Abstract
The field of clinical nanomaterials is enlarging steadily, with more than a billion US dollars of funding allocated to research by US government agencies in the past decade. The first generation of anti-cancer agents using novel nanomaterials has successfully entered widespread use. Newer nanomaterials are garnering increasing interest as potential multifunctional therapeutic agents; these drugs are conferred novel properties, by virtue of their size and shape. The new features of these agents could potentially allow increased cancer selectivity, changes in pharmacokinetics, amplification of cytotoxic effects, and simultaneous imaging capabilities. After attachment to cancer target reactive-ligands, which interact with cell-surface antigens or receptors, these new constructs can deliver cytolytic and imaging payloads. The molecules also introduce new challenges for drug development. While nanoscale molecules are of a similar size to proteins, the paradigms for how cells, tissues and organs of the body react to the non-biological materials are not well understood, because most cellular and metabolic processes have evolved to deal with globular, enzyme degradable molecules. We discuss examples of different materials to illustrate interesting principles for development and future applications of these nanomaterial medicines with emphasis on the possible pharmacologic and safety hurdles for accomplishing therapeutic goals.
Introduction
In his 1959 lecture, “There's plenty of room at the bottom,” which is credited with initiating interest in nano technology, Richard Feynman discussed “the problem of manipulateing and controlling things on a small scale,” including placing “the mechanical surgeon inside the blood vessel” to observe, report, and perform the surgery.1 In the field of nanomedicine we are now successfully approaching solutions to this challenge (Figure 1). Many current systemic therapeutic approaches to cancer lack specificity and most of the actions of these agents cannot be controlled following injection. Some solutions may be found in the ability to create truly intelligent drugs.2,3
Figure 1.
50 years of nanomedicine development for cancer. The timeline is not to scale. Abbreviations: AFM, atomic force microscope; CNT, carbon nanotubes; EPR, enhanced permeability and retention; NCI, National Cancer Institute; NP, nanoparticle; PRINT, particle replication in nonwetting templates; SPIO, superparamagnetic iron oxide.
Anti-cancer agents that utilize novel materials to alter pharmacokinetics (PK), such as emulsified drugs and liposomal constructs, are already in development or approved by the FDA (Supplementary Tables 1 and 2) and many of these agents have been extensively reviewed. The first of a generation of complex, nanoscale, multifunctional medicines have already been designed or are in human use. These include the engineered targetable toxins that bind to cancer cells, enter their cytoplasm and deliver an enzyme capable of shutting down ribosomal synthesis,4 multistep targeting strategies to enhance and control the rate of drug delivery to a tumor;5 enzymes targeted by ligands to cells, which then act on pro-drug substrates to convert them on-site to the active agent,6 a nano-cell that sequentially delivers different drugs to the tumor7 or targetable atomic nanogenerators.8 Other newer multifunctional nanomaterial-based agents in development have reached human testing (Supplementary Table 1).
Here, we restrict the scope of our discussion to a relatively small segment of the novel, systemically administered nanomaterial-based cancer drugs, including polymers, dendrimers, carbon nanotubes, and various metallic and non-metallic nano-particles, in order to illustrate certain interesting features that make nanomaterial-based agents both appealing and problematic (Boxes 1 and 2). These properties, include size, shape, charge, surface patterning, polydispersity, multivalency and multicomponent structures, biocompatibility and biochemical stability.9–13 Due to space considerations, we will not discuss various nanoparticle or liposomal drug formulations or locally implantable devices or depots or detection systems14–18 nor engineered cells, viruses, aptamers or nucleotide agents,19–21 fusion proteins, or antibody–drug conjugates,4 which do display many of the above properties and have been extensively reviewed elsewhere.
Box 1. Definition of nanomaterial cancer drugs.
Size: 1–100 nm in one dimension
Agents are composed of synthetic materials, at least in part
The size and shape confer unique properties
The agent is multifunctional
The agent is multimeric
Box 2. Rationale for developing nanomaterial medicines.
Multifunctionality
Increased potency and multivalency
Increased selectivity for targets
‘Theranostic’ potential (imaging and therapy together)
Altered pharmacokinetics
Controlled syntheses
Controlled agent release and kinetics
Novel properties and interactions
Lack of immunogenicity
Enhanced physical stability
The appeal of nanomaterial-based drugs in cancer is based on two factors. Firstly, the ability to control the synthesis of the agents in a manufacturing process to make multifunctional and multivalent molecules, or to alter PK, allowing changes in potency and safety, with structures arranged in distinct surface patterns exposed to the environment. In cancer cells, the diverse signaling and receptor changes that occur provide targets that might be templates for multivalent and multifunctional targeting ligands and cargo. Secondly, the sizes (up to several hundred nanometers in one of the dimensions) and shapes (hollow spheres, long rods, stars, etc.) of the molecules, which are quite distinct from traditional small molecule or protein-based drugs, yield very large surface to volume ratios or the possibility of containment for various cargo (Figure 2). For example, a single wall carbon nanotube (in which every atom is a surface carbon atom) with a volume similar to a typical large protein (for example, 100–150 kDa), will have more than 15 times the surface area available for ligand attachment or cell interactions than a large protein. Because of the non-biological sources of some materials, and the distinct sizes, valencies and shapes, the pharmacology, such as clearance and biodegradation, toxicology and possible safety of these agents in patients might be very different from current drugs; in order for some of the agents to be useful, it is likely that biocompatible coatings and ligands will be a part of their structure. Unfortunately, gaps in current knowledge about nanomaterial biology, toxico logy and pharmacology, which have led to fears of possible toxic effects and environmental damage, have already put societal, ethical and political constraints on their successful application.22–24 In this regard, we will attempt to address the current benefits and hurdles of these new agents and future directions for these key issues, especially those relevant to cancer therapy.
Figure 2.
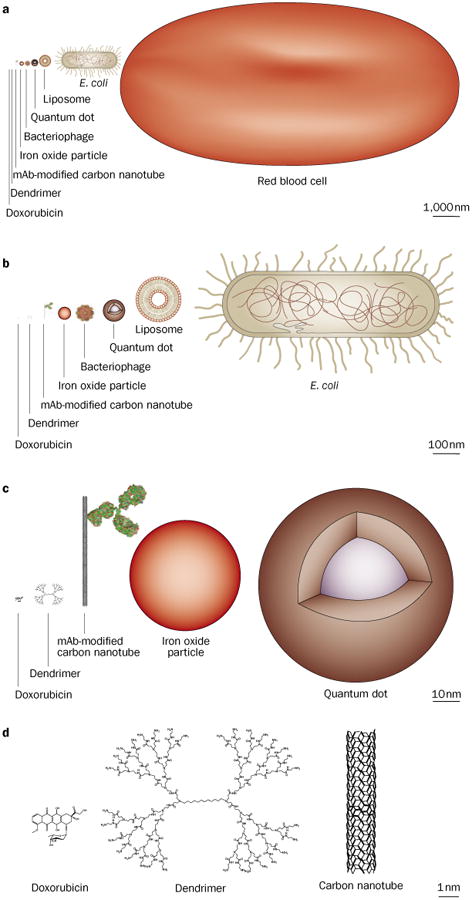
Representation of nanomaterials with comparison to biologics, drawn to scale.
Multiple examples of nanoparticles have emerged as candidates for drugs or have reached FDA approval (Supplementary Tables 1 and 2). These include those with intrinsic medically useful properties to allow robust external imaging25–27 or promote tumor thermolysis28–31 (for example, quantum dots, silica nanoparticles, gold nanoparticles, carbon nanotubes), or which have noncovalent and covalent modifications with encapsulation of therapeutic agents (for example, liposomes, dendrimers, [co]polymers). More details can be found in recent articles.11,12,14,15,17,32–36
Interaction with cells and tissues
The size, shape, and patterns of proteins presented to cells are important factors in their receptor binding and the signaling processes they control. As the physical scale of nanomaterials is of the same order as viruses, proteins, oligonucleic acids and macromolecular cellular machinery (Figure 2), one might expect they also will have distinctive and highly variable interactions with cells and tissues dependent on their size, shape, surface patterning, and charge.37 These interactions will have a role in both the beneficial and potentially toxic effects of these drugs. The great appeal of nanomaterials as cancer drugs is the ability to control these properties, thus improving specificity towards malignant cells and PK properties.
Size
Early studies and modeling involving microscale and nanoscale materials focused on the effect of molecular weight and size on the biologic behaviors of micelles, liposomes, and polymers.17,32 Most organic nanoparticles (such as polymeric particles) have traditionally been synthesized using emulsion-based synthetic techniques that allow for encapsulation of various drugs, while in organic nanoparticles (such as metallic particles) are often produced through controlled nucleation.38 While these techniques allow particle sizes to be controlled, newer synthetic techniques and improved characterization through dynamic light scattering, electron microscopy, and atomic force microscopy, have attempted to provide more precise control over nanoscale sizes, and have allowed delineation of the biological effects of nanometer scale changes. For the same material, size changes as small as a few nanometers can lead to significant differences in cellular uptake, toxic effects, and fate within the cell.39–43 For example, engineered particles of sizes 40–60 nm were able to maximally bind and induce receptor-mediated endocytic processes, while smaller particles had an impaired ability to induce membrane receptor crosslinking.40 Size also plays an important role in directing the fate of particles in the antigen presenting cells of the immune system.44,45 Overall, initial studies suggest that adjustable sizes could offer a means to direct different nanomaterials into different cellular pathways, maximize affinity of appended ligands, modulate uptake into malignant cells, and minimize adverse effects towards normal cells.40,46,47 Size also affects PK and renal clearance.48,49 Consistent with this, renal filtration and nonspecific uptake by the reticuloendothelial system (RES) has been shown to be dependent on particle size.50–55
Shape
Similar to the study of nanomaterial size effects, the study of nanomaterial shapes began with polymers that demonstrated the significant impact of the molecular architecture of these materials on pharmacology and function.56 As with improvement in particle size control, technology for control of shape has rapidly progressed.13,57–59 The advancement in synthetic techniques for non-spherical particles has shown that particle shape significantly impacts the cellular and tissue interactions of nanomaterials. While a variety of shapes (elliptical, spherical, and discoid) of model polystyrene particles can induce endocytosis, endocytosis of shapes that are non-spherical are highly dependent on the local shape at the interface of the cell with the nanomaterial or the tangential angle that the nanomaterial shape makes with the cell.39,60,61 These studies have predicted that when spherical particles bind or rod-like particles align perpendicular to the cellular membrane as apposed to aligning parallel to the surface, there is greater propensity for cellular uptake.62,63 More recently, ‘worm-like’ particles of high aspect ratio (that is, high length to width ratio) were shown to inhibit phagocytosis, and could only be engulfed when cells interacted specifically at particle ends.64 By contrast, studies of particles with diameters >100 nm with varying aspect ratios (up to three) found that higher aspect ratios resulted in increased uptake by phagocytic cells.42 Interestingly, carbon nanotubes, which have unusually large aspect ratios (50–1,000:1) but are of very small diameters (1–2 nm) have highly rapid and efficient cellular uptake. Several mechanisms have been described for these observations.30,63,65,66 Investigators have found that filamentous micelles had circulation times about 10-fold longer than their spherical counterparts.67 By contrast, circulation times of carbon nanotubes of a similar filamentous shape but smaller diameter (<2 nm) were demonstrated to be very short (1–3 h) with rapid renal clearance.68–70 In one example where novel shapes were used to mimic biological structures, discoid nanosheets were able to enhance spreading of platelet thrombi twofold as compared with their spherical counterparts.71
Surface patterning
Another component of nanoparticle structure is the geometric arrangement, or patterning, of surface groups. varying patterning provides a valuable tool to investigate the importance of multivalency and ligand geometry in the targeting and avidity of cancer antigens.72,73 Thus, if targeting ligands were rationally patterned onto nanomaterials of particular shapes, there is potential for designing nanomedicines with ‘intelligent’ inter actions with cancerous and normal tissues and enhancing specificity.
Charge
Biomacromolecules have different cellular and tissue interactions depending on the net charge of the chemical substituents. For example, cell-penetrating-peptides often contain a high density of cationic residues and are known to readily cross cellular membranes.74,75 On the tissue level, engineering the charge on ligands targeted by drugs, such as antibody fragments, results in dramatically altered biodistribution profiles.76 Indeed, a common strategy to avoid RES uptake of nanomaterials is to introduce neutral, hydrophilic polyethylene glycol chains (pegylation) to reduce opsonization.77 Unsurprisingly, surface charge or zeta potential (the electrical potential at the hydro dynamic slipping plane of a particle) is also an important factor influencing the biologic behavior of nanomaterials.78 It seems that negative or neutral surfaces could be preferable to avoid nonspecific cellular uptake.79,80 Furthermore, for nanomaterials that are filtered by the kidney, charge plays a major role in tubular reabsorption, with positive charge leading to increased retention in the renal cortex.81
Cancer targeting
An important goal in nanomedicine is to combine several of the special features of the materials to improve the agent's therapeutic index. The simplest way to achieve this is by combining the functions of targeting, often with specific ligands, and killing with a cytotoxic warhead, with both functions associated with the nanomaterial vehicle (Table 1 and Box 3). This might be achieved via active targeting with specific ligands (antibodies, peptides, etc.), passive targeting that takes advantage of physical interactions between the agents and the tumor microenvironment (blood flow, lymphatic drainage, etc), or more complex interactions with molecules at the tumor site that might serve to activate or release the therapeutic moiety (peptidases, pH, etc). Active (or ligand directed) targeting improves relative tumor localization; if the target is internalized it will also allow efficient cellular accumulation of the agent at specific sites, which can further increase efficacy.82 Effective cancer therapies that are selective for the expressed target are now routinely achieved with monoclonal antibodies specific to antigens overexpressed in cancer cells.83 A large range of other targeting moieties has now become available ranging from macromolecular biologics to synthetic small molecules (Table 1).84–87 Most active targeting approaches in nanomedicine directly append one of these targeting ligands to a nanoparticle scaffold.
Table 1. Ligands for active cancer targeting.
| Type | Mw (kDa) | Diameter (nm) | Features |
|---|---|---|---|
| Monoclonal antibodies | |||
| Whole antibodies | 150 | 15–20 | High affinity, divalent, many clinically approved examples, contains biologically active constant (Fc) region, long circulation |
| Engineered fragments (monovalent) | |||
| ScFv | 25 | 3–5 | Lowered affinity, rapid clearance from circulation, renal retention, reduced stability, reduced immunogenicity |
| Fab' | 50 | 5–10 | Can be produced genetically or enzymatically by cleavage of monoclonal antibodies |
| Nanobody | 15 | 2–3 | Smallest antigen-binding fragment, single domain, can bind cryptic epitopes |
| Engineered fragments (divalent) | |||
| F(ab')2 | 100 | 10–15 | Improved affinity, can be engineered to a variety of sizes and arrangements of protein domains |
| Diabodies | 50–80 | 5–10 | Mono-specific or bi-specific dimer of ScFv |
| Minibodies | 80 | 10 | Can be produced genetically |
| Aptamers | |||
| RNA | 10–30 | 2–3 | Rapid clearance, automated chemical synthesis, susceptible to nucleases without chemical modification |
| DNA | 10–30 | 2–3 | Rapid clearance, automated chemical synthesis, susceptible to nucleases without chemical modification |
| Receptor ligands | |||
| Peptides | 0.5–10 | variable | Facile synthesis and modification, diverse libraries and screening technologies, susceptible to peptidases, renal retention |
| Whole proteins | 30–150 | variable | Produced using recombinant DNA technologies, can be biologically active, susceptible to proteases |
| Small molecules | 0.1–1.0 | 0.5–2.0 | Chemical synthesis, simple modification and coupling chemistries, can be biologically active, highly variable affinities |
Box 3. Examples of cancer-associated targets.
Hematologic antigens
B cells: CD19, CD20, CD21, CD22, CD23
T cells: CD4, CD25, CD30
Myeloid precursors: CD33, CD66
Solid tumor antigens
Colon: Integrins, A33 glycoprotein, Tag72, epithelial cell adhesion molecule (EpCAM)
Breast: HER2/neu, Lewis-Y, carcinoembryonic antigen (CEA)
Prostate: Prostate-specific membrane antigen (PSMA), prostate stem-cell antigen (PSCA)
Other: GD2 ganglioside (glioblastoma), MUC1 mucin-like glycoprotein (pancreatic cancer), folate receptor (FR), epidermal growth factor receptor (EGFR), transferrin receptor (TFR)
Vascular antigens
vascular endothelial cadherin (VE-cadherin), vascularendothelial growth factor receptor (VEGFR), integrins(such as alpha-V-beta-3)
Types of tumor-associated targets
To date, hematopoietic cancer targets (Table 1) have yielded the most clinically successful targeting approaches,83 in part because these malignancies are readily accessible due to their intravascular dispersion and are often more sensitive to chemotherapy and radiotherapy. By contrast, the delivery of nanoparticles to solid tumors is more challenging due to the poor penetration of the relatively large nanoscale particles through the interstitial tumor microenvironment, despite the fact many solid tumor antigens demonstrate great selectivity for cancer cells. One solution to this problem is the use of targets within the tumor vasculature including nascent vessel-associated markers or a variety of integrins.88 Targeting the vessel has the pharmacologic advantage of immediate delivery for hematopoietic cancers, and also the benefit that the tumor vessels are derived from the host and are thus genetically stable (and thus unlikely to become drug resistant). Moreover, tumor vessels display a more uniform antigen profile thus allowing a single agent to target a large variety of tumor types. In some cases, targeting of the tumor vasculature has resulted in remodeling of the vessels to make them more-effective transporters of the therapeutic agent and of oxygen, which might in turn make the tumors more radiosensitive.88,89
The accumulation of nanoparticles in tumors, termed the enhanced permeability and retention effect (EPR)90,91 was initially described over two decades ago, and has been successfully applied to nanoparticles. Approved agents include re-engineered conventional therapeutics that take advantage of EPR to improve the therapeutic index78 (Supplementary Tables 1 and 2). Some of the earliest ‘nano-medicines’, such as Abraxane® or Doxil®, are novel macromolecular reformulations of paclitaxel and doxorubicin, respectively. EPR depends upon the leaky, disorganized, and tortuous nature of tumor neo-vasculature, which allows for selective retention of particles in the range of 60 to 500 nm due to their propensity to leak out of the vascular space more readily than they can permeate back into the circulation These effects occur as a result of the deficient lymphatic drainage of the tumor environment, but the effect may not be fully reproduced in physiologic models such as in transgenic mouse tumors, orthotopic models, or ultimately in humans, especially in non vascularized metastases.92–94
Novel multifunctional agents are being developed such that passive targeting delivers the active agent near the surface of the cancer cell and a second event, such as the release of cargo for diffusion and entry into the cell, follows. While the EPR effect could improve the therapeutic index of nanoscale particles by improving relative accumulation in solid tumors, the most successful nanomedicines for cancer will likely still need to use an active targeting approach in conjunction with this approach to improve tumor-to-normal tissue ratios. Further improvements in therapeutic index can then be achieved by combining the platform with a cytotoxic agent appropriate for the particular cancer being targeted, or an oligonucleotide selective for a particular activated gene product within the cell. While each of the forms of targeting are not truly cancer specific, one could propose agents that provide sequential increases in cancer selectivity, first by EPR or vascular targeting, second by ligand directed delivery, and finally by cell pathway-selective cargo.
PK, manufacturing and regulatory issues
The unusual properties of the proposed nanomaterials, as well as their multivalency and multifunctionality pose challenges to understanding their PK because different components will have different features that affect the distribution, clearance and catabolism of nanomaterials. Typical PK studies examine the absorption, distribution, metabolism, and excretion (ADME) of a drug. These four factors and the administered dose determine the concentration of a drug at its sites of action, and thus, the intensity of its effects as a function of time. After proto type nanomaterials are modified with different cargoes, their ADME profile might change, requiring iterative testing to determine the new profiles. Multiple steps of synthesis and purification and a thorough physico chemical characterization followed by the determination of the PK and the evaluation of tolerability (toxicity and immunogenicity) by the host will require developmental steps. For many of the new materials, both a detailed physicochemical description and PK data in animals or humans are limited and will be expected to vary significantly among different materials and the composition of the structures.
Hydrodynamic diameter and positive charge in general are inversely related to glomerular filtration rate.54 For example, Kobayashi and Brechbiel50 used MRI to demonstrate that varying the size or hydrophobicity of non-targeting Gd-labeled dendrimer constructs altered the route of renal and liver excretion, respectively. Similarly, non-targeted, radiolabeled quantum dots that were modified with metal-ion chelates and a 600 Dalton polyethylene glycol (PEG) moiety were rapidly cleared from the blood and accumulated in the liver within a few minutes.95,96 Similarly, the particle sizes of nanoparticle “C-dots” from Cornell University have been tuned for changes in filtration, or carbon nanotube charge properties were altered to make dramatic changes in their PK.68,69,97–99
Typically a size cut off of about 6 nm is seen for filtration of globular materials. Interestingly, however, much larger carbon nanotubes with high aspect ratios are still filtered.68,69 Particle charge also affects total body clearance, mediated often by interactions with serum proteins and cells, as has been observed with Q-dots.53 Knowledge of the physicochemical parameters and their interactions can allow design of particles, for example Q-dots, with ligand numbers and sizes that permit renal clearance.100
The nature of the ligands and their attachments, and types of cargo will allow interesting approaches to drug design. For example, the intact agent may accumulate at the tumor site by the EPR effect, while its antibody ligand may selectively bind the tumor cell. A large particulate platform may be trapped in the liver and excreted while the released cargo might be cleared via the kidney; each of these processes may proceed at different rates. As a consequence of this complexity, studies of the PK of the intact particle as well as the components, cargo and metabolites may be required. An advantage to these agents might be that cargoes can be designed for triggered release under various conditions, such as those that exist inside the target cell. For example, pH, redox, proteasesensitive or esterase-sensitive linkers or crosslinkers that degrade over time or that loosen under cellular conditions can trigger release of the cargo. Cytotoxicity, therefore, could be focused directly on the cancer cell. One approach that might overcome the slow diffusion and clearance of certain nanomaterial platforms is to use multi-step pre-targeting approaches in which the slower material is administered first, coupled with a unique ligand (for example, avidin) followed later by a rapidly diffusing and clearing, small-molecule cytotoxic agent coupled with a second ligand specifically able to bind to the first ligand with high affinity (for example, biotin).
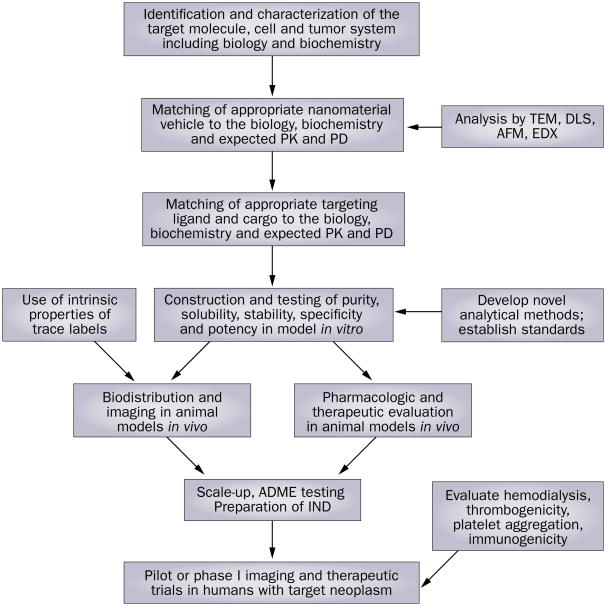
Developing a nanomaterial-based drug will be a complex process (Figure 3). It is anticipated that many of the nanotechnology products that will be regulated by the FDA will span the regulatory boundaries between pharmaceuticals, medical devices, and biologics. As a consequence, these products may be regulated as ‘combination products.’ In part, the FDA attempts to ensure that drugs, drug delivery systems, medical devices, and vaccines that reach the market are safe and effective. The FDA regulates products, not technology.101 Therefore, this distinction will determine the stage at which the FDA regulates a process to manufacture and market novel nanomaterial-based drugs. Because many of the new agents may behave similar to ‘devices’ and have aggregate large surface areas and multivalent moieties that are exposed to the blood stream, they may be expected to have similar effects as other full-scale medically implanted devices (for example grafts, catheters or valves) such as in the promotion of hemolysis, thrombosis, and platelet aggregation, which will need to be studied.102 The multi-component features of these agents might make the approval process complicated and possibly longer than for traditional drugs, for which more is known.
Figure 3.
Some proposed steps in the development of a nanomaterial anti-cancer agent. Abbreviations: ADME, absorption, distribution, metabolism and excretion; AFM, atomic force microscope; DLS, dynamic light scattering; EDX, energy dispersive X-ray; IND, investigational new drug application; TEM, transmission electron microscopy.
Key questions for nanomaterial development will be addressed by the use of animal models, the outcomes of which may not translate well for human use, and ultimately in human subjects.93,103 If possible, these PK experiments can be performed in both animals and humans using classic tracer methodology104 in which a pure and well-characterized product, trace-labeled with a stable moiety should not alter the overall PK profile. Imaging of the agents will most likely be employed increasingly to provide real-time, whole-body bio distributions of the tracers, thus allowing far greater detail as to the possible clearance and toxic effects to be expected, than can be achieved with simple blood measurements.
As new toxicological risks arise from the uses of novel nanomaterials and their varied compositions, new characterization tests will be necessary. In principle, characterization of the ADME of the agent will be required as usual. The specific features of nanomaterial-based agents (large size, relative to small-molecule drugs, multiple component nature, poly-dispersity) will, however, require novel and additional methods of study and new standards.105 The large size of nanomaterials can be seen directly with techniques such as transmission electron microscopy (TEM) and atomic force microscopy (AFM), which often brings insight into their shape, poly dispersity and aggregation. Intrinsic features of the materials permit direct measurement in vitro and in vivo such as by fluorescence in the Q-dots or raman spectroscopy with carbon nanotubes, or by energy dispersive X-ray. Radio-tracing (see imaging modalities section) is also helpful for assessing the properties of these agents in vivo. large, multivalent particles or their appended ligands are possibly immunogenic, in part, due to their poor solubility and because they are often opsonized with antigen-presenting cells and trapped in organs such as the liver, spleen and bone marrow. On the other hand, the immune system is designed to recognize biological peptides and carbohydrates with specific receptors. Thus, nanomaterials may not be recognized by these processes because of their chemical make-up. In addition to improved analytical methodologies, there is a clear need to devise a rational paradigm to characterize nanomaterials in vitro and in vivo.105 Multiple components will need to have each component analyzed separately and also assessed as a final product. One solution to the complexity of evaluating the pharmacology of the materials for regulatory review may be to use components that by themselves are already well-characterized in humans with known toxic effect profiles. Imaging the agents in vivo, in particular by PET, which can provide real-time concentrations of the drugs in tissues, will also aid in describing the PK and potential for toxic effects in greater detail. Early trials that employ biopsies of target tissues can also provide invaluable information on local PK, especially if used in conjunction with full body imaging.
Toxic effects of nanomaterial drugs
Understanding and predicting the toxicities of new nanomaterial agents in humans is complicated by their multicomponent nature, novel structures and polydispersity. Some of the toxic effects of these multicomponent drugs will be attributable to the individual components. However, additional toxic effects may arise because the new PK properties of the materials described above may result in unexpected interactions with target and non-target tissues as well as with the organs involved in clearance. Predictions based on micron-scale particles (such as inhaled particles, asbestos, cells, bacteria or viruses) or from sub-nanoscale molecules (such as typical pharmaceutical drugs) or from nanoscale agents (such as proteins) may not be generalizable to the nanomaterial-based agents discussed here because of large differences in the way cells respond to these novel agents. As a consequence, there may be changes in the magnitude, quality and tissue location of toxic effects compared with the original drugs. For example, the lack of biodegradation of some components could lead to long-term inflammatory problems. Indeed, considerable discussion has ensued about the possible environmental and ecological consequences of widespread nanomaterial uses.106,107
While there has been considerable debate about the possible toxicities of nanomaterial-based drugs, it should be noted that drugs used in cancer patients have typically had low therapeutic indices. Patients have been willing to tolerate significant risks of morbidity and mortality during both the clinical development of the agents and after marketing approval. Data do not suggest that nanomaterial drugs are more toxic than their components (some of which are well-known cytotoxic agents) and in many cases the agents are designed to render the cytotoxic agent less toxic by altering its delivery and clearance. Therefore, in the development of these new agents, it seems reasonable to set the threshold for tolerability and therapeutic index to no higher than that used for anti-cancer agents in current use. In addition, for the cancer applications addressed here, quantities of injected materials are likely to be quite small (mg) so the large-scale manufacturing issues that may be seen with uses of these materials in the electronics or fabrication industries are less troublesome. Furthermore, some of the components of these agents (liposomes, antibodies, chemotherapy drugs, particulate albumin, PEG, super paramagnetic iron oxide, polylactic-co-glutamic acid) have been widely studied in humans for many years and have been approved by the FDA as safe and effective.
The possible toxic effects of carbon nanotubes have been the subject of much speculation, due to their profound chemical stability, which might confer a long life in vivo if these agents are not cleared. Moreover the high aspect ratio (length:width) of carbon nanotubes has prompted some researchers to compare them to asbestos.46 As expected from inhalation studies, insoluble raw carbon nanotubes can cause variable amounts of inflammation (as measured by cytokine release, reactive oxygen species elevations, complement activation, cellular morphology changes) when inhaled or added to cell cultures.108–115 The relevance of these findings to cancer applications is not clear; cancer drugs would be formulated as soluble, intravenous forms, which do not seem to show systemic toxicity.68,69,98,116–118 Importantly, while insoluble micron-scale multi-wall tubes (with sizes larger than could be ingested by macro phages and thus mechanistically similar to asbestos) caused inflammation, nanoscale material caused no inflammation, exudate or granulomas, even in insoluble form.46 Hence, at the nanoscale, these molecules seem to behave in a similar manner to proteins, which are of a similar size. In addition, a new study has shown carbon nanotubes to be biodegradable.119
Other particles under investigation for therapy and imaging such as quantum dots (Q-dots), gold-, iron-, or silica-based nanoparticles and nanoshells have associated toxicities in vitro120,121 including reactive oxygen species activation, inflammation and cytotoxicity.122 Iron oxide particles are safe and are widely used as imaging agents and sources of iron for anemia.25,26 Q-dots are notable for their metallic cores and as a consequence, exposure of the core or its dissolution could result in toxicity, especially from heavy metals such as Cd, Pb, or Se.123–125
The ability to control the generation number of dendrimeric compounds (that is, the number of shells of chemical arms, each shell typically doubling the number of arms and consequently the molecular weight and sizes of the dendrimers from 1 to 20+ nm diameters) allows substantial alterations in charge and valency that can result in changes in cell surface crosslinking, aggregation or activation and thus possible increased toxic effects.47,79,126–129 Pegylation of the dendrimers reduces toxicity and improves the half-life of the agent in the plasma.79,130 These materials are also biodegradable. Although the toxicity potential for these agents is not well understood, there are already extensive efficacy and safety data with nano-formulations such as liposomes, albumin particles, and pegylated molecules, each of which has been used safely in thousands of patients after their FDA approvals. Moreover, recent data with novel nanomaterials are becoming increasingly available; this combination of information should allow design of safe drugs for the drug classes under development.17,32,33
Imaging modalities
Imaging of nanomaterials can have a diagnostic role, dating back 40 years,131 or can be a method to characterize the material in vivo and a means to predict possible toxicity and therapeutic action.132 Nanomaterials are ideally suited as scaffolds to incorporate features (inherent or appended moieties) to report information and these features can result in considerable increases in signal-to-noise ratio relative to conventional imaging molecules. One advantage is that these materials could be designed to possess multimodal means to report information by having combinations of tracer features (for example, fluorescence, magnetic, and radionuclidic). Individually, these conventional imaging techniques have advantages and disadvantages: radionuclides yield high sensitivity, but suffer low spatial resolution; fluorescent labels can provide good spatial resolution in vitro but are difficult to quantify in vivo because of signal attenuation in tissue and high background noise; magnetic particles offer high resolution and good contrast but are not very sensitive. However, used in combination, these different modalities can be built into a single cancer targeting nanomaterial to simultaneously report PK, targeting, and clearance data in vivo and could also be used to report cellular and perhaps subcellular location of a biopsied sample ex vivo.
Another potential advantage of nanomaterials, based upon their size and surface area relative to smaller targeting scaffolds (such as peptides and proteins) is the potential to incorporate multiple copies of fluorophores, chromophores, Gd(III), FeO, and/or radionuclides, thus leading to amplification of signal-to-noise relative to conventional imaging agents.95,98,133 In one interesting example, the nanoscale dimensions and chemical environment of a carbon nanotube encapsulated imaging agent (in this case gadolinium for MRI) resulted in a dramatic improvement in sensitivity of the tracer, in principle allowing smaller tumors to be found.134
Conclusions and future directions
A number of approaches for successful applications of nanomaterial-based cancer drugs will be possible because of their unusual characteristics. The unique pharmacological, biochemical and physicochemical properties will also require novel strategies to overcome the potential hurdles outlined. Control of size, shape, charge, and patterning of nanoscale therapeutic molecules offers potential for optimization of cellular and tissue inter actions. Combining different modes of selective deli very offers the possibility of large increases in therapeutic index and the multivalent capability of the materials will provide higher potency and the possibility of simultaneous imaging and therapy, known as ‘theranostics’. There are already agents capable of multiple functions such as targeting and warhead delivery, dual warhead delivery, or imaging and therapy in trials. Some of the hurdles for future agents and ways to solve potential problems are shown in Table 2. Ultimately, we envision naturally available biomaterials combined with synthetic structures to create multifunctional agents, which are engineered to have defined diffusion, biodegradation and clearance rates, immunologic invisibility, and controlled actions.
Table 2. Specifications and solutions for therapeutic nano-devices for cancer.
| Specification or hurdle | Possible solutions |
|---|---|
| Not detected by human immune system | Use of human-derived antibodies, proteins, receptors, ligands or enzymes for functionalization of platforms. Study of the antigen presentation characteristics of the new materials to understand how to reduce recognition of haptens. |
| Resistance to proteases, nucleases and esterases | Use of aptamers with altered DNA backbone chemistry, peptide ligands with unnatural amino acids, pegylation. Take advantage of the processes to engineer desired clearance and delivery of cargo. |
| Biocompatibilty, catabolism and clearance issues | Engineered sizes, charge, and shape compatible with desired clearance; biofunctionalization to be soluble and biocompatible; use of structures that degrade or release cargo within cells. Preference for degradable components or linkers with small renally cleared agents; controlled degradation after use of agent by enzymatic cleavage. |
| Specificity for or retention near tumors and cancer cells | Platforms contain peptide or small molecule ligands, aptamers or antibodies/fragments; agent takes advantage of enhanced permeability and retention effect to increase selective delivery; multistep pre-targeting approaches are used to increase therapeutic index. |
| Potency | Surface multivalency to increase affinity and cargo delivery; contained spaces for multiple copies of cargo; use of enzymes to multiply effects, such as prodrug conversion at the tumor site. |
| Reporting and accountability | Use of theranostics: radiotraced; MRI active agents, optical or infra-red tracers; caged reporters that signal upon binding, activation or delivery to or into the cell; use of genetic reporters. |
| Controllability | Control by infused second ligands, by focused radio-frequency or magnetic felds; use of intrinsic sensors or tumor-activatable agents; use of artifcial cells or viruses; suicide genes; controlled design of degradation pathways. |
| Intrinsic dynamics and information content | Use of addressable DNA sequences, proteins or sugar polymers; design multivalent ligands to encode avidity for the target. Use of built-in mechanical or enzymatic machinery or pirating such activities from the target cell. |
| Safety and environmental concerns | Science should be built on known agents and components already in use in vivo, then developed individually after pharmacologic study. |
| Political and social issues | Enhanced efforts to educate the public; careful toxicologic analyses. Avoid overstating the promise. |
Supplementary Material
Key points.
Therapeutic uses of novel materials have become widespread; many newer nanoparticles have emerged as candidates for drugs, each with distinctive chemical and biological compositions, and diverse in vivo behaviors
Newer nanomaterials are garnering increasing interest as potential multifunctional therapeutic agents, which by virtue of their size, geometric patterning and shape are conferred novel properties
The synthesis of nanomaterials allows multifunctional and multivalent molecules to be generated, which may enhance potency, therapeutic index or selectivity
The various sizes and shapes of nanomaterials yield very large surface to volume ratios or the possibility of containment for various cargo
The accumulation of nanoparticles in tumors, termed the enhanced permeability and retention effect was initially described over two decades ago, and has been successfully applied to nanoparticles
The unusual properties of nanomaterials pose challenges to understanding their pharmacokinetics as different components will have different features that affect their distributions, clearance and catabolism
Review criteria.
Articles included in the Review were chosen after an exhaustive search on the PubMed, Google Scholar and Science Direct databases for full-text papers published in English without a time restriction. The search terms included each of the relevant nanomaterials by name, cancer antigens and therapeutic drugs and modalities, and more general terms for the fields under study. Recent reviews and papers, many of which are cited in the text, were consulted for additional references.
Footnotes
Competing interests: D. A. Scheinberg declares associations with the following company: Encyse Biosciences Inc. M. R. McDevitt declares associations with the following company: Actinium Pharmaceuticals. See the article online for full details of the relationships. The other authors declare no competing interests.
Supplementary information is linked to the online version of the paper at www.nature.com/nrclinonc
References
- 1.Feynman RP, Robbins J, Dyson FJ. The Pleasure of Finding Things Out: The Best Short Works of Richard P Feynman. Perseus Books; Cambridge, MA: 1999. [Google Scholar]
- 2.Haberzettl CA. Nanomedicine: destination or journey? Nanotechnology. 2002;13:R9. [Google Scholar]
- 3.Whitesides GM. The once and future nanomachine. Sci Am. 2001;285:78–83. doi: 10.1038/scientificamerican0901-78. [DOI] [PubMed] [Google Scholar]
- 4.Pastan I, Hassan R, Fitzgerald DJ, Kreitman RJ. Immunotoxin therapy of cancer. Nat Rev Cancer. 2006;6:559–565. doi: 10.1038/nrc1891. [DOI] [PubMed] [Google Scholar]
- 5.Zhang M, et al. Pretarget radiotherapy with an anti-CD25 antibody-streptavidin fusion protein was effective in therapy of leukemia/lymphoma xenografts. Proc Natl Acad Sci USA. 2003;100:1891–1895. doi: 10.1073/pnas.0437788100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu G, McLeod HL. Strategies for enzyme/prodrug cancer therapy. Clin Cancer Res. 2001;7:3314–3324. [PubMed] [Google Scholar]
- 7.Sengupta S, et al. Temporal targeting of tumour cells and neovasculature with a nanoscale delivery system. Nature. 2005;436:568–572. doi: 10.1038/nature03794. [DOI] [PubMed] [Google Scholar]
- 8.McDevitt MR, et al. Tumor therapy with targeted atomic nanogenerators. Science. 2001;294:1537–1540. doi: 10.1126/science.1064126. [DOI] [PubMed] [Google Scholar]
- 9.National Research Council of the National Academies. A Matter of Size: Triennial Review of the National Nanotechnology Initiative. The National Academies Press; 2006. [Google Scholar]
- 10.Hartman KB, Wilson LJ, Rosenblum MG. Detecting and treating cancer with nanotechnology. Mol Diagn Ther. 2008;12:1–14. doi: 10.1007/BF03256264. [DOI] [PubMed] [Google Scholar]
- 11.Davis ME, Chen ZG, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat Rev Drug Discov. 2008;7:771–782. doi: 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]
- 12.Ferrari M. Cancer nanotechnology: opportunities and challenges. Nat Rev Cancer. 2005;5:161–171. doi: 10.1038/nrc1566. [DOI] [PubMed] [Google Scholar]
- 13.Ferrari M. Nanogeometry: beyond drug delivery. Nat Nanotechnol. 2008;3:131–132. doi: 10.1038/nnano.2008.46. [DOI] [PubMed] [Google Scholar]
- 14.Peer D, et al. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2:751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 15.Allen TM. Ligand-targeted therapeutics in anticancer therapy. Nat Rev Cancer. 2002;2:750–763. doi: 10.1038/nrc903. [DOI] [PubMed] [Google Scholar]
- 16.Minko T, Pakunlu RI, Wang Y, Khandare JJ, Saad M. New generation of liposomal drugs for cancer. Anticancer Agents Med Chem. 2006;6:537–552. doi: 10.2174/187152006778699095. [DOI] [PubMed] [Google Scholar]
- 17.Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4:145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 18.Heath JR, Phelps ME, Hood L. NanoSystems biology. Mol Imaging Biol. 2003;5:312–325. doi: 10.1016/j.mibio.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 19.Tuerk C, Gold L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science. 1990;249:505–510. doi: 10.1126/science.2200121. [DOI] [PubMed] [Google Scholar]
- 20.Simmel FC. Towards biomedical applications for nucleic acid nanodevices. Nanomedicine. 2007;2:817–830. doi: 10.2217/17435889.2.6.817. [DOI] [PubMed] [Google Scholar]
- 21.Cattaneo R, Miest T, Shashkova EV, Barry MA. Reprogrammed viruses as cancer therapeutics: targeted, armed and shielded. Nat Rev Microbiol. 2008;6:529–540. doi: 10.1038/nrmicro1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Resnik DB, Tinkle SS. Ethical issues in clinical trials involving nanomedicine. Contemp Clin Trials. 2007;28:433–441. doi: 10.1016/j.cct.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drexler KE. Engines of Creation. Anchor Press/Doubleday; Garden City, N Y: 1986. [Google Scholar]
- 24.Crichton M. Prey. HarperCollins; New York: 2002. [Google Scholar]
- 25.Stark DD, et al. Superparamagnetic iron oxide: clinical application as a contrast agent for MR imaging of the liver. Radiology. 1988;168:297–301. doi: 10.1148/radiology.168.2.3393649. [DOI] [PubMed] [Google Scholar]
- 26.Weissleder R, et al. Ultrasmall superparamagnetic iron oxide: an intravenous contrast agent for assessing lymph nodes with MR imaging. Radiology. 1990;175:494–498. doi: 10.1148/radiology.175.2.2326475. [DOI] [PubMed] [Google Scholar]
- 27.Eghtedari M, Liopo AV, Copland JA, Oraevsky AA, Motamedi M. Engineering of hetero-functional gold nanorods for the in vivo molecular targeting of breast cancer cells. Nano Lett. 2008;9:287–291. doi: 10.1021/nl802915q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gannon CJ, et al. Carbon nanotube-enhanced thermal destruction of cancer cells in a noninvasive radiofrequency field. Cancer. 2007;110:2654–2665. doi: 10.1002/cncr.23155. [DOI] [PubMed] [Google Scholar]
- 29.Burke A, et al. Long-term survival following a single treatment of kidney tumors with multiwalled carbon nanotubes and near-infrared radiation. Proc Natl Acad Sci USA. 2009;106:12897–12902. doi: 10.1073/pnas.0905195106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kam NW, Dai H. Carbon nanotubes as intracellular protein transporters: generality and biological functionality. J Am Chem Soc. 2005;127:6021–6026. doi: 10.1021/ja050062v. [DOI] [PubMed] [Google Scholar]
- 31.Georgakilas V, et al. Organic functionalization of carbon nanotubes. J Am Chem Soc. 2002;124:760–761. doi: 10.1021/ja016954m. [DOI] [PubMed] [Google Scholar]
- 32.Duncan R. Polymer conjugates as anticancer nanomedicines. Nat Rev Cancer. 2006;6:688–701. doi: 10.1038/nrc1958. [DOI] [PubMed] [Google Scholar]
- 33.Li C, Wallace S. Polymer–drug conjugates: recent development in clinical oncology. Adv Drug Deliv Rev. 2008;60:886–898. doi: 10.1016/j.addr.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schluep T, et al. Preclinical efficacy of the camptothecin–polymer conjugate IT-101 in multiple cancer models. Clin Cancer Res. 2006;12:1606–1614. doi: 10.1158/1078-0432.CCR-05-1566. [DOI] [PubMed] [Google Scholar]
- 35.Scheinberg DA, Strand M, Gansow OA. Tumor imaging with radioactive metal chelates conjugated to monoclonal antibodies. Science. 1982;215:1511–1513. doi: 10.1126/science.7199757. [DOI] [PubMed] [Google Scholar]
- 36.Langer R, Folkman J. Polymers for the sustained release of proteins and other macromolecules. Nature. 1976;263:797–800. doi: 10.1038/263797a0. [DOI] [PubMed] [Google Scholar]
- 37.Whitesides GM. The ‘right’ size in nanobiotechnology. Nat Biotechnol. 2003;21:1161–1165. doi: 10.1038/nbt872. [DOI] [PubMed] [Google Scholar]
- 38.Euliss LE, DuPont JA, Gratton S, DeSimone J. Imparting size, shape, and composition control of materials for nanomedicine. Chem Soc Rev. 2006;35:1095–1104. doi: 10.1039/b600913c. [DOI] [PubMed] [Google Scholar]
- 39.Chithrani BD, Ghazani AA, Chan WC. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006;6:662–668. doi: 10.1021/nl052396o. [DOI] [PubMed] [Google Scholar]
- 40.Jiang W, Kim BY, Rutka J, Chan WC. Nanoparticle-mediated cellular response is size-dependent. Nat Nanotechnol. 2008;3:145–150. doi: 10.1038/nnano.2008.30. [DOI] [PubMed] [Google Scholar]
- 41.Osaki F, Kanamori T, Sando S, Sera T, Aoyama Y. A quantum dot conjugated sugar ball and its cellular uptake. On the size effects of endocytosis in the subviral region. J Am Chem Soc. 2004;126:6520–6521. doi: 10.1021/ja048792a. [DOI] [PubMed] [Google Scholar]
- 42.Gratton SE, et al. The effect of particle design on cellular internalization pathways. Proc Natl Acad Sci USA. 2008;105:11613–11618. doi: 10.1073/pnas.0801763105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rejman J, Oberle V, Zuhorn IS, Hoekstra D. Size-dependent internalization of particles via the pathways of clathrin- and caveolae-mediated endocytosis. Biochem J. 2004;377:159–169. doi: 10.1042/BJ20031253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fifis T, et al. Size-dependent immunogenicity: therapeutic and protective properties of nano-vaccines against tumors. J Immunol. 2004;173:3148–3154. doi: 10.4049/jimmunol.173.5.3148. [DOI] [PubMed] [Google Scholar]
- 45.Tran KK, Shen H. The role of phagosomal pH on the size-dependent efficiency of cross-presentation by dendritic cells. Biomaterials. 2009;30:1356–1362. doi: 10.1016/j.biomaterials.2008.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Poland CA, et al. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat Nanotechnol. 2008;3:423–428. doi: 10.1038/nnano.2008.111. [DOI] [PubMed] [Google Scholar]
- 47.Malik N, et al. Dendrimers: relationship between structure and biocompatibility in vitro, and preliminary studies on the biodistribution of 125I-labelled polyamidoamine dendrimers in vivo. J Control Release. 2000;65:133–148. doi: 10.1016/s0168-3659(99)00246-1. [DOI] [PubMed] [Google Scholar]
- 48.Deen WM. What determines glomerular capillary permeability? J Clin Invest. 2004;114:1412–1414. doi: 10.1172/JCI23577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Deen WM, Lazzara MJ, Myers BD. Structural determinants of glomerular permeability. Am J Physiol Renal Physiol. 2001;281:F579–F596. doi: 10.1152/ajprenal.2001.281.4.F579. [DOI] [PubMed] [Google Scholar]
- 50.Kobayashi H, Brechbiel MW. Dendrimer-based nanosized MRI contrast agents. Curr Pharm Biotechnol. 2004;5:539–549. doi: 10.2174/1389201043376571. [DOI] [PubMed] [Google Scholar]
- 51.Semmler-Behnke M, et al. Biodistribution of 1.4- and 18-nm gold particles in rats. Small. 2008;4:2108–2111. doi: 10.1002/smll.200800922. [DOI] [PubMed] [Google Scholar]
- 52.De Jong WH, et al. Particle size-dependent organ distribution of gold nanoparticles after intravenous administration. Biomaterials. 2008;29:1912–1919. doi: 10.1016/j.biomaterials.2007.12.037. [DOI] [PubMed] [Google Scholar]
- 53.Choi HS, et al. Renal clearance of quantum dots. Nat Biotechnol. 2007;25:1165–1170. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Burns AA, et al. Fluorescent silica nanoparticles with efficient urinary excretion for nanomedicine. Nano Lett. 2009;9:442–448. doi: 10.1021/nl803405h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Joshi A, Vance D, Rai P, Thiyagarajan A, Kane RS. The design of polyvalent therapeutics. Chemistry. 2008;14:7738–7747. doi: 10.1002/chem.200800278. [DOI] [PubMed] [Google Scholar]
- 56.Fox ME, Szoka FC, Fréchet JM. Soluble polymer carriers for the treatment of cancer: the importance of molecular architecture. Acc Chem Res. 2009;42:1141–1151. doi: 10.1021/ar900035f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Champion JA, Katare YK, Mitragotri S. Making polymeric micro- and nanoparticles of complex shapes. Proc Natl Acad Sci USA. 2007;104:11901–11904. doi: 10.1073/pnas.0705326104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gratton SE, Napier ME, Ropp PA, Tian S, DeSimone JM. Microfabricated particles for engineered drug therapies: elucidation into the mechanisms of cellular internalization of PRINT particles. Pharm Res. 2008;25:2845–2852. doi: 10.1007/s11095-008-9654-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jana NR, Gearheart L, Murphy J. Seed-mediated growth approach for shape-controlled synthesis of spheroidal and rod-like gold nanoparticles using a surfactant template. Adv Mater. 2001;13:1389–1393. [Google Scholar]
- 60.Champion JA, Mitragotri S. Role of target geometry in phagocytosis. Proc Natl Acad Sci USA. 2006;103:4930–4934. doi: 10.1073/pnas.0600997103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang K, Fang H, Chen Z, Taylor JS, Wooley KL. Shape effects of nanoparticles conjugated with cell-penetrating peptides (HIV Tat PTD) on CHO cell uptake. Bioconjug Chem. 2008;19:1880–1887. doi: 10.1021/bc800160b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Decuzzi P, Ferrari M. The receptor-mediated endocytosis of nonspherical particles. Biophys J. 2008;94:3790–3797. doi: 10.1529/biophysj.107.120238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jin H, Heller DA, Sharma R, Strano MS. Size-dependent cellular uptake and expulsion of single-walled carbon nanotubes: single particle tracking and a generic uptake model for nanoparticles. ACS Nano. 2009;3:149–158. doi: 10.1021/nn800532m. [DOI] [PubMed] [Google Scholar]
- 64.Champion JA, Mitragotri S. Shape induced inhibition of phagocytosis of polymer particles. Pharm Res. 2009;26:244–249. doi: 10.1007/s11095-008-9626-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wong Shi Kam N, Dai H. Single walled carbon nanotubes for transport and delivery of biological cargos. Physica Status Solidi B. 2006;243:3561–3566. [Google Scholar]
- 66.Kostarelos K, et al. Cellular uptake of functionalized carbon nanotubes is independent of functional group and cell type. Nat Nanotechnol. 2007;2:108–113. doi: 10.1038/nnano.2006.209. [DOI] [PubMed] [Google Scholar]
- 67.Geng Y, et al. Shape effects of filaments versus spherical particles in flow and drug delivery. Nat Nanotechnol. 2007;2:249–255. doi: 10.1038/nnano.2007.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McDevitt MR, et al. PET imaging of soluble yttrium-86-labeled carbon nanotubes in mice. PLoS One. 2007;2:e907. doi: 10.1371/journal.pone.0000907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Singh R, et al. Tissue biodistribution and blood clearance rates of intravenously administered carbon nanotube radiotracers. Proc Natl Acad Sci USA. 2006;103:3357–3362. doi: 10.1073/pnas.0509009103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Villa C, et al. Synthesis and biodistribution of oligonucleotide-functionalized, tumor-targetable carbon nanotubes. Nano Lett. 2008;8:4221–4228. doi: 10.1021/nl801878d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Okamura Y, et al. Novel platelet substitutes: disk-shaped biodegradable nanosheets and their enhanced effects on platelet aggregation. Bioconjug Chem. 2009;20:1958–1965. doi: 10.1021/bc900325w. [DOI] [PubMed] [Google Scholar]
- 72.Joshi A, vance D, Rai P, Thiyagarajan A, Kane RS. The design of polyvalent therapeutics. Chemistry. 2008;14:7738–7747. doi: 10.1002/chem.200800278. [DOI] [PubMed] [Google Scholar]
- 73.Verma A, et al. Surface-structure-regulated cell-membrane penetration by monolayer-protected nanoparticles. Nat Mater. 2008;7:588–595. doi: 10.1038/nmat2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jones SW, et al. Characterisation of cell-penetrating peptide-mediated peptide delivery. Br J Pharmacol. 2005;145:1093–1102. doi: 10.1038/sj.bjp.0706279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Costantini DL, Hu M, Reilly RM. Peptide motifs for insertion of radiolabeled biomolecules into cells and routing to the nucleus for cancer imaging or radiotherapeutic applications. Cancer Biother Radiopharm. 2008;23:3–24. doi: 10.1089/cbr.2007.0430. [DOI] [PubMed] [Google Scholar]
- 76.Kobayashi H, et al. The pharmacokinetic characteristics of glycolated humanized anti-Tac Fabs are determined by their isoelectric points. Cancer Res. 1999;59:422–430. [PubMed] [Google Scholar]
- 77.Faure AC, et al. Control of the in vivo biodistribution of hybrid nanoparticles with different poly(ethylene glycol) coatings. Small. 2009;5:2565–2575. doi: 10.1002/smll.200900563. [DOI] [PubMed] [Google Scholar]
- 78.Alexis F, Pridgen E, Molnar LK, Farokhzad OC. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol Pharm. 2008;5:505–515. doi: 10.1021/mp800051m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Duncan R, Izzo L. Dendrimer biocompatibility and toxicity. Adv Drug Deliv Rev. 2005;57:2215–2237. doi: 10.1016/j.addr.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 80.Yamamoto Y, Nagasaki Y, Kato Y, Sugiyama Y, Kataoka K. Long-circulating poly(ethylene glycol)-poly(D, L-lactide) block copolymer micelles with modulated surface charge. J Control Release. 2001;77:27–38. doi: 10.1016/s0168-3659(01)00451-5. [DOI] [PubMed] [Google Scholar]
- 81.Gotthardt M, et al. Indication for different mechanisms of kidney uptake of radiolabeled peptides. J Nucl Med. 2007;48:596–601. doi: 10.2967/jnumed.106.036020. [DOI] [PubMed] [Google Scholar]
- 82.Bartlett DW, Su H, Hildebrandt IJ, Weber WA, Davis ME. Impact of tumor-specific targeting on the biodistribution and efficacy of siRNA nanoparticles measured by multimodality in vivo imaging. Proc Natl Acad Sci USA. 2007;104:15549–15554. doi: 10.1073/pnas.0707461104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Albrecht H, Denardo S. Recombinant antibodies: from the laboratory to the clinic. Cancer Biother Radiopharm. 2006;21:285–304. doi: 10.1089/cbr.2006.21.285. [DOI] [PubMed] [Google Scholar]
- 84.Oldham RK, Dillman RO. Monoclonal antibodies in cancer therapy: 25 years of progress. J Clin Oncol. 2008;26:1774–1777. doi: 10.1200/JCO.2007.15.7438. [DOI] [PubMed] [Google Scholar]
- 85.Boyiadzis M, Foon KA. Approved monoclonal antibodies for cancer therapy. Expert Opin Biol Ther. 2008;8:1151–1158. doi: 10.1517/14712598.8.8.1151. [DOI] [PubMed] [Google Scholar]
- 86.Castillo J, Winer E, Quesenberry P. Newer monoclonal antibodies for hematological malignancies. Exp Hematol. 2008;36:755–768. doi: 10.1016/j.exphem.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 87.Tassev DV, Cheung NK. Monoclonal antibody therapies for solid tumors. Expert Opin Biol Ther. 2009;9:341–353. doi: 10.1517/14712590802715764. [DOI] [PubMed] [Google Scholar]
- 88.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 89.Shaked Y, et al. Rapid chemotherapy-induced acute endothelial progenitor cell mobilization: implications for antiangiogenic drugs as chemosensitizing agents. Cancer Cell. 2008;14:263–273. doi: 10.1016/j.ccr.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46:6387–6392. [PubMed] [Google Scholar]
- 91.Yuan F, et al. vascular permeability in a human tumor xenograft: molecular size dependence and cutoff size. Cancer Res. 1995;55:3752–3756. [PubMed] [Google Scholar]
- 92.Boven E, et al. Phase II preclinical drug screening in human tumor xenografts: a first European multicenter collaborative study. Cancer Res. 1992;52:5940–5947. [PubMed] [Google Scholar]
- 93.Voskoglou-Nomikos T, Pater JL, Seymour L. Clinical predictive value of the in vitro cell line, human xenograft, and mouse allograft preclinical cancer models. Clin Cancer Res. 2003;9:4227–4239. [PubMed] [Google Scholar]
- 94.Minko T, Kopeckova P, Pozharov V, Jensen KD, Kopecek J. The influence of cytotoxicity of macromolecules and of VEGF gene modulated vascular permeability on the enhanced permeability and retention effect in resistant solid tumors. Pharm Res. 2000;17:505–514. doi: 10.1023/a:1007500412442. [DOI] [PubMed] [Google Scholar]
- 95.Michalet X, et al. Quantum dots for live cells, in vivo imaging, and diagnostics. Science. 2005;307:538–544. doi: 10.1126/science.1104274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gao X, Cui Y, Levenson R, Chung L, Nie S. In vivo cancer targeting and imaging with semiconductor quantum dots. Nat Biotechnol. 2004;22:969–976. doi: 10.1038/nbt994. [DOI] [PubMed] [Google Scholar]
- 97.Liu Z, et al. Drug delivery with carbon nanotubes for in vivo cancer treatment. Cancer Res. 2008;68:6652–6660. doi: 10.1158/0008-5472.CAN-08-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.McDevitt MR, et al. Tumor targeting with antibody-functionalized, radiolabeled carbon nanotubes. J Nucl Med. 2007;48:1180–1189. doi: 10.2967/jnumed.106.039131. [DOI] [PubMed] [Google Scholar]
- 99.Bhirde AA, et al. Targeted killing of cancer cells in vivo and in vitro with EGF-directed carbon nanotube-based drug delivery. ACS Nano. 2009;3:307–316. doi: 10.1021/nn800551s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Choi HS, et al. Design considerations for tumour-targeted nanoparticles. Nat Nanotechnol. 2009;5:42–47. doi: 10.1038/nnano.2009.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.US Department of Health and Human Services. FDA Regulation of Nanotechnology Products. 2009 [online], http://www.fda.gov/ScienceResearch/SpecialTopics/Nanotechnology/default.htm.
- 102.Dobrovolskaia MA, Aggarwal P, Hall JB, McNeil SE. Preclinical studies to understand nanoparticle interaction with the immune system and its potential effects on nanoparticle biodistribution. Mol Pharm. 2008;5:487–495. doi: 10.1021/mp800032f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Eckelman W, Kilbourn MR, Joyal JL, Labiris R, valliant JF. Justifying the number of animals for each experiment. Nucl Med Biol. 2007;34:229–232. doi: 10.1016/j.nucmedbio.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 104.Wilson BJ, editor. The Radiochemical Manual. 2nd. The Radiochemical Centre; Amersham: 1966. [Google Scholar]
- 105.Hall JB, Dobrovolskaia MA, Patri AK, McNeil SE. Characterization of nanoparticles for therapeutics. Nanomedicine. 2007;2:789–803. doi: 10.2217/17435889.2.6.789. [DOI] [PubMed] [Google Scholar]
- 106.Boxall AB, Tiede K, Chaudhry Q. Engineered nanomaterials in soils and water: how do they behave and could they pose a risk to human health? Nanomedicine. 2007;2:919–927. doi: 10.2217/17435889.2.6.919. [DOI] [PubMed] [Google Scholar]
- 107.Nel A, Xia T, Mädler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006;311:622–627. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- 108.De Jong WH, Borm PJ. Drug delivery and nanoparticles: applications and hazards. Int J Nanomedicine. 2008;3:133–149. doi: 10.2147/ijn.s596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Elgrabli D, et al. Induction of apoptosis and absence of inflammation in rat lung after intratracheal instillation of multiwalled carbon nanotubes. Toxicology. 2008;253:131–136. doi: 10.1016/j.tox.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 110.Hamad I, et al. Complement activation by pegylated single-walled carbon nanotubes is independent of C1q and alternative pathway turnover. Mol Immunol. 2008;45:3797–3803. doi: 10.1016/j.molimm.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kagan VE, Bayir H, Shvedova AA. Nanomedicine and nanotoxicology: two sides of the same coin. Nanomedicine. 2005;1:313–316. doi: 10.1016/j.nano.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 112.Magrez A, et al. Cellular toxicity of carbon-based nanomaterials. Nano Lett. 2006;6:1121–1125. doi: 10.1021/nl060162e. [DOI] [PubMed] [Google Scholar]
- 113.Simon-Deckers A, et al. In vitro investigation of oxide nanoparticle and carbon nanotube toxicity and intracellular accumulation in A549 human pneumocytes. Toxicology. 2008;253:137–146. doi: 10.1016/j.tox.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 114.Zhang LW, Zeng L, Barron AR, Monteiro-Riviere NA. Biological interactions of functionalized single-wall carbon nanotubes in human epidermal keratinocytes. Int J Toxicol. 2007;26:103–113. doi: 10.1080/10915810701225133. [DOI] [PubMed] [Google Scholar]
- 115.Lam CW, James JT, McCluskey R, Arepalli S, Hunter RL. A review of carbon nanotube toxicity and assessment of potential occupational and environmental health risks. Crit Rev Toxicol. 2006;36:189–217. doi: 10.1080/10408440600570233. [DOI] [PubMed] [Google Scholar]
- 116.Dumortier H, et al. Functionalized carbon nanotubes are non-cytotoxic and preserve the functionality of primary immune cells. Nano Lett. 2006;6:1522–1528. doi: 10.1021/nl061160x. [DOI] [PubMed] [Google Scholar]
- 117.Lacerda L, et al. Tissue histology and physiology following intravenous administration of different types of functionalized multiwalled carbon nanotubes. Nanomedicine. 2008;3:149–161. doi: 10.2217/17435889.3.2.149. [DOI] [PubMed] [Google Scholar]
- 118.Schipper ML, et al. A pilot toxicology study of single-walled carbon nanotubes in a small sample of mice. Nat Nanotechnol. 2008;3:216–221. doi: 10.1038/nnano.2008.68. [DOI] [PubMed] [Google Scholar]
- 119.Allen BL, et al. Biodegradation of single-walled carbon nanotubes through enzymatic catalysis. Nano Lett. 2008;8:3899–3903. doi: 10.1021/nl802315h. [DOI] [PubMed] [Google Scholar]
- 120.Costigan S. The toxicology of nanoparticles used in healthcare products. 2006 http://www.mhra.gov.uk/home/idcplg?IdcService=SS_GET_PAGE&nodeId=996.
- 121.De Jong WH, Borm PJ. Drug delivery and nanoparticles: applications and hazards. Int J Nanomedicine. 2008;3:133–149. doi: 10.2147/ijn.s596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chang JS, Chang KL, Hwang DF, Kong ZL. In vitro cytotoxicitiy of silica nanoparticles at high concentrations strongly depends on the metabolic activity type of the cell line. Environ Sci Technol. 2007;41:2064–2068. doi: 10.1021/es062347t. [DOI] [PubMed] [Google Scholar]
- 123.Hardman R. A toxicologic review of quantum dots: toxicity depends on physicochemical and environmental factors. Environ Health Perspect. 2006;114:165–172. doi: 10.1289/ehp.8284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hoshino A, et al. Physicochemical properties and cellular toxicity of nanocrystal quantum dots depend on their surface modification. Nano Lett. 2004;4:2163–2169. [Google Scholar]
- 125.Lovric J, et al. Differences in subcellular distribution and toxicity of green and red emitting CdTe quantum dots. J Mol Med. 2005;83:377–385. doi: 10.1007/s00109-004-0629-x. [DOI] [PubMed] [Google Scholar]
- 126.Chen HT, Neerman MF, Parrish AR, Simanek EE. Cytotoxicity, hemolysis, and acut in vivo toxicity of dendrimers based on melamine, candidate vehicles for drug delivery. J Am Chem Soc. 2004;126:10044–10048. doi: 10.1021/ja048548j. [DOI] [PubMed] [Google Scholar]
- 127.Heiden TC, Dengler E, Kao WJ, Heideman W, Peterson RE. Developmental toxicity of low generation PAMAM dendrimers in zebrafish. Toxicol Appl Pharmacol. 2007;225:70–79. doi: 10.1016/j.taap.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Roberts JC, Bhalgat MK, Zera RT. Preliminary biological evaluation of polyamidoamine (PAMAM) Starburst dendrimers. J Biomed Mater Res. 1996;30:53–65. doi: 10.1002/(SICI)1097-4636(199601)30:1<53::AID-JBM8>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 129.Plank C, Mechtler K, Szoka FC, Jr, Wagner E. Activation of the complement system by synthetic DNA complexes: a potential barrier for intravenous gene delivery. Hum Gene Ther. 1996;7:1437–1446. doi: 10.1089/hum.1996.7.12-1437. [DOI] [PubMed] [Google Scholar]
- 130.Kaminskas LM, et al. The impact of molecular weight and PEG chain length on the systemic pharmacokinetics of pegylated poly l-lysine dendrimers. Mol Pharm. 2008;5:449–463. doi: 10.1021/mp7001208. [DOI] [PubMed] [Google Scholar]
- 131.Larson SM, Nelp WB. Radiopharmacology of a simplifield technetium-99m-colloid preparation for photoscanning. J Nucl Med. 1966;7:817–826. [PubMed] [Google Scholar]
- 132.Escorcia FE, McDevitt MR, Villa CH, Scheinberg DA. Targeted nanomaterials for radiotherapy. Nanomedicine. 2007;2:805–815. doi: 10.2217/17435889.2.6.805. [DOI] [PubMed] [Google Scholar]
- 133.Cai W, Chen X. Multimodality molecular imaging of tumor angiogenesis. J Nucl Med. 2008;49(Suppl. 2):113S–128S. doi: 10.2967/jnumed.107.045922. [DOI] [PubMed] [Google Scholar]
- 134.Sitharaman B, et al. Superparamagnetic gadonanotubes are high-performance MRI contrast agents. Chem Commun. 2005;21:3915–3917. doi: 10.1039/b504435a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.