycin-resistant Enterococcus [VRE] and vancomycin sensitive Enterococcu [VSE]) bacteremia, (iii) to investigate the impact of vancomycin resistance and antibiotic therapy in enterococcal bacteremia among trauma patients, and (iv) to calculate the rate of enterococcal bacteremia per 1000 admissions.
METHODOLOGY
Setting and study population
We performed a retrospective cohort study from January 2011 to December 2013 at a level I trauma care center serving a reference population of 16.3–17.8 million inhabitants during the study period. It has 176 functional beds out of a total of 186 beds with average total admissions per year of 4,094 during the study period. The hospital bed occupancy rate was 83% with an average bed turnover rate of 24/day. A total of 50,137 emergency visits and 4,384 major surgical procedures was performed out of a total of 4,850 surgical procedures during the study period per year. Patients with enterococcal bacteremia were identified using the microbiology laboratory database. We have included all the blood culture samples sent for bacterial culture during the study period.
Related information on patient demographics, antimicrobial use, medical procedures, treatment of enterococcal bacteremia, and outcomes of hospitalization was collected through a retrospective review of patient medical records from the hospital database.
Blood culture protocol
Our institution recommends collection of at least two blood samples for evaluation of all episodes of suspected bacteremia. Blood cultures were processed using BacT/ALERT system (bioMieréux, Durham, USA) as per the manufacturer's instructions. Methods for processing positive blood cultures were standard.[12,13]
Definitions
Definitions of enterococcal bacteremia were based upon the definitions for nosocomial infections of the Centers for Disease Control and Prevention.[14] Patients with enterococcal bacteremia included patients classified as having healthcare-associated bacteremia. For our study, enterococcal bacteremia was considered to be healthcare-associated if any one of the following criteria applied:[15,16] (i) One positive blood culture(s) taken more than 48 h after hospital admission; (ii) patient had previous hospital admission for 2 days; (iii) hospitalized patients who developed clinical signs of BSIs along with positive blood culture.
An episode of “significant” bloodstream infection was defined as an episode of bacteremia, in which those pathogens were present in ≥1 blood cultures.[17] In this study, only the number of patients – not the number of blood cultures – was taken into consideration. All microorganisms isolated from blood of the same patient after 72 h of admission but within 1-week were considered a single episode.
Microorganisms recovered from blood cultures were identified using the automated VITEK 2 compact systems (bioMérieux, Durham, US) according to the manufacturer's protocol. Antibiotic sensitivity was done both by disc diffusion according to the Clinical and Laboratory Standards Institute guidelines,[18,19] EUCAST[20] and VITEK 2 in parallel. Throughout the study, Enterococcus faecalis ATCC 29212 (vancomycin sensitive), E. faecalis ATCC 51299 (vancomycin-resistant) and Enterococcus faecium ATCC 700221 (vancomycin-resistant) were used as controls.
Statistical analysis
Descriptive statistics was used in most cases, but test for significance was done wherever feasible. A value of P < 0.05 was considered significant.
RESULTS
Demographic findings
Details regarding demographics, clinical characteristics, and outcomes of patients with enterococcal bacteremia are shown in Table 1. All the possible risk factors and the difference between VRE and VSE have been discussed in the table. It was seen that isolation was more in male (92, 88.4%) compared to the female patients.
Table 1.
Distribution of various demographic and clinical findings in the trauma patients with enterococcal bacteremia
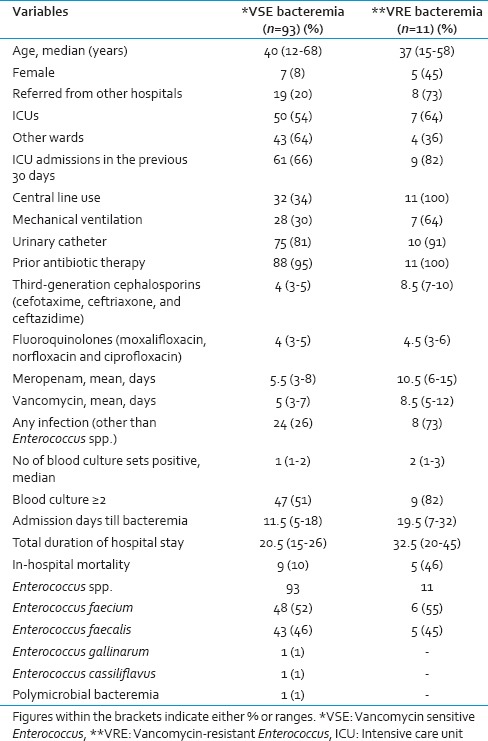
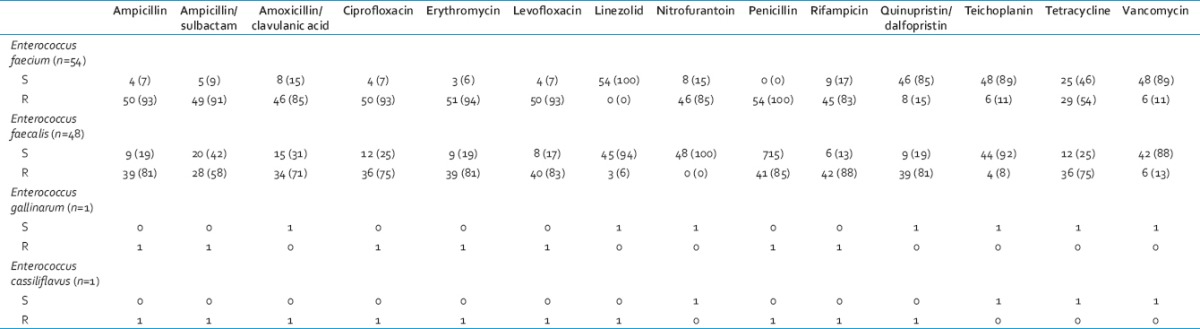
During the study period, a total of 28,237 samples was received, of which 9,636 consists of blood samples for culture. From these, 104 nonrepetitive Enterococcus spp. were isolated from different trauma patients. By nonrepetitive bacterial isolate, we mean in this study inclusion of only one isolate of Enterococcus sp. from one type of sample obtained from a patient from subsequent positive samples for a week. We have used this working definition for this study as typing of the bacterial isolates was not done in this study. Of these, E. faecium (54/104, 52%) was the most common, followed by E. faecalis (48/104, 46%) and 1 (1%) each of Enterococcus gallinarum and Enterococcus cassiliflavus. This shows that Enterococcus sp. bacteremia is very much prevalent among the trauma patients admitted for care. The antimicrobial sensitivity pattern of different Enterococcus spp. is shown in Table 2. It was seen that a low level of vancomycin resistance was present in the Enterococcus spp. at our center. Our study also observed that the number of bacteremia due to E. faecalis was high in 2011, but was gradually overtaken by E. faecium during 2012 and 2013 subsequently.
Table 2.
Distribution of various Enterococcus spp. and its antimicrobial sensitivity pattern
In the patients having bacteremia due to VRE, 7 (55%) were treated with teicoplanin monotherapy, whereas 2 (18%) each with linezolid monotherapy or no antibiotics, respectively, in the beginning. However, a combination (concurrent or in sequence) of teicoplanin, linezolid, or benzylpenicillin as definitive therapy had to be administered to 8 (22%) patients with VRE. In the patients with VSE bacteremia, definitive therapy with IV glycopeptides (vancomycin or teicoplanin) and penicillins (ampicillin/benzylpenicillin/ticarcillin-clavulanic acid/piperacillin-tazobactam) were given to 56 (40%) and 37 (18%) patients, respectively. A combination of vancomycin, teicoplanin, linezolid, ampicillin, or benzylpenicillin was administered to 30 (26%) of the VSE patients either concurrently or in sequence later. Intravenous gentamicin daily was administered in combination with ampicillin in 15 (16%) patients with VSE whereas oral linezolid was administered in 7 (8%) patients.
It was seen that a very high-level of resistance was seen to gentamicin high-level in all the species of Enterococcus causing bacteremia whereas a low level resistance to vancomycin and teichoplanin was observed among the isolates [Table 2].
Overall mortality was more among those patients infected with VRE (5/11, 46%) compared to those with VSE (9/93, 10%); though there was no significant association of mortality with Enterococcus spp. bacteremia (P > 0.05) in our study. Similarly, LOS was more in those patients with VRE infection compared to those with VSE infection but this did not influence the mortality of the patients [Table 1].
Enterococcus faecium bacteremia amounted to rate of 13/1,000 admissions (54/4,094) whereas E. faecalis bacteremia amounted to 12/1,000 admissions (48/4,094) in our center. However, bacteremia due to E. gallinarum and E. cassiliflavus amounted to a rate of 0.2 episodes/1000 admissions (1/4,094) each. Overall, the rate of bacteremia due to Enterococcus spp. was 25.4 episodes/1,000 admissions (104/4,094) during the study period.
DISCUSSION
Our study found that bacteremia due to Enterococcus spp. irrespective of VSE or VRE was more in males compared to females. Prior Intensive Care Unit (ICU) stay was associated with increased bacteremia with VRE in our study. Similar findings have been seen in other studies.[3,4,5,6,7,8,9]
Our study has seen a shift from E. faecalis to that of E. faecium in the later part of the study, which was also seen in another study.[21] This may be explained by the stringent antibiotic policy introduced after an increase in E. faecalis or selecting up of E. faecium after eliminating E. faecalis. Our study found a low level of vancomycin resistance among the Enterococcus isolates which is an encouraging finding. Furthermore, enterococcal bacteremia was not a significant risk factor of mortality in our study though it influenced the morbidity and increased the LOS. This might be explained by the prevalence of low level of VRE in our center. Another study in neutropenic patients also showed that vancomycin resistance does not affect the 7-day and 30-day mortalities in a relatively homogeneous group of patients.[22]
Our study found only one case of polymicrobial bacteremia with E. faecium bacteremia though the frequency is high in other studies.[23] Furthermore, longer duration of hospital stay was seen in VRE patients compared to those of VSE, which might support the fact that longer LOS is an independent risk factor for VRE bacteremia/BSI.[3] Though prior ICU stay was more among VRE bacteremia patients, our study did not observe a significant association of mortality with either VRE or VSE bacteremia. The reverse situation was seen in another study.[3] However, other risk factors like co-infections or co-morbidities, cost of treatment/hospitalization could not be looked into detail due to the retrospective nature of the study.
In this study, a high-level resistance to ampicillin was seen in both E. faecium (50, 93%) and E. faecalis (39, 81%), respectively. Similar picture was also seen in E. faecium (50, 93%) and E. faecalis (36, 75%) to ciprofloxacin as was seen in another study.[24] A high-level of resistance to gentamicin high-level was seen in the Enterococcus spp. responsible for bacteremia; though the reverse was seen with vancomycin and teichoplanin our study. Though the number of bacteremia due to VSE among trauma patients has increased over the years in the study, there was no significant risk of mortality associated with it. However, it might precede the emergence of more and high-level of VRE in future. Such an observation was also noted in another study.[24]
Our study has many strengths. It is a study of enterococci bacteremia/BSI in trauma patients and such studies are rare. Trauma patients generally lack underlying illness/co-morbidities and are usually middle-age. Thus, these infections are undoubtedly nosocomial. The BSI/bacteremia rate was expressed per 1000 patient admissions instead of catheter-days. The changing pattern of Enterococcus sp. was clearly shown in the study and may act as a baseline data for comparison in future. Furthermore, the study tried to look into possible risk factors.
This study is not without limitations. First, it is a single-center study design, which makes extrapolation to other institutions difficult. Second, it is not a randomized study, with the resulting risk of bias due to confounding factors. Furthermore, continuous monitoring of all patients outside the ICUs was not possible due to the retrospective nature of the study. Retrospective data collection and pooling of data may be subject to variability. To ensure precision in data interpretation, criteria for the exposure and outcome were interpreted cautiously. As the majority of the patients had VSE bacteremia during the study period (2011–2013), matching VRE and VSE patients for date of admission was not possible in the study. Furthermore, we were unable to obtain the standardized data on costs of hospitalization due to enterococcal BSI.
CONCLUSION
Enterococcal bacteremia is much prevalent in trauma care facilities especially in the ICUs. Enterococci persist in patient environments for long time periods and are notoriously difficult to eradicate. Here, a microbiologist can act as a sentinel, help in empirical therapeutic decisions and also in preventing such infections. Intensified infection control and proper antibiotic stewardship may help in curtailing this infection and preventing it in the long run.
ACKNOWLEDGMENTS
The authors would like to thank all the Hospital Infection Control staff of JPNATC, AIIMS, New Delhi for their contribution to healthcare and their earnest efforts toward this study without which this study would not have been possible.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Bonten MJ, Willems R, Weinstein RA. Vancomycin-resistant enterococci: Why are they here, and where do they come from? Lancet Infect Dis. 2001;1:314–25. doi: 10.1016/S1473-3099(01)00145-1. [DOI] [PubMed] [Google Scholar]
- 2.Tripathi A, Shukla SK, Singh A, Prasad KN. A new approach of real time polymerase chain reaction in detection of vancomycin-resistant enterococci and its comparison with other methods. Indian J Med Microbiol. 2013;31:47–52. doi: 10.4103/0255-0857.108721. [DOI] [PubMed] [Google Scholar]
- 3.Cheah AL, Spelman T, Liew D, Peel T, Howden BP, Spelman D, et al. Enterococcal bacteraemia: Factors influencing mortality, length of stay and costs of hospitalization. Clin Microbiol Infect. 2013;19:E181–9. doi: 10.1111/1469-0691.12132. [DOI] [PubMed] [Google Scholar]
- 4.McComb MN, Collins CD. Comparative cost-effectiveness of alternative empiric antimicrobial treatment options for suspected enterococcal bacteremia. Pharmacotherapy. 2014;34:537–44. doi: 10.1002/phar.1393. [DOI] [PubMed] [Google Scholar]
- 5.Coombs GW, Pearson JC, Daley DA, Le T, Robinson OJ, Gottlieb T, et al. Molecular epidemiology of enterococcal bacteremia in Australia. J Clin Microbiol. 2014;52:897–905. doi: 10.1128/JCM.03286-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pinholt M, Ostergaard C, Arpi M, Bruun NE, Schønheyder HC, Gradel KO, et al. Incidence, clinical characteristics and 30-day mortality of enterococcal bacteraemia in Denmark 2006-2009: A population-based cohort study. Clin Microbiol Infect. 2014;20:145–51. doi: 10.1111/1469-0691.12236. [DOI] [PubMed] [Google Scholar]
- 7.Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: Analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39:309–17. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 8.Fluit AC, Schmitz FJ, Verhoef J European SENTRY Participant Group. Frequency of isolation of pathogens from bloodstream, nosocomial pneumonia, skin and soft tissue, and urinary tract infections occurring in European patients. Eur J Clin Microbiol Infect Dis. 2001;20:188–91. doi: 10.1007/s100960100455. [DOI] [PubMed] [Google Scholar]
- 9.Barnaud G, Bingen E. Genotypic characterisation of endemic VanA Enterococcus faecium strains isolated in a paediatric hospital. J Med Microbiol. 2000;49:793–9. doi: 10.1099/0022-1317-49-9-793. [DOI] [PubMed] [Google Scholar]
- 10.Mathur P, Kapil A, Chandra R, Sharma P, Das B. Antimicrobial resistance in Enterococcus faecalis at a tertiary care centre of northern India. Indian J Med Res. 2003;118:25–8. [PubMed] [Google Scholar]
- 11.Rajkumari N, Mathur P, Sharma S, Gupta B, Bhoi S, Misra MC. Procalcitonin as a predictor of sepsis and outcome in severe trauma patients: A prospective study. J Lab Physicians. 2013;5:100–8. doi: 10.4103/0974-2727.119852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baron EJ, Weinstein MP, Dunne WM, Yagupsky P, Welch DF, Wilson DM. In: Blood Cultures IV. Yagupsky P, Welch DF, Wilson DM, editors. Washington DC: American Society for Microbiology; 2005. [Google Scholar]
- 13.Collee JG, Miles RS, Watt B. Tests for the identification of bacteria. In: Collee JG, Fraser AG, Marmion BP, Simmons A, editors. Mackie and McCartney Practical Medical Microbiology. 14th ed. New York: Churchill Livingstone; 1996. pp. 131–45. [Google Scholar]
- 14.Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections, 1988. Am J Infect Control. 1988;16:128–40. doi: 10.1016/0196-6553(88)90053-3. [DOI] [PubMed] [Google Scholar]
- 15.Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298:1763–71. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 16.Klevens RM, Morrison MA, Fridkin SK, Reingold A, Petit S, Gershman K, et al. Community-associated methicillin-resistant Staphylococcus aureus and healthcare risk factors. Emerg Infect Dis. 2006;12:1991–3. doi: 10.3201/eid1212.060505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodriguez-Creixems M, Munoz P, Martin-Rabadan P, Cercenado M, Guembe M, Bouza E. Evolution and aetiological shift of catheter-related bloodstream infection in a whole institution: The microbiology department may act as a watchdog. Clin Microbiol Infect. 2013;19:845–51. doi: 10.1111/1469-0691.12050. [DOI] [PubMed] [Google Scholar]
- 18.Wayne, PA: Approved Guideline-Second Edition; 2010. CLSI. Methods for Antimicrobial Dilution and Disk Susceptibility Testing of Infrequently Isolated or Fastidious Bacteria; pp. M45–A2. [Google Scholar]
- 19.Wayne, PA, USA: Clinical and Laboratory Standards Institute; 2012. CLSI. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Second Informational Supplement. Clinical and Laboratory Standard Institute (CLSI) document; pp. M100–S22. [Google Scholar]
- 20.Matuschek E, Brown DF, Kahlmeter G. Development of the EUCAST disk diffusion antimicrobial susceptibility testing method and its implementation in routine microbiology laboratories. Clin Microbiol Infect. 2014;20:O255–66. doi: 10.1111/1469-0691.12373. [DOI] [PubMed] [Google Scholar]
- 21.Weisser M, Capaul S, Dangel M, Elzi L, Kuenzli E, Frei R, et al. Additive effect of Enterococcus faecium on enterococcal bloodstream infections: A 14-year study in a Swiss tertiary hospital. Infect Control Hosp Epidemiol. 2013;34:1109–12. doi: 10.1086/673145. [DOI] [PubMed] [Google Scholar]
- 22.Cho SY, Lee DG, Choi SM, Kwon JC, Kim SH, Choi JK, et al. Impact of vancomycin resistance on mortality in neutropenic patients with enterococcal bloodstream infection: A retrospective study. BMC Infect Dis. 2013;13:504. doi: 10.1186/1471-2334-13-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reigadas E, Rodríguez-Créixems M, Guembe M, Sánchez-Carrillo C, Martín-Rabadán P, Bouza E. Catheter-related bloodstream infection caused by Enterococcus spp. Clin Microbiol Infect. 2013;19:457–61. doi: 10.1111/j.1469-0691.2012.03897.x. [DOI] [PubMed] [Google Scholar]
- 24.Gudiol C, Ayats J, Camoez M, Domínguez MÁ, García-Vidal C, Bodro M, et al. Increase in bloodstream infection due to vancomycin-susceptible Enterococcus faecium in cancer patients: Risk factors, molecular epidemiology and outcomes. PLoS One. 2013;8:e74734. doi: 10.1371/journal.pone.0074734. [DOI] [PMC free article] [PubMed] [Google Scholar]


