Abstract
Background and Aims:
Early graft function is crucial for successful kidney transplantation. The aim of our study was to evaluate the effect of intra-operative central venous pressure (CVP) and mean arterial pressure (MAP) on early graft function and biochemical outcome.
Material and Methods:
This was a retrospective study carried out on patients undergoing renal transplant only from live-related donors between March 2011 and May 2013. We mainly divided the patients into two groups based on CVP and mean MAP. One group had CVP more than 12 and other with CVP <12 mmHg at the time of declamping. Further one group was with mean MAP >100 mmHg and other with mean MAP of <100 mmHg. The graft outcome of genetically related and genetically unrelated donors was also evaluated in early postoperative period. The trend in fall of serum creatinine was studied during the first five post-operative days. The effect of age, dry weight, sex, relation with donor and intraoperative factors like MAP and CVP on early graft function were analysed. Correlation analysis, analysis of variance test (ANOVA) and multivariate analysis technique were used in this study for statistical computation.
Results:
The mean CVP at the time of declamping was 13.91 mmHg. The minimum CVP was 6 mmHg in one patient who had ischemic heart disease with low ejection fraction. All 5 days mean serum creatinine values were comparable in two groups on 1st, 2nd, 3rd and 4th postoperative days. The mean MAP at the time of declamping was 111.22 mmHg. Mean MAP varied from a minimum of 95 mmHg to maximum of 131 mmHg. There was no significant difference in two groups on 1st, 2nd, 3rd, 4th and 5th postoperative days.
Conclusion:
A CVP around 12 mmHg and mean MAP >95 mmHg with good perioperative fluid hydration is associated with good early graft function.
Keywords: Central venous pressure, mean arterial pressure, renal transplant, serum creatinine
Introduction
Renal transplantation is now recognized as a treatment of choice for patients with chronic renal failure with end-stage renal disease. Renal transplantations are associated with better quality of life, better cost/benefit ratio, and possibly longer survival.[1] The grafted kidney's outcome in first few days is generally measured by urine output and serum creatinine values. Early graft malfunction has been associated with decreased graft survival and increased recipient complications. These factors initiated us to study the early graft function (urine output and biochemical parameters) in relation to the fluid dynamics during the surgery. The most important operative intra-operative measure, to improve immediate graft function, is to maintain an adequate intravascular volume.[3,4] Transplantation of organs leads to several physiological changes in chronic renal failure recipients, who usually have increased total body water and electrolyte imbalance. Few studies have shown that intravascular volume must be higher in recipient to counteract several physiological and immunological insults.
With advances in surgical and anesthetic techniques, older and more medically complex patients are being considered for renal transplantation. An aggressive intra-operative volume expansion by keeping central venous pressure (CVP) between 10 and 15 mmHg is still uniformly recommended in the anesthesiological management.[3,4] This volume expansion is associated with increased renal blood flow and improved graft function.[5] However, some authors advocate intra-operative fluid hydration might not be mandatory.[6] Therefore, this retrospective study was aimed to evaluate the effect of intra-operative CVP and mean arterial pressure (MAP) on early graft function and biochemical outcome.
Material and Methods
This was a retrospective study wherein we evaluated 100 patients from a single center who underwent renal transplant from March 2011 to May 2013 from live related donors. All transplants were conducted by a single surgeon with a same team of anesthesiologists. The study was approved by the Institutional Review Committee and conformed to ethical guidelines of 1975 Helsinki declaration.
Age, gender, weight, hemoglobin (Hb), hematocrit, and serum potassium, urea, and creatinine were obtained from the preanesthetic evaluation records. All other parameters were obtained from the anesthesia chart, including those related with the intra-operative monitoring. Data from the medical charts were used whenever necessary. All transplant recipients received hemodialysis within 24 h before surgery. A dedicated intra-operative monitoring of ECG, blood pressure, CVP, urine output, core temperature, pulse oxymetry and end-tidal CO2 (EtCO2) were observed. Invasive arterial blood pressure monitoring was done in patients who were anticipated to have hemodynamic fluctuations. Anesthesia was administered using a standard protocol in all patients. Induction of anesthesia was done using injection thiopentone 3-5 mg/kg, fentanyl 1-2 μg/kg, along with oxygen/air/isoflurane at one minimum alveolar concentration, and muscle relaxation was achieved with atracurium 0.5 mg/kg. Patient's lungs were ventilated with volume control mode of ventilation keeping the positive end-expiratory pressure between 0 and 5 mmHg. Forced air warming was used to maintain a central body temperature around 36°C. Internal jugular vein catheterization was performed after the induction of anesthesia, and fluid administration was done according to CVP which was recorded every half hourly with a special focus at the time of declamping.
Once the vessels were declamped, patients were given 100 mg of injection furosemide and MAP was kept above 60 mmHg. CVP and MAP were documented at the time of decalmping. In the postoperative period fluid, was decided according to the diuresis, blood pressure, and CVP. All the patients underwent daily serum creatinine levels, serum electrolytes, hemogram and input-output charting. The first five postoperative period data were used for analysis.
We divided patients into groups for purpose of analysis based on CVP value of > or < 12 mmHg recorded at declamping. The fall in creatinine trend was analyzed in the first 5 postoperative days. We also analysed data considering MAP values of > or < 100 mmHg. Our study group had a mixture of both genetically related and genetically unrelated living donors. To rule out any bias due to this factor we further divided the study population into those receiving kidney from genetically related donors and other receiving kidney from genetically unrelated donors.
Statistical analysis
We evaluated data using the SPSS software (version 11.5, SPSS Science, Inc.). Correlation analysis and analysis of variance test (ANOVA) technique were used in the study for statistical computation. Multivariate analysis was used to compare various variables.
Results
We evaluated 100 patients who underwent transplant in between March 2011 and May 2013. Out of the 100 patients we had serum creatinine records for all 5 days for 96 patients; serum creatinine values were available for first 3 postoperative days for 97 patients and till 1st postoperative day for 98 patients. Biochemical values were not available for two patients in our study group. Of the total 100 renal transplant recipients, 17 were females, and 83 were males. Sixty-four patients received kidney from genetically related donors (father, mother, brother and sister). Thirty-six patients received kidney from genetically unrelated donors (spouse). The mean age of the renal transplant recipient in our study was 36.19 years (maximum = 64 years, minimum = 11 years). The mean CVP at the time of declamping was 13.91 mmHg. The minimum CVP was 6 mmHg in one of our patients who had ischemic heart disease with low ejection fraction (30%). The mean MAP at the time of declamping was 111.22 mmHg. MAP varied from a minimum of 95 mmHg to maximum of 131 mmHg. The mean core body temperature was 36.08°C. The mean EtCO2 was 31.46 mmHg. The dry weights were available for 97 patients, the records of three patients on dry weight were not available. The mean dry weight was 56.28 kg. The dry weights were comparable in all the groups.
Fall in serum creatinine were analyzed using multivariate analysis taking the parameters of age, dry weight, gender, MAP, CVP and relation with donor as variables. The fall in serum creatinine on 3rd and 4th days were significantly related to dry weight with P value of 0.009 and 0.025 respectively. Fall in serum creatinine on 1st day was related to gender with P value of 0.037 [Table 1].
Table 1.
Influence of various variables on first five days serum creatinine values
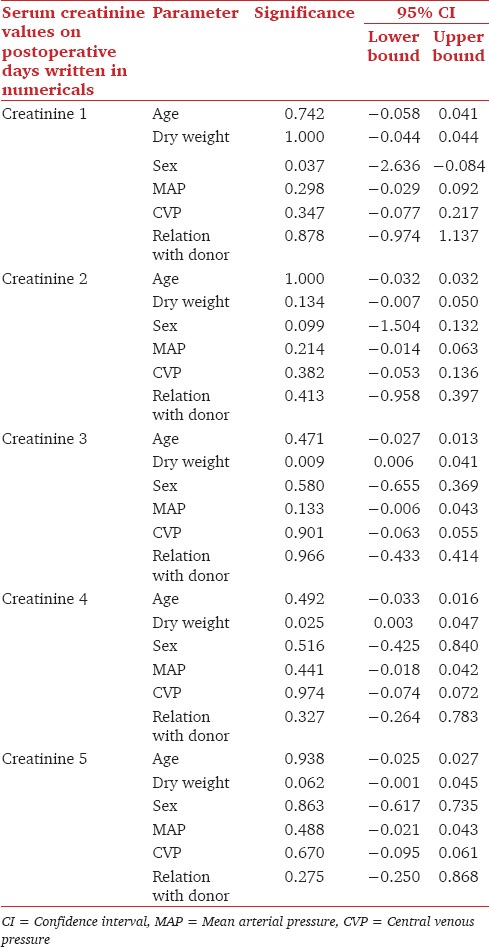
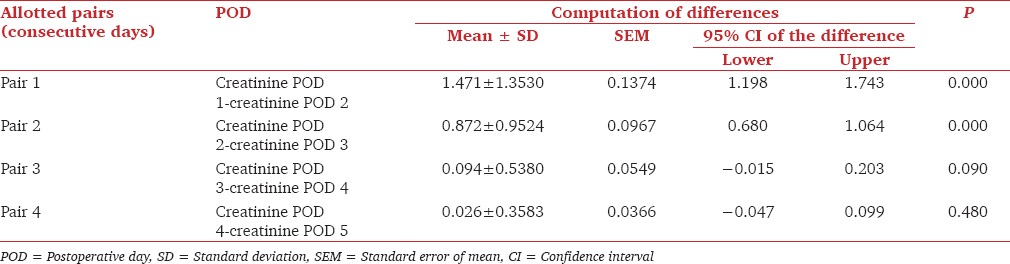
The serum creatinine values on first 5 postoperative days were used for analysis. The mean serum creatinine on postoperative day 1, 2, 3, 4 and 5 were 3.93 ± 2.21 mg/dL, 2.46 ± 1.46 mg/dL, 1.59 ± 0.89 mg/dL, 1.54 ± 1.09 mg/dL and 1.47 ± 1.16 mg/dL. On comparing the trends in fall of creatinine we found a significant drop in serum creatinine on 1st and 2nd postoperative days (P < 0.05). Similarly, there was a significant drop in serum creatinine on 3rd postoperative days when compared to 2nd postoperative days (P < 0.05). The drop in serum creatinine on 4th and 5th postoperative days was not significantly different (P = 0.90 and P = 0.48) [Table 2].
Table 2.
Trends in drop in serum creatinine in first five PODs
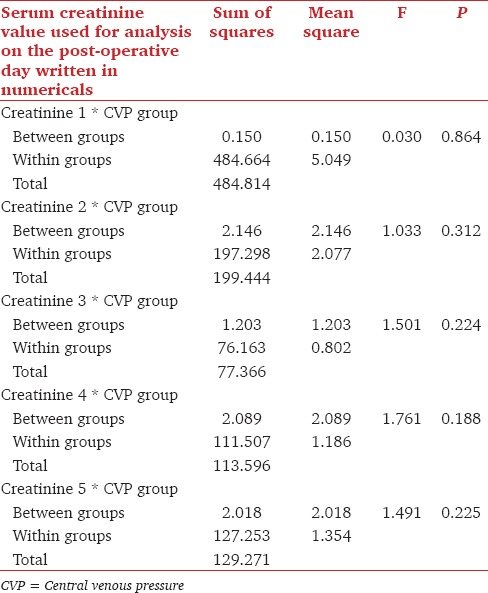
For comparison we created two patient groups of CVP <12 mmHg at declamping (n = 36) versus CVP >12 mmHg at declamping. In the CVP >12 mmHg groups records for the first 3 postoperative days were available for all the 62 patients and the record of one patient was missing for the 4th and 5th postoperative days. Multivariate analysis was done, which compared the various parameters in two groups. The dry weight of patients significantly affected CVP. The mean dry weight in CVP <12 mmHg group was 52.78 ± 12.30. The mean dry weight in CVP >12 mmHg group was 58.45 ± 12.81 [Table 3]. The all 5 days mean serum creatinine values were compared in the two said group using ANOVA. On 1st postoperative day there was no significant difference in the two groups (P = 0.864), Similarly, there was no significant difference in two groups on 2nd, 3rd and 4th postoperative days; P values 0.312, 0.224 and 0.188, respectively [Table 4].
Table 3.
Mean Values of various parameters in the two group
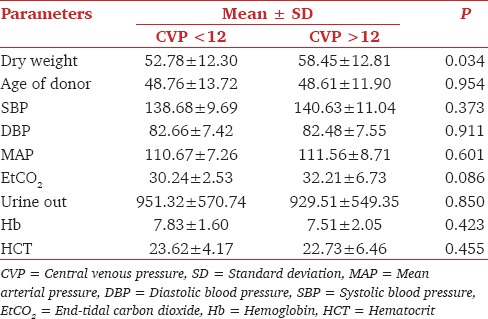
Table 4.
Fall in serum creatinine trend compared in the CVP <12 mmHg and CVP >12 mmHg group
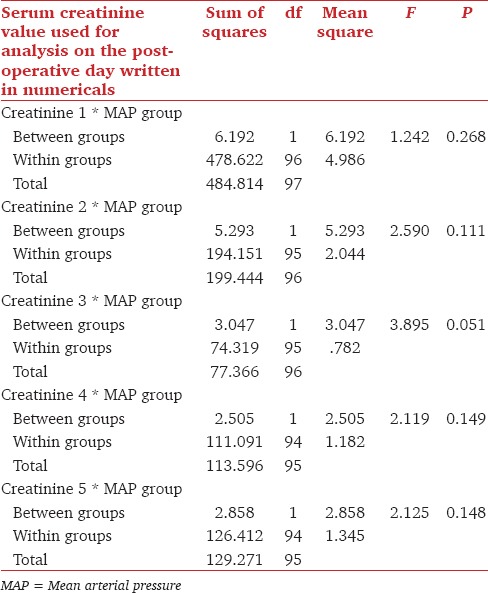
We further divided our patients based on MAP. First group had mean MAP <100 mmHg. In this group records of all the nine patients, were available till 3rd postoperative days and the record of one patient was missing on 4th and 5th postoperative days. The other group had mean MAP of >100 mmHg. In this group records of all the 89 patients, were available till 1st postoperative day and the record of one patient was missing for the following days. Using multivariate analysis, variables such as age, dry weight, age of the donor, EtCO2 showed no significant effect in either group. All the 5 days serum creatinine trend was compared in the two groups of MAP There was no significant difference in 1st postoperative day creatinine in the two groups (P = 0.286). Similarly, there was no significant difference in two groups on 2nd, 3rd, 4th and 5th postoperative days P values 0.111, 0.051, 0.149 and 0.148 respectively [Table 5].
Table 5.
Fall in serum creatinine trend compared in MAP <100 mmHg and MAP >100 mmHg group
In our study, the average Hb was 7.63 g/dL. We invariably transfused our recipients with one to two units of packed cells after declamping. All the 5 days mean serum creatinine values were compared in the genetically related and genetically unrelated groups. There was no significant difference in 1st postoperative day creatinine in the two groups (P = 0.688). Similarly, there was no significant difference in the falling creatinine value on 2nd, 3rd, 4th and 5th postoperative days; P values 0.756, 0.754, 0.202 and 0.10 respectively.
Discussion
Organ demand for renal transplant far exceeds than organ availability. This makes every transplanted kidney precious and optimization of several parameters are needed for a successful outcome. The overall anesthetic goal is to maintain intravascular volume and good perfusion to the transplanted kidney after revascularization. Various studies have suggested that perioperative hemodynamic factors influence immediate and long-term graft survival.[6] Proper perioperative fluid management is one of the most important aspects governing hemodynamic function in these patients resulting in good graft function. Adequate plasma volume is essential in maintaining cardiac output and hence tissue perfusion. Hypotension related acute tubular necrosis (ATN) is a significant factor in graft dysfunction especially in the early period. Hypotension is anticipated at declamping of the renal vessels and reperfusion of the graft. This has been explained to be due to a sudden shift of around 25% of cardiac output to the renal graft and release of vasodilator mediators accumulated during renal ischemia period. The systolic blood pressure is usually maintained between 130 and 160 mmHg, and the CVP is maintained between 12 and 14 mmHg.[7] For the practicing anesthetists it is important to know that the right ventricular preload is determined by end-diastolic volume not pressure and, therefore, without knowledge of the ventricular compliance, an isolated CVP reading is of limited value. Compliance not only varies from patient to patient but varies with time also in the same patient. Thus, dynamic changes in CVP are more useful than absolute values. According to our standard institutional guideline if the CVP rises >7 mmHg in response to a fluid challenge (e.g., 50-200 mL of colloid over 10 min) then the patient is probably maximally filled and any further filling will result in development of pulmonary edema. If, however, the CVP returns to within 3 mmHg of its original value within 10 min then the risk of pulmonary edema is only moderate; nevertheless no further filling is required.
Measurement of CVP is an absolute requirement to ensure good plasma volume expansion. Review of literature revealed two streams of thoughts on maintaining CVP at the time of declamping (reperfusion). Ferris et al.[7] recommends aggressive intra-operative volume expansion to maximize graft's functional recovery, hence a CVP >11 mmHg is accepted value. Wilson et al. favored CVP in the range of 10-12 mmHg for better renal perfusion.[8] Other authors advocate that intra-operative fluid hydration might not be mandatory.[6]
Our study revealed, the target CVP was kept around 7 mm of Hg because the risk of pulmonary edema increases if CVP values are raised.[5] The mean CVP in our study population was 13.9 mm of Hg thereby showing that all patients were well hydrated. The CVP of 12 mmHg was taken as the cut-off value in our study. We then compared serum creatinine trend in the first 5 days after renal transplant. The results in our study further showed that keeping CVP more than 12 mmHg or <12 mmHg has no statistical significant difference on the fall in serum creatinine levels in first 5 days (P > 0.05). Gasperi et al. also showed no increase in delayed graft function with more restrictive hydration regime (CVP 7-9 mmHg). Our study focused on the early biochemical outcomes of the recipient. The early biochemical function has a reflection on delayed outcome of the graft and recipient complication.[4,9]
Central venous pressure invariably declines in the late intra-operative and immediate postoperative period despite positive fluid balance and vigorous fluid resuscitation. The etiology is unclear but may be due to increased vascular permeability and alterations in vascular tone accompanying surgery. Fluid redistribution in different compartments as a result of preexisting vascular permeability may also be responsible for decrease in CVP. The maximum decrease in CVP values occurred between the operating room and intensive care unit.[7] Graft ATN has been reported to be lower in patients who are vigorously hydrated.[10] Ferris et al. found no correlation between the decrease in CVP and fluid balance although the fluid balance of recipients correlated strongly with immediate postoperative graft function.
The MAP is an important parameter deciding organ perfusion. A MAP above 60 mmHg is important for vital organ perfusion like brain, heart and kidney. We further divided our patient into two groups based on MAP. First group had MAP <100 and second group had MAP >100 mmHg. In our study, the MAP varied between 95 and 131 mmHg. We did not find any difference in the biochemical outcome (drop in serum creatinine levels) in both the groups. A study by Tóth et al. showed that the rise in serum creatinine levels with MAP below 80 mmHg, and serum creatinine decreased in patients with MAP above 100 mmHg; whereas it remained stable in patients with MAP of 80–100 mmHg.[11] Campos et al.[6] also showed lower graft survival (P = 0.04) in patients with MAP <93 mmHg. The results in our study did not show any change in serum creatinine levels probably because at no point of time our MAP dropped below 95 mmHg. We maintained MAP >95 mmHg with the judicious use of fluids and vasopressors in form of dopamine (2-5 mcg/kg/min).
In our study, we evaluated only serum creatinine as the marker of graft function, diuresis (immediate or delayed) was not taken into account in our study. All the transplants were from live related donors, and none of the patient required early postoperative dialysis. In our study, the average Hb was 7.63 g/dL. We invariably transfused our recipients with one to two units of packed cell after declamping. Several studies have recommended albumin as the colloid of choice for use in case of renal transplant as it is likely to produce intravascular volume expansion and achieve a prompt restoration of blood flow. Transfusion of blood in such situations has been reported to have a higher rate of rejection and chronic graft dysfunction.[9] Our patient population had low preoperative Hb and considering the intra-operative blood loss transfusing blood was essential. In our study normal saline (2-2.5 L) was the fluid used for raising the CVP and injection furosemide 100 mg was given at the time of declamping to aid early graft function. Loop diuretics inhibit NaK-ATPase, decreasing tubular oxygen consumption, which may offer some protection against ischemic injury.[12] Osmotic and loop diuretics (mannitol and furosemide), dopamine, and dobutamine have been proposed as aiding kidney function after reperfusion. Of these, only mannitol has been shown to decrease the incidence of ATN.[7] Normal saline is the intra-operative intravenous fluid of choice in renal transplant recipients. Potassium-containing fluids, such as lactated Ringer's solution, are avoided in order to minimize the risk of development of hyperkalemia. Metabolic acidosis may develop following the administration of large volumes of normal saline.[13]
Our study population had both genetically related and genetically unrelated renal donors. Hence, we further divided our patients into two groups and observed for the fall in creatinine trends. All the 5 days mean serum creatinine values were compared in these two genetically related and genetically unrelated renal donor groups. There was no significant difference in 5 postoperative day creatinine in the two groups. The limitations of this study were its retrospective nature and its associated sample bias.
Conclusion
We conclude that the patient should be adequately hydrated throughout renal transplant surgery with normal saline (2-2.5 L) in preparation for reperfusion of the graft. Close monitoring of the CVP, maintaining MAP above 95 mmHg can prevent hypotension and hence graft dysfunction. Hence optimizing the CVP at the time of declamping to 12 mmHg ensures good early graft function. However, this conclusion should be interpreted with caution because of lack of long-term follow-up data and variable duration of cold ischemia time in the presence of multiple vessel anastomosis or any coincident unexpected surgical problems.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341:1725–30. doi: 10.1056/NEJM199912023412303. [DOI] [PubMed] [Google Scholar]
- 2.So SK, Chang PN, Najarian JS, Mauer SM, Simmons RL, Nevins TE. Growth and development in infants after renal transplantation. J Pediatr. 1987;110:343–50. doi: 10.1016/s0022-3476(87)80491-2. [DOI] [PubMed] [Google Scholar]
- 3.Sprung J, Kapural L, Bourke DL, O’Hara JF., Jr Anesthesia for kidney transplant surgery. Anesthesiol Clin North America. 2000;18:919–51. doi: 10.1016/s0889-8537(05)70202-9. [DOI] [PubMed] [Google Scholar]
- 4.De Gasperi A, Narcisi S, Mazza E, Bettinelli L, Pavani M, Perrone L, et al. Perioperative fluid management in kidney transplantation: Is volume overload still mandatory for graft function? Transplant Proc. 2006;38:807–9. doi: 10.1016/j.transproceed.2006.01.072. [DOI] [PubMed] [Google Scholar]
- 5.Lemmens HL. Kidney transplantation: Recent developments and recommendations for anesthetic management. Anesthesiol Clin North America. 2004;22:651–62. doi: 10.1016/j.atc.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 6.Campos L, Parada B, Furriel F, Castelo D, Moreira P, Mota A. Do intraoperative hemodynamic factors of the recipient influence renal graft function? Transplant Proc. 2012;44:1800–3. doi: 10.1016/j.transproceed.2012.05.042. [DOI] [PubMed] [Google Scholar]
- 7.Ferris RL, Kittur DS, Wilasrusmee C, Shah G, Krause E, Ratner L. Early hemodynamic changes after renal transplantation: Determinants of low central venous pressure in the recipients and correlation with acute renal dysfunction. Med Sci Monit. 2003;9:CR61–6. [PubMed] [Google Scholar]
- 8.Wilson WC, Aronson S. Oliguria. A sign of renal success or impending renal failure? Anesthesiol Clin North America. 2001;19:841–83. [PubMed] [Google Scholar]
- 9.Dawidson IJ, Sandor ZF, Coorpender L, Palmer B, Peters P, Lu C, et al. Intraoperative albumin administration affects the outcome of cadaver renal transplantation. Transplantation. 1992;53:774–82. doi: 10.1097/00007890-199204000-00014. [DOI] [PubMed] [Google Scholar]
- 10.Hestin D, Mertes PM, Hubert J, Claudon M, Mejat E, Renoult E, et al. Relationship between blood pressure and renin, angiotensin II and atrial natriuretic factor after renal transplantation. Clin Nephrol. 1997;48:98–103. [PubMed] [Google Scholar]
- 11.Tóth M, Réti V, Gondos T. Effect of recipients’ peri-operative parameters on the outcome of kidney transplantation. Clin Transplant. 1998;12:511–7. [PubMed] [Google Scholar]
- 12.Esson ML, Schrier RW. Diagnosis and treatment of acute tubular necrosis. Ann Intern Med. 2002;137:744–52. doi: 10.7326/0003-4819-137-9-200211050-00010. [DOI] [PubMed] [Google Scholar]
- 13.Schmid S, Jungwirth B. Anaesthesia for renal transplant surgery: An update. Eur J Anaesthesiol. 2012;29:552–8. doi: 10.1097/EJA.0b013e32835925fc. [DOI] [PubMed] [Google Scholar]


