Abstract
ATP is released in an activity-dependent manner from different cell types in the brain, fulfilling different roles as a neurotransmitter, neuromodulator, in astrocyte-to-neuron communication, propagating astrocytic responses and formatting microglia responses. This involves the activation of different ATP P2 receptors (P2R) as well as adenosine receptors upon extracellular ATP catabolism by ecto-nucleotidases. Notably, brain noxious stimuli trigger a sustained increase of extracellular ATP, which plays a key role as danger signal in the brain. This involves a combined action of extracellular ATP in different cell types, namely increasing the susceptibility of neurons to damage, promoting astrogliosis and recruiting and formatting microglia to mount neuroinflammatory responses. Such actions involve the activation of different receptors, as heralded by neuroprotective effects resulting from blockade mainly of P2X7R, P2Y1R and adenosine A2A receptors (A2AR), which hierarchy, cooperation and/or redundancy is still not resolved. These pleiotropic functions of ATP as a danger signal in brain damage prompt a therapeutic interest to multi-target different purinergic receptors to provide maximal opportunities for neuroprotection.
Keywords: ATP, adenosine, P2 receptors, P1 receptors, ecto-nucleotidases, P2X7 receptor, P2Y1 receptor, A2A receptor
Introduction
Intracellular adenosine 5′-triphosphate (ATP) plays several pivotal roles, namely in energy transfer (Lipmann, 1941). Hence, the proposal by Burnstock (1972) that ATP was released to function as an extracellular signal was controversial. However, this concept is supported by the identification of mechanisms of ATP release, of ecto-enzymes metabolizing ATP named ecto-nucleotidases, and of purinergic receptors. ATP can trigger biological effects per se through the activation of P2 receptors (P2R) or through its ecto-nucleotidase metabolites ADP activating some P2R and adenosine through P1R activation (Ralevic and Burnstock, 1998). Cloning identified seven P2XR subunits P2X1-7, forming functional homomeric or heteromeric ionotropic receptors activated by ATP (Khakh and North, 2012) and eight different metabotropic P2YR (P2Y1,2,4,6,11,12,13,14) exhibiting a different sensitivity to ATP (P2Y11), ADP (P2Y1,12,13), UTP/ATP (P2Y2,4), UDP (P2Y6), or UDP-glucose (P2Y14) (Abbracchio et al., 2006), whereas adenosine P1R family comprises A1, A2A, A2B, and A3 metabotropic receptors, identified by convergent molecular, biochemical and pharmacological data (Fredholm et al., 2011).
ATP is stored in synaptic and in astrocyte vesicles, but it can be released from different cell types, namely nerve terminals, dendrites, and axons from neurons (Pankratov et al., 2006; Fields, 2011), astrocytes (Koizumi, 2010) and microglia (Imura et al., 2013; George et al., 2015) through multiple pathways (Bodin and Burnstock, 2001). Also, purinergic receptors display a widespread brain expression both in neuronal or non-neuronal cells such as astrocytes, microglia or endothelial cells (Fredholm et al., 2005; Fields and Burnstock, 2006). Accordingly, multiple roles have been attributed to extracellular ATP. ATP can act as a neurotransmitter, since P2XR-mediated ATPergic transmission has been found in central synapses (Edwards et al., 1992; Bardoni et al., 1997; Nieber et al., 1997; Pankratov et al., 1998, 2002; Mori et al., 2001). ATP is also a controller of inflammation (Idzko et al., 2014), with multiple actions on microglia (Koizumi et al., 2013) and its consequences on astrocytes and neurons. ATP and adenosine both regulate oligodendrocyte differentiation and myelination (Agresti et al., 2005; Rivkees and Wendler, 2011) in an activity-dependent manner (Fields, 2006). Moreover, purines modulate astrocytic function and sustain Ca2+-waves, the substrate of glial excitability and intercellular communication (Guthrie et al., 1999; Koizumi, 2010) to influence synaptic activity (Zhang et al., 2003; Jourdain et al., 2007; Franke et al., 2012). In fact, it is mostly concluded that ATP acts as a synaptic neuromodulator through presynaptic regulation of neurotransmitter release, by postsynaptic regulation of other receptors or of intrinsic neuronal excitability, with an impact in synaptic plasticity (Cunha and Ribeiro, 2000; Khakh, 2001; Halassa et al., 2009).
The variety of purinergic receptors and their widespread region- and cell-specific expression pattern and actions places purinergic signaling as a major system for integration of functional activity between neurons, glial and vascular cells in the brain as heralded by the role of purines (ATP and adenosine) in neuron-neuron, astrocyte-neuron, oligodendrocyte-neuron and/or microglia/neuron bi-directional communication (Fields and Burnstock, 2006; Butt, 2011). Moreover, the different sensitivities of the different receptors to their different ligands (ATP, ADP, adenosine) displaying spatial and temporal fine-tuned gradients (Zhang et al., 2003; Cunha, 2008), endows purinergic signaling with unique features adapted to control brain networks. Not surprisingly, the dysfunction of this purinergic system is closely associated with brain disorders and we will now exploit the concept that ATP acts as a danger signal, implying an abnormal and sustained elevation of extracellular ATP levels in brain dysfunction and the involvement of purine receptors, namely P2X7R (ATP), P2Y1R (ADP) and A2AR (adenosine), in brain damage.
Sustained increase of extracellular ATP levels in brain pathology
There is growing evidence for a rapid increase of the extracellular ATP levels upon noxious brain conditions such as trauma (Wang et al., 2004; Davalos et al., 2005; Franke et al., 2006; Choo et al., 2013), hypoxia/ischemia (Lutz and Kabler, 1997; Jurányi et al., 1999; Melani et al., 2005) or epilepsy-associated seizures (Wieraszko et al., 1989; see Dale and Frenguelli, 2009). The sustained nature of the enhanced extracellular levels of purines (ATP and adenosine) in brain dysfunction is indicative of regulated mechanisms of ATP release rather than simple ATP leakage. However, neither the cellular source nor the mechanism of ATP release upon noxious brain conditions has yet been clarified. Neurons can release ATP either through a vesicular release (White, 1977; Pankratov et al., 2006) mostly occurring at high frequency of firing (Wieraszko et al., 1989; Cunha et al., 1996a) or upon anoxic or spreading depolarization (Frenguelli et al., 2007). Astrocytes (Florian et al., 2011; Bennett et al., 2012) and microglia (Kim et al., 2007; Sanz et al., 2009) can also release purines upon brain dysfunction through vesicular release (Coco et al., 2003; Bowser and Khakh, 2007; Imura et al., 2013) and/or other mechanisms namely pannexin and/or connexin channels (Bao et al., 2004; Reigada et al., 2008; Iwabuchi and Kawahara, 2011), which have been proposed as a target for neuroprotection (Shestopalov and Slepak, 2014). In other cells, ATP release through lysosomal-dependent vesicles (Zhang et al., 2007) and/or from pannexin channels (Bennett et al., 2012) from autophagic (Wang et al., 2013) or apoptotic cells (Sandilos et al., 2012; Xiao et al., 2012) acts as a find-me signal (Elliott et al., 2009) (Figure 1).
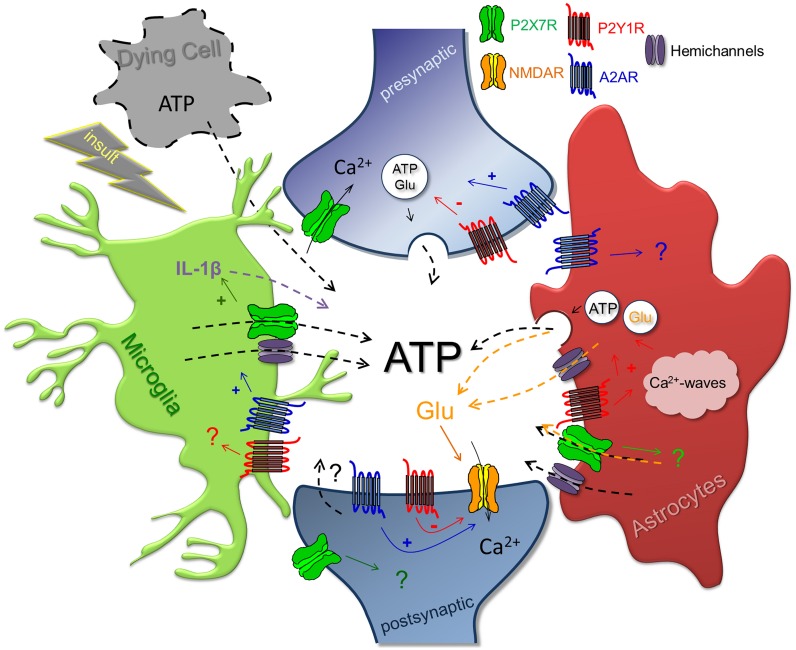
Figure 1.
Integrated view of the purinergic signaling in brain disorders. In addition to the leakage of ATP through damaged cell membrane from injured or dying cells, evolution has assured multiple mechanisms from different sources to place ATP in the extracellular milieu as a danger signal in the brain. Interestingly, this increase is self-sustained: activation of P2X7R induces the release of ATP either directly through its channel or by exocytotic or non-exocytotic mechanisms (e.g., hemichannels); P2Y1R induces the release of ATP from astrocytes; A2AR controls the release of ATP from microglia and presynaptic terminals. Once in the extracellular millieu, ATP seems to contribute to neurotoxicity through an integrated action through P2X7R, P2Y1R, and A2AR. P2X7R: it is well-established that P2X7R antagonism is beneficial by preventing the neurotoxic processing and release of IL-1β from microglia; yet a deleterious action through astrocytes namely through the regulation of glutamate levels or pro-inflammatory cytokines, or a direct neurotoxic action cannot be discarded. P2Y1R: the contribution of P2Y1R to brain demises has been mainly associated to astrocytic reactivity through Ca2+-waves and through an astrocytic-driven release of glutamate; this may be further promoted by direct actions on neuronal and synaptic function. A2AR: there is gain of function of A2AR particularly targeted to synapses in different brain disorders, where A2AR either with a presynaptic or postsynaptic locus of action, has been associated to synaptic dysfunction/loss; the precise mechanisms remain to be identified.
Purinergic receptors in brain pathology
The concept of ATP as a danger signal implies the release of ATP but also the involvement of purinergic receptors in brain disorders, which has mostly been documented for P2X7R, P2Y1R, and A2AR.
P2X7 receptor
P2X7R have a lower affinity for ATP (0.1–1 mM) compared to other P2XR (EC50 = 1–10 μ M) (Surprenant and North, 2009), suggesting that their activation mostly occurs in pathological conditions associated to enhanced extracellular ATP levels. This is supported by the well-documented increase of P2X7R levels and P2X7R gain-of-function to control different brain disorders, from trauma or metabolic stress (Cavaliere et al., 2004; Franke et al., 2004; Melani et al., 2006; Arbeloa et al., 2012; Kimbler et al., 2012) to Alzheimer's disease (AD; Parvathenani et al., 2003; McLarnon et al., 2006; Díaz-Hernández et al., 2012; Murphy et al., 2012), Parkinson's disease (PD; Marcellino et al., 2010; Carmo et al., 2014a), Huntington's disease (HD; Díaz-Hernández et al., 2009) epilepsy (Solle et al., 2001; Vianna et al., 2002; Rappold et al., 2006; Avignone et al., 2008; Doná et al., 2009; Engel et al., 2012; Jimenez-Pacheco et al., 2013), prion disease (Takenouchi et al., 2007), and multiple sclerosis (MS, Matute et al., 2007; Sharp et al., 2008; Grygorowicz et al., 2011). Increased P2X7R levels have been also reported in human brain tissue of patients with temporal lobe epilepsy (Fernandes et al., 2009; Padrão et al., 2011), MS or AD (Narcisse et al., 2005; McLarnon et al., 2006; Yiangou et al., 2006).
P2X7R up-regulation has been mainly associated with microgliosis, since P2X7R promote neuronal death through microglia-derived interleukin-1β (IL-1β) (Ferrari et al., 1996; Chakfe et al., 2002; Skaper et al., 2006; Bernardino et al., 2008; Takenouchi et al., 2009) or production of reactive oxygen species (Parvathenani et al., 2003; Skaper et al., 2006; Lee et al., 2011). In AD, P2X7R are predominantly up-regulated in microglia around β-amyloid (Aβ) plaques in mice (Parvathenani et al., 2003; Lee et al., 2011) and humans (McLarnon et al., 2006) and Aβ triggers IL-1β secretion from microglia in a P2X7R-dependent manner (Sanz et al., 2009). A similar gain of function of P2X7R in formatting microglia responsiveness has been observed after ischemia (Franke et al., 2004), MS (Yiangou et al., 2006), prion disease (Takenouchi et al., 2007), PD (Marcellino et al., 2010) or upon status epilepticus (Rappold et al., 2006; Avignone et al., 2008; Kim et al., 2009; Choi et al., 2012; Engel et al., 2012), where P2X7R blockade/deletion reduces seizure severity during status epilepticus (Solle et al., 2001; Engel et al., 2012; Jimenez-Pacheco et al., 2013). P2X7R have also been linked to psychiatric disorders, as heralded by the association of P2X7R polymorphisms with major depression (Lucae et al., 2006; Hejjas et al., 2009) and by the anti-depressive behavior of P2X7R KO mice (Basso et al., 2009; Csölle et al., 2013), in line with the ability of IL-1β to induce depression-like behavioral changes (Pollak and Yirmiya, 2002; Anisman et al., 2005).
Besides this major role on overactivation of microglia, P2X7R are also up-regulated in reactive astrocytes and in neurons in the diseased brain (Franke et al., 2004; Doná et al., 2009; Engel et al., 2012). Astrocytic and neuronal P2X7R may also contribute to neuronal damage by inducing the release of glutamate and GABA from astrocytes (Wang et al., 2002; Duan et al., 2003; Fu et al., 2013) or from neurons (Wirkner et al., 2005; Marcoli et al., 2008; Cho et al., 2010; Cervetto et al., 2012), unbalancing excitability (Tian et al., 2005) and/or causing a direct neurotoxicity (Volonté et al., 2003) involving either the dilation of P2X7R pore (Di Virgilio et al., 1998; Khadra et al., 2013) or the recruitment of pannexin-1 hemichannels (Suadicani et al., 2012). Accordingly neuronal P2X7R are required for neurotoxicity in HD (Díaz-Hernández et al., 2009), PD (Carmo et al., 2014a) or ischemic conditions (Arbeloa et al., 2012). A direct toxic action of ATP through P2X7R activation has also been shown in oligodendrocytes (Matute et al., 2007), which may be relevant to the contribution of P2X7R to MS (Amadio et al., 2011).
In summary, the observed gain of function of P2X7R in pathological conditions, suggests that P2X7R may essentially act as a danger sensor shared by different brain disorders, contributing to the progression of brain diseases through a combined neurotoxic overactivation of microglia, also involving astrocytic-mediated or direct neurotoxic actions (Figures 1, 2).
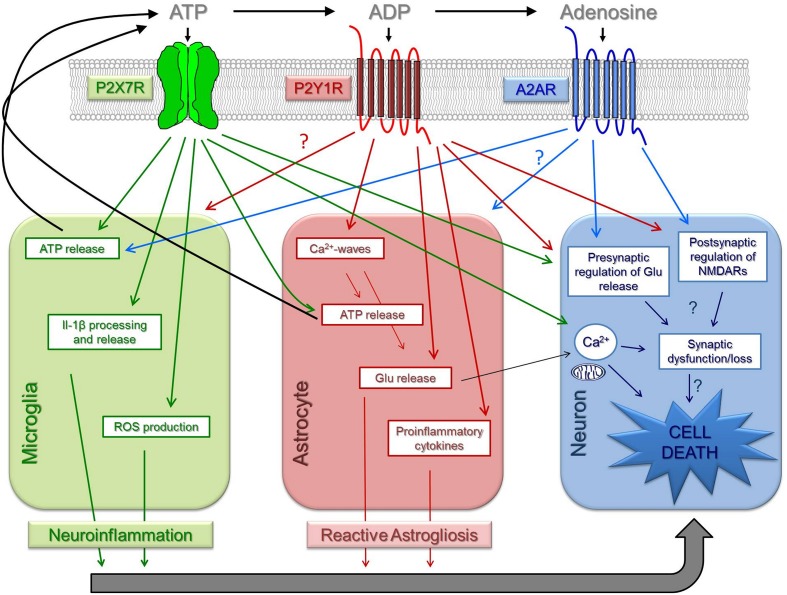
Figure 2.
Schematic diagram of the actions of P2X7R, P2Y1R and A2AR in brain pathologies. Extracellular ATP, both directly through the activation of P2X7R and indirectly through the activation of P2Y1R and A2AR upon its extracellular catabolism into ADP and adenosine, seems to be a key signal in brain pathologies, being endowed with the unique capacity to promote and integrate neuroinflammation, reactive astrogliosis, synaptic dysfunction/loss, and increased susceptibility of neurons to damage. Here, it is summarized the different mechanisms reported for each receptor that are or may be contributing to neurodegeneration. The knowledge of the precise mechanisms and the challenging characterization of the temporal and spatial hierarchy of these different actions, perhaps as a common neurodegenerative pathway to different brain disorders, will most likely unravel an opportunity for multi-drug target therapeutics.
P2Y1 receptor
P2Y1R is a metabotropic receptor preferentially activated by ADP, which pharmacological or genetic blockade affords neuroprotection in ischemic conditions (Sun et al., 2008; Kuboyama et al., 2011; Chin et al., 2013; Carmo et al., 2014b) or trauma (Choo et al., 2013). P2Y1R have a widespread cellular distribution and modulate neurons (Bowser and Khakh, 2004; Guzman et al., 2010), astrocytes (Fam et al., 2003; Fumagalli et al., 2003; Zheng et al., 2013) and microglia (Boucsein et al., 2003; Ballerini et al., 2005; Bianco et al., 2005). However, the pathological role of P2Y1R has been predominantly associated to reactive astrocytes since P2Y1R play a key role in entraining the propagation of calcium waves throughout the astrocyte network (Fam et al., 2003; Neary et al., 2003; Bowser and Khakh, 2007) and promote astrocytic hyperactivity and astrogliosis upon mechanical injury (Franke et al., 2001), ischemic conditions (Sun et al., 2008) or AD (Delekate et al., 2014), which is known to interfere with neuronal repair and regeneration (McKeon et al., 1999; Tian et al., 2006). The neuroprotection resulting from P2Y1R blockade might also involve the ability of P2Y1R to control GABA uptake (Jacob et al., 2014) and glutamate release (Domercq et al., 2006) impacting on synaptic function (Jourdain et al., 2007; Santello et al., 2011), and to regulate inflammatory/trophic factors expression in astrocytes (Kuboyama et al., 2011). However, in line with the existence of multiple populations of P2Y1R with different functions in astrocytes operating different transducing pathways (Fam et al., 2003; Sun et al., 2008; Kuboyama et al., 2011; Zheng et al., 2013), the blockade or the stimulation of P2Y1R in astrocytes can cause paradoxical effects; thus, the exogenous overactivation of P2Y1R can prevent astrocytic damage (Shinozaki et al., 2006) and protect against neuronal damage induced by oxidative stress through IL-6 release (Fujita et al., 2009). This apparently paradoxical effect might also result from the up-regulation of P2Y1R in pathological conditions, such as epilepsy (Fernandes et al., 2009; Padrão et al., 2011), mechanical injury (Franke et al., 2004), ischemia (Kuboyama et al., 2011) or AD (Moore et al., 2000), which might trigger a time-dependent gain of noxious function of P2Y1R under non-acute pathological conditions.
Neuronal P2Y1R may also directly affect brain function and damage (Carmo et al., 2014b). P2Y1R are located in central synapses, where they control glutamate release (Mendonza-Fernández et al., 2000; Rodrigues et al., 2005) and NMDA receptors (Luthardt et al., 2003). P2Y1R also control calcium and potassium conductances (Gerevich et al., 2004; Filippov et al., 2006; Coppi et al., 2012) and inhibitory transmission (Bowser and Khakh, 2004; Kawamura et al., 2004), but it is unclear how these different effects impact on the functioning and viability of neuronal networks; in fact, brain insults trigger an up-regulation of neuronal P2Y1R (Moore et al., 2000) coupled to a noxious gain of function, as heralded by the selective ability of P2Y1R to inhibit cortical LTD only in hypoxic conditions (Guzman et al., 2010) and to normalize neurotransmission upon anoxic depolarization (Traini et al., 2011). Finally, microglia P2Y1R are also expected to be involved in the neuroprotection associated with P2Y1R blockade since P2Y1R modulate neuroinflammatory responses (Ballerini et al., 2005). Thus, the role of P2Y1R in neurodegeneration is likely to involve a trans-cellular network, as illustrated by the evidence that activated microglia is capable to modulate synaptic function through ATP release, which in turn stimulates astrocytic P2Y1R controlling glutamatergic gliotransmission that feeds-back to impact on synaptic activity (Pascual et al., 2012) (Figures 1, 2).
In summary, it seems that, in addition to P2X7R, P2Y1R also contribute to brain dysfunction and damage, further arguing for the role of extracellular ATP as a danger signal in brain pathology. This is further heralded by the neurotoxicity of exogenously added ATP (Ryu et al., 2002; Amadio et al., 2005; Resta et al., 2005) and by the neuroprotection afforded by non-selective P2R antagonists (Krügel et al., 2001; Lämmer et al., 2006), supporting that P2R might be valuable targets for neuroprotection (Volonté et al., 2003; Franke et al., 2006).
A2A receptor
Apart from a direct effect of ATP acting through P2X7R and P2Y1R, ATP may also impact on brain dysfunction upon its extracellular catabolism by ecto-nucleotidases (Cunha, 2001; Zimmermann et al., 2012) into adenosine, followed by activation of adenosine receptors (Cunha, 2005; Chen et al., 2007, 2013; Gomes et al., 2011). In fact, there is robust evidence showing that the pharmacological or genetic deletion of adenosine A2A receptors (A2AR) diminishes neurodegeneration and brain dysfunction in animal models of aging (Prediger et al., 2005), PD (Schwarzschild et al., 2006), AD (Canas et al., 2009; Laurent et al., 2014), epilepsy (El Yacoubi et al., 2008, 2009; Cognato et al., 2010), Machado-Joseph's disease (Gonçalves et al., 2013), chronic stress (Batalha et al., 2013) or ADHD (Pires et al., 2009; Pandolfo et al., 2013). This remarkably agrees with the impact of the regular consumption of the non-selective A2AR antagonist, caffeine, on age and AD-related memory impairment (Cunha and Agostinho, 2010), PD (Ascherio et al., 2003), and major depression (Lucas et al., 2011). The observation that A2AR are mostly located in synapses (Rebola et al., 2005a), A2AR selectively control NMDA receptor (Rebola et al., 2008) and synaptic plasticity phenomena (d'Alcantara et al., 2001; Costenla et al., 2011) and the deletion of neuronal A2AR is sufficient to afford neuroprotection (Kachroo et al., 2005; Shen et al., 2008; Wei et al., 2014), prompts the hypothesis that the control of synaptotoxicity is at the core of A2AR neuroprotection (Cunha and Agostinho, 2010). However, the possible role of A2AR in astrocytes (Matos et al., 2012a, 2015; Orr et al., 2015) and in microglia (Orr et al., 2009; Rebola et al., 2011; Gomes et al., 2013) still remains to be determined, especially since A2AR undergo a marked up-regulation in neurodegenerative and neuropsychiatric disorders in glial cells (Yu et al., 2008; Matos et al., 2012b) but mainly in synapses (Rebola et al., 2005b; Cunha et al., 2006; Duarte et al., 2012), which is associated with a shift of function of A2AR (reviewed in Cunha et al., 2008; Rial et al., 2014) (Figures 1, 2).
Notably, it has been established that the adenosine activating A2AR is derived from the activity of ecto-5′-nucleotidase (Cunha et al., 1996b; Rebola et al., 2008; Augusto et al., 2013), the final step in the ATP catabolism into adenosine. Furthermore, unpublished work from our group has documented that the blockade of ecto-5′-nucleotidase or of A2AR affords comparable neuroprotection, further heralding the concept that A2AR activation is part of the signaling operated by extracellular ATP as a danger signal.
P2X7R-P2Y1R-A2AR: an hazardous orchestra
The sustained increase of extracellular ATP levels upon brain dysfunction/damage together with the compelling evidence that the pharmacological blockade or genetic deletion of P2X7R or P2Y1R or A2AR prevents or attenuates neuronal injury or the onset/evolution of brain diseases, supports a role for ATP both as a warning and harmful signal in the brain. It will now be important to understand the time-dependent involvement of these three purinoceptors and their inter-play. In fact, the activation of A2AR or P2X7R may constitute an auto-stimulatory loop (Verderio and Matteoli, 2001; Cunha et al., 2012) since they can trigger ATP release from astrocytes, neurons or microglia (George et al., 2015), either directly through the P2X7R pore (Duan and Neary, 2006), through interaction with pannexin channels (Locovei et al., 2007; Iglesias et al., 2008; Bennett et al., 2012), or by exocytotic release (Gutiérrez-Martín et al., 2011). Furthermore, P2X7R synergistically regulate P2Y1R activation (Locovei et al., 2006), particularly in pathological conditions (Traini et al., 2011; Vessey et al., 2011; Choo et al., 2013). Finally, emerging evidence indicates a synergic interplay between ATP and its metabolite adenosine (Gerwins and Fredholm, 1992; Neary et al., 1998; Chevrier et al., 2006; Färber et al., 2008; Koizumi et al., 2013; George et al., 2015), namely between A2AR and P2X7R (Chen et al., 2004; Pellegatti et al., 2011) and P2Y1R (Stafford et al., 2007; Doengi et al., 2008; Suzuki et al., 2011), which highlights the possible key role of ecto-nucleotides in regulating the integration of purinergic responses. Thus, the action of individual purinergic receptors may be part of a time-dependent orchestrated response triggered by the increase of extracellular ATP levels in brain pathology (Figure 2). The understanding of the hierarchy and integration/redundancy of their actions will be paramount to develop multi-target therapeutics to exploit this role of ATP as a danger signal in the brain.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The research activity of the authors has been supported by funding from Fundação para a Ciência e a Tecnologia (FCT; EXPL/NEU-NMC/0671/2012, Pest-C/SAU/LA0001/2013-2014), FP7-EU Marie Curie actions (M.Curie:Cycle4-2013-PT-07), QREN (CENTRO-07-ST24-FEDER-002006) and COMPETE. The authors also thank Joana M. Marques for critical reading of the manuscript.
References
- Abbracchio M. P., Burnstock G., Boeynaems J. M., Barnard E. A., Boyer J. L., Kennedy C., et al. (2006). International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol. Rev. 58, 281–341. 10.1124/pr.58.3.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agresti C., Meomartini M. E., Amadio S., Ambrosini E., Volonté C., Aloisi F., et al. (2005). ATP regulates oligodendrocyte progenitor migration, proliferation, and differentiation: involvement of metabotropic P2 receptors. Brain Res. Rev. 48, 157–165. 10.1016/j.brainresrev.2004.12.005 [DOI] [PubMed] [Google Scholar]
- Amadio S., Apolloni S., D'Ambrosi N., Volonté C. (2011). Purinergic signalling at the plasma membrane: a multipurpose and multidirectional mode to deal with amyotrophic lateral sclerosis and multiple sclerosis. J. Neurochem. 116, 796–805. 10.1111/j.1471-4159.2010.07025.x [DOI] [PubMed] [Google Scholar]
- Amadio S., D'Ambrosi N., Trincavelli M. L., Tuscano D., Sancesario G., Bernadi G., et al. (2005). Differences in the neurotoxicity profile induced by ATP and ATPγS in cultured cerebellar granule neurons. Neurochem. Int. 47, 334–342. 10.1016/j.neuint.2005.05.008 [DOI] [PubMed] [Google Scholar]
- Anisman H., Merali Z., Poulter M. O., Hayley S. (2005). Cytokines as a precipitant of depressive illness: animal and human studies. Curr. Pharm. Des. 11, 963–972. 10.2174/1381612053381701 [DOI] [PubMed] [Google Scholar]
- Arbeloa J., Pérez-Samartín A., Gottlieb M., Matute C. (2012). P2X7 receptor blockade prevents ATP excitotoxicity in neurons and reduces brain damage after ischemia. Neurobiol. Dis. 45, 954–961. 10.1016/j.nbd.2011.12.014 [DOI] [PubMed] [Google Scholar]
- Ascherio A., Chen H., Schwarzschild M. A., Zhang S. M., Colditz G. A., Speizer F. E. (2003). Caffeine, postmenopausal estrogen, and risk of Parkinson's disease. Neurology 60, 790–795. 10.1212/01.WNL.0000046523.05125.87 [DOI] [PubMed] [Google Scholar]
- Augusto E., Matos M., Sévigny J., El-Tayeb A., Bynoe M. S., Müller C. E., et al. (2013). Ecto-5′-nucleotidase (CD73)-mediated formation of adenosine is critical for the striatal adenosine A2A receptor functions. J. Neurosci. 33, 11390–11399. 10.1523/JNEUROSCI.5817-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avignone E., Ulmann L., Levavasseur F., Rassendren F., Audinat E. (2008). Status epilepticus induces a particular microglial activation state characterized by enhanced purinergic signaling. J. Neurosci. 28, 9133–9144. 10.1523/JNEUROSCI.1820-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballerini P., Di Iorio P., Ciccarelli R., Caciagli F., Poli A., Beraudi A., et al. (2005). P2Y1 and cysteinyl leukotriene receptors mediate purine and cysteinyl leukotriene co-release in primary cultures of rat microglia. Int. J. Immunopathol. Pharmacol. 18, 255–268. [DOI] [PubMed] [Google Scholar]
- Bao L., Sachs F., Dahl G. (2004). Connexins are mechanosensitive. Am. J. Physiol. Cell Physiol. 287, C1389–C1395. 10.1152/ajpcell.00220.2004 [DOI] [PubMed] [Google Scholar]
- Bardoni R., Goldstein P. A., Lee C. J., Gu J. G., MacDermott A. B. (1997). ATP P2X receptors mediate fast synaptic transmission in the dorsal horn of the rat spinal cord. J. Neurosci. 17, 5297–5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso A. M., Bratcher N. A., Harris R. R., Jarvis M. F., Decker M. W., Rueter L. E. (2009). Behavioral profile of P2X7 receptor knockout mice in animal models of depression and anxiety: relevance for neuropsychiatric disorders. Behav. Brain Res. 198, 83–90. 10.1016/j.bbr.2008.10.018 [DOI] [PubMed] [Google Scholar]
- Batalha V. L., Pego J. M., Fontinha B. M., Costenla A. R., Valadas J. S., Baqi Y., et al. (2013). Adenosine A2A receptor blockade reverts hippocampal stress-induced deficits and restores corticosterone circadian oscillation. Mol. Psychiatry 18, 320–331. 10.1038/mp.2012.8 [DOI] [PubMed] [Google Scholar]
- Bennett M. V., Garré J. M., Orellana J. A., Bukauskas F. F., Nedergaard M., Giaume C., et al. (2012). Connexin and pannexin hemichannels in inflammatory responses of glia and neurons. Brain Res. 1487, 3–15. 10.1016/j.brainres.2012.08.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardino L., Balosso S., Ravizza T., Marchi N., Ku G., Randle J. C., et al. (2008). Inflammatory events in hippocampal slice cultures prime neuronal susceptibility to excitotoxic injury: a crucial role of P2X7 receptor-mediated IL-1β release. J. Neurochem. 106, 271–280. 10.1111/j.1471-4159.2008.05387.x [DOI] [PubMed] [Google Scholar]
- Bianco F., Fumagalli M., Pravettoni E., D'Ambrosi N., Volonté C., Matteoli M., et al. (2005). Pathophysiological roles of extracellular nucleotides in glial cells: differential expression of purinergic receptors in resting and activated microglia. Brain Res. Rev. 48, 144–156. 10.1016/j.brainresrev.2004.12.004 [DOI] [PubMed] [Google Scholar]
- Bodin P., Burnstock G. (2001). Purinergic signalling: ATP release. Neurochem. Res. 26, 959–969. 10.1023/A:1012388618693 [DOI] [PubMed] [Google Scholar]
- Boucsein C., Zacharias R., Färber K., Pavlovic S., Hanisch U. K., Kettenmann H. (2003). Purinergic receptors on microglial cells: functional expression in acute brain slices and modulation of microglial activation in vitro. Eur. J. Neurosci. 17, 2267–2276. 10.1046/j.1460-9568.2003.02663.x [DOI] [PubMed] [Google Scholar]
- Bowser D. N., Khakh B. S. (2004). ATP excites interneurons and astrocytes to increase synaptic inhibition in neuronal networks. J. Neurosci. 24, 8606–8620. 10.1523/JNEUROSCI.2660-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowser D. N., Khakh B. S. (2007). Vesicular ATP is the predominant cause of intercellular calcium waves in astrocytes. J. Gen. Physiol. 129, 485–491. 10.1085/jgp.200709780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. (1972). Purinergic nerves. Pharmacol. Rev. 24, 509–581. [PubMed] [Google Scholar]
- Butt A.M. (2011). ATP: a ubiquitous gliotransmitter integrating neuron-glial networks. Semin. Cell Dev. Biol. 22, 205–213. 10.1016/j.semcdb.2011.02.023 [DOI] [PubMed] [Google Scholar]
- Canas P. M., Porciúncula L. O., Cunha G. M., Silva C. G., Machado N. J., Oliveira J. M., et al. (2009). Adenosine A2A receptor blockade prevents synaptotoxicity and memory dysfunction caused by β-amyloid peptides via p38 mitogen-activated protein kinase pathway. J. Neurosci. 29, 14741–14751. 10.1523/JNEUROSCI.3728-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmo M. R., Menezes A. P., Nunes A. C., Pliássova A., Rolo A. P., Palmeira C. M., et al. (2014a). The P2X7 receptor antagonist Brilliant Blue G attenuates contralateral rotations in a rat model of Parkinsonism through a combined control of synaptotoxicity, neurotoxicity and gliosis. Neuropharmacology 81, 142–152. 10.1016/j.neuropharm.2014.01.045 [DOI] [PubMed] [Google Scholar]
- Carmo M. R., Simões A. P., Fonteles A. A., Souza C. M., Cunha R. A., Andrade G. M. (2014b). ATP P2Y1 receptors control cognitive deficits and neurotoxicity but not glial modifications induced by brain ischemia in mice. Eur. J. Neurosci. 39, 614–622. 10.1111/ejn.12435 [DOI] [PubMed] [Google Scholar]
- Cavaliere F., Amadio S., Sancesario G., Bernardi G., Volonté C. (2004). Synaptic P2X7 and oxygen/glucose deprivation in organotypic hippocampal cultures. J. Cereb. Blood Flow Metab. 24, 392–398. 10.1097/00004647-200404000-00004 [DOI] [PubMed] [Google Scholar]
- Cervetto C., Mazzotta M. C., Frattaroli D., Alloisio S., Nobile M., Maura G., et al. (2012). Calmidazolium selectively inhibits exocytotic glutamate release evoked by P2X7 receptor activation. Neurochem. Int. 60, 768–772. 10.1016/j.neuint.2012.02.034 [DOI] [PubMed] [Google Scholar]
- Chakfe Y., Seguin R., Antel J. P., Morissette C., Malo D., Henderson D., et al. (2002). ADP and AMP induce interleukin-1β release from microglial cells through activation of ATP-primed P2X7receptor channels. J. Neurosci. 22, 3061–3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. F., Eltzschig H. K., Fredholm B. B. (2013). Adenosine receptors as drug targets-what are the challenges? Nat. Rev. Drug Discov. 12, 265–286. 10.1038/nrd3955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. F., Sonsalla P. K., Pedata F., Melani A., Domenici M. R., Popoli P., et al. (2007). Adenosine A2A receptors and brain injury: broad spectrum of neuroprotection, multifaceted actions and “fine tuning” modulation. Prog. Neurobiol. 83, 310–331. 10.1016/j.pneurobio.2007.09.002 [DOI] [PubMed] [Google Scholar]
- Chen Y., Shukla A., Namiki S., Insel P. A., Junger W. G. (2004). A putative osmoreceptor system that controls neutrophil function through the release of ATP, its conversion to adenosine, and activation of A2 adenosine and P2 receptors. J. Leukoc. Biol. 76, 245–253. 10.1189/jlb.0204066 [DOI] [PubMed] [Google Scholar]
- Chevrier C., Bourdon L., Canini F. (2006). Cosignaling of adenosine and adenosine triphosphate in hypobaric hypoxia-induced hypothermia. Am. J. Physiol. 290, R595–R600. 10.1152/ajpregu.00241.2005 [DOI] [PubMed] [Google Scholar]
- Chin Y., Kishi M., Sekino M., Nakajo F., Abe Y., Terazono Y., et al. (2013). Involvement of glial P2Y1 receptors in cognitive deficit after focal cerebral stroke in a rodent model. J. Neuroinflammation 10, 95. 10.1186/1742-2094-10-95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho J. H., Choi I. S., Jang I. S. (2010). P2X7 receptors enhance glutamate release in hippocampal hilar neurons. Neuroreport 21, 865–870. 10.1097/WNR.0b013e32833d9142 [DOI] [PubMed] [Google Scholar]
- Choi H. K., Ryu H. J., Kim J. E., Jo S. M., Choi H. C., Song H. K., et al. (2012). The roles of P2X7 receptor in regional-specific microglial responses in the rat brain following status epilepticus. Neurol. Sci. 33, 515–525. 10.1007/s10072-011-0740-z [DOI] [PubMed] [Google Scholar]
- Choo A. M., Miller W. J., Chen Y. C., Nibley P., Patel T. P., Goletiani C., et al. (2013). Antagonism of purinergic signalling improves recovery from traumatic brain injury. Brain 136, 65–80. 10.1093/brain/aws286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coco S., Calegari F., Pravettoni E., Pozzi D., Taverna E., Rosa P., et al. (2003). Storage and release of ATP from astrocytes in culture. J. Biol. Chem. 278, 1354–1362. 10.1074/jbc.M209454200 [DOI] [PubMed] [Google Scholar]
- Cognato G. P., Agostinho P. M., Hockemeyer J., Müller C. E., Souza D. O., Cunha R. A. (2010). Caffeine and an adenosine A2A receptor antagonist prevent memory impairment and synaptotoxicity in adult rats triggered by a convulsive episode in early life. J. Neurochem. 112, 453–462. 10.1111/j.1471-4159.2009.06465.x [DOI] [PubMed] [Google Scholar]
- Coppi E., Pedata F., Gibb A. J. (2012). P2Y1 receptor modulation of Ca2+-activated K+ currents in medium-sized neurons from neonatal rat striatal slices. J. Neurophysiol. 107, 1009–1021. 10.1152/jn.00816.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costenla A. R., Diógenes M. J., Canas P. M., Rodrigues R. J., Nogueira C., Maroco J., et al. (2011). Enhanced role of adenosine A2A receptors in the modulation of LTP in the rat hippocampus upon ageing. Eur. J. Neurosci. 34, 12–21. 10.1111/j.1460-9568.2011.07719.x [DOI] [PubMed] [Google Scholar]
- Csölle C., Baranyi M., Zsilla G., Kittel A., Gölöncsér F., Illes P., et al. (2013). Neurochemical changes in the mouse hippocampus underlying the antidepressant effect of genetic deletion of P2X7 receptors. PLoS ONE 8:e66547. 10.1371/journal.pone.0066547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha G. M., Canas P. M., Oliveira C. R., Cunha R. A. (2006). Increased density and synapto-protective effect of adenosine A2A receptors upon sub-chronic restraint stress. Neuroscience 141, 1775–1781. 10.1016/j.neuroscience.2006.05.024 [DOI] [PubMed] [Google Scholar]
- Cunha R. A. (2001). Regulation of the ecto-nucleotidase pathway in rat hippocampal nerve terminals. Neurochem. Res. 26, 979–991. 10.1023/A:1012392719601 [DOI] [PubMed] [Google Scholar]
- Cunha R. A. (2005). Neuroprotection by adenosine in the brain: from A1 receptor activation to A2A receptor blockade. Purinergic Signal. 1, 111–134. 10.1007/s11302-005-0649-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha R. A. (2008). Different cellular sources and different roles of adenosine: A1 receptor-mediated inhibition through astrocytic-driven volume transmission and synapse-restricted A2A receptor-mediated facilitation of plasticity. Neurochem. Int. 52, 65–72. 10.1016/j.neuint.2007.06.026 [DOI] [PubMed] [Google Scholar]
- Cunha R. A., Agostinho P. M. (2010). Chronic caffeine consumption prevents memory disturbance in different animal models of memory decline. J. Alzheimer's Dis. 20, S95–S116. 10.3233/JAD-2010-1408 [DOI] [PubMed] [Google Scholar]
- Cunha R. A., Correia-de-Sá P., Sebastião A. M., Ribeiro J. A. (1996b). Preferential activation of excitatory adenosine receptors at rat hippocampal and neuromuscular synapses by adenosine formed from released adenine nucleotides. Br. J. Pharmacol. 119, 253–260. 10.1111/j.1476-5381.1996.tb15979.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha R. A., Ferré S., Vaugeois J. M., Chen J. F. (2008). Potential therapeutic interest of adenosine A2A receptors in psychiatric disorders. Curr. Pharm. Des. 14, 1512–1524. 10.2174/138161208784480090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha R. A., Queiroz F., Agostinho P., Silva H. B., Tomé A. R. (2012). Synaptic plasticity is controlled by a feed-forward loop between ATP release and adenosine A2A receptors, in Program No. 643.02. 2012 Neuroscience Meeting Planner (New Orleans, LA: Society for Neuroscience; ). [Google Scholar]
- Cunha R. A., Ribeiro J. A. (2000). ATP as a presynaptic modulator. Life Sci. 68, 119–137. 10.1016/S0024-3205(00)00923-1 [DOI] [PubMed] [Google Scholar]
- Cunha R. A., Vizi E. S., Ribeiro J. A., Sebastião A. M. (1996a). Preferential release of ATP and its extracellular catabolism as a source of adenosine upon high- but not low-frequency stimulation of rat hippocampal slices. J. Neurochem. 67, 2180–2187. 10.1046/j.1471-4159.1996.67052180.x [DOI] [PubMed] [Google Scholar]
- d'Alcantara P., Ledent C., Swillens S., Schiffmann S. N. (2001). Inactivation of adenosine A2A receptor impairs long term potentiation in the accumbens nucleus without altering basal synaptic transmission. Neuroscience 107, 455–464. 10.1016/S0306-4522(01)00372-4 [DOI] [PubMed] [Google Scholar]
- Dale N., Frenguelli B. G. (2009). Release of adenosine and ATP during ischemia and epilepsy. Curr. Neuropharmacol. 7, 160–179. 10.2174/157015909789152146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davalos D., Grutzendler J., Yang G., Kim J. V., Zuo Y., Jung S., et al. (2005). ATP mediates rapid microglial response to local brain injury in vivo. Nat. Neurosci. 8, 752–758. 10.1038/nn1472 [DOI] [PubMed] [Google Scholar]
- Delekate A., Füchtemeier M., Schumacher T., Ulbrich C., Foddis M., Petzold G. C. (2014). Metabotropic P2Y1 receptor signalling mediates astrocytic hyperactivity in vivo in an Alzheimer's disease mouse model. Nat. Commun. 5, 5422. 10.1038/ncomms6422 [DOI] [PubMed] [Google Scholar]
- Díaz-Hernández J. I., Gomez-Villafuertes R., León-Otegui M., Hontecillas-Prieto L., Del Puerto A., Trejo J. L., et al. (2012). In vivo P2X7 inhibition reduces amyloid plaques in Alzheimer's disease through GSK3β and secretases. Neurobiol. Aging 33, 1816–1828. 10.1016/j.neurobiolaging.2011.09.040 [DOI] [PubMed] [Google Scholar]
- Díaz-Hernández M., Díez-Zaera M., Sánchez-Nogueiro J., Gómez-Villafuertes R., Canals J. M., Alberch J., et al. (2009). Altered P2X7-receptor level and function in mouse models of Huntington's disease and therapeutic efficacy of antagonist administration. FASEB J. 23, 1893–1906. 10.1096/fj.08-122275 [DOI] [PubMed] [Google Scholar]
- Di Virgilio F., Chiozzi P., Falzoni S., Ferrari D., Sanz J. M., Venketaraman V., et al. (1998). Cytolytic P2X purinoceptors. Cell Death Differ. 5, 191–199. 10.1038/sj.cdd.4400341 [DOI] [PubMed] [Google Scholar]
- Doengi M., Deitmer J. W., Lohr C. (2008). New evidence for purinergic signaling in the olfactory bulb: A2A and P2Y1 receptors mediate intracellular calcium release in astrocytes. FASEB J. 22, 2368–2378. 10.1096/fj.07-101782 [DOI] [PubMed] [Google Scholar]
- Domercq M., Brambilla L., Pilati E., Marchaland J., Volterra A., Bezzi P. (2006). P2Y1 receptor-evoked glutamate exocytosis from astrocytes: control by tumor necrosis factor-alpha and prostaglandins. J. Biol. Chem. 281, 30684–30696. 10.1074/jbc.M606429200 [DOI] [PubMed] [Google Scholar]
- Doná F., Ulrich H., Persike D. S., Conceição I. M., Blini J. P., Cavalheiro E. A., et al. (2009). Alteration of purinergic P2X4 and P2X7 receptor expression in rats with temporal-lobe epilepsy induced by pilocarpine. Epilepsy Res. 83, 157–167. 10.1016/j.eplepsyres.2008.10.008 [DOI] [PubMed] [Google Scholar]
- Duan S., Anderson C. M., Keung E. C., Chen Y., Chen Y., Swanson R. A. (2003). P2X7 receptor-mediated release of excitatory amino acids from astrocytes. J. Neurosci. 23, 1320–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan S., Neary J. T. (2006). P2X7 receptors: properties and relevance to CNS function. Glia 54, 738–746. 10.1002/glia.20397 [DOI] [PubMed] [Google Scholar]
- Duarte J. M., Agostinho P. M., Carvalho R. A., Cunha R. A. (2012). Caffeine consumption prevents diabetes-induced memory impairment and synaptotoxicity in the hippocampus of NONcZNO10/LTJ mice. PLoS ONE 7:e21899. 10.1371/journal.pone.0021899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards F. A., Gibb A. J., Colquhoun D. (1992). ATP receptor-mediated synaptic currents in the central nervous system. Nature 359, 144–147. 10.1038/359144a0 [DOI] [PubMed] [Google Scholar]
- Elliott M. R., Chekeni F. B., Trampont P. C., Lazarowski E. R., Kadl A., Walk S. F., et al. (2009). Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature 461, 282–286. 10.1038/nature08296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Yacoubi M., Ledent C., Parmentier M., Costentin J., Vaugeois J. M. (2008). Evidence for the involvement of the adenosine A2A receptor in the lowered susceptibility to pentylenetetrazol-induced seizures produced in mice by long-term treatment with caffeine. Neuropharmacology 55, 35–40. 10.1016/j.neuropharm.2008.04.007 [DOI] [PubMed] [Google Scholar]
- El Yacoubi M., Ledent C., Parmentier M., Costentin J., Vaugeois J. M. (2009). Adenosine A2A receptor deficient mice are partially resistant to limbic seizures. Naunyn Schmiedebergs Arch. Pharmacol. 380, 223–232. 10.1007/s00210-009-0426-8 [DOI] [PubMed] [Google Scholar]
- Engel T., Gómez-Villafuertes R., Tanaka K., Mesuret G., Sanz-Rodriguez A., Garcia-Huerta P., et al. (2012). Seizure suppression and neuroprotection by targeting the purinergic P2X7 receptor during status epilepticus in mice. FASEB J. 26, 1616–1628. 10.1096/fj.11-196089 [DOI] [PubMed] [Google Scholar]
- Fam S. R., Gallagher C. J., Kalia L. V., Salter M. W. (2003). Differential frequency dependence of P2Y1- and P2Y2- mediated Ca2+ signaling in astrocytes. J. Neurosci. 23, 4437–4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Färber K., Markworth S., Pannasch U., Nolte C., Prinz V., Kronenberg G., et al. (2008). The ectonucleotidase cd39/ENTPDase1 modulates purinergic-mediated microglial migration. Glia 56, 331–341. 10.1002/glia.20606 [DOI] [PubMed] [Google Scholar]
- Fernandes M. S., Speciali D. S., Blini J., Canzian M., Cavalheiro E. A., Ulrich H., et al. (2009). Purinergic P2 receptors are up-regulated In the hippocampus of patients with temporal lobe epilepsy associated with hippocampal sclerosis. Epilepsia 50, 78 10.1111/j.1528-1167.2009.02320.x [DOI] [Google Scholar]
- Ferrari D., Villalba M., Chiozzi P., Falzoni S., Ricciardi-Castagnoli P., Di Virgilio F. (1996). Mouse microglial cells express a plasma membrane pore gated by extracellular ATP. J. Immunol. 156, 1531–1539. [PubMed] [Google Scholar]
- Fields R. D. (2006). Nerve impulses regulate myelination through purinergic signalling. Novartis Found. Symp. 276, 148–158. 10.1002/9780470032244.ch12 [DOI] [PubMed] [Google Scholar]
- Fields R. D. (2011). Nonsynaptic and nonvesicular ATP release from neurons and relevance to neuron-glia signaling. Semin. Cell Dev. Biol. 22, 14–219. 10.1016/j.semcdb.2011.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields R. D., Burnstock G. (2006). Purinergic signalling in neuron-glia interactions. Nat. Rev. Neurosci. 7, 423–436. 10.1038/nrn1928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippov A. K., Choi R. C. Y., Simon J., Barnard E. A., Brown D. A. (2006). Activation of P2Y1 nucleotide receptors induces inhibition of the M-type K+ current in rat hippocampal pyramidal neurons. J. Neurosci. 26, 9340–9348. 10.1523/JNEUROSCI.2635-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florian C., Vecsey C. G., Halassa M. M., Haydon P. G., Abel T. (2011). Astrocyte-derived adenosine and A1 receptor activity contribute to sleep loss-induced deficits in hippocampal synaptic plasticity and memory in mice. J. Neurosci. 31, 6956–6962. 10.1523/JNEUROSCI.5761-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke H., Grosche J., Schädlich H., Krügel U., Allgaier C., Illes P. (2001). P2X receptor expression on astrocytes in the nucleus accumbens of rats. Neuroscience 108, 421–429. 10.1016/S0306-4522(01)00416-X [DOI] [PubMed] [Google Scholar]
- Franke H., Günther A., Grosche J., Schmidt R., Rossner S., Reinhardt R., et al. (2004). P2X7 receptor expression after ischemia in the cerebral cortex of rats. J. Neuropathol. Exp. Neurol. 63, 686–699. [DOI] [PubMed] [Google Scholar]
- Franke H., Krügel U., Illes P. (2006). P2 receptors and neuronal injury. Pflugers Arch. 452, 622–644. 10.1007/s00424-006-0071-8 [DOI] [PubMed] [Google Scholar]
- Franke H., Verkhratsky A., Burnstock G., Illes P. (2012). Pathophysiology of astroglial purinergic signalling. Purinergic Signal. 8, 629–657. 10.1007/s11302-012-9300-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholm B. B., IJzerman A. P., Jacobson K. A., Linden J., Müller C. E. (2011). International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and classification of adenosine receptors - an update. Pharmacol. Rev. 63, 1–34. 10.1124/pr.110.003285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholm B. B., Chen J. F., Cunha R. A., Svenningsson P., Vaugeois J. M. (2005). Adenosine and brain function. Int. Rev. Neurobiol. 63, 191–270. 10.1016/S0074-7742(05)63007-3 [DOI] [PubMed] [Google Scholar]
- Frenguelli B. G., Wigmore G., Llaudet E., Dale N. (2007). Temporal and mechanistic dissociation of ATP and adenosine release during ischaemia in the mammalian hippocampus. J. Neurochem. 101, 1400–1413. 10.1111/j.1471-4159.2006.04425.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu W., Ruangkittisakul A., MacTavish D., Baker G. B., Ballanyi K., Jhamandas J. H. (2013). Activity and metabolism-related Ca2+ and mitochondrial dynamics in co-cultured human fetal cortical neurons and astrocytes. Neuroscience 250, 520–535. 10.1016/j.neuroscience.2013.07.029 [DOI] [PubMed] [Google Scholar]
- Fujita T., Tozaki-Saitoh H., Inoue K. (2009). P2Y1 receptor signaling enhances neuroprotection by astrocytes against oxidative stress via IL-6 release in hippocampal cultures. Glia 57, 244–257. 10.1002/glia.20749 [DOI] [PubMed] [Google Scholar]
- Fumagalli M., Brambilla R., D'Ambrosi N., Volonté C., Matteoli M., Verderio C., et al. (2003). Nucleotide-mediated calcium signalling in rat cortical astrocytes: role of P2X and P2Y receptors. Glia 43, 218–303. 10.1002/glia.10248 [DOI] [PubMed] [Google Scholar]
- George J., Gonçalves F. Q., Cristovão G., Rodrigues L., Fernandes J. R. M., Gonçalves T., et al. (2015). Different danger signals differently impact on microglial proliferation through alterations of ATP release and extracellular metabolism. Glia. [Epub ahead of print]. 10.1002/glia.22833 [DOI] [PubMed] [Google Scholar]
- Gerevich Z., Borvendeg S. J., Schröeder W., Franke H., Wirkner K., Nörenberg W., et al. (2004). Inhibition of N-type voltage-activated calcium channels in rat dorsal root ganglion neurons by P2Y receptors is a possible mechanism of ADP-induced analgesia. J. Neurosci. 24, 797–807. 10.1523/JNEUROSCI.4019-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerwins P., Fredholm B. B. (1992). ATP and its metabolite adenosine act synergistically to mobilize intracellular calcium via the formation of inositol 1,4,5-trisphosphate in a smooth muscle cell line. J. Biol. Chem. 267, 16081–16087. [PubMed] [Google Scholar]
- Gomes C., Ferreira R., George J., Sanches R., Rodrigues D. I., Gonçalves N., et al. (2013). Activation of microglial cells triggers a release of brain-derived neurotrophic factor (BDNF) inducing their proliferation in an adenosine A2A receptor-dependent manner: A2A receptor blockade prevents BDNF release and proliferation of microglia. J. Neuroinflammation 10, 16. 10.1186/1742-2094-10-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes C. V., Kaster M. P., Tomé A. R., Agostinho P. M., Cunha R. A. (2011). Adenosine receptors and brain diseases: neuroprotection and neurodegeneration. Biochim. Biophys. Acta 1808, 1380–1399. 10.1016/j.bbamem.2010.12.001 [DOI] [PubMed] [Google Scholar]
- Gonçalves N., Simões A. T., Cunha R. A., de Almeida L. P. (2013). Caffeine and adenosine A2A receptor inactivation decrease striatal neuropathology in a lentiviral-based model of Machado-Joseph disease. Ann. Neurol. 73, 655–666. 10.1002/ana.23866 [DOI] [PubMed] [Google Scholar]
- Grygorowicz T., Sulejczak D., Struzynska L. (2011). Expression of purinergic P2X7 receptor in rat brain during the symptomatic phase of experimental autoimmune encephalomyelitis and after recovery of neurological deficits. Acta Neurobiol. Exp. (Wars.) 71, 65–73. [DOI] [PubMed] [Google Scholar]
- Guthrie P. B., Knappenberger J., Segal M., Bennett M. V., Charles A. C., Kater S. B. (1999). ATP released from astrocytes mediates glial calcium waves. J. Neurosci. 19, 520–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez-Martín Y., Bustillo D., Gómez-Villafuertes R., Sánchez-Nogueiro J., Torregrosa-Hetland C., Binz T., et al. (2011). P2X7 receptors trigger ATP exocytosis and modify secretory vesicle dynamics in neuroblastoma cells. J. Biol. Chem. 286, 11370–11381. 10.1074/jbc.M110.139410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman S. J., Schmidt H., Franke H., Krügel U., Eilers J., Illes P., et al. (2010). P2Y1 receptors inhibit long-term depression in the prefrontal cortex. Neuropharmacology 59, 406–415. 10.1016/j.neuropharm.2010.05.013 [DOI] [PubMed] [Google Scholar]
- Halassa M. M., Fellin T., Haydon P. G. (2009). Tripartite synapses: roles for astrocytic purines in the control of synaptic physiology and behavior. Neuropharmacology 57, 343–346. 10.1016/j.neuropharm.2009.06.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hejjas K., Szekely A., Domotor E., Halmai Z., Balogh G., Schilling B., et al. (2009). Association between depression and the Gln460Arg polymorphism of P2RX7 gene: a dimensional approach. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 150B, 295–299. 10.1002/ajmg.b.30799 [DOI] [PubMed] [Google Scholar]
- Idzko M., Ferrari D., Eltzschig H. K. (2014). Nucleotide signalling during inflammation. Nature 509, 310–317. 10.1038/nature13085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias R., Locovei S., Roque A., Alberto A. P., Dahl G., Spray D. C., et al. (2008). P2X7 receptor-Pannexin1 complex: pharmacology and signaling. Am. J. Physiol. Cell Physiol. 295, C752–C760. 10.1152/ajpcell.00228.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imura Y., Morizawa Y., Komatsu R., Shibata K., Shinozaki Y., Kasai H., et al. (2013). Microglia release ATP by exocytosis. Glia 61, 1320–1330. 10.1002/glia.22517 [DOI] [PubMed] [Google Scholar]
- Iwabuchi S., Kawahara K. (2011). Functional significance of the negative-feedback regulation of ATP release via pannexin-1 hemichannels under ischemic stress in astrocytes. Neurochem. Int. 58, 376–384. 10.1016/j.neuint.2010.12.013 [DOI] [PubMed] [Google Scholar]
- Jacob P. F., Vaz S. H., Ribeiro J. A., Sebastião A. M. (2014). P2Y1 receptor inhibits GABA transport through a calcium signalling-dependent mechanism in rat cortical astrocytes. Glia 62, 1211–1226. 10.1002/glia.22673 [DOI] [PubMed] [Google Scholar]
- Jimenez-Pacheco A., Mesuret G., Sanz-Rodriguez A., Tanaka K., Mooney C., Conroy R., et al. (2013). Increased neocortical expression of the P2X7 receptor after status epilepticus and anticonvulsant effect of P2X7 receptor antagonist A-438079. Epilepsia 54, 1551–1561. 10.1111/epi.12257 [DOI] [PubMed] [Google Scholar]
- Jourdain P., Bergersen L. H., Bhaukaurally K., Bezzi P., Santello M., Domercq M., et al. (2007). Glutamate exocytosis from astrocytes controls synaptic strength. Nat. Neurosci. 10, 331–339. 10.1038/nn1849 [DOI] [PubMed] [Google Scholar]
- Jurányi Z., Sperlágh B., Vizi E. S. (1999). Involvement of P2 purinoceptors and the nitric oxide pathway in [3H]purine outflow evoked by short-term hypoxia and hypoglycemia in rat hippocampal slices. Brain Res. 823, 183–190. 10.1016/S0006-8993(99)01169-5 [DOI] [PubMed] [Google Scholar]
- Kachroo A., Orlando L. R., Grandy D. K., Chen J. F., Young A. B., Schwarzschild M. A. (2005). Interactions between metabotropic glutamate 5 and adenosine A2A receptors in normal and parkinsonian mice. J. Neurosci. 25, 10414–10419. 10.1523/JNEUROSCI.3660-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura M., Gachet C., Inoue K., Kato F. (2004). Direct excitation of inhibitory interneurons by extracellular ATP mediated by P2Y1 receptors in the hippocampal slice. J. Neurosci. 24, 10835–10845. 10.1523/JNEUROSCI.3028-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khadra A., Tomiæ M., Yan Z., Zemkova H., Sherman A., Stojilkovic S. S. (2013). Dual gating mechanism and function of P2X7 receptor channels. Biophys. J. 104, 2612–2621. 10.1016/j.bpj.2013.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakh B. S. (2001). Molecular physiology of P2X receptors and ATP signalling at synapses. Nat. Rev. Neurosci. 2, 165–174. 10.1038/35058521 [DOI] [PubMed] [Google Scholar]
- Khakh B. S., North R. A. (2012). Neuromodulation by extracellular ATP and P2X receptors in the CNS. Neuron 76, 51–69. 10.1016/j.neuron.2012.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. E., Kwak S. E., Jo S. M., Kang T. C. (2009). Blockade of P2X receptor prevents astroglial death in the dentate gyrus following pilocarpine-induced status epilepticus. Neurol. Res. 31, 982–988. 10.1179/174313209X389811 [DOI] [PubMed] [Google Scholar]
- Kim S. Y., Moon J. H., Lee H. G., Kim S. U., Lee Y. B. (2007). ATP released from β-amyloid-stimulated microglia induces reactive oxygen species production in an autocrine fashion. Exp. Mol. Med. 39, 820–827. 10.1038/emm.2007.89 [DOI] [PubMed] [Google Scholar]
- Kimbler D. E., Shields J., Yanasak N., Vender J. R., Dhandapani K. M. (2012). Activation of P2X7 promotes cerebral edema and neurological injury after traumatic brain injury in mice. PLoS ONE 7:e41229. 10.1371/journal.pone.0041229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi S. (2010). Synchronization of Ca2+ oscillations: involvement of ATP release in astrocytes. FEBS J. 277, 286–292. 10.1111/j.1742-4658.2009.07438.x [DOI] [PubMed] [Google Scholar]
- Koizumi S., Ohsawa K., Inoue K., Kohsaka S. (2013). Purinergic receptors in microglia: functional modal shifts of microglia mediated by P2 and P1 receptors. Glia 61, 47–54. 10.1002/glia.22358 [DOI] [PubMed] [Google Scholar]
- Krügel U., Kittner H., Franke H., Illes P. (2001). Accelerated functional recovery after neuronal injury by P2 receptor blockade. Eur. J. Pharmacol. 420, R3–R4. 10.1016/S0014-2999(01)01001-9 [DOI] [PubMed] [Google Scholar]
- Kuboyama K., Harada H., Tozaki-Saitoh H., Tsuda M., Ushijima K., Inoue K. (2011). Astrocytic P2Y1 receptor is involved in the regulation of cytokine/chemokine transcription and cerebral damage in a rat model of cerebral ischemia. J. Cereb. Blood Flow Metab. 31, 1930–1941. 10.1038/jcbfm.2011.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lämmer A., Günther A., Beck A., Krügel U., Kittner H., Schneider D., et al. (2006). Neuroprotective effects of the P2 receptor antagonist PPADS on focal cerebral ischaemia-induced injury in rats. Eur. J. Neurosci. 23, 2824–2828. 10.1111/j.1460-9568.2006.04825.x [DOI] [PubMed] [Google Scholar]
- Laurent C., Burnouf S., Ferry B., Batalha V. L., Coelho J. E., Baqi Y., et al. (2014). A2A adenosine receptor deletion is protective in a mouse model of Tauopathy. Mol. Psychiatry. [Epub ahead of print]. 10.1038/mp.2014.151 [DOI] [PubMed] [Google Scholar]
- Lee H. G., Won S. M., Gwag B. J., Lee Y. B. (2011). Microglial P2X7 receptor expression is accompanied by neuronal damage in the cerebral cortex of the APPswe/PS1dE9 mouse model of Alzheimer's disease. Exp. Mol. Med. 43, 7–14. 10.3858/emm.2011.43.1.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipmann F. (1941). Metabolic generation and utilization of phosphate bond energy. Adv. Enzymol. 1, 99–162. [Google Scholar]
- Locovei S., Scemes E., Qiu F., Spray D. C., Dahl G. (2007). Pannexin1 is part of the pore forming unit of the P2X(7) receptor death complex. FEBS Lett. 581, 483–488. 10.1016/j.febslet.2006.12.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locovei S., Wang J., Dahl G. (2006). Activation of pannexin 1 channels by ATP through P2Y receptors and by cytoplasmic calcium. FEBS Lett. 580, 239–244. 10.1016/j.febslet.2005.12.004 [DOI] [PubMed] [Google Scholar]
- Lucae S., Salyakina D., Barden N., Harvey M., Gagné B., Labbé M., et al. (2006). P2RX7, a gene coding for a purinergic ligand-gated ion channel, is associated with major depressive disorder. Hum. Mol. Genet. 15, 2438–2445. 10.1093/hmg/ddl166 [DOI] [PubMed] [Google Scholar]
- Lucas M., Mirzaei F., Pan A., Okereke O. I., Willett W. C., O'Reilly É. J., et al. (2011). Coffee, caffeine, and risk of depression among women. Arch. Intern. Med. 171, 1571–1578. 10.1001/archinternmed.2011.393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthardt J., Borvendeg S. J., Sperlagh B., Poelchen W., Wirkner K., Illes P. (2003). P2Y1 receptor activation inhibits NMDA receptor-channels in layer V pyramidal neurons of the rat prefrontal and parietal cortex. Neurochem. Int. 42, 161–172. 10.1016/S0197-0186(02)00069-4 [DOI] [PubMed] [Google Scholar]
- Lutz P. L., Kabler S. (1997). Release of adenosine and ATP in the brain of the freshwater turtle (Trachemys scripta) during long-term anoxia. Brain Res. 769, 281–286. 10.1016/S0006-8993(97)00719-1 [DOI] [PubMed] [Google Scholar]
- Marcellino D., Suárez-Boomgaard D., Sánchez-Reina M. D., Aguirre J. A., Yoshitake T., Yoshitake S., et al. (2010). On the role of P2X7 receptors in dopamine nerve cell degeneration in a rat model of Parkinson's disease: studies with the P2X7 receptor antagonist A-438079. J. Neural Transm. 117, 681–687. 10.1007/s00702-010-0400-0 [DOI] [PubMed] [Google Scholar]
- Marcoli M., Cervetto C., Paluzzi P., Guarnieri S., Alloisio S., Thellung S., et al. (2008). P2X7 pre-synaptic receptors in adult rat cerebrocortical nerve terminals: a role in ATP-induced glutamate release. J. Neurochem. 105, 2330–2342. 10.1111/j.1471-4159.2008.05322.x [DOI] [PubMed] [Google Scholar]
- Matos M., Augusto E., Machado N. J., dos Santos-Rodrigues A., Cunha R. A., Agostinho P. (2012b). Astrocytic adenosine A2A receptors control the amyloid-β peptide-induced decrease of glutamate uptake. J. Alzheimers Dis. 31, 555–567. 10.3233/JAD-2012-120469 [DOI] [PubMed] [Google Scholar]
- Matos M., Augusto E., Santos-Rodrigues A. D., Schwarzschild M. A., Chen J. F., Cunha R. A., et al. (2012a). Adenosine A2A receptors modulate glutamate uptake in cultured astrocytes and gliosomes. Glia 60, 702–716. 10.1002/glia.22290 [DOI] [PubMed] [Google Scholar]
- Matos M., She H. Y., Augusto E., Wang Y., Wei C. J., Wang Y. T., et al. (2015). Deletion of adenosine A2A receptors from astrocytes disrupts glutamate homeostasis leading to psychomotor and cognitive impairment: relevance to schizophrenia. Biol. Psychiatry. [Epub ahead of print]. 10.1016/j.biopsych.2015.02.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matute C., Torre I., Pérez-Cerdá F., Pérez-Samartín A., Alberdi E., Etxebarria E., et al. (2007). P2X7 receptor blockade prevents ATP excitotoxicity in oligodendrocytes and ameliorates experimental autoimmune encephalomyelitis. J. Neurosci. 27, 9525–9533. 10.1523/JNEUROSCI.0579-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeon R. J., Jurynec M. J., Buck C. R. (1999). The chondroitin sulfate proteoglycans neurocan and phosphacan are expressed by reactive astrocytes in the chronic CNS glial scar. J. Neurosci. 19, 10778–10788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLarnon J. G., Ryu J. K., Walker D. G., Choi H. B. (2006). Upregulated expression of purinergic P2X7 receptor in Alzheimer disease and amyloid-beta peptide-treated microglia and in peptide-injected rat hippocampus. J. Neuropathol. Exp. Neurol. 65, 1090–1097. 10.1097/01.jnen.0000240470.97295.d3 [DOI] [PubMed] [Google Scholar]
- Melani A., Amadio S., Gianfriddo M., Vannucchi M. G., Volonté C., Bernardi G., et al. (2006). P2X7 receptor modulation on microglial cells and reduction of brain infarct caused by middle cerebral artery occlusion in rat. J. Cereb. Blood Flow Metab. 26, 974–982. 10.1038/sj.jcbfm.9600250 [DOI] [PubMed] [Google Scholar]
- Melani A., Turchi D., Vannucchi M. G., Cipriani S., Gianfriddo M., Pedata F. (2005). ATP extracellular concentrations are increased in the rat striatum during in vivo ischemia. Neurochem. Int. 47, 442–448. 10.1016/j.neuint.2005.05.014 [DOI] [PubMed] [Google Scholar]
- Mendonza-Fernández V., Andrew R. D., Barajas-López C. (2000). ATP inhibits glutamate synaptic release by acting at P2Y receptors in pyramidal neurons of hippocampal slices. J. Pharmacol Exp. Ther. 293, 172–179. [PubMed] [Google Scholar]
- Moore D., Iritani S., Chambers J., Emson P. (2000). Immunohistochemical localization of the P2Y1 purinergic receptor in Alzheimer's disease. Neuroreport 11, 3799–3803. 10.1097/00001756-200011270-00041 [DOI] [PubMed] [Google Scholar]
- Mori M., Heuss C., Gähwiler B. H., Gerber U. (2001). Fast synaptic transmission mediated by P2X receptors in CA3 pyramidal cells of rat hippocampal slice cultures. J. Physiol. 535, 115–123. 10.1111/j.1469-7793.2001.t01-1-00115.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy N., Cowley T. R., Richardson J. C., Virley D., Upton N., Walter D., et al. (2012). The neuroprotective effect of a specific P2X7 receptor antagonist derives from its ability to inhibit assembly of the NLRP3 inflammasome in glial cells. Brain Pathol. 22, 295–306. 10.1111/j.1750-3639.2011.00531.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narcisse L., Scemes E., Zhao Y., Lee S. C., Brosnan C. F. (2005). The cytokine IL-1β transiently enhances P2X7 receptor expression and function in human astrocytes. Glia 49, 245–258. 10.1002/glia.20110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neary J. T., Kang Y., Willoughby K. A., Ellis E. F. (2003). Activation of extracellular signal-regulated kinase by stretch-induced injury in astrocytes involves extracellular ATP and P2 purinergic receptors. J. Neurosci. 23, 2348–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neary J. T., McCarthy M., Kang Y., Zuniga S. (1998). Mitogenic signaling from P1 and P2 purinergic receptors to mitogen-activated protein kinase in human fetal astrocyte cultures. Neurosci. Lett. 242, 159–162. 10.1016/S0304-3940(98)00067-6 [DOI] [PubMed] [Google Scholar]
- Nieber K., Poelchen W., Illes P. (1997). Role of ATP in fast excitatory synaptic potentials in locus coeruleus neurones of the rat. Br. J. Pharmacol. 122, 423–430. 10.1038/sj.bjp.0701386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr A. G., Hsiao E. C., Wang M. M., Ho K., Kim D. H., Wang X., et al. (2015). Astrocytic adenosine receptor A2A and Gs-coupled signaling regulate memory. Nat. Neurosci. 18, 423–434. 10.1038/nn.3930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr A. G., Orr A. L., Li X. J., Gross R. E., Traynelis S. F. (2009). Adenosine A2A receptor mediates microglial process retraction. Nat. Neurosci. 12, 872–878. 10.1038/nn.2341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padrão R. A., Ariza C. B., Canzian M., Porcionatto M., Araffljo M. G. L., Cavalheiro E. A., et al. (2011). The P2 purinergic receptors are increased in thehippocampus of patients with temporal lobe epilepsy: what is the relevance to the epileptogenesis? Purinergic Signal. 7, 127 10.1007/s11302-010-9208-5 [DOI] [Google Scholar]
- Pandolfo P., Machado N. J., Köfalvi A., Takahashi R. N., Cunha R. A. (2013). Caffeine regulates frontocorticostriatal dopamine transporter density and improves attention and cognitive deficits in an animal model of attention deficit hyperactivity disorder. Eur. Neuropsychopharmacol. 23, 317–328. 10.1016/j.euroneuro.2012.04.011 [DOI] [PubMed] [Google Scholar]
- Pankratov Y., Castro E., Miras-Portugal M. T., Krishtal O. (1998). A purinergic component of the excitatory postsynaptic current mediated by P2X receptors in the CA1 neurons of the rat hippocampus. Eur. J. Neurosci. 10, 3898–3902. 10.1046/j.1460-9568.1998.00419.x [DOI] [PubMed] [Google Scholar]
- Pankratov Y., Lalo U., Krishtal O., Verkhratsky A. (2002). Ionotropic P2X purinoreceptors mediate synaptic transmission in rat pyramidal neurones of layer II/III of somato-sensory cortex. J. Physiol. 542, 529–536. 10.1113/jphysiol.2002.021956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankratov Y., Lalo U., Verkhratsky A., North R. A. (2006). Vesicular release of ATP at central synapses. Pflugers Arch. 452, 589–597. 10.1007/s00424-006-0061-x [DOI] [PubMed] [Google Scholar]
- Parvathenani L. K., Tertyshnikova S., Greco C. R., Roberts S. B., Robertson B., Posmantur R. (2003). P2X7 mediates superoxide production in primary microglia and is up-regulated in a transgenic mouse model of Alzheimer's disease. J. Biol. Chem. 278, 13309–13317. 10.1074/jbc.M209478200 [DOI] [PubMed] [Google Scholar]
- Pascual O., Ben-Achour S., Rostaing P., Triller A., Bessis A. (2012). Microglia activation triggers astrocyte-mediated modulation of excitatory neurotransmission. Proc. Natl. Acad. Sci. U.S.A. 109, E197–E205. 10.1073/pnas.1111098109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegatti P., Falzoni S., Donvito G., Lemaire I., Di, Virgilio F. (2011). P2X7 receptor drives osteoclast fusion by increasing the extracellular adenosine concentration. FASEB J. 25, 1264–1274. 10.1096/fj.10-169854 [DOI] [PubMed] [Google Scholar]
- Pires V. A., Pamplona F. A., Pandolfo P., Fernandes D., Prediger R. D., Takahashi R. N. (2009). Adenosine receptor antagonists improve short-term object-recognition ability of spontaneously hypertensive rats: a rodent model of attention-deficit hyperactivity disorder. Behav. Pharmacol. 20, 134–145. 10.1097/FBP.0b013e32832a80bf [DOI] [PubMed] [Google Scholar]
- Pollak Y., Yirmiya R. (2002). Cytokine-induced changes in mood and behaviour: implications for “depression due to a general medical condition”, immunotherapy and antidepressive treatment. Int. J. Neuropsychopharmacol. 5, 389–399. 10.1017/S1461145702003152 [DOI] [PubMed] [Google Scholar]
- Prediger R. D., Batista L. C., Takahashi R. N. (2005). Caffeine reverses age-related deficits in olfactory discrimination and social recognition memory in rats. Involvement of adenosine A1 and A2A receptors. Neurobiol. Aging 26, 957–964. 10.1016/j.neurobiolaging.2004.08.012 [DOI] [PubMed] [Google Scholar]
- Ralevic V., Burnstock G. (1998). Receptors for purines and pyrimidines. Pharmacol. Rev. 50, 413–492. [PubMed] [Google Scholar]
- Rappold P. M., Lynd-Balta E., Joseph S. A. (2006). P2X7 receptor immunoreactive profile confined to resting and activated microglia in the epileptic brain. Brain Res. 1089, 171–178. 10.1016/j.brainres.2006.03.040 [DOI] [PubMed] [Google Scholar]
- Rebola N., Canas P. M., Oliveira C. R., Cunha R. A. (2005a). Different synaptic and subsynaptic localization of adenosine A2A receptors in the hippocampus and striatum of the rat. Neuroscience 132, 893–903. 10.1016/j.neuroscience.2005.01.014 [DOI] [PubMed] [Google Scholar]
- Rebola N., Lujan R., Cunha R. A., Mulle C. (2008). Adenosine A2A receptors are essential for long-term potentiation of NMDA-EPSCs at hippocampal mossy fiber synapses. Neuron 57, 121–134. 10.1016/j.neuron.2007.11.023 [DOI] [PubMed] [Google Scholar]
- Rebola N., Porciúncula L. O., Lopes L. V., Oliveira C. R., Soares-da-Silva P., Cunha R. A. (2005b). Long-term effect of convulsive behavior on the density of adenosine A1 and A2A receptors in the rat cerebral cortex. Epilepsia 46(Suppl. 5), 159–165 10.1111/j.1528-1167.2005.01026.x [DOI] [PubMed] [Google Scholar]
- Rebola N., Simões A. P., Canas P. M., Tomé A. R., Andrade G. M., Barry C. E., et al. (2011). Adenosine A2A receptors control neuroinflammation and consequent hippocampal neuronal dysfunction. J. Neurochem. 117, 100–111. 10.1111/j.1471-4159.2011.07178.x [DOI] [PubMed] [Google Scholar]
- Reigada D., Lu W., Zhang M., Mitchell C. H. (2008). Elevated pressure triggers a physiological release of ATP from the retina: Possible role for pannexin hemichannels. Neuroscience 157, 396–404. 10.1016/j.neuroscience.2008.08.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resta V., Novelli E., Di Virgilio F., Galli-Resta L. (2005). Neuronal death induced by endogenous extracellular ATP in retinal cholinergic neuron density control. Development 132, 2873–2882. 10.1242/dev.01855 [DOI] [PubMed] [Google Scholar]
- Rial D., Lara D. R., Cunha R. A. (2014). The adenosine neuromodulation system in schizophrenia. Int. Rev. Neurobiol. 119, 395–449. 10.1016/B978-0-12-801022-8.00016-7 [DOI] [PubMed] [Google Scholar]
- Rivkees S. A., Wendler C. C. (2011). Adverse and protective influences of adenosine on the newborn and embryo: implications for preterm white matter injury and embryo protection. Pediatr. Res. 69, 271–278. 10.1203/PDR.0b013e31820efbcf [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues R. J., Almeida T., Richardson P. J., Oliveira C. R., Cunha R. A. (2005). Dual presynaptic control by ATP of glutamate release via facilitatory P2X1, P2X2/3 and P2X3, and inhibitory P2Y1, P2Y2 and/or P2Y4 receptors in the rat hippocampus. J. Neurosci. 27, 6286–6295 10.1523/JNEUROSCI.0628-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu J. K., Kim J., Choi S. H., Oh Y. J., Lee Y. B., Kim S. U., et al. (2002). ATP-induced in vivo neurotoxicity in the rat striatum via P2 receptors. Neuroreport 13, 1611–1615. 10.1097/00001756-200209160-00008 [DOI] [PubMed] [Google Scholar]
- Sandilos J. K., Chiu Y. H., Chekeni F. B., Armstrong A. J., Walk S. F., Ravichandran K. S., et al. (2012). Pannexin 1, an ATP release channel, is activated by caspase cleavage of its pore-associated C-terminal autoinhibitory region. J. Biol. Chem. 287, 11303–11311. 10.1074/jbc.M111.323378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santello M., Bezzi P., Volterra A. (2011). TNFα controls glutamatergic gliotransmission in the hippocampal dentate gyrus. Neuron 69, 988–1001. 10.1016/j.neuron.2011.02.003 [DOI] [PubMed] [Google Scholar]
- Sanz J. M., Chiozzi P., Ferrari D., Colaianna M., Idzko M., Falzoni S., et al. (2009). Activation of microglia by amyloid {beta} requires P2X7 receptor expression. J. Immunol. 182, 4378–4385. 10.4049/jimmunol.0803612 [DOI] [PubMed] [Google Scholar]
- Schwarzschild M. A., Agnati L., Fuxe K., Chen J. F., Morelli M. (2006). Targeting adenosine A2A receptors in Parkinson's disease. Trends Neurosci. 29, 647–654. 10.1016/j.tins.2006.09.004 [DOI] [PubMed] [Google Scholar]
- Sharp A. J., Polak P. E., Simonini V., Lin S. X., Richardson J. C., Bongarzone E. R., et al. (2008). P2x7 deficiency suppresses development of experimental autoimmune encephalomyelitis. J. Neuroinflammation 5, 33. 10.1186/1742-2094-5-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H. Y., Coelho J. E., Ohtsuka N., Canas P. M., Day Y. J., Huang Q. Y., et al. (2008). A critical role of the adenosine A2A receptor in extrastriatal neurons in modulating psychomotor activity as revealed by opposite phenotypes of striatum and forebrain A2A receptor knock-outs. J. Neurosci. 28, 2970–2975. 10.1523/JNEUROSCI.5255-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shestopalov V. I., Slepak V. Z. (2014). Molecular pathways of pannexin1-mediated neurotoxicity. Front. Physiol. 5:23. 10.3389/fphys.2014.00023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki Y., Koizumi S., Ohno Y., Nagao T., Inoue K. (2006). Extracellular ATP counteracts the ERK1/2-mediated death-promoting signaling cascades in astrocytes. Glia 54, 606–618. 10.1002/glia.20408 [DOI] [PubMed] [Google Scholar]
- Skaper S. D., Facci L., Culbert A. A., Evans N. A., Chessell I., Davis J. B., et al. (2006). P2X7 receptors on microglial cells mediate injury to cortical neurons in vitro. Glia 54, 234–242. 10.1002/glia.20379 [DOI] [PubMed] [Google Scholar]
- Solle M., Labasi J., Perregaux D. G., Stam E., Petrushova N., Koller B. H., et al. (2001). Altered cytokine production in mice lacking P2X7 receptors. J. Biol. Chem. 276, 125–132. 10.1074/jbc.M006781200 [DOI] [PubMed] [Google Scholar]
- Stafford M. R., Bartlett P. F., Adams D. J. (2007). Purinergic receptor activation inhibits mitogen-stimulated proliferation in primary neurospheres from the adult mouse subventricular zone. Mol. Cell Neurosci. 35, 535–548. 10.1016/j.mcn.2007.04.013 [DOI] [PubMed] [Google Scholar]
- Suadicani S. O., Iglesias R., Wang J., Dahl G., Spray D. C., Scemes E. (2012). ATP signaling is deficient in cultured Pannexin1-null mouse astrocytes. Glia 60, 1106–1116. 10.1002/glia.22338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J. J., Liu Y., Ye Z. R. (2008). Effects of P2Y1 receptor on glial fibrillary acidic protein and glial cell line-derived neurotrophic factor production of astrocytes under ischemic condition and the related signaling pathways. Neurosci. Bull. 24, 231–243. 10.1007/s12264-008-0430-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surprenant A., North R. A. (2009). Signaling at purinergic P2X receptors. Annu. Rev. Physiol. 71, 333–359. 10.1146/annurev.physiol.70.113006.100630 [DOI] [PubMed] [Google Scholar]
- Suzuki T., Obara Y., Moriya T., Nakata H., Nakahata N. (2011). Functional interaction between purinergic receptors: effect of ligands for A2A and P2Y12 receptors on P2Y1 receptor function. FEBS Lett. 585, 3978–3984. 10.1016/j.febslet.2011.10.050 [DOI] [PubMed] [Google Scholar]
- Takenouchi T., Iwamaru Y., Imamura M., Kato N., Sugama S., Fujita M., et al. (2007). Prion infection correlates with hypersensitivity of P2X7 nucleotide receptor in a mouse microglial cell line. FEBS Lett. 581, 3019–3026. 10.1016/j.febslet.2007.05.057 [DOI] [PubMed] [Google Scholar]
- Takenouchi T., Sugama S., Iwamaru Y., Hashimoto M., Kitani H. (2009). Modulation of the ATP-lnduced release and processing of IL-1beta in microglial cells. Crit. Rev. Immunol. 29, 335–345. 10.1615/CritRevImmunol.v29.i4.40 [DOI] [PubMed] [Google Scholar]
- Tian D. S., Yu Z. Y., Xie M. J., Bu B. T., Witte O. W., Wang W. (2006). Suppression of astroglial scar formation and enhanced axonal regeneration associated with functional recovery in a spinal cord injury rat model by the cell cycle inhibitor olomoucine. J. Neurosci. Res. 84, 1053–1063. 10.1002/jnr.20999 [DOI] [PubMed] [Google Scholar]
- Tian G. F., Azmi H., Takano T., Xu Q., Peng W., Lin J., et al. (2005). An astrocytic basis of epilepsy. Nat. Med. 11, 973–981. 10.1038/nm1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traini C., Pedata F., Cipriani S., Mello T., Galli A., Giovannini M. G., et al. (2011). P2 receptor antagonists prevent synaptic failure and extracellular signal-regulated kinase 1/2 activation induced by oxygen and glucose deprivation in rat CA1 hippocampus in vitro. Eur. J. Neurosci. 33, 2203–2215. 10.1111/j.1460-9568.2011.07667.x [DOI] [PubMed] [Google Scholar]
- Verderio C., Matteoli M. (2001). ATP mediates calcium signaling between astrocytes and microglial cells: modulation by IFN-gamma. J. Immunol. 166, 6383–6391. 10.4049/jimmunol.166.10.6383 [DOI] [PubMed] [Google Scholar]
- Vessey D. A., Li L., Kelley M. (2011). P2X7 receptor agonists pre- and postcondition the heart against ischemia-reperfusion injury by opening pannexin-1/P2X7 channels. Am. J. Physiol. Heart Circ. Physiol. 301, H881–H887. 10.1152/ajpheart.00305.2011 [DOI] [PubMed] [Google Scholar]
- Vianna E. P. M., Ferreira A. T., Naffah-Mazzacoratti M. G., Sanabria E. R. G., Funke M., Cavalheiro E. A., et al. (2002). Evidence that ATP participates in the pathophysiology of pilocarpine-induced temporal lobe epilepsy: fluorimetric, immunohistochemical, and Western blot studies. Epilepsia 43(Suppl. 5), 227–229. 10.1046/j.1528-1157.43.s.5.26.x [DOI] [PubMed] [Google Scholar]
- Volonté C., Amadio S., Cavaliere F., D'Ambrosi N., Vacca F., Bernardi G. (2003). Extracellular ATP and neurodegeneration. Curr. Drug Targets CNS Neurol. Disord. 2, 403–412. 10.2174/1568007033482643 [DOI] [PubMed] [Google Scholar]
- Wang C. M., Chang Y. Y., Kuo J. S., Sun S. H. (2002). Activation of P2X7 receptors induced [3H]GABA release from the RBA-2 type-2 astrocyte cell line through a Cl−/HCO−3-dependent mechanism. Glia 37, 8–18. 10.1002/glia.10004 [DOI] [PubMed] [Google Scholar]
- Wang X., Arcuino G., Takano T., Lin J., Peng W. G., Wan P., et al. (2004). P2X7 receptor inhibition improves recovery after spinal cord injury. Nat. Med. 10, 821–827. 10.1038/nm1082 [DOI] [PubMed] [Google Scholar]
- Wang Y., Martins I., Ma Y., Kepp O., Galluzzi L., Kroemer G. (2013). Autophagy-dependent ATP release from dying cells via lysosomal exocytosis. Autophagy 9, 1624–1625. 10.4161/auto.25873 [DOI] [PubMed] [Google Scholar]
- Wei C. J., Augusto E., Gomes C. A., Singer P., Wang Y., Boison D., et al. (2014). Regulation of fear responses by striatal and extrastriatal adenosine A2A receptors in forebrain. Biol. Psychiatry 75, 855–863. 10.1016/j.biopsych.2013.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White T. D. (1977). Direct detection of depolarisation-induced release of ATP from synaptosomal preparation. Nature 267, 67–68. 10.1038/267067a0 [DOI] [PubMed] [Google Scholar]
- Wieraszko A., Goldsmith G., Seyfried T. N. (1989). Stimulation dependent release of adenosine triphosphate from hippocampal slices. Brain Res. 485, 244–250. 10.1016/0006-8993(89)90567-2 [DOI] [PubMed] [Google Scholar]
- Wirkner K., Köfalvi A., Fischer W., Günther A., Franke H., Gröger-Arndt H., et al. (2005). Supersensitivity of P2X receptors in cerebrocortical cell cultures after in vitro ischemia. J. Neurochem. 95, 1421–1437. 10.1111/j.1471-4159.2005.03465.x [DOI] [PubMed] [Google Scholar]
- Xiao F., Waldrop S. L., Khimji A. K., Kilic G. (2012). Pannexin1 contributes to pathophysiological ATP release in lipoapoptosis induced by saturated free fatty acids in liver cells. Am. J. Physiol. Cell Physiol. 303, C1034–C1044. 10.1152/ajpcell.00175.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yiangou Y., Facer P., Durrenberger P., Chessell I. P., Naylor A., Bountra C., et al. (2006). COX-2, CB2 and P2X7-immunoreactivities are increased in activated microglial cells/macrophages of multiple sclerosis and amyotrophic lateral sclerosis spinal cord. BMC Neurol. 6:12. 10.1186/1471-2377-6-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L., Shen H. Y., Coelho J. E., Araújo I. M., Huang Q. Y., Day Y. J., et al. (2008). Adenosine A2A receptor antagonists exert motor and neuroprotective effects by distinct cellular mechanisms. Ann. Neurol. 63, 338–346. 10.1002/ana.21313 [DOI] [PubMed] [Google Scholar]
- Zhang J. M., Wang H. K., Ye C. O., Ge W., Chen Y., Jiang Z. L., et al. (2003). ATP released by astrocytes mediates glutamatergic activity-dependent heterosynaptic suppression. Neuron 40, 971–982. 10.1016/S0896-6273(03)00717-7 [DOI] [PubMed] [Google Scholar]
- Zhang Z., Chen G., Zhou W., Song A., Xu T., Luo Q., et al. (2007). Regulated ATP release from astrocytes through lysosome exocytosis. Nat. Cell Biol. 9, 945–953. 10.1038/ncb1620 [DOI] [PubMed] [Google Scholar]
- Zheng W., Watts L. T., Holstein D. M., Wewer J., Lechleiter J. D. (2013). P2Y1R-initiated, IP3R-dependent stimulation of astrocyte mitochondrial metabolism reduces and partially reverses ischemic neuronal damage in mouse. J. Cereb. Blood Flow Metab. 33, 600–611. 10.1038/jcbfm.2012.214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann H., Zebisch M., Sträter N. (2012). Cellular function and molecular structure of ecto-nucleotidases. Purinergic Signal. 8, 437–502. 10.1007/s11302-012-9309-4 [DOI] [PMC free article] [PubMed] [Google Scholar]