Abstract
Although IgA is the most abundantly produced immunoglobulin in humans, its role in preventing HIV-1 acquisition, which occurs mostly via mucosal routes, remains unclear. In our passive mucosal immunizations of rhesus macaques (RMs), the anti-HIV-1 neutralizing monoclonal antibody (nmAb) HGN194, given either as dimeric IgA1 (dIgA1) or dIgA2 intrarectally (i.r.), protected 83% or 17% of the RMs against i.r. simian-human immunodeficiency virus (SHIV) challenge, respectively. Data from the RV144 trial implied that vaccine-induced plasma IgA counteracted the protective effector mechanisms of IgG1 with the same epitope specificity. We thus hypothesized that mucosal dIgA2 might diminish the protection provided by IgG1 mAbs targeting the same epitope.
To test our hypothesis, we administered HGN194 IgG1 intravenously (i.v.) either alone or combined with i.r. HGN194 dIgA2. We enrolled SHIV-exposed, persistently aviremic RMs protected by previously administered nmAbs; RM anti-human IgG responses were undetectable. However, low-level SIV Gag-specific proliferative T-cell responses were found. These animals resemble HIV-exposed, uninfected humans, in which local and systemic cellular immune responses have been observed.
HGN194 IgG1 and dIgA2 used alone and the combination of the two neutralized the challenge virus equally well in vitro. All RMs given only i.v. HGN194 IgG1 became infected. In contrast, all RMs given HGN194 IgG1 + dIgA2 were completely protected against high-dose i.r. SHIV-1157ipEL-p challenge. These data imply that combining suboptimal defenses at the mucosal and systemic levels can completely prevent virus acquisition. Consequently, active vaccination should focus on defense-in-depth, a strategy that seeks to build up defensive fall-back positions well behind the fortified frontline.
Keywords: IgA, IgG, Complete protection, Passive immunization, Rhesus monkey, SHIV-C mucosal challenge
1. Introduction
The partially successful RV144 trial has opened new horizons for HIV-1 vaccine design while posing new challenges for researchers [1]. Post-trial analyses revealed an inverse correlation between IgG antibodies (Abs) specific for the variable loops 1 and 2 (V1V2) of the HIV-1 envelope (Env) and the risk of HIV-1 infection. A direct correlation between plasma Env-specific IgA and the risk of HIV-1 infection was also observed [2], although vaccinees with high Env-specific plasma IgA were not more likely to become infected than placebo recipients. Additionally, antibody-dependent cellular cytotoxicity (ADCC) responses in the presence of low plasma concentration of anti-Env IgA correlated with reduced risk of infection. These findings suggested that Env-specific circulating IgA impeded the protective effects of IgG Abs. Secondary analyses showed that vaccinees with plasma IgA specific to the first conserved region (C1) of HIV-1 Env gp120 had a higher risk of infection than vaccinees without C1-specific IgAs [2]. C1-specific monoclonal Abs (mAbs) isolated from RV144 vaccinees and expressed as IgG1 showed HIV-1-specific ADCC-mediated cell killing [3]. Of note, two of these ADCC-mediating IgG1 mAbs, namely CH29 and CH38, were originally of IgA2 and IgA1 isotypes, respectively. Later, Tomaras et al. [4] demonstrated that the C1 epitope recognized by total plasma IgA and mAbs CH29 and CH38 expressed as IgA2 overlapped with the epitopes of IgG1 mAbs within the same Env region. Remarkably, mAb CH38 expressed as IgA2 (originally IgA1) inhibited ADCC activity of C1-specific IgG1 mAbs isolated from RV144 vaccinees, while mAb CH29 expressed as IgA2 (originally IgA2) did not [4]. Since mucosal samples had not been collected during the RV144 trial, the question remains as to how IgA and IgG1 with the same epitope specificity would interact in the mucosal compartment.
Most existing vaccines are administered intramuscularly or subcutaneously and induce both systemic IgG and IgA antibody responses. However, robust mucosal IgA responses with such vaccines are rarely generated (reviewed in [5]). In contrast, intranasal and oral vaccination strategies induce strong mucosal IgA as well as serum IgG responses and have been successfully implemented against the number of infectious agents (reviewed in [5,6]). Mucosal immunization of rhesus monkeys (RMs) with HIV or SIV antigens led to the development of specific IgA responses in vaginal and rectal fluids [7–9]. RMs immunized via both the intramuscular and intranasal routes with HIV-1 gp41-subunit antigens grafted onto virosomes were completely protected from persistent systemic infection with SHIV-SF162P3 and showed gp41-specific vaginal transcytosis-blocking IgAs as well as vaginal IgGs with neutralizing and/or ADCC activities [10]. In separate experiments, Lehner et al. [11] used targeted iliac lymph node immunization with SIV antigens to induce mucosal and systemic Ab responses in macaques. IgG and IgA responses were induced in both compartments. These authors could link vaccine protection with IgA-secreting cells in the iliac lymph nodes. In contrast to active immunization that induces both systemic and mucosal responses, passive mucosal immunization with different IgA isotypes can provide important insights into the mechanisms of protection at the mucosa and the role of IgAs in the prevention of virus acquisition.
The RM/primate immunodeficiency virus model is widely used for HIV-1 vaccine research to reflect vaccination of HIV-1-naïve individuals. However, it is likely that most people are exposed to live HIV-1 without becoming systemically infected. Thus, the ability of the naïve RM model to predict possible outcomes in humans with a history of exposure to live HIV-1 is unknown. In this regard, a non-human primate model using simian-human immunodeficiency virus (SHIV)-exposed but uninfected macaques will reflect the real-life situation where prospective recipients of an AIDS vaccine are not naïve but have a history of HIV-1 exposure that did not result in seroconversion.
In our recent RM study, passive intrarectal (i.r.) immunization with a dimeric IgA1 (dIgA1) version of the anti-V3 loop crown mAb, HGN194 [12], completely protected 83% of RMs against i.r. SHIV challenge [13]. In contrast, the dIgA2 form of the same mAb protected only 17% of RMs. In the same study, the IgG1 version of HGN194 given i.r. prevented infection in 33% of passively immunized animals.
The ratio of IgA1 and IgA2 varies in different human mucosal fluids, with IgA1 percentages in male genital secretions and nasal fluids reaching 80–90% and 60% in saliva. Female genital secretions and rectal fluids contain approximately 60% IgA2 (reviewed in [14]). Among primates, only some great apes have two IgA isotypes, and all macaques, including RMs, possess only one IgA version, which is structurally similar to human IgA2 [15,16]. In this regard, assessing the relationship between mucosal dIgA2 and systemic IgG with the same epitope specificity and elucidating the role of this combination against immunodeficiency virus acquisition is important and timely. Here, we used passive immunization – the administration of pre-formed antibodies to a naïve host – as a classical research tool to dissect the role of different antibodies and their interaction in the prevention of virus acquisition.
The potentially negative role of IgA responses in the RV144 trial mentioned above led us to hypothesize that mucosal dimeric IgA2 could compromise the protective effect of IgG1 of the same epitope specificity. We present the results of a study using systemic infusion of IgG1 and i.r. application of dIgA2 mAbs with the same epitope specificity performed in RMs that had been previously exposed to SHIV but remained aviremic and seronegative. Unexpectedly, all RMs treated with the combination of HGN194 IgG1 + dIgA2 were completely protected against mucosal SHIV challenge.
2. Materials and methods
2.1. Cell lines, reagents, and virus
The following reagent was obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH: TZM-bl cells from Dr. John C. Kappes, Dr. Xiaoyun Wu and Tranzyme Inc. A3R5 cells were kindly provided by Dr. David Montefiori. MAb Fm-6 and VRC01 were kindly provided by Drs. Wayne Marasco (Dana-Farber Cancer Institute) and John Mascola (Vaccine Research Center, NIH), respectively. The SHIV-1157ipEL-p stock (grown in RM PBMC) had a p27 concentration of 792 ng/ml and 7.8 × 105 50% tissue culture infectious doses (TCID50)/ml as measured in TZM-bl cells. Recombinant HGN194 mAb forms were prepared as described previously [13].
2.2. Animals
RMs were housed at the Yerkes National Primate Research Center (YNPRC, Atlanta, GA, USA) in accordance with standards of the National Institutes of Health Guide for the Care and Use of Laboratory Animals. YNPRC is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC). Animal experiments were approved by the Institutional Animal Care and Use Committees at Emory University and the Dana-Farber Cancer Institute (DFCI) via a Collaborating Institution Animal Use Agreement. Blood was collected under ketamine or Telazol anesthesia.
2.3. Lymphocyte proliferation assay
PBMC were stained with CFSE (CellTrace™ CFSE Cell Proliferation Kit, Invitrogen) and incubated with or without SIVmac239 Gag peptides (2 μg/ml for each peptide). The peptides (obtained through ARRRP) were 15-mers with an 11-amino acid overlap between sequential peptides and represented the complete protein sequence. Cells without any stimuli were used to determine background proliferation. After incubation for 5 days at 37 °C, cells were stained with anti-CD3-Alexa Fluor 700 (clone SP34-2), anti-CD4-PerCP (clone L200), and anti-CD8-PE (clone RPA-T8) Abs (all from BD Pharmingen). After fixation, at least 10,000 CD3+ cells were acquired by flow cytometry, and data were analyzed using FACS-Diva (BD Biosciences) software. The percentages of proliferating CD3+CD4+ and CD3+CD8+ cells were determined by CFSE dilution; background proliferation (without stimulation) was subtracted.
2.4. ELISAs
To evaluate HGN194 IgG1 pharmacokinetics, ELISA plates (Nunc) were coated with 1 μg/ml of SHIV-1157ip gp120 in PBS. After washing, plates were blocked with 4% non-fat dry milk (Bio-Rad), 0.05% Tween-PBS (blocking buffer). Plates were then incubated with serial dilutions of RM plasma samples in triplicates. HGN194 IgG1 was included as a standard ranging from 0.1 to 31 ng/ml. After washing, plates were developed by incubation for 1 h with goat anti-human IgG HRP-conjugated Ab (Jackson Immunoresearch) or goat anti-human HRP-conjugated Ab that had been adsorbed with monkey IgG (Southern Biotech) to avoid cross-reactivity with monkey anti-gp120 Abs. Color reaction was performed with TMB solution (Life Technologies).
Analysis of RM plasma binding to SHIV-1157ip gp120 was done essentially as described above. Plates were coated with gp120, blocked and incubated with RM plasma samples at different dilutions. To detect binding, plates were incubated with mouse monoclonal anti-monkey IgG HRP-conjugated Ab with no cross-reactivity to human IgG (Southern Biotech) and developed with TMB solution.
To evaluate RM anti-human IgG responses, plates were coated with 1 μg/ml of HGN194 IgG1 in carbonate buffer, pH 9.6. After blocking and incubation with RM plasma samples, plates were probed with mouse monoclonal anti-monkey IgG HRP-conjugated Ab with no cross-reactivity to human IgG (Southern Biotech) and developed with TMB solution.
2.5. Passive immunization and mucosal SHIV-1157ipEL-p challenge
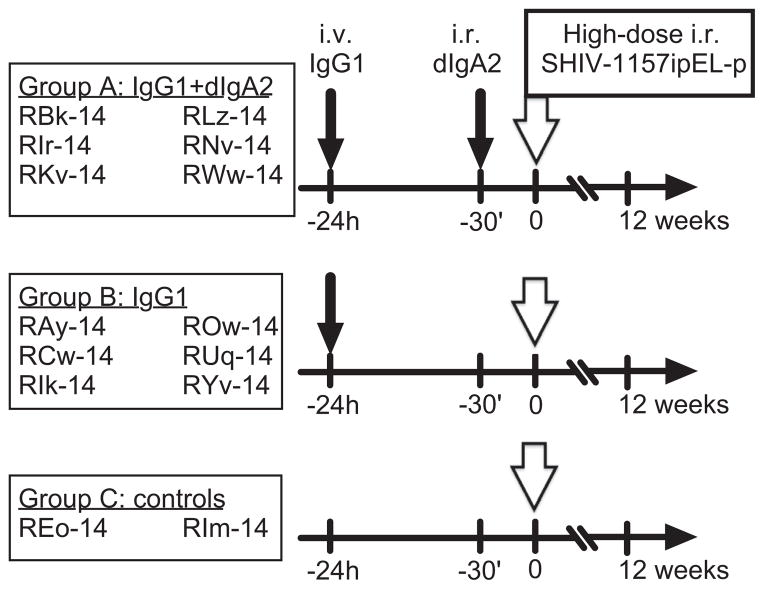
All RMs were Mamu B*008 and B*017 negative and aged between 12 to 16 months at the time of challenge. Mamu A*001-positive animals were evenly distributed in each group (Table 1), as were RMs with different FcγRIIIa genotypes (data not shown). As depicted on Fig. 3, Group A RMs (n = 6) were treated i.v. with 1.45 mg/kg of HGN194 IgG1 at −24 h, and i.r. with 1.25 mg (in 2.1 ml of PBS) of HGN194 dIgA2 30 min before challenge. The six macaques of Group B were treated i.v. with 1.45 mg/kg of HGN194 IgG1 only at −24 h. The control Group C consisted of two untreated animals. All monkeys were challenged i.r. with 31.5 50% animal infectious doses (AID50) of the R5 SHIV-1157ipEL-p, a biological isolate [17].
Table 1.
Group reassignment of virus-experienced uninfected RMs.
| Groups | Animal | HGN194 version | Type of previous mAb treatment | MHC allele | TRIM5α genotype restriction | Reference | ||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| A*001 | B*008 | B*017 | ||||||
| Group A (IgG1 + dIgA2) | RBk-14 | IgG1wt | i.v. | – | – | – | Moderate | – |
| RIr-14 | IgG1kif | i.v. | – | – | – | High | – | |
| RKv-14 | IgG1wt | i.r. | – | – | – | High | [13] | |
| RLz-14 | dIgA1 | i.r. | – | – | – | High | [13] | |
| RNv-14 | dIgA1 | i.r. | + | – | – | Moderate | [13] | |
| RWw-14 | dIgA1 | i.r. | – | – | – | High | [13] | |
| Group B (IgG1) | RAy-14 | dIgA1 | i.r. | – | – | – | Moderate | [13] |
| RCw-14 | IgG1wt | i.r. | – | – | – | Moderate | [13] | |
| RIk-14 | IgG1wt | i.v. | + | – | – | Moderate | – | |
| ROw-14 | dIgA1 | i.r. | – | – | – | High | [13] | |
| RUq-14 | IgG1kif | i.v. | – | – | – | High | – | |
| RYv-14 | dIgA2 | i.r. | – | – | – | High | [13] | |
| Group C (controls) | REo-14 | IgG1wt | i.v. | + | – | – | Moderate | – |
| RIm-14 | IgG1kif | i.v. | – | – | – | Moderate | – | |
All RMs had no anti-HIV Env Ab responses at the time of the 2nd virus challenge. IgG1wt, wild type of HGN194 IgG1; IgG1kif, afucosylated version of HGN194 IgG1.
Fig. 3.
Study timeline and design. Three groups of RMs were enrolled. Group A (n = 6) received the combination of i.v. HGN194 IgG1 (1.45 mg/kg); and i.r. HGN194 dIgA2 (1.25 mg). Group B RMs (n = 6) received i.v. HGN194 IgG1 (1.45 mg/kg) only. Group C (n = 2) RMs served as virus-only controls. Small arrow, mAb administrations; big open arrow, 24 h after IgG1 administration and 30 min after dIgA2 topical application (Group A only) animals were challenged i.r. with 31.5 AID50 of SHIV-1157ipEL-p.
2.6. Plasma viral RNA levels
Plasma vRNA was isolated by QiaAmp Viral RNA Mini-Kits (Qiagen, Germantown, MD, USA); vRNA levels were measured by quantitative reverse-transcriptase polymerase chain reaction (RT-PCR) for SIV gag sequences [18,19]. Assay sensitivity was 50 vRNA copies/ml. Time to first detection of viremia was analyzed by Kaplan–Meier analysis.
2.7. In vitro neutralization assays
For all the assays, mAbs were incubated with virus for 1 h at 37°C and then the cells were added to the mixture. The TZM-bl assay was performed as described [20]. In brief, virus was added to cells in the presence of DEAE-dextran (Sigma), washed 1x on day 1 and luminescence was measured on day 2 using luciferase substrate Bright-Glo (Promega). The A3R5 cell-based assay was performed as described [21] with NL.LucR-1157ipEL virus encoding the env gene of SHIV-1157ip-EL [22] and Renilla luciferase [23]. Human PBMC-based assays were performed as described [24].
2.8. Inhibition of transcytosis
HEC-1A cell (ATCC) monolayers were created on 0.4 μm polyethylene terephthalate (PET) membrane hanging transwell inserts (Millipore). Electrical resistance of >400 Ohms across the membrane confirmed monolayer integrity. Cell-free SHIV-1157ipEL-p (2 ng/ml of p27) was preincubated for 1 h at 37 °C alone or with various concentrations of HGN194 dIgA1, HGN194 dIgA2, or IgG1, or control IgG1 Fm-6. Next, virus or virus/mAb mixtures were added to the apical surface of the cell monolayer in the upper chamber. After 12 h, fluid in the lower chamber (“subnatant fluid”) was collected and used to measure viral RNA copy numbers by RT-PCR [18,19].
2.9. Statistical analysis
Statistical analyses were performed using Graph Pad Prism for Windows, version 6 (Graph Pad Software Inc., San Diego, CA).
3. Results
3.1. Animal selection and analysis of immune responses
The current study used RMs that had remained aviremic and seronegative during two separate, earlier experiments involving passive immunization with mAb HGN194 followed by i.r. SHIV challenge. The human IgG1 neutralizing mAb (nmAb) HGN194, isolated from a long-term non-progressor infected with HIV-1 clade AG, targets the V3-loop crown and protects against cross-clade SHIV challenge in vivo [12,24]. The use of previously exposed animals recapitulates the common scenario in humans, where any given HIV-1 exposure results in a low incidence of systemic infection and where non-transmitting exposures result in local and systemic immune responses in some individuals.
The first study involved topical (i.r.) application of HGN194 dIgA1, dIgA2 or IgG1 [13]. A second, unpublished experiment sought to elucidate the role of nmAb effector functions in protection against i.r. SHIV challenge. In this second study, RMs had been treated intravenously (i.v.) with wild-type HGN194 IgG1 (IgG1wt), its LALA mutant (IgG1LALA) in which binding to the Fcγ receptor (FcγR) was abrogated thereby deleting effector functions, or with an afucosylated version (IgG1kif) of HGN194 IgG1 that had increased binding to FcγRIII, respectively (unpublished data). In both studies, the macaques had been challenged i.r. with 31.5 50% animal infectious doses (AID50) of the R5 clade C SHIV-1157ipEL-p [17].
All RMs selected for the current study were persistently aviremic (as measured by a sensitive RT-PCR assay [19]) and were seronegative by SIV Gag ELISA (data not shown). We assessed preselected animals for cellular and humoral immune responses to viral proteins. SIV Gag-specific proliferative CD4+ and CD8+ T-cell responses were measured at four to eight weeks after the earlier viral challenge (Fig. 1). Although persistently aviremic, 9 out of 14 macaques had measurable proliferation of CD4+ cells, and 11 animals showed CD8+ cell proliferation in response to stimulation with SIV Gag peptides; three RMs had marginal responses and animal RBk-14 showed no reaction. In general, proliferation of CD8+ cells was weaker than that of CD4+ lymphocytes.
Fig. 1.
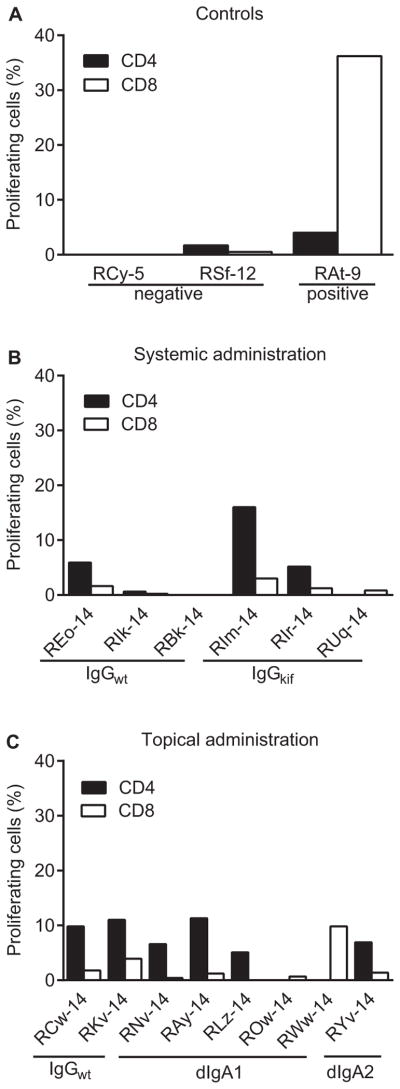
Antiviral T-cell responses after previous SHIV-1157ipEL-p challenge (unpublished data and [13]). PBMC were stimulated with overlapping peptides representing SIVmac239 Gag and proliferation of CD4+ and CD8+ cells was measured using the CFSE dilution method as described in Materials and Methods. The y-axis indicates % proliferating cells. PBMC isolated from two naïve macaques (RCy-5 and RSf-12) were used as a negative control and PBMC from a previously vaccinated, aviremic animal RAt-9 [47] served as a positive control, respectively. (A) Positive (RAt-9) [47] and negative (RCy-5 and RSf-12) controls. (B) T-cell responses of RMs that had received wild-type (IgG1wt ) or afucosylated (IgG1kif ) versions of HGN194 IgG1 systemically (i.v.) (unpublished data). (C) T-cell responses of animals that had previously received HGN194 IgG1, dimeric IgA1 or dimeric IgA2 topically (i.r.) (unpublished data and [13]).
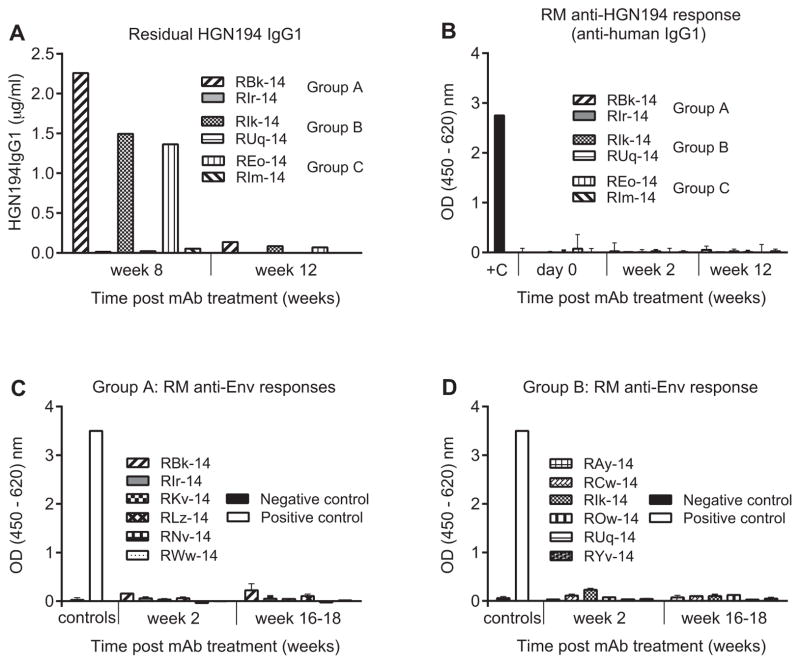
To analyze possible humoral immune responses among the preselected RMs, we first analyzed the residual plasma concentration of HGN194 IgG1 in all animals that had received the HGN194 mAbs systemically in a previous, unpublished study (Fig. 2A). At week 8 after the mAb passive transfer and virus challenge, the concentration of HGN194 IgG1wt was about 2 μg/ml and the IgG1kif concentration was marginally above background. Four weeks later, IgG1wt was detected as low as at 0.14, 0.07 and 0.08 μg/ml in the plasma of RMs RBk-14, REo-14, and RIk-14, respectively. At the same time, the mAb concentration in the plasma of HGN194 IgG1kif -treated macaques fell below the detection limit. Of note, the average in vivo 90% plasma inhibitory concentration (IC90) of HGN194 IgG1 was estimated at 2.15 μg/ml [24]. To allow complete clearance of previously infused mAbs, the current experiment was scheduled at 16–18 weeks after the initial mAb administration. Additionally, we tested plasma samples of animals RBk-14, REo-14 and RIk-14 collected on the day of new mAb administration for any remaining HGN194 IgG1. As expected, no mAb was detected just before re-administration (not shown).
Fig. 2.
Antibody responses in RMs previously given passive immunization with different forms of HGN194 (unpublished data and [13]). (A) and (B) Only animals that had received HGN194 systemically were analyzed. Mucosally treated RMs had been tested earlier and HGN194 had not been detected in the plasma (data not shown). (A) Residual concentration of HGN194 IgG1 at different time points after administration. HGN194 IgG1 was used as a standard. Secondary goat anti-monkey HRP-conjugated Ab was RM IgG adsorbed. (B) RM anti-human IgG responses at different time points after HGN194 IgG1 i.v. administration. +C, positive control (goat anti-human Ab HRP-conjugated). (C) and (D) HIV Env binding ELISA analysis of RM plasma samples collected at different time points after virus challenge. (C) Anti-Env plasma responses of Group A RMs. Black bar, pooled naïve RM plasma was used as a negative control; open bar, plasma of RRi-11 [48] was used as positive control. SHIV-1157ip gp120 served as antigen. The secondary Ab was mouse anti-monkey HRP-conjugated secondary Ab with minimal cross-reactivity to human IgG. (D) Anti-Env plasma responses of Group B RMs. Experimental conditions are same as for (C).
Next, we analyzed the RMs that had received the human mAbs HGN194 IgG1wt and IgG1kif systemically for possible anti-human IgG antibody responses. During recurrent administrations of human IgG, these anti-species Abs, if developed, might cause adverse reactions and rapid elimination of human mAbs from the circulation. Importantly, none of the HGN194-treated RMs had developed any RM anti-human IgG Ab responses (Fig. 2B) as a consequence of their prior treatment with HGN194 IgG1.
Using ELISA, we next confirmed that the passively immunized, protected RMs had not mounted any anti-HIV-1 Env Ab responses of their own (Fig. 2C and D). The secondary Ab in the ELISA was specific for RM IgGs only. No reactivity was seen, as expected from the negative SIV Gag ELISA data (not shown). Thus, the animals did not have any Ab responses that may have skewed the new passive immunization/SHIV challenge study.
3.2. Group assignment and study design
Animals that had earlier received different versions of HGN194 mAb through different routes were distributed evenly between two new experimental groups (Table 1). Each group contained two RMs that had received HGN194 IgG1 systemically and four RMs treated topically. The control group consisted of two macaques that had received HGN194 IgG1 systemically. MHC alleles and TRIM5α genotypes also were distributed evenly among the new groups (Table 1).
The experimental timeline of the current study is depicted in Fig. 3. RMs in both Groups A and B received HGN194 IgG1 i.v. at 1.45 mg/kg 24 h before the viral challenge. RMs of Group A were additionally treated with 1.25 mg of HGN194 dIgA2 applied i.r. (1.25 mg of mAb in 2.1 ml of phosphate-buffered saline (PBS)) 30 min before the virus challenge. Control Group C macaques were left untreated. All animals were challenged i.r. with 31.5 AID50 of SHIV-1157ipEL-p [17], an R5 clade C SHIV, and monitored prospectively by measuring of plasma viral RNA (vRNA) loads.
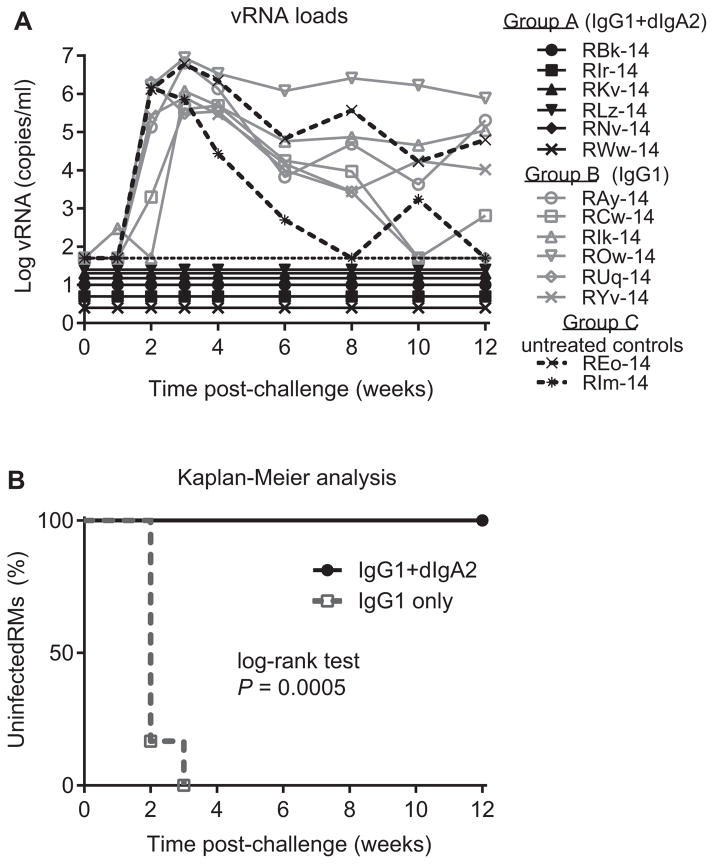
3.3. The combination of IgG1 + dIgA2 versions of HGN194 completely protects RMs from single high-dose SHIV challenge
The single mucosal high-dose challenge with SHIV-1157ipEL-p resulted in systemic infection of all macaques of Group B (HGN194 IgG1 only) by week 3 (Fig. 4A). Control animals (Group C) were viremic as well. Surprisingly, all Group A RMs, which had received the combination of IgG1 + dIgA2, remained aviremic. The time to vRNA load >50 copies/ml for Groups A and B animals was compared by Kaplan–Meier analysis using the log-rank test with two-sided P-values (Fig. 4B). The combination of IgG1 + dIgA2 demonstrated significantly better protection against mucosal SHIV-1157ipEL-p challenge compared with IgG1 alone (P = 0.0005). The shorter half-life of IgG1 in Group B RMs can be explained by absorption and removal of IgG1 from the circulation by newly replicating virus.
Fig. 4.
The combination of HGN194 IgG1 + dIgA2 completely protected RMs from high-dose mucosal virus challenge. (A) Gray open symbols, viral RNA loads for individual RMs for Group A (IgG1 + dIgA2); black filled symbols, vRNA loads for Group B (IgG1) RMs; dashed black lines, vRNA loads for Group C (controls) RMs. (B) Kaplan–Meier analysis of time until vRNA load exceeded 50 copies/ml. Log rank test significance P value is indicated. Gray dashed line, Group A; black solid line, Group B.
In our previous experiment, the same virus challenge caused systemic infection of five out six RM treated with the same dose of dIgA2 i.r. [13]. The results of another, yet unpublished study with different IgG1 versions of HGN194 demonstrated infection of four out seven macaques infused i.v. with 1 mg/kg of IgG1wt. Taken together, these results indicate that the combination of systemic IgG1 and topical dIgA2 treatments yielded better protection compared with individual mAb treatment alone.
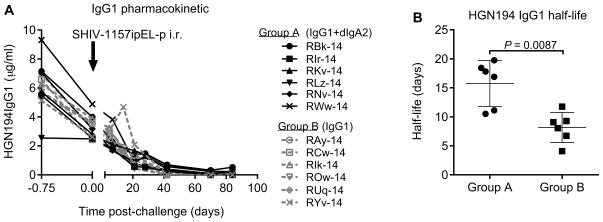
3.4. HGN194 IgG1 pharmacokinetics and plasma neutralization capacity
The IgG1 pharmacokinetics were analyzed by ELISA. The infused IgG1 mAb showed the classical circulation profile in both groups of macaques (Fig. 5A) following virus challenge. RMs of Group B cleared IgG1 faster compared with animals of Group A (Fig. 5B). Mean half-lives of HGN194 IgG1 were calculated at 15.8 ± 3.9 days for Group A and 8.2 ± 2.6 days for Group B RMs (P = 0.0087, Mann–Whitney test).
Fig. 5.
Analysis of HGN194 IgG1 levels in plasma. (A) HGN194 IgG1 pharmacokinetics in RM groups. Black arrow indicates SHIV-1157ipEL-p challenge; black filled symbols, RM of Group A; gray open symbols, RMs of Group B. (B) Analysis of HGN194 IgG1 half-life in RMs. Circles, RMs of Group A; squares, RMs of Group B. Statistical analysis was performed by Mann–Whitney test (P < 0.05).
Although HGN194 IgG1 demonstrated a shorter half-life in Group B RMs, mAb concentrations on the day 0, the day of virus challenge, were similar for Groups A and B animals (Table 2). These concentrations were comparable to that reported previously [24] as well as to the IgG1 concentration observed during the previous experiment (unpublished data). Mean HGN194 IgG1 concentrations were 3.3 ± 0.9 μg/ml for Group A and 3.1 ± 0.5 μg/ml for Group B, respectively.
Table 2.
Concentration and IC50 of HGN194 IgG1 in RM plasma on the day of virus challenge.
| Groups | Animal # | IgG1 concentration, μg/ml | Plasma IC50, μg/ml |
|---|---|---|---|
| Group A (IgG1 + dIgA2) | RBk-14 | 4.0 | 0.54 |
| RIr-14 | 2.5 | ND | |
| RKv-14 | 2.8 | ND | |
| RLz-14 | 2.5 | 0.32 | |
| RNv-14 | 3.2 | 0.86 | |
| RWw-14 | 4.9 | 0.30 | |
| Group B (IgG1) | RAy-14 | 3.5 | 0.53 |
| RCw-14 | 2.4 | 0.41 | |
| RIk-14 | 3.9 | ND | |
| ROw-14 | 3.6 | 0.56 | |
| RUq-14 | 3.2 | 0.40 | |
| RYv-14 | 2.6 | 0.32 |
Plasma IC50 concentrations were determined using the concentration of mAb in RM plasma on the day of challenge and the dilution of this plasma sample showing 50% neutralization in the TZM-bl assay. Calculations were performed with respect to neutralization obtained with the plasma sample from the same RM taken before the mAb administration at the same dilution. ND, not determined. The experiment was performed in triplicate.
Plasma samples of Group A and B RMs collected on the day of virus challenge were able to neutralize SHIV-1157ipEL-p, the challenge virus, with the same efficiency as demonstrated by TZM-bl cell-based neutralization assays (Table 2). There was no difference between mean 50% plasma inhibitory concentration (IC50) values of Groups A (0.5 ± 0.2 μg/ml) and B (0.4 ± 0.08 μg/ml) measured in RM plasmas on the day of virus challenge. These results are in line with in vivo IC50 values observed previously for HGN194 IgG administered to infant RMs at 1 mg/kg dose [24]. Also, these data clearly demonstrate that, in spite of faster clearance of mAb by RMs of Group B, RMs of Groups A and B maintained equal concentrations of IgG1 in plasma on the day of SHIV-1157ipEL-p challenge and that mAb was able to neutralize the challenge virus with the same efficiency. Of note, all RMs in Group B with the shorter half-life were viremic, which probably resulted in faster clearance post-challenge due to immune complex formation.
3.5. Individual mAbs and combination of IgG1 + dIgA2 show the same neutralization profiles in vitro
To understand why the combination was more protective than treatment with individual mAbs, we examined the neutralization of the challenge virus in vitro by the combination of IgG1 + dIgA2. Toward this end, we used TZM-bl, A3R5 and human peripheral blood mononuclear cell (PBMC)-based assays (Fig. 6A–C). For all three assays, the differences between neutralization curves were not significant as evaluated by multiple t-tests and two-way ANOVA test for multiple comparisons (not shown).
Fig. 6.
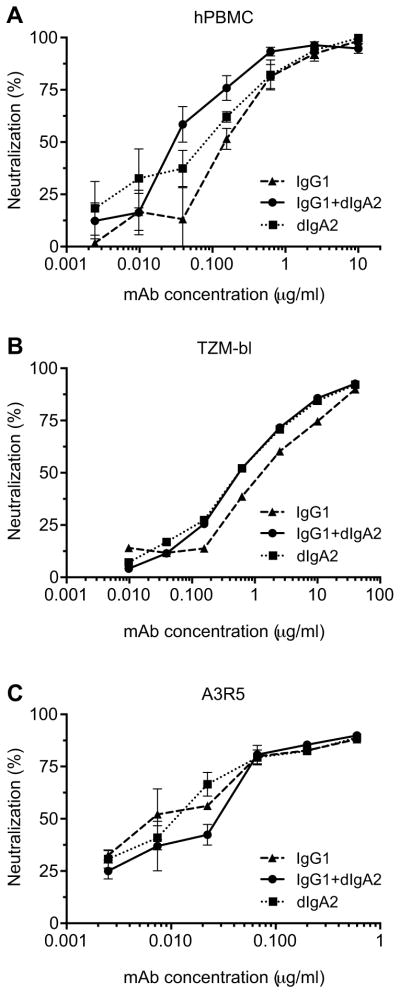
The combination of HGN194 IgG1 + dIgA2 neutralized virus equally well as the individual mAbs. The concentration of IgG1 + dIgA2 combination is the sum of concentrations of individual mAbs. MAbs VRC01 and Fm-6 were used as positive and negative controls, respectively (not shown). (A) Human PBMC-based assay; (B) TZM-bl cell assay; and (C) A3R5 cell assay.
It should be noted that in the neutralization assays, dIgA2 and IgG1 were used at the same mass concentrations. The concentration of the IgG + dIgA2 combination was the sum of mass concentrations of individual mAbs. Dimeric IgA2 has a molecular weight of ~315 kDa, compared to ~150 kDa for IgG1; thus, the molar concentration of dIgA2 taken at the same mass concentration as IgG1 is twofold lower than for IgG1. However, dIgA2 bears four Fab regions, and IgG1 has only two. Therefore, the dIgA2 solution with a twofold lower molar concentration than the IgG1 solution contained the same molar concentration of antigen combining sites as the IgG1 solution. These considerations explain the similar neutralization curves for HGN194 IgG1, dIgA2, and combination of both.
3.6. The combination of IgG1 + dIgA2 does not inhibit virus transcytosis in vitro
As we previously reported [13], only HGN194 dIgA1, but not dIgA2 or IgG1 as single agent, was able to inhibit virus transcytosis in vitro. We evaluated whether the combination of HGN194 IgG1 + dIgA2 could inhibit the transcytosis of SHIV-1157ipEL-p at pH 6. A low pH has been reported to enhance antibody-mediated virus transcytosis due to the pH-dependence of the neonatal Fc receptor (FcRn) and IgG1 interaction [25]. Additionally, the pH of colonic rectal fluid for Macaca species was reported to range between 5.1 and 7.8 [26,27]. As shown previously, the mean concentration of dIgA2 administered i.r. was 231.6 μg/ml 30 min after topical application [13].
To reflect the concentration of dIgA2 observed in vivo, we performed the transcytosis assay at 200 μg/ml of dIgA2. We used dIgA1, previously shown to effectively inhibit transcytosis [13], as a positive control. Dimeric IgA2 or dIgA1 were mixed with different concentrations of HGN194 IgG1 or the isotype control IgG1 Fm-6 (Fig. 7). As expected [23], HGN194 IgG1 alone enhanced transcytosis of SHIV-1157ipEL-p across HEC-1A cells, known to express FcRn, at pH 6 in a dose-dependent manner. DIgA1, used as a positive control, not only prevented the IgG1-mediated enhancement of transcytosis, but almost completely inhibited transcytosis at any concentration of HGN194 or isotype control IgG1 tested. DIgA2 could not abrogate the enhancement of IgG1-mediated transcytosis. The fact that the IgG1 + dIgA2 combination was unable to prevent enhanced virus transcytosis in vitro suggests that this combination is not likely to exert its protective effect in vivo through inhibition of transcytosis.
Fig. 7.
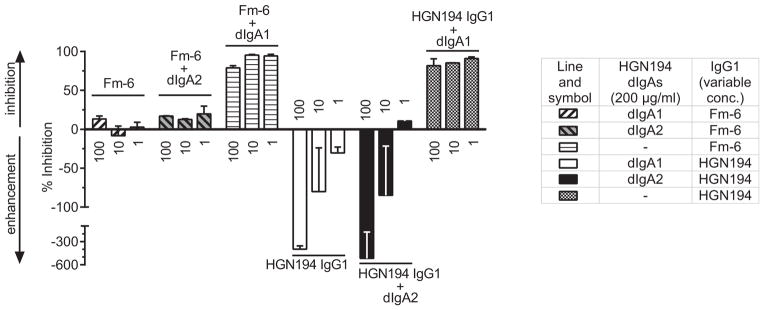
Blocking of IgG1-mediated transcytosis enhanced by low pH. Virus was incubated with the IgG1 mAbs HGN194 or Fm-6 (isotype control) ranging from 1 to 100 μg/ml. The IgG1 mAbs were used alone or in combinations with 200 μg/ml of dIgA2. Experiments involving dIgA1 served as positive control. Next, virus or virus/mAb mixtures were added to the top well of a transwell/transcytosis system (Methods). Percent of transcytosis inhibition was calculated in comparison with the number of vRNA copies determined for wells with virus alone. Negative values on the y-axis represent enhanced transcytosis. All experiments were repeated at least twice.
4. Discussion
We have shown that HIV-1 Env-specific IgG1 plasma Abs in combination with mucosal dIgA2 of the same epitope specificity completely protected RMs against high-dose mucosal challenge with SHIV-1157ipEL-p. Initially, we demonstrated that RMs that had remained aviremic in previous passive immunization experiments had developed low-level, virus-specific cell-mediated immune responses and thus represent a relevant model to assess passive immunization efficacy among HIV-1-exposed, uninfected individuals. The striking 100% protection we observed here in RMs with the combination of i.v. IgG1 plus i.r. dIgA2 mAbs was unexpected – given that the group treated with the IgG1 version alone had 0% protection and that i.r. dIgA2 had protected only 17% of RMs challenged with the same clade C SHIV earlier. Lastly, we demonstrated that inhibition of virus transcytosis, as suggested for dIgA1 [13], was unlikely to be the mechanism of protection by the combination of IgG1 + dIgA2. Thus, our findings suggest that mucosal dimeric IgA2, if generated by active immunization, will complement HIV-1-specific plasma IgG1 in preventing virus acquisition rather than diminishing the protective role of plasma IgG1.
The gastrointestinal mucosa is the largest mucosal surface in the human body, and it represents the major portal of HIV-1 entry during mother-to-child transmission via breastfeeding, sexual transmission in men who have sex with men, as well as during heterosexual anal intercourse (reviewed [28]). The risk of HIV-1 transmission through receptive anal sex was estimated by meta-analysis at 1.4% (an average of one transmission event occurred for every 71 exposures), which is at least 10 times higher than for unprotected vaginal intercourse (reviewed [29]). On another note, the distribution of IgG and IgA varies considerably between different body compartments (reviewed in [16,30]). While serum contains 3.5–14 times more IgG than IgA, IgAs are more prevalent in gastrointestinal tract secretions. Within the intestine, secretions of the digestive part contain more IgA1 than IgA2, whereas secretions of the colon generally possess slightly more IgA2 than IgA1 [30]. During chronic immunodeficiency virus infection, antiviral IgA responses tend to be low; the majority of HIV-1-infected humans as well as HIV-1-infected chimpanzees and macaques infected with SIV or SHIV have minimal IgA responses in mucosal secretions and the circulation [31–40]. Furthermore, the anti-HIV-1 IgA subtypes were frequently not defined (reviewed in [16]).
In the current study, after topical administration, dIgA2 remained localized at the rectal mucosa, because there is no IgA back-transfer from the intestinal lumen across the epithelial barrier. In contrast, IgG1 was administered i.v. and thus was distributed systemically as well as into some mucosal fluids. The Fc neonatal receptor (FcRn) can shuttle IgG in both directions; it unloads IgG or IgG-immune complex cargo in a pH-dependent manner (reviewed in [41]). Thus, our passive immunization study reflected the compartmentalization of Ab responses and addressed the vulnerability of the rectal mucosa for HIV-1 infection.
While a negative correlation was established between circulating anti-HIV-1 Env IgA in the RV144 trial, our data strongly suggest that a successful HIV-1 vaccine must generate both mucosal IgA and systemic IgG responses. Such a defensive strategy is best described by the military term “defense-in-depth” – an approach to defend a vital core by pre-planned, well-armed, multiple lines of defense that can provide backup in case the frontline is breached. Defense-in-depth against mucosal HIV-1 transmission can be described as follows. When HIV-1 virions first encounter mucus in the mucosal cavity, they are exposed to secretory IgAs (SIgAs), which may either crosslink the virions, prevent virus transcytosis across the epithelial barrier, or simply neutralize virus. In case some virions remain free, they can penetrate the mucus and the epithelium by transcytosis or other mechanism. Such virions then face ubiquitous IgG and may be neutralized by it. The neutralization capacity of antiviral IgG is limited by its affinity and by the accessibility of HIV-1 Env epitopes. In other words, the tissue and/or plasma concentration of neutralizing IgG must be high enough to neutralize incoming virus as it penetrates the mucosa.
In our current study, the concentration of HGN194 IgG1 used for i.v. administration (1.45 mg/kg) was expected to protect approximately half of the RMs from SHIV challenge based on data from our previous study, where 50% of RMs were protected with 1 mg/kg of HGN194 IgG1 [24]. However, the RMs used for the current study had developed low-level cellular immune responses as a consequence of their earlier experience to live virus; in fact, proliferative responses among CD4+ lymphocytes tended to predominate. Thus, the HGN194 IgG1 dose of 1.45 mg/kg was not protective, and all the animals of Group B became systemically infected. When applied mucosally (i.r.) as single mAbs in an earlier study [13], IgG1 protected only 33% and dIgA2 17% of RMs, respectively, against the same challenge virus. However, in the current experiment, the combination of i.v. IgG1 plus i.r. dIgA2, in which both mAbs were administered at sub-protective doses, completely prevented virus acquisition. It is reasonable to suggest that in this case, part of the challenge virus was neutralized in the rectal lumen by dIgA2 and to a lesser extent by transudated IgG1; the residual virus, which was not neutralized in the lumen and crossed the epithelium, was met and neutralized by IgG1 in tissues or in the circulation.
In order to preserve the integrity of RM rectal mucosa before the virus challenge, we did not measure the rectal concentration of IgG1 24 h after the i.v. administration. However, the approximate level of mAb in rectal mucosal secretions can be extrapolated using the ratio between plasma and rectal concentrations observed in RMs at 24 h post-administration of 3BNC117, an IgG1 nmAb, [42]. Thus, the rectal concentration of HGN194 IgG1 at the time of virus challenge, i.e., 24 h post-administration, was estimated to be ~0.05 μg/ml. This value is significantly lower than the concentration of topically administered dIgA2, which was approximately 231.6 μg/ml at the time of virus inoculation [13]. Based on the fact that free secretory component exists in mucosal secretions [43,44], it is reasonable to assume that topically administered dIgA2 binds to the endogenous free secretory component, thus representing SIgA2.
The affinity of HGN194 IgG1 and dIgA2 to the V3 loop of gp120 was comparable as shown by ELISA and Biacore analysis [13]. Both of these HGN194 versions also had the same neutralization profiles in different assays and showed the same epitope specificity by competition ELISA with V3 peptides [13]. Therefore, the phenomenon of altered mAb epitope specificity in the context of different constant regions, which had been reported for mAbs targeting other epitopes [45,46], does not apply to HGN194 dIgA2 and IgG1. This further supports the defense-in-depth provided by dIgA2 and IgG1versions of HGN194 rather than indicating an additive effect of HGN194 isotypes in the rectal lumen. Our RM model data of passive immunization with the combination of topically applied dIgA2 and systemically administered IgG1 followed by mucosal SHIV challenge imply that a successful HIV-1 vaccine should induce both systemic IgG and strong IgA responses in external secretions, where most current immunization protocols or HIV-1 infections have resulted only in low responses.
The results of the current study, taken together with our previous findings regarding the protective role of dIgA1 [13], provide a rational explanation of a possible way of preventing HIV-1 acquisition if active vaccination were to generate both mucosal IgA and systemic antibody responses. In this regard, to achieve the complete prevention of HIV-1 infection, a successful vaccine must induce different immune effectors, including HIV-1 Env-specific Abs in mucosal secretions as frontline defense, with back-up provided by neutralizing Abs in tissues and in the circulation and cytotoxic T cells, thus generating optimal defense-in-depth.
Acknowledgments
We thank Dr. J. Mascola for providing mAb VRC01, Dr. S.-L. Hu for providing SHIV-1157ip Env proteins, and Dr. W. Marasco for providing mAb Fm-6. We thank Dr. K. Rogers and K. Kinsley for TRIM5α genotype analysis, Dr. S. Lee for assistance in statistical analysis, V. Shanmuganathan for technical assistance, and Juan Esquivel for assistance with the preparation of the manuscript. This was work supported by the Bill and Melinda Gates Foundation Collaboration for AIDS Vaccine Discovery (CAVD) UCL-VDC Grant 38637 (R.A.W.). This project was also funded in part by NIH grants P01 AI048240, R01 AI100703 and R37 AI034266 to RMR. Base grant P51 OD011132 provided support to the Yerkes National Primate Research Center. The Southwest National Primate Research Center is supported by an NIH primate center base grant (previously NCRR grant P51 RR013986; currently Office of Research Infrastructure Programs/OD P51 OD011133).
Abbreviations
- Ab
antibody
- mAb
monoclonal antibody
- nmAb
neutralizing monoclonal antibody
- ADCC
antibody-dependent cellular cytotoxic activity
- AID50
50% animal infectious doses
- C1
first conserved region
- CTL
cytotoxic T lymphocyte
- dIgA
dimeric IgA
- Env
envelope
- FcγR
Fcγ receptor
- FcRn
Fc neonatal receptor
- PBS
phosphate buffered saline
- HIV-C
HIV-1 clade C
- IC50
50% inhibitory concentration
- IC90
90% inhibitory concentration
- Ig
immunoglobulin
- i.r
intrarectal
- i.v
intravenous
- RM
rhesus monkey
- SHIV
simian-human immunodeficiency virus
- TCID50
50% tissue culture infectious dose
- V1V2
variable loops 1 and 2
- vRNA
viral RNA
References
- 1.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209–20. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 2.Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med. 2012;366:1275–86. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonsignori M, Pollara J, Moody MA, Alpert MD, Chen X, Hwang KK, et al. Antibody-dependent cellular cytotoxicity-mediating antibodies from an HIV-1 vaccine efficacy trial target multiple epitopes and preferentially use the VH1 gene family. J Virol. 2012;86:11521–32. doi: 10.1128/JVI.01023-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tomaras GD, Ferrari G, Shen X, Alam SM, Liao HX, Pollara J, et al. Vaccine-induced plasma IgA specific for the C1 region of the HIV-1 envelope blocks binding and effector function of IgG. Proc Natl Acad Sci U S A. 2013;110:9019–24. doi: 10.1073/pnas.1301456110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neutra MR, Kozlowski PA. Mucosal vaccines: the promise and the challenge. Nat Rev Immunol. 2006;6:148–58. doi: 10.1038/nri1777. [DOI] [PubMed] [Google Scholar]
- 6.Ryan EJ, Daly LM, Mills KH. Immunomodulators and delivery systems for vaccination by mucosal routes. Trends Biotechnol. 2001;19:293–304. doi: 10.1016/s0167-7799(01)01670-5. [DOI] [PubMed] [Google Scholar]
- 7.Belyakov IM, Hel Z, Kelsall B, Kuznetsov VA, Ahlers JD, Nacsa J, et al. Mucosal AIDS vaccine reduces disease and viral load in gut reservoir and blood after mucosal infection of macaques. Nat Med. 2001;7:1320–6. doi: 10.1038/nm1201-1320. [DOI] [PubMed] [Google Scholar]
- 8.Kubota M, Miller CJ, Imaoka K, Kawabata S, Fujihashi K, McGhee JR, et al. Oral immunization with simian immunodeficiency virus p55gag and cholera toxin elicits both mucosal IgA and systemic IgG immune responses in nonhuman primates. J Immunol. 1997;158:5321–9. [PubMed] [Google Scholar]
- 9.Vajdy M, Singh M, Kazzaz J, Soenawan E, Ugozzoli M, Zhou F, et al. Mucosal and systemic anti-HIV responses in rhesus macaques following combinations of intranasal and parenteral immunizations. AIDS Res Hum Retrovir. 2004;20:1269–81. doi: 10.1089/aid.2004.20.1269. [DOI] [PubMed] [Google Scholar]
- 10.Bomsel M, Tudor D, Drillet AS, Alfsen A, Ganor Y, Roger MG, et al. Immunization with HIV-1 gp41 subunit virosomes induces mucosal antibodies protecting nonhuman primates against vaginal SHIV challenges. Immunity. 2011;34:269–80. doi: 10.1016/j.immuni.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 11.Lehner T, Wang Y, Cranage M, Bergmeier LA, Mitchell E, Tao L, et al. Protective mucosal immunity elicited by targeted iliac lymph node immunization with a subunit SIV envelope and core vaccine in macaques. Nat Med. 1996;2:767–75. doi: 10.1038/nm0796-767. [DOI] [PubMed] [Google Scholar]
- 12.Corti D, Langedijk JP, Hinz A, Seaman MS, Vanzetta F, Fernandez-Rodriguez BM, et al. Analysis of memory B cell responses and isolation of novel monoclonal antibodies with neutralizing breadth from HIV-1-infected individuals. PLoS ONE. 2010;5:e8805. doi: 10.1371/journal.pone.0008805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watkins JD, Sholukh AM, Mukhtar MM, Siddappa NB, Lakhashe SK, Kim M, et al. Anti-HIV IgA isotypes: differential virion capture and inhibition of transcytosis are linked to prevention of mucosal R5 SHIV transmission. AIDS. 2013;27:F13–20. doi: 10.1097/QAD.0b013e328360eac6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woof JM, Russell MW. Structure and function relationships in IgA. Mucosal Immunol. 2011;4:590–7. doi: 10.1038/mi.2011.39. [DOI] [PubMed] [Google Scholar]
- 15.Scinicariello F, Engleman CN, Jayashankar L, McClure HM, Attanasio R. Rhesus macaque antibody molecules: sequences and heterogeneity of alpha and gamma constant regions. Immunology. 2004;111:66–74. doi: 10.1111/j.1365-2567.2003.01767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou M, Ruprecht RM. Are anti-HIV IgAs good guys or bad guys? Retrovirology. 2014;11:109. doi: 10.1186/s12977-014-0109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siddappa NB, Watkins JD, Wassermann KJ, Song R, Wang W, Kramer VG, et al. R5 clade C SHIV strains with tier 1 or 2 neutralization sensitivity: tools to dissect env evolution and to develop AIDS vaccines in primate models. PLoS ONE. 2010;5:e11689. doi: 10.1371/journal.pone.0011689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cline AN, Bess JW, Piatak M, Jr, Lifson JD. Highly sensitive SIV plasma viral load assay: practical considerations, realistic performance expectations, and application to reverse engineering of vaccines for AIDS. J Med Primatol. 2005;34:303–12. doi: 10.1111/j.1600-0684.2005.00128.x. [DOI] [PubMed] [Google Scholar]
- 19.Hofmann-Lehmann R, Swenerton RK, Liska V, Leutenegger CM, Lutz H, McClure HM, et al. Sensitive and robust one-tube real-time reverse transcriptase-polymerase chain reaction to quantify SIV RNA load: comparison of one- versus two-enzyme systems. AIDS Res Hum Retrovir. 2000;16:1247–57. doi: 10.1089/08892220050117014. [DOI] [PubMed] [Google Scholar]
- 20.Montefiori DC. Evaluating neutralizing antibodies against HIV, SIV, and SHIV in luciferase reporter gene assays. Curr Protoc Immunol. 2005;Chapter 12(Unit 12.1) doi: 10.1002/0471142735.im1211s64. [DOI] [PubMed] [Google Scholar]
- 21.McLinden RJ, Labranche CC, Chenine AL, Polonis VR, Eller MA, Wieczorek L, et al. Detection of HIV-1 neutralizing antibodies in a human CD4(+)/CXCR4(+)/CCR5(+) T-lymphoblastoid cell assay system. PLoS ONE. 2013;8:e77756. doi: 10.1371/journal.pone.0077756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sholukh AM, Byrareddy SN, Shanmuganathan V, Hemashettar G, Lakhashe SK, Rasmussen RA, et al. Passive immunization of macaques with polyclonal anti-SHIV IgG against a heterologous tier 2 SHIV: outcome depends on IgG dose. Retrovirology. 2014;11:8. doi: 10.1186/1742-4690-11-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edmonds TG, Ding H, Yuan X, Wei Q, Smith KS, Conway JA, et al. Replication competent molecular clones of HIV-1 expressing Renilla luciferase facilitate the analysis of antibody inhibition in PBMC. Virology. 2010;408:1–13. doi: 10.1016/j.virol.2010.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watkins JD, Siddappa NB, Lakhashe SK, Humbert M, Sholukh A, Hemashettar G, et al. An anti-HIV-1 V3 loop antibody fully protects cross-clade and elicits T-cell immunity in macaques mucosally challenged with an R5 clade C SHIV. PLoS ONE. 2011;6:e18207. doi: 10.1371/journal.pone.0018207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gupta S, Gach JS, Becerra JC, Phan TB, Pudney J, Moldoveanu Z, et al. The Neonatal Fc receptor (FcRn) enhances human immunodeficiency virus type 1 (HIV-1) transcytosis across epithelial cells. PLoS Pathog. 2013;9:e1003776. doi: 10.1371/journal.ppat.1003776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patton DL, Sweeney YC, Cummings PK, Meyn L, Rabe LK, Hillier SL. Safety and efficacy evaluations for vaginal and rectal use of BufferGel in the macaque model. Sex Transm Dis. 2004;31:290–6. doi: 10.1097/01.olq.0000124614.91448.d4. [DOI] [PubMed] [Google Scholar]
- 27.Kararli TT. Comparison of the gastrointestinal anatomy, physiology, and biochemistry of humans and commonly used laboratory animals. Biopharm Drug Dispos. 1995;16:351–80. doi: 10.1002/bdd.2510160502. [DOI] [PubMed] [Google Scholar]
- 28.Cavarelli M, Scarlatti G. HIV-1 infection: the role of the gastrointestinal tract. Am J Reprod Immunol. 2014;71:537–42. doi: 10.1111/aji.12245. [DOI] [PubMed] [Google Scholar]
- 29.Baggaley RF, White RG, Boily MC. HIV transmission risk through anal intercourse: systematic review, meta-analysis and implications for HIV prevention. Int J Epidemiol. 2010;39:1048–63. doi: 10.1093/ije/dyq057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woof JM, Mestecky J. Mucosal immunoglobulins. Immunol Rev. 2005;206:64–82. doi: 10.1111/j.0105-2896.2005.00290.x. [DOI] [PubMed] [Google Scholar]
- 31.Artenstein AW, VanCott TC, Sitz KV, Robb ML, Wagner KF, Veit SC, et al. Mucosal immune responses in four distinct compartments of women infected with human immunodeficiency virus type 1: a comparison by site and correlation with clinical information. J Infect Dis. 1997;175:265–71. doi: 10.1093/infdis/175.2.265. [DOI] [PubMed] [Google Scholar]
- 32.Mestecky J, Jackson S, Moldoveanu Z, Nesbit LR, Kulhavy R, Prince SJ, et al. Paucity of antigen-specific IgA responses in sera and external secretions of HIV-type 1-infected individuals. AIDS Res Hum Retrovir. 2004;20:972–88. doi: 10.1089/aid.2004.20.972. [DOI] [PubMed] [Google Scholar]
- 33.Raux M, Finkielsztejn L, Salmon-Ceron D, Bouchez H, Excler JL, Dulioust E, et al. Comparison of the distribution of IgG and IgA antibodies in serum and various mucosal fluids of HIV type 1-infected subjects. AIDS Res Hum Retrovir. 1999;15:1365–76. doi: 10.1089/088922299310070. [DOI] [PubMed] [Google Scholar]
- 34.Wright PF, Kozlowski PA, Rybczyk GK, Goepfert P, Staats HF, VanCott TC, et al. Detection of mucosal antibodies in HIV type 1-infected individuals. AIDS Res Hum Retrovir. 2002;18:1291–300. doi: 10.1089/088922202320886334. [DOI] [PubMed] [Google Scholar]
- 35.Belec L, Meillet D, Gaillard O, Prazuck T, Michel E, Ngondi Ekome J, et al. Decreased cervicovaginal production of both IgA1 and IgA2 subclasses in women with AIDS. Clin Exp Immunol. 1995;101:100–6. doi: 10.1111/j.1365-2249.1995.tb02284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu FX. Predominate HIV1-specific IgG activity in various mucosal compartments of HIV1-infected individuals. Clin Immunol. 2000;97:59–68. doi: 10.1006/clim.2000.4910. [DOI] [PubMed] [Google Scholar]
- 37.Schafer F, Kewenig S, Stolte N, Stahl-Hennig C, Stallmach A, Kaup FJ, et al. Lack of simian immunodeficiency virus (SIV) specific IgA response in the intestine of SIV infected rhesus macaques. Gut. 2002;50:608–14. doi: 10.1136/gut.50.5.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Israel ZR, Marx PA. Nonclassical mucosal antibodies predominate in genital secretions of HIV-1 infected chimpanzees. J Med Primatol. 1995;24:53–60. doi: 10.1111/j.1600-0684.1995.tb00146.x. [DOI] [PubMed] [Google Scholar]
- 39.Fiore JR, Laddago V, Lepera A, La Grasta L, Di Stefano M, Saracino A, et al. Limited secretory-IgA response in cervicovaginal secretions from HIV-1 infected, but not high risk seronegative women: lack of correlation to genital viral shedding. N Microbiol. 2000;23:85–92. [PubMed] [Google Scholar]
- 40.Qiao X, He B, Chiu A, Knowles DM, Chadburn A, Cerutti A. Human immunodeficiency virus 1 Nef suppresses CD40-dependent immunoglobulin class switching in bystander B cells. Nat Immunol. 2006;7:302–10. doi: 10.1038/ni1302. [DOI] [PubMed] [Google Scholar]
- 41.Rath T, Kuo TT, Baker K, Qiao SW, Kobayashi K, Yoshida M, et al. The immunologic functions of the neonatal Fc receptor for IgG. J Clin Immunol. 2013;33(Suppl 1):S9–17. doi: 10.1007/s10875-012-9768-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shingai M, Donau OK, Plishka RJ, Buckler-White A, Mascola JR, Nabel GJ, et al. Passive transfer of modest titers of potent and broadly neutralizing anti-HIV monoclonal antibodies block SHIV infection in macaques. J Exp Med. 2014;211:2061–74. doi: 10.1084/jem.20132494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johansen FE, Kaetzel CS. Regulation of the polymeric immunoglobulin receptor and IgA transport: new advances in environmental factors that stimulate pIgR expression and its role in mucosal immunity. Mucosal Immunol. 2011;4:598–602. doi: 10.1038/mi.2011.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Merrill WW, Goodenberger D, Strober W, Matthay RA, Naegel GP, Reynolds HY. Free secretory component and other proteins in human lung lavage. Am Rev Respir Dis. 1980;122:156–61. doi: 10.1164/arrd.1980.122.1.156. [DOI] [PubMed] [Google Scholar]
- 45.Sela-Culang I, Kunik V, Ofran Y. The structural basis of antibody–antigen recognition. Front Immunol. 2013;4:302. doi: 10.3389/fimmu.2013.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tudor D, Yu H, Maupetit J, Drillet AS, Bouceba T, Schwartz-Cornil I, et al. Isotype modulates epitope specificity, affinity, and antiviral activities of anti-HIV-1 human broadly neutralizing 2F5 antibody. Proc Natl Acad Sci U S A. 2012;109:12680–5. doi: 10.1073/pnas.1200024109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rasmussen RA, Siddappa NB, Lakhashe SK, Watkins J, Villinger F, Ibegbu C, et al. High-level, lasting antiviral immunity induced by a bimodal AIDS vaccine and boosted by live-virus exposure: prevention of viremia. AIDS. 2012;26:149–55. doi: 10.1097/QAD.0b013e32834d3c4f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lakhashe SK, Wang W, Siddappa NB, Hemashettar G, Polacino P, Hu SL, et al. Vaccination against heterologous R5 clade C SHIV: prevention of infection and correlates of protection. PLoS ONE. 2011;6:e22010. doi: 10.1371/journal.pone.0022010. [DOI] [PMC free article] [PubMed] [Google Scholar]