Abstract
Hospitalized patients with diabetes pose numerous clinical challenges, including hyperglycemia, which may often be difficult to control. The therapeutic challenges are further accentuated by the difficulty in practical application of existing guidelines among Indian and South Asian patients. The present review highlights the various clinical challenges encountered during management of different diabetic hospitalized populations, and attempts to collate a set of practical, patient and physician friendly recommendations to manage hyperglycemia in such patients.
Keywords: Diabetes mellitus, hyperglycemia, inpatients, insulin analogues, insulin therapy
Introduction and Epidemiology
Diabetes mellitus (DM) has acquired epidemic proportions with increasing likelihood of people with diabetes being subjected to prolonged hospitalization.[1,2] It has been established that diabetic patients usually have a poorer outcome when compared to general population.[3,4] Various landmark studies and clinical trials in this field are at variance, and have shown inconsistent results with regards to intensive control of hyperglycemia.[5,6] Globally, these results have created a lot of variations over the adoption of precise therapeutic interventions for the control of hyperglycemia.
A commonly observed feature in our hospitals is that a large number of DM cases are diagnosed for the first time during routine check-up in the outpatient department or when they are admitted for some other co-morbidity, which may or may not be related to DM. Though various surveys reveal that every sixth patient getting admitted to hospital is diabetic, in reality the number may be much larger as diabetic patients are more frequently hospitalized.[7]
Evidence Based Need for Formulation of Recommendations
Available literary evidence with regards to control of hyperglycemia pertains mostly to developed nations, as the data from developing nations is scarce and sporadic. It has been established that there do exist numerous racial and ethnic differences in insulin resistance, dietary pattern, glucose metabolism and genetic makeup, which makes it extremely difficult to uniformly apply and extrapolate the guidelines of the western nations on Indian and South Asian population.[8] This field of medicine, known as ethno pharmacy, has important ramifications for diabetes management. Most guidelines assume availability of adequate staff and ancillary resources for glycemic management, which may not always be the case in resource limited hospitals. The scarcity of medical staff, resources, economic considerations and lack of awareness among the medical fraternity are important factors that pose difficulty in implementation of these guidelines in totality. A continuous need is felt for formulation and adoption of user friendly guidelines, relevant not only for India, but also for other developing nations in order to promote rational control of hyperglycemia amongst in patients.
These recommendations are based upon evidence derived from available published literature pertaining to the domain of inpatient hyperglycemia and its management. Though based upon predominantly western literature, they include suggestions based upon experience in resource challenged settings. Evidence and experience based knowledge has been adapted for use after consensus between multiple specialties, in indoor medical set-ups, including wards, operation theatres, and Intensive Care Units (ICUs). The core writing group consisting of the authors of this article prepared a draft article which was subjected to scrutiny by a panel of experts consisting of four endocrinologists having at least a decade of experience in this field. The grading of recommendations were done by expert panel consisting of five endocrinologists, one internist and two intensivists based on the a method suggested by Frid et al., which includes an ABC scale of recommendation and 123 scale for supporting scientific evidence [Table 1].[9] For each recommendation, the postfixed capital letter in bold indicates how much weight a recommendation would carry in daily practice, and the number defines its degree of support in the medical literature. A total of 42 important recommendations are put forward, and explained, in these guidelines.
Table 1.
Criteria for grading and rating recommendations
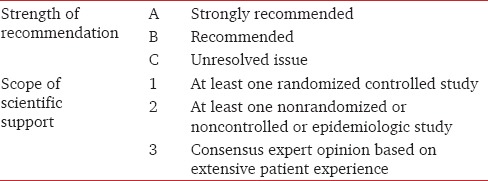
Table 1 shows evidence based grading and rating of proposed recommendations for control of inpatient hyperglycemia.
There will definitely be scope for further improvement in these recommendations, based upon results of future multicenter, randomized studies as well as upon practical suggestions received after use of these recommendations.
Definitions
Hyperglycemia
Hyperglycemia can be defined as blood glucose (BG) values >140 mg/dL (>7.8 mmol/L) in hospitalized patients. However, these elevated readings cannot be taken as evidence of DM as numerous clinical conditions can cause stress hyperglycemia. The value of HbA1c >6.5% is a substantial evidence to label the patient as diabetic.[10]
Hypoglycemia
It can be defined as BG values below 70 mg/dL (<3.9 mmol/L).[11] Though clinicians are at variance, BG reading <40-50 mg/dL (2.2-2.8 mmol/L) is labeled as severe hypoglycemia as it is invariably accompanied with clinical evidence of cognitive impairment.[11]
Current Recommendations for Glycemic Control
Various recommendations have been formulated and modified from time to time on the basis of available literary evidence. Management of hospitalized diabetic patients can be considered into three broad categories:
Patients with diabetes in noncritical areas including preoperative patients
ICU patients with diabetes
Treatment on discharge.
Patients hospitalized in noncritical locations
Hyperglycemia can have detrimental effects in hospitalized patients who had been admitted for various medical and surgical indications.[12] However, it is mandatory to distinguish whether hyperglycemia has occurred as a marker of illness, or is a potential mediator of enhanced morbidity. In less critical patients, hyperglycemia is invariably overlooked as the physician focus upon management of the primary pathology which prompted the hospitalization. Poorly controlled hyperglycemia in these settings may lead to prolonged hospitalization, delayed/impaired wound healing, potential risk of polyneuropathy, a greater incidence of systemic infections, urinary tract infections, acute renal failure (ARF) and increased cardiac morbidity.[13,14]
It is mandatory to try and achieve optimal glycemic control in all indoor patients to minimize or avoid these complications. Intravenous (IV) insulin is the best form of therapeutic modality to control hyperglycemia in general wards.[15] However, IV insulin should be used only when adequately trained staff is available to regularly monitor BG especially in postoperative wards, or in patients with persistently uncontrolled hyperglycemia (BG >300 mg/dL).[16] Subcutaneous (SC) insulin, especially rapid acting insulin analogues, can be a better option to control meal related hyperglycemia as IV insulin runs the risk of causing hypoglycemia due to transiently increased BG levels.
The predefined target (American Association of Clinical Endocrinologists-American Diabetes Association [AACE-ADA]) of premeal BG <140 and casual (random) BG <180 mg/dL is globally acceptable. Stable patients with the preadmission history of tight glycemic control can be treated to maintain BG lower than these thresholds. The infusion pumps can be self-managed if the patient is psychologically sound, cognition is not impaired, and is willing to learn.
Insulin should always be preferred over oral hypoglycaemic agents (OHAs) as the latter are inflexible and have numerous contraindications such as heart failure for thiazolidinediones, renal insufficiency for metformin, and nil per orally (NPO) status for sulphonylureas in preoperative patients.[2,12]
Randomized Study of Basal-Bolus Insulin Therapy-2 study has established the superiority of insulin analogues over sliding scale insulin (SSI) in maintaining a predefined target of BG (<140 mg/dL). Further, the values of mean fasting glucose and mean random glucose during the hospital stay were significantly lower in the insulin analogue group. Similarly, basal insulin analogue exerts better glycemic control in diabetic patients receiving enteral nutrition as compared to SSI.[17]
Insulin analogues are more effective in controlling peri-operative hyperglycemia when compared with regular SSI.[18] Not only BG control but also the incidence of wound infections, respiratory tract infections, respiratory failure, bacteremia and ARF were much lower in patients treated with insulin analogues. However, incidence of hypoglycemia was higher in patients treated with basal-bolus regimen as compared to SSI. Ultra long acting basal insulin analogue (degludec) is now available which allows for greater flexibility of use, while causing less hypoglycemia.
Recommendations
Premeal target should be 110-130 mg/dL, and postmeal target should be 140-180 mg/dL. Less stringent target should be set for patients with significant advanced chronic macrovascular or microvascular medical co-morbidity, and elderly patients, who are at higher risk of hypoglycemia. Tighter targets should be kept for patients with acute medical co-morbidity such as foot infection or tuberculosis.
Critically ill in patients with diabetes, on enteral nutrition, should be preferably managed with insulin [A1]. Basal insulin to cover the basal and correctional need and prandial rapid acting insulin to cover the nutritional need are preferred the choice. SSI is not recommended [A1].
Insulin analogues should be preferred in indoor patients as they are associated with less hypoglycemia, better therapeutic outcomes, and are more flexible to use [A1].
Hospitalized intensive care unit patients
Critically ill patients pose unique challenge for the intensivist and the endocrinologist with regards to glycemic control as these patients invariably have multi-organ dysfunction. One needs to maintain a balance between correction of hyperglycemia and avoidance of hypoglycemia. Recent research strongly recommends IV intensive insulin therapy (IIT) to achieve tight glycemic control (blood sugar [BS] <110 mg/dL) so as to decrease the mortality and morbidity due to hyperglycemia.[3] Although many recent studies are at variance with the recommendations of Leuven study, the data furnished is not conclusive enough to support or refute a choice of conventional versus IIT.[12,19] The most important conclusion drawn from such studies is the higher incidence of hypoglycemia in patients treated with IIT, which may be responsible for a higher relative risk of mortality.[12,19]
The AACE-ADA updated their recommendations in 2009 as the number of conflicting observations went higher with the newer studies. It is currently recommended that BG in critically ill patients be kept between 110 and 140 mg/dL provided that facilities are available safely to monitor through strict, narrow range. These updated guidelines further recommend the use of IV insulin infusion if the BG level touches 180 mg/dL. Aiming for BG levels below 110 mg/dL carries the risk of iatrogenic hypoglycemia and hence are not recommended. However, the protocols of IV insulin infusion should take into account the preinfusion BG level. The only acceptable modality of treatment is continuous IV insulin infusion, which should be initiated when BG levels >180 mg/dL [Tables 2, 3 and Flow Chart 1]. There are quite a few IV insulin infusion regimens available, but preferred regimens take into account both current BG values and rate of change of BG.[12,20,21,22]
Table 2.
Estimated initial dose of insulin in noncritically ill hospitalized patients
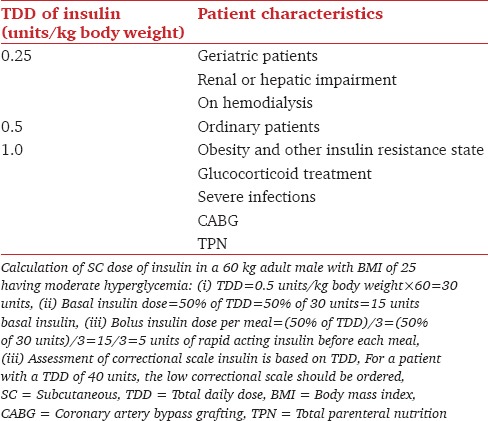
Table 3.
Suggested protocol for insulin infusion in ICU

Flow Chart 1.

A simple algorithm of point of care monitoring╬ of blood glucose in critically ill in patients. ╬Capillary blood sample can be used except in situations like hypotension, hypothermia, shock, use of vasoconstrictors and vasopressors where venous samples are preferred
Recommendations
-
4.
Maintain BG level at a range of 140-180 mg/dL for majority of patients with medical morbidity, and 110 mg/dL-140 mg/dL for those with surgical morbidity [A1].
-
5.
IV insulin is the only recommended therapy as a drug delivery. SC regimens with premixed insulin, intermediate acting or long acting insulins and SSI are not recommended [A1].
-
6.
Regular insulin or rapid acting insulin analogs (aspart, lispro, glulisine) can be used as IV infusion. Glulisine should be used only with normal saline [Table 4][A1].
-
7.
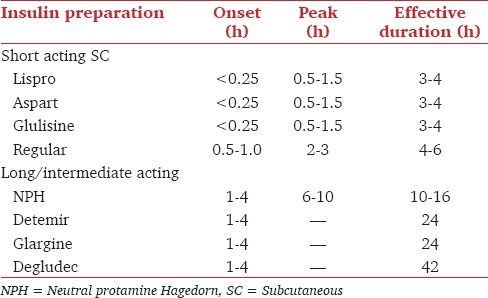
Transition to SC insulin from IV insulin should have an overlapping period of 1-2 h. The overlap can be reduced to 15-30 min if rapid analogs are used [Table 5][B2].
Table 4.
Titration of insulin dose according to BG levels
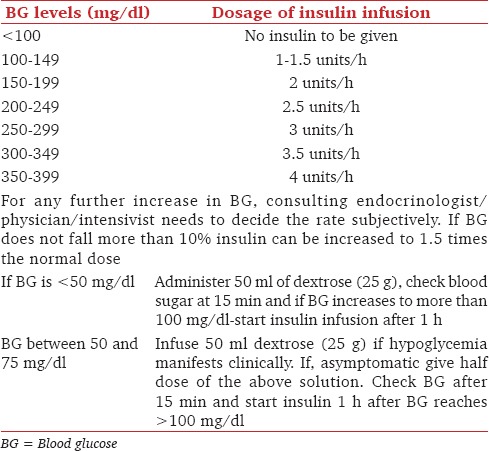
Table 5.
The onset, peak and total effective duration of action of various preparations of Insulin
This involves close monitoring of BG levels, focused attention on clinical status of the patient, nutritional status and any significant variation in these states along with appropriate titration of insulin therapy involving a multi-disciplinary approach. The therapy should also take into account the possible potential complications of DM.
Oral hypoglycemic agents
Various long and short acting OHAs such as the sulfonylurea, biguanides, thiazolidinediones, meglitinides and α-glucosidase inhibitors are available, but they are more suitable for ambulatory patients.
Recommendation
-
8.
Avoid the use of OHAs in sick inpatients [A1].
Noninsulin injectables
Noninsulin injectables such as pramlintide, liraglutide, and exenatide are not appropriate for use in ill people with diabetes with reduced oral intake. A higher incidence of nausea and vomiting associated with pramlintide and exenatide injections is also responsible for their limited popularity for inpatient use.[23]
Recommendation
-
9.
Avoid the use of noninsulin injectables in sick inpatients [A1].
Pump therapy
The use of continuous SC insulin administration through infusion pump is recommended in diabetic inpatients provided they have sound mental and physical capacity.[2,24] A precise titration and a controlled administration of insulin can be achieved through infusion pumps at any given point of time. The binding of insulin to variety of surfaces like glass, plastic and filters necessitates an initial priming and coating of the infusion set to minimize insulin absorption phenomenon so as to administer right dose of insulin to control hyperglycemia. Studies have established that without priming, the concentration of insulin delivered to patients is 15.8% lower than the set values (95% confidence interval [CI] = 9.1-22.6%) whereas after priming with 20 ml of solution the discrepancy is reduced to unrecognized values (3.4% absorption, 95% CI = 0.2-7.1%). The availability of trained staff in the hospital setting is necessary if pump therapy is to be used.
Recommendations
-
10.
Use infusion pumps whenever available for continuous SC or IV insulin infusion to achieve precise control of BG [A2].
-
11.
Initial priming of the infusion pumps should be done with 20 mL of solution containing insulin so as to minimize the “absorption phenomenon”[C3].
-
12.
Selection of the patient is very important considering the sound mental and physical well-being in stable patients, while trained staff is essential in ICU to take care of such equipment [B2].
-
13.
The lack of insulin pump facilities does not preclude attempts at optimal glycemic control [A3].
Monitoring of blood glucose
Fasting BG measurement has been the major diagnostic test for screening purposes.[25,26] However, capillary glucose measurement has become more popular than venous BG estimation tests due to its ease of use, agility, rapidity and safety not only in general population, but in hospital settings as well.[27] These features of capillary BG measurement make it extremely useful for the routine intensive monitoring of critically ill patients in ICUs and emergency departments. Many inpatients can get easily diagnosed for the first time by incorporating capillary BG measurement as a routine screening test in all the hospitalized patients. This intervention can help to a great extent in timely diagnosis and initiation of appropriate therapy and can thus avoid many complications associated with untreated DM.[28,29] The question of accuracy of capillary source of blood vis-ä-vis venous source has become redundant with the advent of newer glucometers that allow the blood sample from either source to be measured. The resulting advantages are minimal needle pricks (less injury, less contamination, cost cutting), and elimination of factitious reporting.[30,31] However, to minimize errors and discrepancy, the glucometers readings should always be tested and compared intermittently with laboratory glucose values (internal quality control). Another Food and Drug Administration — approved monitoring technique is continuous glucose monitoring system, which can monitor glucose levels continuously up to 72 h. Such a system is useful in emergency and ICUs as it exhibit various glycemic trends and patterns, help in timely detection of hypoglycemia, and assesses efficacy of ongoing therapy. However, the high cost and delay in obtaining the results (after 72 h) and the limitation of real time display are a few of the disadvantages associated with its use.[32]
Recommendations
-
14.
Mandatory BG testing for every patient on admission and at least two readings in the next 24 h to rule out hyperglycemia [A1].
-
15.
Glycated hemoglobin should be obtained in the patient with hyperglycemia without the prior history of DM, and with persistent hyperglycemia of uncertain etiology. This test is to be avoided in patients receiving massive blood transfusions [B2].
-
16.
Point of care monitoring of BG to be done preferably with capillary method. In cases of hypotension, hypothermia, shock, use of vasoconstrictors and vasopressors use venous sampling instead [A1].
-
17.
Initial monitoring should be done on an hourly basis and the interval of testing can be increased when three consecutive readings are consistently around the target of control [B2].
-
18.
Postprandial testing should be included during monitoring glycemia in patients on oral feed [C2].
Special Populations
Management of diabetes and hyperglycemia during peri-operative period
Initial studies which reported the benefits of tight glycemic control during peri-operative period and hospital stay have been followed by various clinical trials and meta-analytical reviews with variable results.[21] These variations raise questions such as the safety and efficacy of IIT compared with conventional insulin therapy, impact of hyperglycemia on surgical outcome and targeted range of BG levels,.[6,33,34] Besides these facts, the type of surgery (elective or emergency), the duration of surgical procedure, degree of surgery (major or minor), associated co-morbidities, duration of DM and degree of control of hyperglycemia are important factors that determine surgical outcome. Insulin is the preferred therapy in the majority of surgical patients. In spite of various conflicting reports in literature, various international advisory committees have supported the role of tight glycemic control in improving the surgical outcome.[35,36] However, it must be stressed that hypoglycemia must be avoided at all costs.
The most commonly used insulin regimens in India and other South Asian countries are GIK (glucose, insulin and potassium) and variable insulin infusion regimen.[8,22,24,30] The cost associated with the pump infusion system prohibits the use of such regimens in low resource setting. However as an alternative, the use of micro-drip set attached to dextrose solution containing therapeutic insulin and potassium is not only cost effective but also can be used in any of the settings where monitoring of BG is possible as and when required. Evidence has established good surgical outcome with adequate control of hyperglycemia in cardiac surgery, cardio-thoracic surgery, neuro-surgery besides general surgical procedures.[31,32,34] Glycemic control (BG <150 mg/dL) with IV infusion of insulin is considered one of the most important factors in reducing the cardiac morbidity and sterna wound infection in patients undergoing cardiac surgery by the results of many observational and prospective studies.[37,38] Apart from its glucose lowering properties, insulin has other significant beneficial actions such as normalization of cholesterol levels, profound effect on protein metabolism, modulation of nitric oxide activity and antiinflammatory effects.[39,40]
Recommendations
-
19.
Tight glycemic control with insulin is advocated for a better surgical outcome, with targets of BG between 110 mg/dL and 140 mg/dL [A1].
-
20.
Glucose and insulin should be given through separate IV routes serum potassium should be monitored and supplemented [B2].
-
21.
Patients who need not remain NPO for prolonged periods, during or after surgery, such as cataract surgery, may continue on OHAs if they are well controlled [A2].
Diabetic patients with trauma
Scarce data is available to substantiate the evidence of poor outcome in trauma patients with associated hyperglycemia. However, stress hyperglycemia (BG >135 mg/dL) and preexisting DM are associated with longer hospital stay and greater in hospital morbidity from infectious and noninfectious complications in patients admitted with trauma.[41] The contributory factors in this subset of the population may include but are not limited to alterations in the immune system, degree of glycemic control and preexisting associated co-morbidities besides DM. A higher incidence of acute respiratory distress syndrome and nosocomial pneumonia has been observed in trauma patients with preexisting diabetes.[41,42,43] Hyperglycemia, either due to DM or due to stress response, controlled with IIT is associated with improved clinical outcome in trauma patients.[41,44] As such in hospital trauma patients with DM require a higher level of care as they are highly prone to develop complications. When dealing with such clinical situations, cost involved in the overall treatment of such patients is also a significant aspect in the low resource economies.[41]
Recommendations
-
22.
Control of stress hyperglycemia and preexisting DM should always be given priority after initial resuscitation so as to improve the outcome in trauma patients [B2].
-
23.
Preferred therapy is the administration of IV insulin to prevent all potential complications associated with trauma [A1].
-
24.
The cognitive changes associated with head injuries and fears of induced hypoglycemia with aggressive insulin therapy are important considerations during management of trauma patients with DM [B2].
Patients on enteral nutrition
The control of hyperglycemia is challenging in patients on enteral nutrition as BG levels show marked variability with type and duration of enteral nutrition (i.e., Ryles’ tube or gastrostomy tube). Furthermore, insulin may exert hypoglycemic effect on sudden discontinuation of enteral nutrition.[45,46]
Recommendations
-
25.
Insulin analogs should be preferred to control hyperglycemia in indoor patients on enteral nutrition [A2].
-
26.
Basal plus multiple SC prandial boluses are preferred over SSI [A1].
Patients receiving parenteral nutrition
Parenteral nutrition can be extremely detrimental in critically ill diabetic patients as the large amount of glucose in these solutions results in severe hyperglycemia. Uncontrolled hyperglycemia is responsible for higher incidence of complications and mortality in this subset of population.[47]
Recommendations
-
27.
Intravenous insulin is the preferred treatment for control of hyperglycemia in patients receiving parenteral nutrition [A1].
-
28.
Glucose targets should be based on the severity of underlying illness [A1].
Critically ill pediatric patients with diabetes mellitus
Management of hyperglycemia in pediatric patients is challenging, especially among critically sick patients.[48] Factors such as longer duration of hyperglycemia and higher peak BG values can lead to 3.5-fold increase in mortality risk.[48] Peak glucose levels of >180 mg/dL has been shown to be associated with higher risk of death in children with sepsis.[49] High glycemic variability is also associated with a higher incidence of mortality, increased length of hospital stay and increased risk of nosocomial infections.[50,51] Management of hyperglycamia in ICU to achieve glycemic control with IV insulin infusion is definitely a safer option in pediatric patients.[52] The target of BG (110-150 mg/dL) should be modest and insulin therapy should be used judiciously so as to avoid hypoglycemia.[53] There exists a physiological insulin resistance during pubertal growth, which may be perceived as increased insulin requirement in patients of this age group.[54] Prevention of hypoglycemia, glycemic fluctuations, and ketoacidosis are of paramount importance.[50,51,52,53]
Recommendations
-
29.
Insulin is the preferred therapy in pediatric ICU with optimal BG target of 110-150 mg/dL [A2].
-
30.
Insulin analogs are preferred over conventional insulin. Recommended age limit for use of aspart is >2 years, lispro >3 years, glulisine >4 years, detemir >2 years, and glargine >6 years [A2].
Transplant patients with diabetes mellitus
Transplant surgery in diabetic patients is a challenging task as uncontrolled hyperglycemia is associated with increased cardiac morbidity, risk of organ failure, acute graft versus host disease (Grade II-IV) and increased risk of nonrelapse related mortality.[55] Each 10 mg/dL increase of BG is associated with 1-1.5 fold increase in the odds ratio of bacteremia in neutropenic patients who are not treated with glucocorticoids.[56] Hyperglycemia, hypoglycemia and fluctuation in glycemic levels are associated with nonrelapse related mortality within 6 months after transplantation procedure. Administration of immunosuppressants in posttransplant period is associated with hyperglycemia in diabetic patients. As such, insulin requirement is much higher in these patients. On the other hand, patient's dietary changes and warrant the use of analogs due to their flexibility.
Recommendations
-
31.
The BG target in posttransplant patients should be sensibly put, preferably in the line of surgical inpatients [A2].
-
32.
IV infusion is preferred in patients who are NPO. Basal-bolus regimen with analogs should be preferred in patients on regular meals. High doses of bolus insulin will be required [A2].
Transition therapy
Discharge and outdoor management of the diabetic patient should be done only after prior stabilization of BG levels. The patient should be handed over a simplified treatment plan which should include various drug regimens and their appropriate use, BG monitoring schedule, prompt recognition of hypoglycemic symptoms and their management and contact number of primary care physician whom they can contact during any major complaint or emergency. [Table-6] Patients should be shifted to more convenient insulin regimes, such as premixed insulin twice daily, if possible, before discharge from hospital and concordance of meals and SC insulin should be ensured. Overlap of IV and SC insulin is not needed if SC rapid acting analogues are used. They should be monitored on this regime for a few days in hospital if possible and the first follow-up should be done within 10-14 days time period.[57]
Table 6.
Insulin dose calculation during transition from IV to SC regimen

Recommendations
-
33.
Patients being discharged from the hospital may be prescribed basal-bolus premixed or basal insulin regimen as required [A2].
-
34.
Insulin analogues have the advantage of lesser hypoglycemia and greater flexibility [A1].
-
35.
Education regarding the insulin technique, self-monitoring of BG, hypoglycemia, and self-adjustment of doses should be provided before discharge and on an ongoing basis. Elaborate, clear instructions written in comprehensible language should be provide [B2].
Basal-bolus insulin
Administration of bed time basal-bolus insulin (long and intermediate acting) has been associated with improved nocturnal and fasting BG level. Long acting insulin analogues not only have the advantage of effectively decreasing HbA1c levels but also do not cause any sustained peak action which makes them highly useful for IIT in ICU. SC basal insulin can be combined with OHAs to achieve a more precise control of hyperglycemia. BG target in the general ward patients should be set to deliver preprandial BD of 90-150 mg/dL. A wide range of these target levels can easily overcome the various factors responsible for any discrepancy such as degree of hyperglycemia, nutritional status, insulin sensitivity and the severity of the underlying illness. Improvement in the clinical status warrants switching over to basal-bolus insulin and prandial regimens with proper overlap between IV and SC regimens. SSI is generally considered inappropriate.[58,59,60]
Recommendations
-
36.
Basal-bolus insulin with long and intermediate acting insulin prevents the fluctuation of nocturnal BG levels and improves fasting BG levels.
-
37.
Insulin analogues are considered better alternative to conventional insulin therapy as they decrease the HbA1c levels and do not cause hypoglycemia.
-
38.
Basal-bolus insulin can be combined with oral OHAs for precise control of BG in stable patients.
-
39.
Basal-bolus therapies such as intermediate to long acting insulin combined with short and rapid acting preprandial insulin regimen or rapid acting insulin analogues given 30 min prior to meal achieve an almost ideal physiological glucose control by blunting the postprandial spikes in BS.
-
40.
Basal insulin dose can be simply initiated with 0.2-0.3 U/kg/day, using detemir, glargine or ultra-long acting degludec insulin.
-
41.
Intermediate acting insulin such as neutral protamine Hagedorn can be administered 12 hourly or one can switch to insulin detemir 12-24 h, inj. Insulin glargine every 24 h or insulin degludec every 24 h. Insulin degludec provides the advantage of flexibility in time of administration.
-
42.
The general consensus is to keep casual BG levels as close to 90-130 mg/dL as possible and postprandial levels <180 mg/dL.
Supportive treatment
Besides pharmacological therapies, management of diabetes also involves numerous nonpharmacological approaches including medical nutrition therapy, physical activity and exercise, behavioral therapy and cessation of smoking. Enteral and parenteral nutrition in diabetic inpatients as per the requirement can be designed by a dietetics department on an individual basis depending upon the severity of the underlying illness and associated co-morbidities.[61] In set-up where dietetics department is not functional, diet charts can help to outline the nutritional needs. Hypoglycemia can be a resulting complication of an aggressive approach to control hyperglycemia with IV insulin and insulin analogues. However, diabetic complications can be timely detected, and appropriate therapeutic interventions can be carried out by proper education and training of the attending staff members. One simple strategy is to find out team leaders and champions among the present staff and train them thoroughly so as to create a dedicated learning atmosphere in the hospital. To set a leading example, the onus will be on the endocrinologists, anesthesiologists, intensivists and other clinical associates. A well co-ordinated teamwork can go a long way in establishing various simple protocols to manage the increasing menace of uncontrolled hyperglycemia in the hospital setting.
Conclusion
The management of diabetes in hospitalized patients can be made more effective and safer by following these guidelines provided that there is good co-ordination among various specialties and super specialties. Appropriate use of insulin and insulin analogs, using IV or SC regimes, as required, to target euglycemia, ensures better therapeutic outcomes. Along with this, training of health workers, education of support staff, use of simple protocols, auditing of mortality and morbidity statistics and a good feedback system are essential for the successful management of hyperglycemia in low resource health set-up.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.American Diabetes Association. Economic costs of diabetes in the U.S. In 2007. Diabetes Care. 2008;31:596–615. doi: 10.2337/dc08-9017. [DOI] [PubMed] [Google Scholar]
- 2.Clement S, Braithwaite SS, Magee MF, Ahmann A, Smith EP, Schafer RG, et al. Management of diabetes and hyperglycemia in hospitals. Diabetes Care. 2004;27:553–91. doi: 10.2337/diacare.27.2.553. [DOI] [PubMed] [Google Scholar]
- 3.van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345:1359–67. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 4.Malmberg K, Norhammar A, Wedel H, Rydén L. Glycometabolic state at admission: Important risk marker of mortality in conventionally treated patients with diabetes mellitus and acute myocardial infarction: Long-term results from the Diabetes and Insulin-Glucose Infusion in Acute Myocardial Infarction (DIGAMI) study. Circulation. 1999;99:2626–32. doi: 10.1161/01.cir.99.20.2626. [DOI] [PubMed] [Google Scholar]
- 5.Wiener RS, Wiener DC, Larson RJ. Benefits and risks of tight glucose control in critically ill adults: A meta-analysis. JAMA. 2008;300:933–44. doi: 10.1001/jama.300.8.933. [DOI] [PubMed] [Google Scholar]
- 6.Brunkhorst FM, Engel C, Bloos F, Meier-Hellmann A, Ragaller M, Weiler N, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358:125–39. doi: 10.1056/NEJMoa070716. [DOI] [PubMed] [Google Scholar]
- 7.Bajwa SJ. Intensive care management of critically sick diabetic patients. Indian J Endocrinol Metab. 2011;15:349–50. doi: 10.4103/2230-8210.85603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bajwa SJ, Kalra S, Baruah M, Bajwa SK. Insulin injection guidelines for peri-operative and critically ill patients. J Sci Soc. 2013;40:68–75. [Google Scholar]
- 9.Frid A, Hirsch L, Gaspar R, Hicks D, Kreugel G, Liersch J, et al. New injection recommendations for patients with diabetes. Diabetes Metab. 2010;36(Suppl 2):S3–18. doi: 10.1016/S1262-3636(10)70002-1. [DOI] [PubMed] [Google Scholar]
- 10.Saudek CD, Herman WH, Sacks DB, Bergenstal RM, Edelman D, Davidson MB. A new look at screening and diagnosing diabetes mellitus. J Clin Endocrinol Metab. 2008;93:2447–53. doi: 10.1210/jc.2007-2174. [DOI] [PubMed] [Google Scholar]
- 11.Kalra S, Bajwa SJ, Baruah M, Sehgal V. Hypoglycaemia in anesthesiology practice: Diagnostic, preventive, and management strategies. Saudi J Anaesth. 2013;7:447–52. doi: 10.4103/1658-354X.121082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moghissi ES, Korytkowski MT, DiNardo M, Einhorn D, Hellman R, Hirsch IB, et al. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Care. 2009;32:1119–31. doi: 10.2337/dc09-9029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pasquel FJ, Spiegelman R, McCauley M, Smiley D, Umpierrez D, Johnson R, et al. Hyperglycemia during total parenteral nutrition: An important marker of poor outcome and mortality in hospitalized patients. Diabetes Care. 2010;33:739–41. doi: 10.2337/dc09-1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frisch A, Chandra P, Smiley D, Peng L, Rizzo M, Gatcliffe C, et al. Prevalence and clinical outcome of hyperglycemia in the perioperative period in noncardiac surgery. Diabetes Care. 2010;33:1783–8. doi: 10.2337/dc10-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smiley D, Rhee M, Peng L, Roediger L, Mulligan P, Satterwhite L, et al. Safety and efficacy of continuous insulin infusion in noncritical care settings. J Hosp Med. 2010;5:212–7. doi: 10.1002/jhm.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cao SG, Ren JA, Shen B, Chen D, Zhou YB, Li JS. Intensive versus conventional insulin therapy in type 2 diabetes patients undergoing D2 gastrectomy for gastric cancer: A randomized controlled trial. World J Surg. 2011;35:85–92. doi: 10.1007/s00268-010-0797-5. [DOI] [PubMed] [Google Scholar]
- 17.Umpierrez GE, Smiley D, Zisman A, Prieto LM, Palacio A, Ceron M, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial) Diabetes Care. 2007;30:2181–6. doi: 10.2337/dc07-0295. [DOI] [PubMed] [Google Scholar]
- 18.Umpierrez GE, Smiley D, Jacobs S, Peng L, Temponi A, Mulligan P, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 surgery) Diabetes Care. 2011;34:256–61. doi: 10.2337/dc10-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scurlock C, Raikhelkar J, Mechanick JI. Critique of normoglycemia in intensive care evaluation: Survival using glucose algorithm regulation (NICE-SUGAR) — A review of recent literature. Curr Opin Clin Nutr Metab Care. 2010;13:211–4. doi: 10.1097/MCO.0b013e32833571f4. [DOI] [PubMed] [Google Scholar]
- 20.Reider J, Donihi A, Korytkowski MT. Practical implications of the revised guidelines for inpatient glycemic control. Pol Arch Med Wewn. 2009;119:801–9. [PubMed] [Google Scholar]
- 21.Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, et al. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354:449–61. doi: 10.1056/NEJMoa052521. [DOI] [PubMed] [Google Scholar]
- 22.Bajwa SJ, Sehgal V, Kalra S, Baruah MP. Management of diabetes mellitus type-2 in the geriatric population: Current perspectives. J Pharm Bioall Sci. 2014;6:151–7. doi: 10.4103/0975-7406.130956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krentz AJ, Patel MB, Bailey CJ. New drugs for type 2 diabetes mellitus: What is their place in therapy? Drugs. 2008;68:2131–62. doi: 10.2165/00003495-200868150-00005. [DOI] [PubMed] [Google Scholar]
- 24.Bailon RM, Partlow BJ, Miller-Cage V, Boyle ME, Castro JC, Bourgeois PB, et al. Continuous subcutaneous insulin infusion (insulin pump) therapy can be safely used in the hospital in select patients. Endocr Pract. 2009;15:24–9. doi: 10.4158/EP.15.1.24. [DOI] [PubMed] [Google Scholar]
- 25.American Diabetes Association. Standards of medical care in diabetes. Diabetes Care. 2008;31:S5–11. doi: 10.2337/dc14-S005. [DOI] [PubMed] [Google Scholar]
- 26.American Diabetes Association. Screening for type 2 diabetes. Diabetes Care. 2003;26:S21–4. doi: 10.2337/diacare.26.2007.s21. [DOI] [PubMed] [Google Scholar]
- 27.Geneva: WHO; 1999. Worldworld Health Organization. Definition, diagnosis and classification of diabetes mellitus and its complications. Report of WHO Consultation. Part 1: Diagnosis and Classification of Diabetes Mellitus. [Google Scholar]
- 28.Harris MI, Eastman RC. Early detection of undiagnosed diabetes mellitus: A US perspective. Diabetes Metab Res Rev. 2000;16:230–6. doi: 10.1002/1520-7560(2000)9999:9999<::aid-dmrr122>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 29.Engelgau MM, Narayan KM, Herman WH. Screening for type 2 diabetes. Diabetes Care. 2000;23:1563–80. doi: 10.2337/diacare.23.10.1563. [DOI] [PubMed] [Google Scholar]
- 30.Vidhya K, Sudhir R, Mohan V. Continuous glucose monitoring system — Useful but expensive tool in management of diabetes. J Assoc Physicians India. 2004;52:587–90. [PubMed] [Google Scholar]
- 31.Chaney MA, Nikolov MP, Blakeman BP, Bakhos M. Attempting to maintain normoglycemia during cardiopulmonary bypass with insulin may initiate postoperative hypoglycemia. Anesth Analg. 1999;89:1091–5. doi: 10.1213/00000539-199911000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Latham R, Lancaster AD, Covington JF, Pirolo JS, Thomas CS., Jr The association of diabetes and glucose control with surgical-site infections among cardiothoracic surgery patients. Infect Control Hosp Epidemiol. 2001;22:607–12. doi: 10.1086/501830. [DOI] [PubMed] [Google Scholar]
- 33.Bajwa SJ, Sethi E, Kaur R. Nutritional risk factors in endocrine diseases. J Med Nutr Nutraceuticals. 2013;2:86–90. [Google Scholar]
- 34.Gandhi GY, Nuttall GA, Abel MD, Mullany CJ, Schaff HV, O’Brien PC, et al. Intensive intraoperative insulin therapy versus conventional glucose management during cardiac surgery: A randomized trial. Ann Intern Med. 2007;146:233–43. doi: 10.7326/0003-4819-146-4-200702200-00002. [DOI] [PubMed] [Google Scholar]
- 35.Garber AJ, Moghissi ES, Bransome ED, Jr, Clark NG, Clement S, Cobin RH, et al. American College of Endocrinology position statement on inpatient diabetes and metabolic control. Endocr Pract. 2004;10(Suppl 2):4–9. doi: 10.4158/EP.10.S2.4. [DOI] [PubMed] [Google Scholar]
- 36.Skyler JS, Bergenstal R, Bonow RO, Buse J, Deedwania P, Gale EA, et al. Intensive glycemic control and the prevention of cardiovascular events: Implications of the ACCORD, ADVANCE, and VA diabetes trials: A position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association. Circulation. 2009;119:351–7. doi: 10.1161/CIRCULATIONAHA.108.191305. [DOI] [PubMed] [Google Scholar]
- 37.Krinsley JS. Effect of an intensive glucose management protocol on the mortality of critically ill adult patients. Mayo Clin Proc. 2004;79:992–1000. doi: 10.4065/79.8.992. [DOI] [PubMed] [Google Scholar]
- 38.Lazar HL, Chipkin SR, Fitzgerald CA, Bao Y, Cabral H, Apstein CS. Tight glycemic control in diabetic coronary artery bypass graft patients improves perioperative outcomes and decreases recurrent ischemic events. Circulation. 2004;109:1497–502. doi: 10.1161/01.CIR.0000121747.71054.79. [DOI] [PubMed] [Google Scholar]
- 39.Mesotten D, Swinnen JV, Vanderhoydonc F, Wouters PJ, Van den Berghe G. Contribution of circulating lipids to the improved outcome of critical illness by glycemic control with intensive insulin therapy. J Clin Endocrinol Metab. 2004;89:219–26. doi: 10.1210/jc.2003-030760. [DOI] [PubMed] [Google Scholar]
- 40.Bajwa SS, Sehgal V. Psycho-social and clinical aspects of diabeto-criticare. J Soc Health Diabetes. 2013;1:70–4. [Google Scholar]
- 41.Ahmad R, Cherry RA, Lendel I, Mauger DT, Service SL, Texter LJ, et al. Increased hospital morbidity among trauma patients with diabetes mellitus compared with age-and injury severity score-matched control subjects. Arch Surg. 2007;142:613–8. doi: 10.1001/archsurg.142.7.613. [DOI] [PubMed] [Google Scholar]
- 42.Tejada Artigas A, Bello Dronda S, Chacón Vallés E, Muñoz Marco J, Villuendas Usón MC, Figueras P, et al. Risk factors for nosocomial pneumonia in critically ill trauma patients. Crit Care Med. 2001;29:304–9. doi: 10.1097/00003246-200102000-00015. [DOI] [PubMed] [Google Scholar]
- 43.Leal-Noval SR, Marquez-Vácaro JA, García-Curiel A, Camacho-Laraña P, Rincón-Ferrari MD, Ordoñez-Fernández A, et al. Nosocomial pneumonia in patients undergoing heart surgery. Crit Care Med. 2000;28:935–40. doi: 10.1097/00003246-200004000-00004. [DOI] [PubMed] [Google Scholar]
- 44.Bajwa SS, Kalra S. Endocrine anesthesia: A rapidly evolving anesthesia specialty. Saudi J Anaesth. 2014;8:1–3. doi: 10.4103/1658-354X.125890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pancorbo-Hidalgo PL, García-Fernandez FP, Ramírez-Pérez C. Complications associated with enteral nutrition by nasogastric tube in an internal medicine unit. J Clin Nurs. 2001;10:482–90. doi: 10.1046/j.1365-2702.2001.00498.x. [DOI] [PubMed] [Google Scholar]
- 46.Sehgal V, Bajwa SS, Khaira U, Sehgal R, Bajaj A. Challenging aspects of and solutions to diagnosis, prevention, and management of hypoglycemia in critically ill geriatric patients. J Sci Soc. 2013;40:128–34. [Google Scholar]
- 47.Nylen ES, Muller B. Endocrine changes in critical illness. J Intensive Care Med. 2004;19:67–82. doi: 10.1177/0885066603259551. [DOI] [PubMed] [Google Scholar]
- 48.Srinivasan V, Spinella PC, Drott HR, Roth CL, Helfaer MA, Nadkarni V. Association of timing, duration, and intensity of hyperglycemia with intensive care unit mortality in critically ill children. Pediatr Crit Care Med. 2004;5:329–36. doi: 10.1097/01.pcc.0000128607.68261.7c. [DOI] [PubMed] [Google Scholar]
- 49.Branco RG, Garcia PC, Piva JP, Casartelli CH, Seibel V, Tasker RC. Glucose level and risk of mortality in pediatric septic shock. Pediatr Crit Care Med. 2005;6:470–2. doi: 10.1097/01.PCC.0000161284.96739.3A. [DOI] [PubMed] [Google Scholar]
- 50.Wintergerst KA, Buckingham B, Gandrud L, Wong BJ, Kache S, Wilson DM. Association of hypoglycemia, hyperglycemia, and glucose variability with morbidity and death in the pediatric intensive care unit. Pediatrics. 2006;118:173–9. doi: 10.1542/peds.2005-1819. [DOI] [PubMed] [Google Scholar]
- 51.Hirshberg E, Larsen G, Van Duker H. Alterations in glucose homeostasis in the pediatric intensive care unit: Hyperglycemia and glucose variability are associated with increased mortality and morbidity. Pediatr Crit Care Med. 2008;9:361–6. doi: 10.1097/PCC.0b013e318172d401. [DOI] [PubMed] [Google Scholar]
- 52.Preissig CM, Hansen I, Roerig PL, Rigby MR. A protocolized approach to identify and manage hyperglycemia in a pediatric critical care unit. Pediatr Crit Care Med. 2008;9:581–8. doi: 10.1097/PCC.0b013e31818d36cb. [DOI] [PubMed] [Google Scholar]
- 53.Jeschke MG, Kraft R, Emdad F, Kulp GA, Williams FN, Herndon DN. Glucose control in severely thermally injured pediatric patients: What glucose range should be the target? Ann Surg. 2010;252:521–7. doi: 10.1097/SLA.0b013e3181f2774c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Allard P, Delvin EE, Paradis G, Hanley JA, O’Loughlin J, Lavallée C, et al. Distribution of fasting plasma insulin, free fatty acids, and glucose concentrations and of homeostasis model assessment of insulin resistance in a representative sample of Quebec children and adolescents. Clin Chem. 2003;49:644–9. doi: 10.1373/49.4.644. [DOI] [PubMed] [Google Scholar]
- 55.Davidson JA, Wilkinson A. International Expert Panel on New-Onset Diabetes after. Transplantation. New-Onset Diabetes after Transplantation 2003 International Consensus Guidelines: An endocrinologist's view. Diabetes Care. 2004;27:805–12. doi: 10.2337/diacare.27.3.805. [DOI] [PubMed] [Google Scholar]
- 56.Derr RL, Hsiao VC, Saudek CD. Antecedent hyperglycemia is associated with an increased risk of neutropenic infections during bone marrow transplantation. Diabetes Care. 2008;31:1972–7. doi: 10.2337/dc08-0574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moghissi ES. Addressing hyperglycemia from hospital admission to discharge. Curr Med Res Opin. 2010;26:589–98. doi: 10.1185/03007990903566822. [DOI] [PubMed] [Google Scholar]
- 58.Deedwania P, Kosiborod M, Barrett E, Ceriello A, Isley W, Mazzone T, et al. Hyperglycemia and acute coronary syndrome: A scientific statement from the American Heart Association Diabetes Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2008;117:1610–9. doi: 10.1161/CIRCULATIONAHA.107.188629. [DOI] [PubMed] [Google Scholar]
- 59.Pinto DS, Skolnick AH, Kirtane AJ, Murphy SA, Barron HV, Giugliano RP, et al. U-shaped relationship of blood glucose with adverse outcomes among patients with ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2005;46:178–80. doi: 10.1016/j.jacc.2005.03.052. [DOI] [PubMed] [Google Scholar]
- 60.Svensson AM, McGuire DK, Abrahamsson P, Dellborg M. Association between hyper- and hypoglycaemia and 2 year all-cause mortality risk in diabetic patients with acute coronary events. Eur Heart J. 2005;26:1255–61. doi: 10.1093/eurheartj/ehi230. [DOI] [PubMed] [Google Scholar]
- 61.Bajwa SJ, Kalra S. Diabeto-anaesthesia: A subspecialty needing endocrine introspection. Indian J Anaesth. 2012;56:513–7. doi: 10.4103/0019-5049.104564. [DOI] [PMC free article] [PubMed] [Google Scholar]


