Abstract
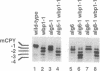
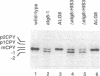
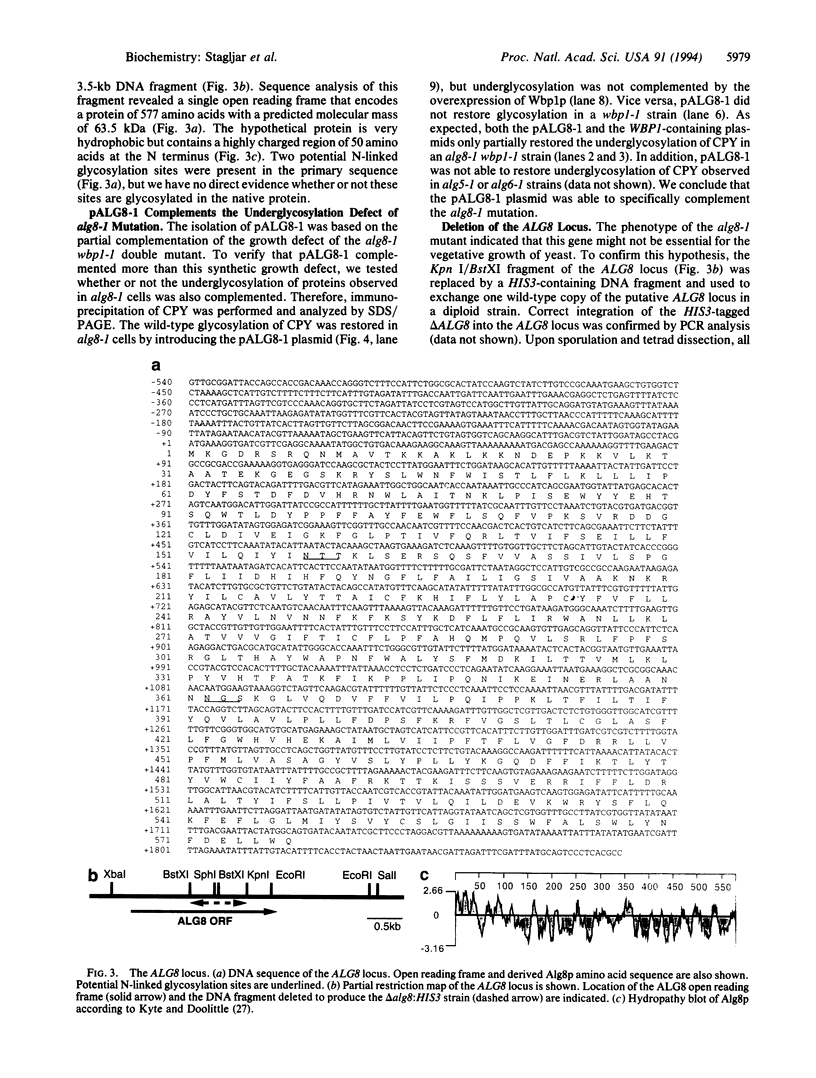
Glc3Man9GlcNAc2 is the preferred substrate of the oligosaccharyltransferase of N-linked glycosylation of proteins, but nonglucosylated oligosaccharides can be transferred to proteins in Saccharomyces cerevisiae. Mutations affecting the addition of the three terminal glucose residues lead to accumulation of Man9GlcNAc2 or Glc1Man9GlcNAc2 in vivo but do not show any detectable growth defect. When these mutations were introduced into a strain with reduced oligosaccharyltransferase activity (due to the wbp1-1 mutation), a severe growth defect was observed: accumulation of suboptimal lipid-linked oligosaccharide and reduced oligosaccharyltransferase activity resulted in a severe underglycosylation of secreted proteins. This new synthetic phenotype made it possible to isolate the ALG8 locus, encoding a potential glucosyltransferase of the endoplasmic reticulum. The ALG8 protein is a 63.5-kDa hydrophobic protein that is not essential for the vegetative growth of yeast. However, the lack of this protein resulted in underglycosylation of secreted proteins.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeijon C., Hirschberg C. B. Topography of glycosylation reactions in the endoplasmic reticulum. Trends Biochem Sci. 1992 Jan;17(1):32–36. doi: 10.1016/0968-0004(92)90424-8. [DOI] [PubMed] [Google Scholar]
- Albright C. F., Orlean P., Robbins P. W. A 13-amino acid peptide in three yeast glycosyltransferases may be involved in dolichol recognition. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7366–7369. doi: 10.1073/pnas.86.19.7366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amberg D. C., Fleischmann M., Stagljar I., Cole C. N., Aebi M. Nuclear PRP20 protein is required for mRNA export. EMBO J. 1993 Jan;12(1):233–241. doi: 10.1002/j.1460-2075.1993.tb05649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballou L., Gopal P., Krummel B., Tammi M., Ballou C. E. A mutation that prevents glucosylation of the lipid-linked oligosaccharide precursor leads to underglycosylation of secreted yeast invertase. Proc Natl Acad Sci U S A. 1986 May;83(10):3081–3085. doi: 10.1073/pnas.83.10.3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza-Schorey C., Elbein A. D. Partial purification and properties of a glucosyltransferase that synthesizes Glc1Man9(GlcNAc)2-pyrophosphoryldolichol. J Biol Chem. 1993 Mar 5;268(7):4720–4727. [PubMed] [Google Scholar]
- Datta A. K., Lehrman M. A. Both potential dolichol recognition sequences of hamster GlcNAc-1-phosphate transferase are necessary for normal enzyme function. J Biol Chem. 1993 Jun 15;268(17):12663–12668. [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hasilik A., Tanner W. Carbohydrate moiety of carboxypeptidase Y and perturbation of its biosynthesis. Eur J Biochem. 1978 Nov 15;91(2):567–575. doi: 10.1111/j.1432-1033.1978.tb12710.x. [DOI] [PubMed] [Google Scholar]
- Herscovics A., Orlean P. Glycoprotein biosynthesis in yeast. FASEB J. 1993 Apr 1;7(6):540–550. doi: 10.1096/fasebj.7.6.8472892. [DOI] [PubMed] [Google Scholar]
- Hill J. E., Myers A. M., Koerner T. J., Tzagoloff A. Yeast/E. coli shuttle vectors with multiple unique restriction sites. Yeast. 1986 Sep;2(3):163–167. doi: 10.1002/yea.320020304. [DOI] [PubMed] [Google Scholar]
- Huffaker T. C., Robbins P. W. Yeast mutants deficient in protein glycosylation. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7466–7470. doi: 10.1073/pnas.80.24.7466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lehrman M. A. Biosynthesis of N-acetylglucosamine-P-P-dolichol, the committed step of asparagine-linked oligosaccharide assembly. Glycobiology. 1991 Dec;1(6):553–562. doi: 10.1093/glycob/1.6.553. [DOI] [PubMed] [Google Scholar]
- Murphy L. A., Spiro R. G. Transfer of glucose to oligosaccharide-lipid intermediates by thyroid microsomal enzymes and its relationship to the N-glycosylation of proteins. J Biol Chem. 1981 Jul 25;256(14):7487–7494. [PubMed] [Google Scholar]
- Rothblatt J. A., Deshaies R. J., Sanders S. L., Daum G., Schekman R. Multiple genes are required for proper insertion of secretory proteins into the endoplasmic reticulum in yeast. J Cell Biol. 1989 Dec;109(6 Pt 1):2641–2652. doi: 10.1083/jcb.109.6.2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runge K. W., Huffaker T. C., Robbins P. W. Two yeast mutations in glucosylation steps of the asparagine glycosylation pathway. J Biol Chem. 1984 Jan 10;259(1):412–417. [PubMed] [Google Scholar]
- Runge K. W., Robbins P. W. A new yeast mutation in the glucosylation steps of the asparagine-linked glycosylation pathway. Formation of a novel asparagine-linked oligosaccharide containing two glucose residues. J Biol Chem. 1986 Nov 25;261(33):15582–15590. [PubMed] [Google Scholar]
- Sathe G. M., O'Brien S., McLaughlin M. M., Watson F., Livi G. P. Use of polymerase chain reaction for rapid detection of gene insertions in whole yeast cells. Nucleic Acids Res. 1991 Sep 11;19(17):4775–4775. doi: 10.1093/nar/19.17.4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma C. B., Lehle L., Tanner W. N-Glycosylation of yeast proteins. Characterization of the solubilized oligosaccharyl transferase. Eur J Biochem. 1981 May;116(1):101–108. doi: 10.1111/j.1432-1033.1981.tb05306.x. [DOI] [PubMed] [Google Scholar]
- Staneloni R. J., Ugalde R. A., Leloir L. F. Addition of glucose to dolichyl diphosphate oligosaccharide and transfer to protein. Eur J Biochem. 1980 Apr;105(2):275–278. doi: 10.1111/j.1432-1033.1980.tb04498.x. [DOI] [PubMed] [Google Scholar]
- Stevens T., Esmon B., Schekman R. Early stages in the yeast secretory pathway are required for transport of carboxypeptidase Y to the vacuole. Cell. 1982 Sep;30(2):439–448. doi: 10.1016/0092-8674(82)90241-0. [DOI] [PubMed] [Google Scholar]
- Struhl K. Nucleotide sequence and transcriptional mapping of the yeast pet56-his3-ded1 gene region. Nucleic Acids Res. 1985 Dec 9;13(23):8587–8601. doi: 10.1093/nar/13.23.8587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimble R. B., Byrd J. C., Maley F. Effect of glucosylation of lipid intermediates on oligosaccharide transfer in solubilized microsomes from Saccharomyces cerevisiae. J Biol Chem. 1980 Dec 25;255(24):11892–11895. [PubMed] [Google Scholar]
- Turco S. J., Stetson B., Robbins P. W. Comparative rates of transfer of lipid-linked oligosaccharides to endogenous glycoprotein acceptors in vitro. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4411–4414. doi: 10.1073/pnas.74.10.4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verostek M. F., Atkinson P. H., Trimble R. B. Glycoprotein biosynthesis in the alg3 Saccharomyces cerevisiae mutant. I. Role of glucose in the initial glycosylation of invertase in the endoplasmic reticulum. J Biol Chem. 1993 Jun 5;268(16):12095–12103. [PubMed] [Google Scholar]
- Verostek M. F., Atkinson P. H., Trimble R. B. Glycoprotein biosynthesis in the alg3 Saccharomyces cerevisiae mutant. II. Structure of novel Man6-10GlcNAc2 processing intermediates on secreted invertase. J Biol Chem. 1993 Jun 5;268(16):12104–12115. [PubMed] [Google Scholar]
- Vijayraghavan U., Company M., Abelson J. Isolation and characterization of pre-mRNA splicing mutants of Saccharomyces cerevisiae. Genes Dev. 1989 Aug;3(8):1206–1216. doi: 10.1101/gad.3.8.1206. [DOI] [PubMed] [Google Scholar]
- Zimmerman J. W., Robbins P. W. The hydrophobic domain of dolichyl-phosphate-mannose synthase is not essential for enzyme activity or growth in Saccharomyces cerevisiae. J Biol Chem. 1993 Aug 5;268(22):16746–16753. [PubMed] [Google Scholar]
- te Heesen S., Janetzky B., Lehle L., Aebi M. The yeast WBP1 is essential for oligosaccharyl transferase activity in vivo and in vitro. EMBO J. 1992 Jun;11(6):2071–2075. doi: 10.1002/j.1460-2075.1992.tb05265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- te Heesen S., Knauer R., Lehle L., Aebi M. Yeast Wbp1p and Swp1p form a protein complex essential for oligosaccharyl transferase activity. EMBO J. 1993 Jan;12(1):279–284. doi: 10.1002/j.1460-2075.1993.tb05654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]