Abstract
Importance
Risk of end-stage renal disease (ESRD) in kidney donors has been compared with risk faced by the general population, but the general population represents an unscreened, high-risk comparator. A comparison to similarly screened healthy nondonors would more properly estimate the sequelae of kidney donation.
Objectives
To compare the risk of ESRD in kidney donors with that of a healthy cohort of nondonors who are at equally low risk of renal disease and free of contraindications to live donation and to stratify these comparisons by patient demographics.
Design, Settings, and Participants
A cohort of 96 217 kidney donors in the United States between April 1994 and November 2011 and a cohort of 20 024 participants of the Third National Health and Nutrition Examination Survey (NHANES III) were linked to Centers for Medicare & Medicaid Services data to ascertain development of ESRD, which was defined as the initiation of maintenance dialysis, placement on the waiting list, or receipt of a living or deceased donor kidney transplant, whichever was identified first. Maximum follow-up was 15.0 years; median follow-up was 7.6 years (interquartile range [IQR], 3.9-11.5 years) for kidney donors and 15.0 years (IQR, 13.7-15.0 years) for matched healthy nondonors.
Main Outcomes and Measures
Cumulative incidence and lifetime risk of ESRD.
Results
Among live donors, with median follow-up of 7.6 years (maximum, 15.0), ESRD developed in 99 individuals in a mean (SD) of 8.6 (3.6) years after donation. Among matched healthy nondonors, with median follow-up of 15.0 years (maximum, 15.0), ESRD developed in 36 nondonors in 10.7 (3.2) years, drawn from 17 ESRD events in the unmatched healthy nondonor pool of 9364. Estimated risk of ESRD at 15 years after donation was 30.8 per 10 000 (95% CI, 24.3-38.5) in kidney donors and 3.9 per 10 000 (95% CI, 0.8-8.9) in their matched healthy nondonor counterparts (P < .001). This difference was observed in both black and white individuals, with an estimated risk of 74.7 per 10 000 black donors (95% CI, 47.8-105.8) vs 23.9 per 10 000 black nondonors (95% CI, 1.6-62.4; P < .001) and an estimated risk of 22.7 per 10 000 white donors (95% CI, 15.6-30.1) vs 0.0 white nondonors (P < .001). Estimated lifetime risk of ESRD was 90 per 10 000 donors, 326 per 10 000 unscreened nondonors (general population), and 14 per 10 000 healthy nondonors.
Conclusions and Relevance
Compared with matched healthy nondonors, kidney donors had an increased risk of ESRD over a median of 7.6 years; however, the magnitude of the absolute risk increase was small. These findings may help inform discussions with persons considering live kidney donation.
Every year in the United States, approximately 6000 healthy adults accept the risks of donor nephrectomy to help family members, friends, or even strangers improve survival and quality of life.1 It is imperative that the transplant community, in due diligence to donors, understands the risk of donation to the fullest extent possible and communicates known risks to those considering donation.2,3 To date, studies have shown that perioperative death is extremely rare and subsequent survival rates are comparable with healthy nondonors.4-6
However, physiologic sequelae resulting from kidney donation remain less well characterized. Recent single-center7-9 and nationwide10 studies suggest that live donors do not have increased risk of end-stage renal disease (ESRD) compared with the general population; however, the general population is unscreened and, as such, at higher inherent risk of ESRD than carefully screened donors. Limited by small sample sizes and lack of a healthy comparison group, previous studies have been unable to compare the risk of ESRD that a healthy individual faces after donation with the risk that individual would have faced had he or she not donated.11 Also, although studies show higher risk of ESRD in donor subgroups including black donors10 compared with nonblack donors and male donors8,9 compared with female donors, these studies comparing donors with other donors cannot account for the fact that race and sex are associated with ESRD (and chronic kidney disease) in nondonors as well.12-16
The goal of this study was to better understand the risk of ESRD following live donation by comparing the incidence of ESRD in live donors with their healthy nondonor counterparts.
Methods
Live Kidney Donors
By national mandate, all kidney donations in the United States are reported to the Organ Procurement and Transplantation Network (OPTN). Through this reporting, all adult live donors between April 1, 1994, and November 30, 2011, were included in this study. End-stage renal disease outcomes were ascertained by linkage to the Centers for Medicare & Medicaid Services' (CMS's) medical evidence Form 2728 (certification of ESRD), the transplant network's kidney waiting list transplant databases (including records through November 30, 2011) using a combination of Social Security number, last name, first, middle name, or all 3; date of birth; and sex. End-stage renal disease was defined as the initiation of maintenance dialysis, placement on the waiting list, or receipt of a living or deceased donor kidney transplant, whichever was identified first.
Matched Nondonors
The matched nondonor population was drawn from the Third National Health and Nutrition Examination Survey (NHANES III). In this cohort, medical information was obtained from patient self-report, physical examination, and radiologic and laboratory test results at NHANES III enrollment between 1988 and 1994. A healthy, screened nondonor population was derived from adult NHANES III participants by excluding those with identified contraindications to kidney transplantation (eAppendix 1 in the Supplement). Nondonors were individually matched with replacement to live donors using iterative expanding radius matching.6,17-20 Matching was based on age, sex, self-identified race, educational background, body mass index (BMI), smoking history, and systolic blood pressure (eAppendix 2 in the Supplement). Similar to the process outlined above for live donors, ESRD outcomes were ascertained by linkage to the CMS medical evidence Form 2728 and to the CMS patient profile and death notification Form 2746 (including records through September 30, 2008).
Cumulative Incidence of ESRD
Kaplan-Meier methods were used to estimate cumulative incidence of ESRD, with a time scale of years since study entry (time of donation for donors, and enrollment into NHANES for nondonors).21 Participants were censored at death or at the end of the study (November 30, 2011, for donors, and September 30, 2008, for nondonors).
Estimated Lifetime Risk of ESRD
Kaplan-Meier methods were used to estimate lifetime risk of ESRD, with a time scale of age in years and left truncation of age prior to study entry. Time at risk was accrued from age at donation for live donors and from age at enrollment into NHANES for nondonors.22 In other words, we estimated risk of ESRD across the life scale by splicing together observed incidence at younger ages (accrued by individuals who were young while they were part of the study population) with observed incidence at older ages (accrued by individuals who were older while they were part of the study population). For example, an individual who donated a kidney at age 45 years and was followed up for 7 years contributed to the estimate of ESRD accrued by donors ages 45 years to 52 years. Lifetime risk was estimated for 3 populations: live donors; matched healthy nondonors; and demographically matched unscreened nondonors (general population).
Absolute Risk Increase
The difference in cumulative incidence between the live donors (ie, those exposed to donor nephrectomy) and the nondonor comparator populations was reported as the absolute risk increase.
Statistical Analysis
Donor and nondonor characteristics at baseline were compared using ordinary least squares regression for continuous variables and logistic regression for categorical variables. The P values were estimated using bootstrap methods to account for resampling of participants in the nondonor population necessitated by the difference in sample size between the donor and nondonor cohorts. Risk of ESRD within live donor subgroups was compared using log-rank tests. Risk of ESRD between live donors and healthy nondonors was compared using bootstrap procedures tailored to the structure of our data.23 We calculated 95% confidence intervals for ESRD incidence using separate bootstraps for the live donors and healthy nondonors. For assessment of effect-modification by race/ ethnicity, we calculated 83.4% confidence intervals for ESRD incidence to arrive at a type I error probability of 5%.24 Each bootstrap repetition for the live donors and healthy nondonors drew with replacement from the original population. However, the probability that a given record would be drawn was proportional to the number of times it appeared in the data set, and, if drawn, all copies of that record were added to the bootstrapped sample. Selection continued in this way until the bootstrapped sample was the size of the original sample. All analyses were performed using Stata 12.0/MP for Linux (Stata Corp). All hypothesis tests were 2 sided (α = .05).
Results
Study Populations
Among 96 217 live donors, 78.3% were younger than 50 years, 59.0% were women, 74.6% were white, and 63.7% had attended college at some point; 67.6% of live donors were biologically related to their recipient, 25.2% were obese (BMI>30, calculated as weight in kilograms divided by height in meters squared), 9.0% had a systolic blood pressure greater than 140 mm Hg, and 24.2% smoked cigarettes at the time of donation. Among 20 024 unscreened adult NHANES III participants, 9364 (47%) had no identified contraindication to kidney donation and were matched 1:1 to donors to create a healthy nondonor cohort of 96 217 (Table 1).
Table 1. Characteristics of Live Kidney Donors in the United States at the Time of Donation and Matched Healthy Nondonors in the United States at the Time of NHANES Enrollment.
| Characteristicsa | Live Kidney Donors, % (n = 96 217) | Matched Healthy Nondonors, % (n = 96 217)b | P Value |
|---|---|---|---|
| Age, mean (SD), y | 40.2 (11.1) | 40.2 (11.1) | .90 |
| 18-39 | 48.2 | 48.0 | .70 |
| 40-49 | 30.1 | 29.8 | |
| 50-59 | 17.5 | 17.9 | |
| ≥60 | 4.2 | 4.3 | |
| Women | 59.0 | 59.0 | >.99 |
| Race/ethnicityc | |||
| White/other | 74.6 | 74.6 | >.99 |
| Black | 12.9 | 12.9 | |
| Hispanic | 12.5 | 12.5 | |
| Educational statusd | |||
| ≤High school | 36.3 | 42.5 | <.001 |
| Attended college | 28.4 | 25.8 | |
| College graduate | 25.1 | 21.0 | |
| Post college | 10.2 | 10.7 | |
| BMIe | |||
| Mean (SD) | 26.7 (7.5) | 26.2 (4.8) | <.001 |
| <24 | 33.0 | 40.1 | <.001 |
| 25-29 | 41.8 | 38.6 | |
| ≥30 | 25.2 | 21.3 | |
| Blood pressure, mm Hgf | |||
| Systolic | |||
| Mean (SD) | 121.0 (16.3) | 119.2 (12.5) | <.001 |
| <120 | 44.3 | 51.1 | <.001 |
| 120-139 | 46.7 | 43.0 | |
| ≥140 | 9.0 | 5.9 | |
| Diastolic | |||
| Mean (SD) | 73.6 (11.5) | 75.5 (10.2) | <.001 |
| <80 | 69.0 | 62.1 | <.001 |
| 80-89 | 26.6 | 30.3 | |
| ≥90 | 4.4 | 7.6 | |
| Smokerg | 24.2 | 10.4 | <.001 |
| Creatinine, mean (SD), mg/dL | 0.9 (0.2) | 1.0 (0.2) | <.001 |
| eGFR, mL/min/1.73 m2,h | |||
| Mean (SD) | 100.7 (23.7) | 86.4 (24.6) | <.001 |
| <80 | 22.1 | 41.1 | <.001 |
| 80-89 | 7.2 | 10.2 | |
| ≥90 | 70.7 | 48.7 | |
| Urine albumin:creatinine ratio, mean (SD), μg/mg | 0.04 (0.6) | ||
| Biologically related to recipienti | 67.6 | ||
| Year of donation | |||
| 1994-1997 | 13.8 | ||
| 1998-2001 | 21.5 | ||
| 2002-2005 | 26.9 | ||
| 2006-2009 | 25.8 | ||
| 2010-2011 | 12.0 |
Abbreviations: Blank cells, not applicable; BMI, body mass index, calculated as weight in kilograms divided by height in meters squared; eGFR, estimated glomerular filtration rate; NHANES, National Health and Nutrition Examination Survey.
SI conversion: To convert creatinine from mg/dL to μmol/L, multiply by 88.4.
Characteristics at the time of donation (April 1994-November 2011) and at enrollment (January 1988-December 1994) are shown; age, sex, and race/ethnicity were available throughout the study period.
Matched healthy nondonors were identified among participants in the NHANES III survey and were drawn with replacement in light of a larger population of donors than of healthy nondonors. Participants with missing data were excluded from this study.
For race/ethnicity, the category of “Other” included American Indian, Native Hawaiian, Alaskan Native, Pacific Islander, and multiracial.
For donors, education was only available after 1998 (42% missing between 2000-2004; 21% missing between 2005-2009, 9% missing 2010-2011).
Body mass index was only available after 2003 (33% missing between 2005-2009; 19% missing between 2010-2011).
Blood pressure was only available after 1999 (24% missing between 2000-2004; 8% missing between 2005-2009; 4% missing between 2010-2011).
Smoking status was only available after 2004 (5% missing between 2005-2009; 1% missing between 2010-2011).
Creatinine and eGFR (estimated using the chronic kidney disease– epidemiology collaboration equation) were only available after 1997 (34% missing between 1998-2001; 5% missing between 2002-2005; 1% missing after 2006).
The Relationship to recipient was missing for 0.25% records between 1994-2011.
Frequency and Timing of ESRD
Among live donors, with median follow-up of 7.6 years (maximum, 15.0 years), ESRD developed in 99 individuals in a mean (SD) of 8.6 (3.6) years after donation. Of donors who subsequently developed ESRD, 50 were 18 to 39 years old at the time of donation, 57 were men, 50 were white, and 83 were biologically related to the recipient (Table 2). By contrast, among matched healthy nondonors, with median follow-up of 15.0 years (maximum, 15.0 years), ESRD developed in 17 individuals among the 9364 individuals in the nondonor pool, resulting in 36 ESRD events in matched nondonors in a mean (SD) of 10.7 (3.2) years after enrollment.
Table 2. Development of End-Stage Renal Disease in Subgroups of Live Kidney Donors in the United States, 1994-2011.
| No. of Donors | Cases of ESRD | Cumulative Incidence of ESRD at 15 Years per 10 000 (95% CI) | |
|---|---|---|---|
| All donorsa | 96 217 | 99 | 30.8 (24.3-38.5) |
| Age at donation, y | |||
| 18-39 | 46 344 | 50 | 29.4 (21.4-40.2) |
| 40-49 | 28 994 | 17 | 17.4 (10.1-30.0) |
| 50-59 | 16 840 | 25 | 54.6 (34.8-85.4) |
| ≥60 | 4039 | 7 | 70.2 (30.4-161.8) |
| Sex | |||
| Women | 56 775 | 42 | 21.1 (14.9-29.9) |
| Men | 39 442 | 57 | 44.1 (32.9-59.1) |
| Race | |||
| White/other | 71 769 | 50 | 22.7 (15.6-30.1) |
| Black | 12 387 | 36 | 74.7 (47.8-105.8) |
| Hispanic | 12 061 | 13 | 32.6 (17.9-59.1) |
| Relationship to recipientb | |||
| Biological | 64 897 | 83 | 34.1 (26.9-43.3) |
| Nonbiological | 31 081 | 16 | 15.1 (08.7-26.3) |
In a mean (SD) of 8.6 (3.6) years after donation, 99 donors who were aged 50 (13) years developed end-stage renal disease (ESRD).
Relationship to recipient was missing for 0.25% of the records between 1994-2011.
Absolute Risk Increase
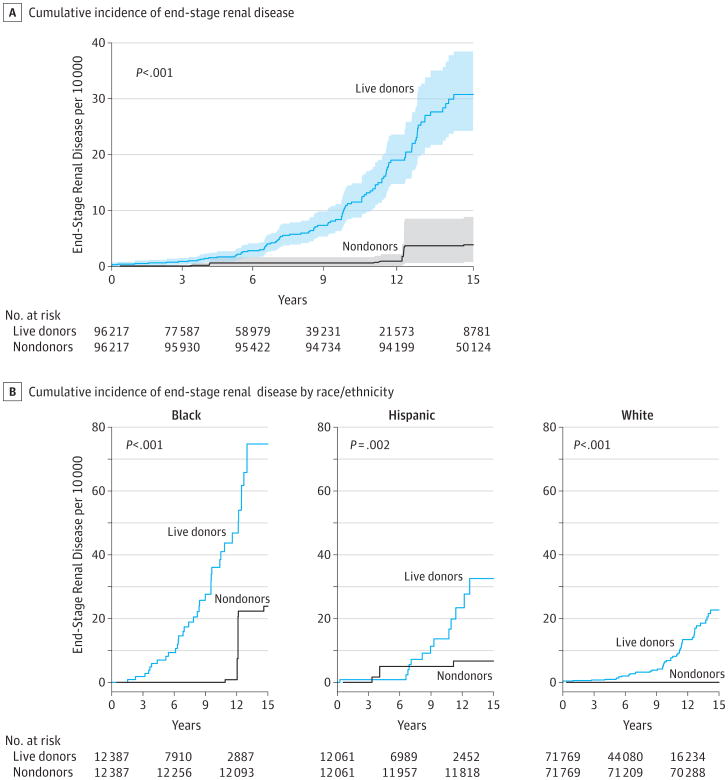
Estimated cumulative incidence of ESRD at 15 years after donation was 30.8 per 10 000 (95% CI, 24.3-38.5) in donors and 3.9 per 10 000 (95% CI, 0.8-8.9) in healthy nondonors (P < .001; Figure 1A). Absolute risk of ESRD was highest among both black donors at 74.7 per 10 000 (95% CI, 47.8-105.8) and black nondonors at 23.9 per 10 000 (95% CI, 1.6-62.4), and the absolute risk increase was also highest in this race group (50.8 per 10 000, P < .001). Risk among Hispanic donors was 32.6 per 10 000 (95% CI, 17.9-59.1) and for Hispanic nondonors, it was 6.7 per 10 000 (95% CI, 0.0-15.0), for an absolute risk increase of 25.9 per 10 000 (P = .002). Absolute risk was lowest among both white donors at 22.7 per 10 000 (95% CI, 15.6-30.1) and white nondonors at 0.0 per 10 000 (95% CI, 0.0-0.0), and absolute risk increase was also lowest in this group (22.7 per 10 000; P < .001; Figure 1B).
Figure 1. Cumulative Incidence of End-Stage Renal Disease in Live Kidney Donors and Matched Healthy Nondonors.
A, The shaded areas indicate 95% confidence intervals obtained by bootstrapping. Matched healthy nondonors were identified among participants in the third National Health and Nutrition Examination Survey and were drawn with replacement in light of a larger population of donors compared with healthy nondonors by bootstrapping (see the Methods section).
Cumulative Incidence by Subgroup
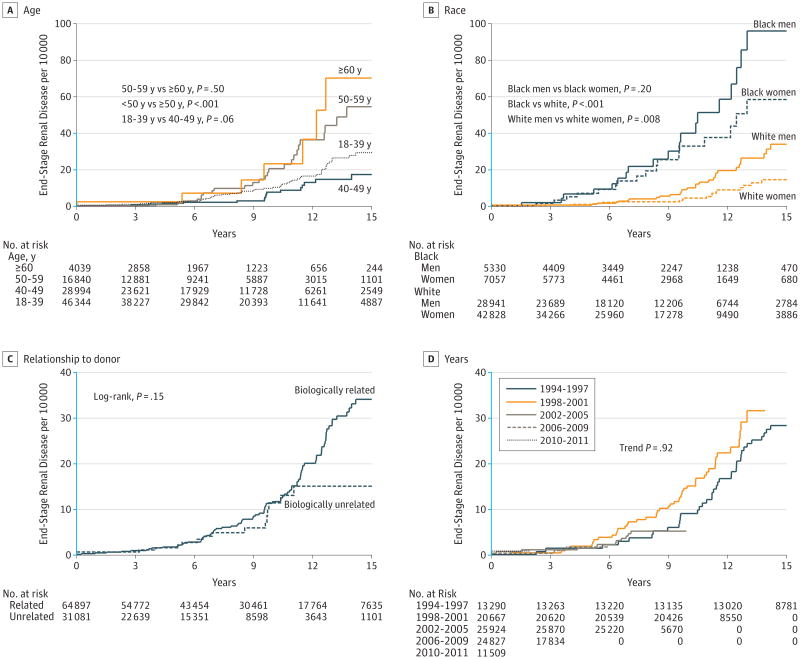
Although low among live donors, cumulative incidence of ESRD per 10 000 at 15 years varied significantly by age: 29.4 (95% CI, 21.4-40.2) among those aged 18 through 39 years; 17.4 (95% CI, 10.1-30.0) among those 40 through 49 years; 54.6 (95% CI, 34.8-85.4) among those 50 through 59 years; and 70.2 (95% CI, 30.4-161.8) among those 60 years or older (P < .001; Figure 2A) and differed per 10 000 at 15 years by race and sex: 96.0 (95% CI, 58.0-158.8) among black men vs 58.5 (95% CI, 34.2-100.0) among black women and 34.0 (95% CI, 22.7-51.0) among white men vs 14.6 (95% CI, 8.8-24.2) among white women (P < .001; Figure 2B). The difference in ESRD incidence per 10 000 at 15 years between biologically related and unrelated donors was not statistically significant: 34.1 (95% CI, 26.9-43.3) for biologically related donors vs 15.1 (95% CI, 8.7-26.3) for biologically unrelated donors (P = .15; Figure 2C). There was no observed temporal trend in risk of ESRD between 1994 and 2011 (trend P = .92; Figure 2D).
Figure 2. Cumulative Incidence of End-Stage Renal Disease in Live Kidney Donors.
Estimates obtained using Kaplan-Meier methods and compared using log-rank tests. The y-axis scale shown in blue indicates the range from 0 to 40 events per 10 000.
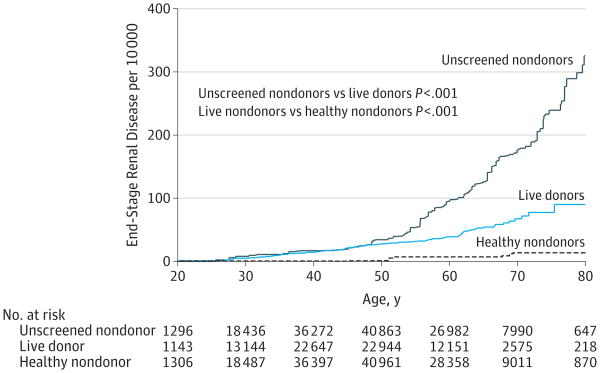
Estimated Lifetime Risk
Live donors had a higher estimated risk of ESRD than healthy nondonors across all ages (Figure 3). Those who had donated at some point before the age of 30 years had an estimated risk of 5 per 10 000 compared with healthy nondonors who had estimated risk of 0 per 10 000. Similarly, by age 50 years, estimated risk in donors was 28 per 10 000 vs 1 per 10 000 in nondonors, and by age 80 years, the estimated risk was 90 per 10 000 in donors vs 14 per 10 000 in nondonors, representing an estimated lifetime absolute risk increase of 76 per 10 000. Nevertheless, live donors had much lower estimated lifetime risk of ESRD than did the general population by age 80 years, 90 per 10 000 in donors vs 326 per 10 000 in the general population.
Figure 3. Estimated Lifetime Risk of End-Stage Renal Disease in Matched But Unscreened Nondonors, Live Kidney Donors, and Matched Healthy Nondonors.
Nondonors were identified among participants in the third National Health and Nutrition Examination Survey. Healthy nondonors were a subset of unscreened nondonors. Comparisons were made by bootstrapping.
Discussion
In this national study of 96 217 live kidney donors linked to CMS data for reliable ascertainment of ESRD, we estimated that approximately 23 white, 33 Hispanic, and 75 black donors per 10 000 developed ESRD after kidney donation; however, ESRD occurred in 23 white, 26 Hispanic, and 51 black individuals because they donated a kidney, whereas the remaining cases resulted from the inherent risk of ESRD. We also determined that kidney donors had a somewhat higher estimated risk of developing ESRD throughout their lifetimes (90 per 10 000) than similarly healthy individuals who did not donate (14 per 10 000), but still a much lower risk than the general population (326 per 10 000).
Our findings reaffirm the prevailing belief that lifetime risk of ESRD in live donors is no higher than in the general demographics-matched US population,7,8,10 and our estimate of population-based risk of ESRD (derived from unscreened NHANES III participants) was comparable with a recent estimate of 360 per 10 000 in the general US population.25 Although, to our knowledge, no association between donor nephrectomy and risk of ESRD has been reported before, this association in our study was strong and was statistically significant within each race/ethnicity stratum. Our findings are an extension of those by Ibrahim et al7 who observed a decline in renal reserve in as many as 1400 per 10 000 carefully selected white donors, from a mean (SD) predonation glomerular filtration rate (GFR) of 84 (9.2) mL/min/1.73 m2 to a post-donation GFR of less than 60 mL/min/1.73 m2 (a decline of greater than 24 mL/min/1.73 m2) in 12.2 (9.2) years after donation. Ibrahim et al further noted development of ESRD in 30 per 10 000 of these donors 22.5 (10.4) years after donation.
The primary strengths of our approach were the inclusion of every kidney donor in the United States over nearly 2 decades, the highly reliable linkage-based ESRD ascertainment, and the comparison with a healthy nondonor cohort matched on a wide range of demographic and clinical variables. Because of the large sample size of our study populations, we were able to estimate the incidence of a relatively rare event and to make inferences specific to race/ethnicity subgroups, providing critical information not only for those considering donation but also for the nearly 100 000 individuals in the United States living after a donor nephrectomy. An additional strength of our approach was the inclusion of an unscreened nondonor population demographically matched to the donor population. In showing that risk of ESRD in donors was no higher (and, in fact, much lower) than in this unscreened nondonor population, our findings are consistent with previous reports of risk of ESRD in donors.7-10
Despite these strengths, some limitations of this study are important to note. First, our inferences were based on 2 cohorts of healthy individuals from the United States and may generalize imperfectly to donors in other countries.26 Second, donors are meticulously screened, and it is possible that the donors were healthier than the healthy nondonors, even after screening by NHANES history, physical, and laboratory testing. Third, the follow-up in our study, although long enough to identify a risk of ESRD in donors, was limited to 15 years and may not have permitted us to fully understand the long-term risk of donation; however, our lifetime risk estimates enable inferences generalizable to individuals of all ages irrespective of the number of years after donation.
It is also worth noting that the donors in this study donated between 1994-2011, whereas the nondonors to whom they were matched entered NHANES III between 1988-1994. With increasing incidence of ESRD over the last 2 decades,27 one might wonder if the more recent cohort (ie, donors) had a higher risk of ESRD just by virtue of these secular trends. However, secular trends in the general population were mediated by conditions such as morbid obesity, diabetes, and hypertension28; these conditions have increased substantially over the last 2 decades in the general population, but much less so in carefully screened donors, for whom many of these conditions are contraindications to donation. As such, not surprisingly, the ESRD rate in donors did not change over time. Furthermore, our study screened for more than 30 medical conditions, thereby attenuating the possibility that the increased rates of ESRD in donors were attributable to secular trends rather than to donation.
Conclusion
Compared with a matched cohort of healthy nondonors, kidney donors had an increased risk of ESRD; however, the magnitude of the absolute risk increase was small. These findings may help inform discussions with persons considering live kidney donation.
Supplementary Material
Acknowledgments
Funding/Support: This study was supported by grant R01DK096008 from the Health Resources and Services Administration contract 234-2005-370011C.
Role of the Sponsors: The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Supplemental content at jama.com
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Wainright reported that she is an employee of the United Network for Organ Sharing. Dr Segev reported institutional grant support from the National Institutes of Health. No other disclosures were reported.
Additional Contributions: United Network for Organ Sharing staff, including Dr Wainright, performed all Social Security number linkages to Social Security Death Master File and Centers for Medicare & Medicaid Services data to ensure confidentiality of Social Security number data provided to the OPTN. Likewise, Research Data Center staff at the National Center for Health Statistics performed all NHANES social security number linkages to Centers for Medicare and Medicaid Services data.
Disclaimer: The analyses described herein are the responsibility of the authors alone and do not necessarily reflect the views or policies of the US Department of Health and Human Services, nor does the mention of trade names, commercial products, or organizations imply endorsement by the US government.
References
- 1. [Accessed April 17, 2013];Organ Procurement and Transplant Network website. http://optn.transplant.hrsa.gov/latestData/step2.asp.
- 2.Delmonico FL, Dew MA. Living donor kidney transplantation in a global environment. Kidney Int. 2007;71(7):608–614. doi: 10.1038/sj.ki.5002125. [DOI] [PubMed] [Google Scholar]
- 3.Poggio ED, Braun WE, Davis C. The science of stewardship: due diligence for kidney donors and kidney function in living kidney donation— evaluation, determinants, and implications for outcomes. Clin J Am Soc Nephrol. 2009;4(10):1677–1684. doi: 10.2215/CJN.02740409. [DOI] [PubMed] [Google Scholar]
- 4.Bia MJ, Ramos EL, Danovitch GM, et al. Evaluation of living renal donors: the current practice of US transplant centers. Transplantation. 1995;60(4):322–327. doi: 10.1097/00007890-199508270-00003. [DOI] [PubMed] [Google Scholar]
- 5.Matas AJ, Bartlett ST, Leichtman AB, Delmonico FL. Morbidity and mortality after living kidney donation, 1999-2001: survey of United States transplant centers. Am J Transplant. 2003;3(7):830–834. [PubMed] [Google Scholar]
- 6.Segev DL, Muzaale AD, Caffo BS, et al. Perioperative mortality and long-term survival following live kidney donation. JAMA. 2010;303(10):959–966. doi: 10.1001/jama.2010.237. [DOI] [PubMed] [Google Scholar]
- 7.Ibrahim HN, Foley R, Tan L, et al. Long-term consequences of kidney donation. N Engl J Med. 2009;360(5):459–469. doi: 10.1056/NEJMoa0804883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fehrman-Ekholm I, Nordén G, Lennerling A, et al. Incidence of end-stage renal disease among live kidney donors. Transplantation. 2006;82(12):1646–1648. doi: 10.1097/01.tp.0000250728.73268.e3. [DOI] [PubMed] [Google Scholar]
- 9.Wafa EW, Refaie AF, Abbas TM, et al. End-stage renal disease among living-kidney donors: single-center experience. Exp Clin Transplant. 2011;9(1):14–19. [PubMed] [Google Scholar]
- 10.Cherikh WS, Young CJ, Kramer BF, Taranto SE, Randall HB, Fan PY. Ethnic and gender related differences in the risk of end-stage renal disease after living kidney donation. Am J Transplant. 2011;11(8):1650–1655. doi: 10.1111/j.1600-6143.2011.03609.x. [DOI] [PubMed] [Google Scholar]
- 11.Garg AX, Muirhead N, Knoll G, et al. Donor Nephrectomy Outcomes Research (DONOR) Network. Proteinuria and reduced kidney function in living kidney donors: a systematic review, meta-analysis, and meta-regression. Kidney Int. 2006;70(10):1801–1810. doi: 10.1038/sj.ki.5001819. [DOI] [PubMed] [Google Scholar]
- 12.Eriksen BO, Ingebretsen OC. The progression of chronic kidney disease: a 10-year population-based study of the effects of gender and age. Kidney Int. 2006;69(2):375–382. doi: 10.1038/sj.ki.5000058. [DOI] [PubMed] [Google Scholar]
- 13.Hsu CY, Iribarren C, McCulloch CE, Darbinian J, Go AS. Risk factors for end-stage renal disease: 25-year follow-up. Arch Intern Med. 2009;169(4):342–350. doi: 10.1001/archinternmed.2008.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brancati FL, Whittle JC, Whelton PK, Seidler AJ, Klag MJ. The excess incidence of diabetic end-stage renal disease among blacks: a population-based study of potential explanatory factors. JAMA. 1992;268(21):3079–3084. [PubMed] [Google Scholar]
- 15.Klag MJ, Whelton PK, Randall BL, Neaton JD, Brancati FL, Stamler J. End-stage renal disease in African-American and white men: 16-year MRFIT findings. JAMA. 1997;277(16):1293–1298. [PubMed] [Google Scholar]
- 16.Lentine KL, Schnitzler MA, Xiao H, et al. Racial variation in medical outcomes among living kidney donors. N Engl J Med. 2010;363(8):724–732. doi: 10.1056/NEJMoa1000950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Montgomery RA, Lonze BE, King KE, et al. Desensitization in HLA-incompatible kidney recipients and survival. N Engl J Med. 2011;365(4):318–326. doi: 10.1056/NEJMoa1012376. [DOI] [PubMed] [Google Scholar]
- 18.Berger JC, Muzaale AD, James N, et al. Living kidney donors ages 70 and older: recipient and donor outcomes. Clin J Am Soc Nephrol. 2011;6(12):2887–2893. doi: 10.2215/CJN.04160511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muzaale AD, Dagher NN, Montgomery RA, Taranto SE, McBride MA, Segev DL. Estimates of early death, acute liver failure, and long-term mortality among live liver donors. Gastroenterology. 2012;142(2):273–280. doi: 10.1053/j.gastro.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 20.Van Arendonk KJ, Orandi BJ, James NT, Segev DL, Colombani PM. Living unrelated renal transplantation: a good match for the pediatric candidate? J Pediatr Surg. 2013;48(6):1277–1282. doi: 10.1016/j.jpedsurg.2013.03.023. [DOI] [PubMed] [Google Scholar]
- 21.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):25. [Google Scholar]
- 22.Vasan RS, Beiser A, Seshadri S, et al. Residual lifetime risk for developing hypertension in middle-aged women and men: the Framingham Heart Study. JAMA. 2002;287(8):1003–1010. doi: 10.1001/jama.287.8.1003. [DOI] [PubMed] [Google Scholar]
- 23.Efron B. Bootstrap methods: another look at the jackknife. Ann Stat. 1979;7(1):1–27. [Google Scholar]
- 24.Knol MJ, Pestman WR, Grobbee DE. The (mis)use of overlap of confidence intervals to assess effect modification. Eur J Epidemiol. 2011;26(4):253–254. doi: 10.1007/s10654-011-9563-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grams ME, Chow EK, Segev DL, Coresh J. Lifetime incidence of CKD stages 3-5 in the United States. Am J Kidney Dis. 2013;62(2):245–252. doi: 10.1053/j.ajkd.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horvat LD, Shariff SZ Garg AX; Donor Nephrectomy Outcomes Research (DONOR) Network. Global trends in the rates of living kidney donation. Kidney Int. 2009;75(10):1088–1098. doi: 10.1038/ki.2009.20. [DOI] [PubMed] [Google Scholar]
- 27.US Renal Data Systems. USRDS 2006 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2007. [Google Scholar]
- 28.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298(17):2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.