Abstract
Background
We previously showed that a water extract of the medicinal plant Centella asiatica (CAW) attenuates β-amyloid (Aβ)-induced cognitive deficits in vivo, and prevents Aβ-induced cytotoxicity in vitro. Yet the neuroprotective mechanism of CAW is unknown.
Objective
The goal of this study was to identify biochemical pathways altered by CAW using in vitro models of Aβ toxicity.
Methods
The effects of CAW on aberrations in antioxidant response, calcium homeostasis and mitochondrial function induced by Aβ were evaluated in MC65 and SH-SY5Y neuroblastoma cells.
Results
CAW decreased intracellular ROS and calcium levels elevated in response to Aβ, and induced the expression of antioxidant response genes in both cell lines. In SH-SY5Y cells, CAW increased basal and maximal oxygen consumption without altering spare capacity, and attenuated Aβ-induced decreases in mitochondrial respiration. CAW also prevented Aβ –induced decreases in ATP and induced the expression of mitochondrial genes and proteins in both cell types. Caffeoylquinic acids from CAW were shown to have a similar effect on antioxidant and mitochondrial gene expression in neuroblastoma cells. Primary rat hippocampal neurons treated with CAW also showed an increase in mitochondrial and antioxidant gene expression.
Conclusions
These data suggest an effect of CAW on mitochondrial biogenesis, which in conjunction with activation of antioxidant response genes and normalizing calcium homeostasis, likely contributes to its neuroprotective action against Aβ toxicity.
Keywords: β-amyloid toxicity, calcium homeostasis, Centella asiatica, mitochondrial dysfunction, neuroprotection, reactive oxygen species
Introduction
Alzheimer’s disease (AD) affects more than 5 million people in the United States alone [1]. While the hallmarks of AD, beta-amyloid (Aβ) plaques and neurofibrillary tangles, are well established, the molecular mechanisms underlying the progression of the disease remain unknown. This incomplete understanding of the etiology of AD has limited the development of effective therapeutic agents.
Mitochondrial dysfunction and oxidative stress increase with age and are thought to contribute to cognitive decline and neuronal cell death in AD[2]. AD patients show deficits in proteins in the electron transport chain (ETC) [3] as well as impaired mitochondrial function [4, 5]. Mitochondrial dysfunction causes lower ATP production, calcium mishandling, and increased reactive oxygen species (ROS), which are also observed in AD[6, 7].
These effects have been recapitulated in various models of AD. Synaptic Aβ accumulation in primary neurons, particularly within mitochondria, leads to mitochondrial dysfunction and synaptic degradation [8]. Cell culture experiments have similarly shown that Aβ treatment generates ROS, decreases ATP production, and disrupts mitochondrial membrane potential [9, 10]. Increased oxidative stress and mitochondrial defects are also observed in mouse models of Aβ toxicity [11–13]. This evidence implicating mitochondrial dysfunction and oxidative stress in AD pathology has sparked an interest in these pathways as therapeutic targets.
The plant Centella asiatica (L) Urban, (Apiaceae), known in the United States as Gotu Kola, is used in traditional Chinese and Ayurvedic medicine to improve cognitive function [14]. The neuroprotective and cognitive enhancing effects of Centella asiatica have been confirmed in human studies [15–17] as well as in vitro and in vivo model systems [18–20].
Our earlier studies have shown that a water extract of Centella asiatica (CAW) can attenuate the cognitive impairments in the Tg2576 mouse model of Aβ accumulation without altering plaque burden [21] and can prevent Aβ toxicity in vitro [22]. Although the mechanism remains unknown, studies in other models of neurotoxicity show that Centella asiatica possesses antioxidant activity and can alter mitochondrial function [23, 24].
In the present study we investigated the mechanism by which CAW protects against Aβ toxicity using the MC65 and the SH-SY5Y neuroblastoma cell lines. MC65 cells conditionally express amyloid β precursor protein (APP) [25] and are a model of intracellular Aβ toxicity while SH-SY5Y cells are widely used to model the effects of exogenous Aβ treatment. We examined the effects of CAW on mitochondrial function and antioxidant response in both of these cellular systems.
Materials and Methods
Aqueous extract of Centella asiatica
Dried Centella asiatica was purchased (StarWest Botanicals, Lot #45158) and its identity was confirmed by comparing its thin layer chromatographic profile with that reported in the literature [26] and the Centella asiatica samples used in our previous studies [21]. The water extract of Centella asiatica (CAW) was prepared by refluxing Centella asiatica (60g) with water (750mL) for 2 hours, filtering the solution and freeze drying to yield a powder (~6–8g). Voucher specimens of the dried plant material [22] and extract are deposited in our laboratory.
Cell culture MC65
MC65 neuroblastoma cells express the C-terminal fragment of APP (APP-C99) under the control of a tetracycline responsive promoter. Following tetracycline withdrawal, endogenous Aβ accumulates and cell death occurs within 72 hours [25]. MC65 cells were cultured in MEMα supplemented with 10% FBS (Gibco), 2mM L-glutamine (Sigma-Aldrich) and 0.1% tetracycline (Sigma-Aldrich). For experiments cells were trypsinized and resuspended in OptiMEM without phenol red (Gibco). Cells were treated with vehicle or CAW (100ug/mL) in the absence of tetracycline. All endpoints were compared to those for tetracycline-treated cells with or without the addition of CAW. Cells were plated at 15,000 cells/well in 96 well plates. Intracellular calcium was measured at 6, 24 and 48h and intracellular ROS was measured at 48 hours. Cells were plated at 60,000 cells/well in 12 well plates for gene expression or 120,000 cells/well in 6 well plates for protein expression as well as ATP determination and were harvested 48h post-treatment.
Cell Culture SH-SY5Y
SH-SY5Y neuroblastoma cells were cultured in DMEM/F12 media supplemented with 10% FBS (GIBCO) and 1% penicillin-streptomycin (Sigma-Aldrich). For gene expression and ATP determination cells were plated at 200,000 cells/well in 12-well plates whereas for protein expression they were plated at 400,000 cells/well in 6-well plates. For intracellular calcium and ROS measurements cells were plated at 25,000 cells/well in 96 well plates. Three days after plating cells were washed with PBS and switched to serum free DMEM/F12 containing 1% N-2 growth supplement (Gibco) and CAW (100ug/mL). The following day, 50μM Aβ25–35 (American Peptide Company) was added to the cells. This fragment of full-length Aβ has been shown to mediate its toxic effects in vitro [27]. Aβ solution was prepared by incubating at 37C for 72h prior to addition to the cell cultures. All endpoints were assessed after 48h of treatment unless otherwise noted.
Caffeoylquinic acid treatment in MC65 and SH-SY5Y cells
The purified forms of 1,5-dicaffeoylquinic acid (1,5dCQA) and isocholorogenic acid A (IsoA also called 3,5-dicaffeoylquinic acid) (Chromadex), two compounds that we have previously determined to contribute to the neuroprotective effects of CAW [22], were used to treat MC65 and SH-SY5Y cells in place of CAW in some experiments. They were used at a concentration of 1.5uM which is similar to their concentration in 100ug/mL CAW which we previously reported to be approximately 1uM [22].
Cell culture primary neurons
Hippocampal neurons were isolated from embryonic rats as previously described by Kaech and Banker [28]. Briefly, embryos were harvested at 18 days of gestation and hippocampi isolated. Dissociated hippocampal cells were plated at 200,000 per well in 12-well poly-l-lysine coated plates in MEM media (GIBCO), 5% FBS (Atlanta Biologicals) and 0.6% glucose (Sigma Aldrich). After 4h media was removed and replaced with Neurobasal Media (Gibco) supplemented with 1x GlutaMAX (Gibco) and 1x GS21 (Global Stem). After 7 days in culture cells were treated with CAW (50ug/mL) and 48h later were harvested for qPCR analysis.
Intracellular ROS quantification
ROS levels were determined using OxiSelect Intracellular ROS Assay kit (Cell biolabs) as per the manufacturer’s instructions. Cells were incubated with the fluorogenic probe for 1h at 37C prior to measurement. Values were normalized to protein content determined by a bicinchoninic acid (BCA) protein assay as per the manufacturer’s instructions (Pierce Biotechnology).
Quantitative Real Time PCR
Cells were harvested and RNA was extracted using Tri-Reagent (Molecular Research Center). RNA was reverse transcribed with the Superscript III First Strand Synthesis kit (Invitrogen) to generate cDNA as per the manufacturer’s instructions. Relative gene expression was determined using TaqMan Gene Expression Master Mix (Invitrogen) and commercially available TaqMan primers (Invitrogen) for nuclear factor (erythroid-derived 2)-like 2 (NFE2L2), NAD(P)H dehydrogenase-quinone oxidoreductase 1 (NQO1), glutamate-cysteine ligase, catalytic subunit (GCLC), heme oxygenase 1 (HMOX1), mitochondrially encoded NADH dehydrogenase 1 (Mt-ND1), mitochondrially encoded ATP synthase 6 (Mt-ATP6), mitochondrially encoded cytochrome c oxidase 1 (Mt-CO1), mitochondrially encoded cytochrome B (Mt-CYB) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Quantitative PCR (qPCR) was performed on a StepOne Plus Machine (Applied Biosystems) and analyzed using the delta-delta Ct method.
Cell number determination
Cell number was determined, for data normalization, using the CellTiter 96 Aqueous Non-Radioactive Cell Proliferation Assay (Promega) as per the manufacturer’s instructions. Cells were incubated with the reagent for 2h at 37C prior to spectrophotometric reading.
Intracellular Calcium Determination
Intracellular calcium was measured using the Fluo-4 Direct Calcium Assay Kit (Invitrogen) as per the manufacturer’s instructions. Cells were incubated with the fluo-4 direct reagent for 1h at 37C prior to measurement. Values were normalized to cell number.
ATP Quantification
ATP was quantified using ATP determination kit (Invitrogen) as per the manufacturer’s instructions. Cells were lysed with 0.1% Triton X and incubated with the reaction solution for 15 minutes at room temperature prior to measurement. Values were normalized to protein content determined by BCA.
Western Blots
Cells were harvested, sonicated and boiled in Laemmli buffer. Samples were separated electrophoretically on an SDS gel, transferred onto nitrocellulose membranes and immunoblotted using antibodies for Porin (also known as VDAC1) (Abcam) and GAPDH (Cell Signaling). The optical density of the bands was quantified using Image J software (http://rsbweb.nih.gov/ij) and normalized to GAPDH.
Analysis of Mitochondrial Function
Mitochondrial function was assessed using the Seahorse Bioscience XF24 Extracellular Flux Analyzer. SH-SY5Y cells were plated on Seahorse XF culture plates (Seahorse Bioscience) in DMEM/F12 containing N2 growth supplement and either CAW (100ug/mL), 1,5dCQA(1.5uM) or IsoA (1.5uM), at 60,000 cells/well, which was determined to be the optimum density for basal O2 consumption rate (OCR). The following day Aβ25–35 (50uM) was added. Two days later cells were rinsed in assay medium (pH 7.4) containing XF Base medium (Seahorse Bioscience), 5.5mM glucose and 1mM sodium-pyruvate. Cells remained in assay medium 1h at 37 C in a non-CO2 incubator prior to initializing the Seahorse24XF analysis. Using the MitoStress Kit as previously described [29], OCR was measured under varying conditions. After three initial baseline measurements of OCR, the ATP synthase inhibitor oligomycin (1 μM) was added and three subsequent measurements were taken. Next an ETC accelerator, p-trifluoromethoxy carbonyl cyanide phenyl hydrazone (FCCP at 1.5 μM), was added and after 3 measurements were taken, mitochondrial inhibitors rotenone (1 μM) and antimycin (1 μM) were added, and three final measurements were taken. Data was normalized to total DNA content, which was determined from each well using the CyQuant kit (Invitrogen) as per the manufacturer’s instructions.
Statistics
Statistical significance was determined using one- or two-way analysis of variance with appropriate t-tests. Bonferroni post-hoc tests were also conducted. Significance was defined as p ≤0.05. Analyses were performed using Excel or GraphPad Prism 6.
Results
CAW Prevents Aβ-induced increases in intracellular ROS
MC65 neuroblastoma cells conditionally express APP-C99 under a tetracycline responsive promoter. When tetracycline is present in the media (Tet+) the gene is repressed. However, when tetracycline is withdrawn (Tet−) Aβ accumulates with significant levels observed by 48h [25].
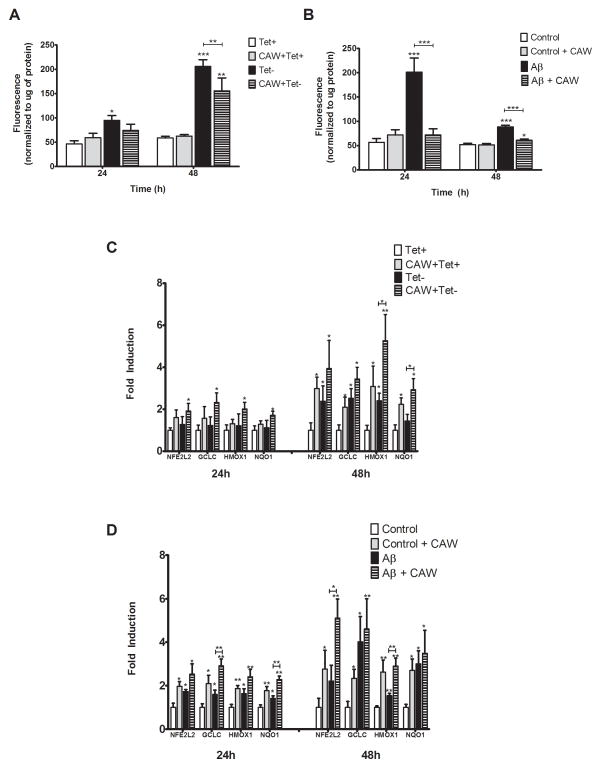
An increase in intracellular ROS was seen 24h post-tetracycline withdrawal that did not occur in CAW-treated cells (Figure 1A). An even greater increase in ROS levels occurred 48h after tetracycline withdrawal that was attenuated by CAW. Treatment with CAW in the presence of tetracycline had no effect on ROS levels.
Figure 1. CAW induces anti-oxidant response in MC65 and SH-SY5Y cells.
A) Aβ accumulation, in MC65 cells grown without tetracycline (Tet−), significantly increased intracellular ROS levels at both 24h and 48h. CAW treatment (100ug/mL) attenuated that increase (n=12–16 per treatment condition). B) Treatment with Aβ25–35 peptide (50uM) induced intracellular ROS in SH-SY5Y cells at 24h and 48h. CAW (100ug/mL) significantly reduced that increase (n=12–16 per treatment condition). C) At 24h, CAW treatment following tetracycline withdrawal significantly induced the expression of a panel of antioxidant response genes in MC65 cells. After 48h induction was even greater in the Tet− condition as well as cells treated with CAW in the absence of tetracycline. CAW treatment in the presence of tetracycline also significantly induced antioxidant gene expression but only at the 48h time point. (n=6–7 per treatment condition) D) In SH-SY5Y cells Aβ treatment significantly induced the expression of NFE2L2 and its target genes at both 24h and 48h. CAW treatment potentiated this increase at both time points. CAW treatment also significantly induced expression of the antioxidant response genes at both time points but to a lesser extent than CAW and Aβ together (n=6 per treatment condition). *p<0.05, **p<0.01, ***p<0.001 relative to either Tet+ or Control unless otherwise indicated.
A similar effect was observed in SH-SY5Y cells treated with CAW 24h prior to the administration of Aβ25–35, the fragment of the peptide reported to mediate the toxic effects of full-length Aβ in vitro [27]. Aβ treatment robustly increased intracellular ROS levels at 24h and 48h (Figure 1B), with the greatest induction observed at 24h. CAW treatment reduced this induction at both time points. In the absence of Aβ, CAW treatment had no effect on intracellular ROS levels.
CAW induces antioxidant response genes
The expression of the antioxidant response gene NFE2L2 and its target genes, GCLC, NQO1 and HMOX1, was evaluated 24h and 48h after tetracycline removal in MC65 cells. While no change in expression was observed at 24h, the expression of all four genes was significantly induced 48h post-tetracycline withdrawal (Figure 1C). CAW treatment, in the absence of tetracycline, further increased expression of NFE2L2 and its target genes beyond what was observed in the Tet− condition alone at both time points. In the presence of tetracycline CAW also induced the expression of the antioxidant response genes after 48h but had no effect at 24h.
In SH-SY5Y cells antioxidant response gene expression was induced 24h after Aβ administration (Figure 1D). CAW treatment prior to Aβ administration further increased this expression. CAW also increased expression these genes in the absence of Aβ although to a lesser degree. The Aβ-induced expression of these genes remained increased 48h post-treatment. The effect of CAW pre-treatment was even greater at 48h than was seen at 24h both in the presence and absence of Aβ.
CAW increases ATP production
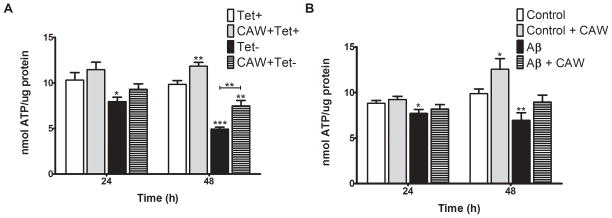
ATP was measured 24h and 48h after tetracycline withdrawal in MC65 cells. There was a slight decrease in ATP 24h post-tetracycline removal, which became more pronounced after 48h (Figure 2A). CAW treatment attenuated this decrease at 24h and 48h. In the presence of tetracycline there was a trend towards increased ATP levels in CAW-treated cells at 24h but it was not significant until 48h.
Figure 2. CAW treatment attenuates the decline in ATP caused by Aβ in MC65 and SH-SY5Y cells.
A) Following tetracycline withdrawal (Tet−) ATP levels in MC65 cells decreased significantly at 24h and further decreased at 48h. CAW treatment attenuated this decrease. CAW treatment in the presence of tetracycline significantly increased ATP levels after 48h. (n=9–12 per treatment condition). B) In SH-SY5Y cells Aβ treatment resulted in a slight but significant decrease in ATP at 24h and a greater decrease at 48h. At both time points CAW treatment with Aβ did not decrease ATP relative to controls. At 48h CAW treatment in the absence of Aβ robustly increased ATP levels relative to controls. (n=8–12 per treatment condition). *p<0.05, **p<0.01, ***p<0.001 relative to either Tet+ or Control unless otherwise indicated.
Aβ treatment resulted in decreased ATP levels in SH-SY5Y cells 24h and 48h after its administration (Figure 2B). CAW prevented this decrease at both time points. Consistent with what was observed in MC65 cells, CAW treatment in the absence of Aβ also increased intracellular ATP at the 48h time point.
CAW increases OCR and attenuates bioenergetic deficits caused by Aβ treatment
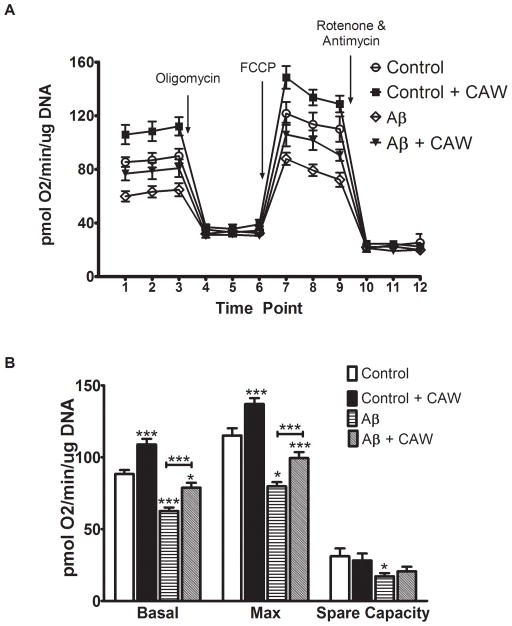
The bioenergetic profile of SH-SY5Y cells, treated with CAW for 72h and Aβ for the final 48h, was determined using the Seahorse XF Analyzer (Figure 3A). Under basal conditions CAW-treated cells exhibited a higher oxygen consumption rate (OCR) compared to controls while Aβ-treated cells displayed a significantly lower OCR. CAW treatment attenuated the decreased metabolic rate caused by Aβ. While there were no differences in OCR between treatment groups after the addition of oligomycin or rotenone and antimycin, CAW significantly increased the maximal respiratory rate, following FCCP treatment, which was reduced by Aβ treatment.
Figure 3. CAW increases mitochondrial respiration in SH-SY5Y cells.
A) Aβ treatment significantly reduced oxygen consumption rate (OCR) in SH-SY5Y cells at baseline and after FCCP stimulation. CAW attenuated this decrease under both basal and stimulated conditions. CAW treatment without Aβ resulted in significantly higher basal and FCCP-induced OCR. There were no differences in OCR between treatment groups after either oligomycin or rotenone and antimycin treatment (n=9–10 per treatment condition). B) Aβ decreased both basal and maximal OCR and CAW treatment attenuated these decreases. In the absence of Aβ, CAW significantly increased both basal and maximal OCR. Aβ also reduced the spare capacity of mitochondria and CAW prevented this decrease. CAW treatment alone had no effect on spare capacity (n=9–10 per treatment condition). *p<0.05, **p<0.01, ***p<0.001 relative to Control unless otherwise indicated.
By averaging the three measurements taken sequentially at baseline and after FCCP stimulation, average basal and maximal OCR were calculated. Aβ reduced both basal and maximal OCR and CAW treatment partially attenuated these decreases (Figure 3B). CAW treatment by itself significantly increased both basal and maximal OCR as well. The difference between the maximal OCR and the basal OCR is the spare capacity of the cell and reflects the amount of extra ATP that can be generated in response to a sudden increase in energy demand. Aβ decreased spare capacity and CAW prevented this decrease but did not improve spare capacity under control conditions (Figure 3B).
CAW induces mitochondrial gene and protein expression
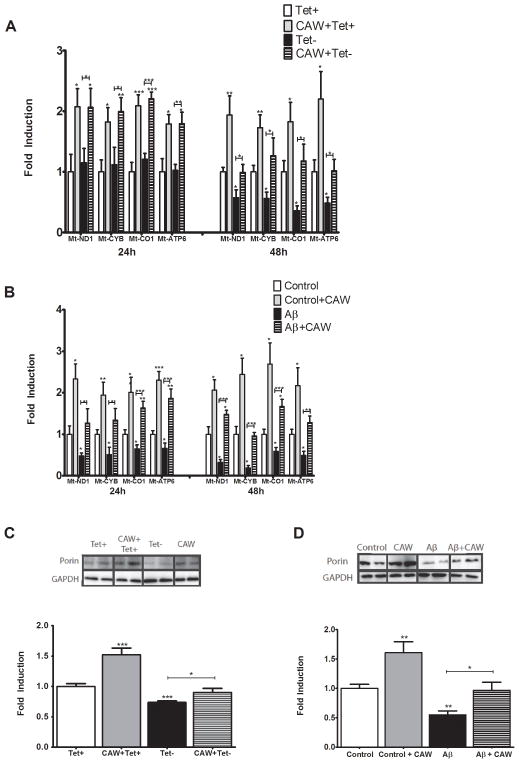
The expression of mitochondrial DNA-derived genes Mt-ND1, Mt-CYB, Mt-CO1 and Mt-ATP6, encoding proteins in complexes I, III, IV and V of the ETC respectively, was evaluated in MC65 cells 24h and 48h after tetracycline withdrawal. At 24h, tetracycline withdrawal had no effect on the expression of any of the genes whereas CAW treatment robustly increased the expression of all four genes in both the Tet+ and Tet− conditions (Figure 4A). At 48h all four genes were repressed in the Tet− condition but CAW treatment attenuated this effect. In the presence of tetracycline CAW coordinately increased the expression of all the mitochondrial genes 48h post-treatment as well.
Figure 4. CAW induces the expression of mitochondrial proteins in MC65 and SH-SY5Y cells.
A) In MC65 cells 24h of Aβ accumulation (Tet−) had no effect on gene expression of ETC enzymes but after 48h resulted in a coordinate reduction in expression of all genes. CAW treatment significantly attenuated this decrease at 48h. At 24h and 48h, CAW treatment robustly increased the expression of all four mitochondrial genes both in the presence and absence of tetracycline (n=6–8 per treatment condition). B) Aβ treatment reduced mitochondrial gene expression at both 24h and 48h in SH-SY5Y cells. CAW treatment prevented this decrease. In the absence of Aβ, CAW significantly increased mitochondrial gene expression at both time points (n=5–7 per treatment condition). C) In the absence of tetracycline (Tet−) Aβ accumulation also decreased the expression of the mitochondrial protein porin in MC65 cells and CAW treatment restored expression to control (Tet+) levels. CAW treatment also induced porin expression in the presence of tetracycline. (n=10 per treatment condition) D) In SH-SY5Y cells Aβ treatment reduced porin expression and CAW treatment prevented this decrease. CAW treatment significantly increased porin expression in the absence of Aβ (n=12 per treatment condition). *p<0.05, **p<0.01, ***p<0.001 relative to either Tet+ or Control unless otherwise indicated.
In SH-SY5Y cells Aβ treatment significantly repressed the expression of the mitochondrial genes at 24h and this expression was even further reduced at 48h. CAW treatment prevented this decrease at 24h, restoring the expression of Mt-ND1 and Mt-CYB to control levels and slightly increasing the expression of Mt-CO1 and Mt-ATP6 (Figure 4B). At 48h CAW treatment prevented the Aβ-induced repression in mitochondrial gene expression for all genes and slightly induced the expression of Mt-ND1 and Mt-CO1. Without Aβ, CAW induced the expression of all four genes to a similar degree at both 24h and 48h.
The protein expression of the mitochondrial membrane ion channel porin (also known as VDAC1) was assessed in MC65 and SH-SY5Y cells 48h after tetracycline withdrawal and Aβ administration respectively. In MC65 cells porin expression was reduced in the Tet− condition and CAW treatment restored expression to control levels (Figure 4C). CAW treatment in the presence of tetracycline induced porin expression. In SH-SY5Y cells Aβ administration significantly reduced porin expression (Figure 4D) while CAW prevented this decrease. In the absence of Aβ, CAW treatment significantly increased porin expression.
CAW prevents Aβ-induced alterations in calcium homeostasis in MC65 and SH-SY5Y cells
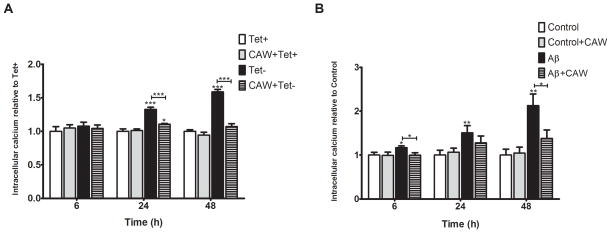
Intracellular calcium was quantified 6h, 24h and 48h post-tetracycline withdrawal in MC65 cells. At 6h there was no change in calcium levels but at 24h there was a significant increase in the Tet− condition (Figure 5A). CAW attenuated this increase, although the calcium levels remained slightly elevated as compared to cells grown with tetracycline. In the Tet− condition calcium levels were also elevated at 48h and CAW treatment reduced this increase. There was no effect of CAW treatment on calcium levels in the presence of tetracycline alone.
Figure 5. CAW treatment significantly attenuates Aβ-induced increases in intracellular calcium in MC65 and SH-SY5Y cells.
A) In MC65 cells there were no differences in intracellular calcium levels after 6 hours of treatment however, there was a significant increase in intracellular calcium at 24h in the absence of tetracycline (Tet−) which continued at 48h. CAW treatment significantly reduced the increase in calcium in the Tet− condition but had no effect on calcium levels in the presence of tetracycline (n=8 per treatment condition). B) Aβ treatment slightly increased intracellular calcium in SH-SY5Y cells after 6h, with progressively larger increases at 24h and 48h. CAW treatment (100ug/mL) prevented Aβ-induced increase at all time points. CAW treatment had no effect on intracellular calcium in the absence of Aβ. (n=10–12 per treatment condition). *p<0.05, **p<0.01, ***p<0.001 relative to either Tet+ or Control unless otherwise indicated.
Similarly, in SH-SY5Y cells Aβ treatment increased intracellular calcium (Figure 5B). This induction was detectable at 6h and continued to increase up to 48h. CAW treatment prevented the increase caused by Aβ at all time points but had no effect in the absence of Aβ.
Caffeoylquinic acids (CQAs) from CAW result in similar changes in gene expression and mitochondrial function
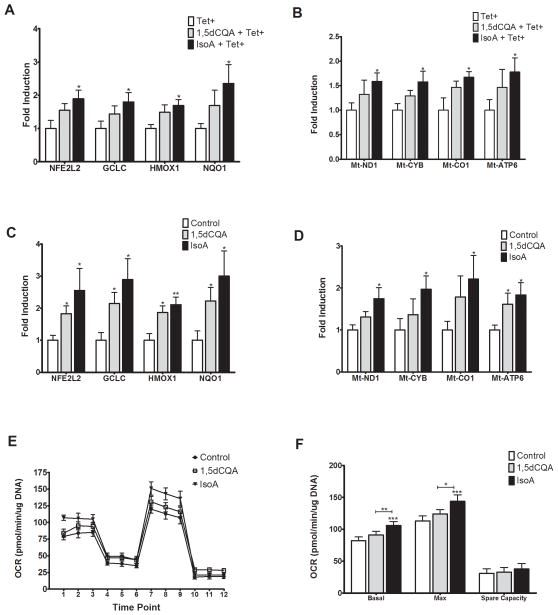
We have previously identified several caffeoylquinic acids (CQAs) present in CAW [22]. To determine if these CQAs could be contributing to the mitochondrial and antioxidant effects of CAW, expression of antioxidant and ETC genes was quantified in MC65 and SH-SY5Y cells treated with 1,5dCQA or IsoA. In MC65 cells IsoA (1.5uM) treatment for 48h significantly increased the expression of NFE2L2 and its target genes (Figure 6A) as well the genes of the ETC (Figure 6B). Treatment with 1,5dCQA (1.5uM) for 48h resulted in a consistent but non-significant increase in all genes measured. In SH-SY5Y cells both 1,5dCQA and IsoA both significantly increased expression of antioxidant response genes (Figure 6C). IsoA also significantly increased the expression of all ETC genes while 1,5dCQA only significantly increased Mt-ATP6. (Figure 6D).
Figure 6. Treatment with IsoA induces antioxidant and mitochondrial gene expression and alters mitochondrial respiration in neuroblastoma cell lines.
A) In MC65 cells treatment with IsoA (1.5μM) significantly increased the expression of NFE2L2 and its target genes. Treatment with 1,5dCQA (1.5μM) did not induce expression relative to control treated cells (n=5–8 per treatment condition). B) IsoA treatment also induced the expression of ETC genes in MC65 cells while 1,5dCQA did not (n=5–7 per treatment condition). C) In SH-SY5Y cells 48h of treatment with IsoA increased expression of NEF2L2 and its target genes as did treatment with 1,5dCQA (n=8 per treatment condition). D) IsoA also increased expression of ETC genes in SH-SY5Y cells while 1,5dCQA did not (n=5–8 per treatment condition). E) In SH-SY5Y cells IsoA treatment resulted in a significantly higher basal and FCCP-induced OCR. There were no differences in OCR between treatment groups after either oligomycin or rotenone and antimycin treatment. 1,5dCQA had no effect on OCR relative to controls (n=7–8 per treatment condition). F) IsoA significantly increased both basal and maximal OCR but had no effect on spare capacity in SH-SY5Y cells. 1,5 dCQA did not alter basal or maximal OCR or spare capacity in these cells (n=7–8 per treatment condition). ). *p<0.05, **p<0.01, ***p<0.001 relative to Control unless otherwise indicated.
IsoA also significantly altered the bioenergetic profile of SH-SY5Y cells (Figure 6E) similarly to what was observed with CAW treatment while 1,5dCQA did not significantly alter OCR compared to control treatment. Consistent with what was observed with CAW treatment, IsoA significantly increased both basal and maximal OCR but had no effect on spare capacity (Figure 6F).
CAW induces antioxidant response genes as well as mitochondrial genes in primary hippocampal neurons
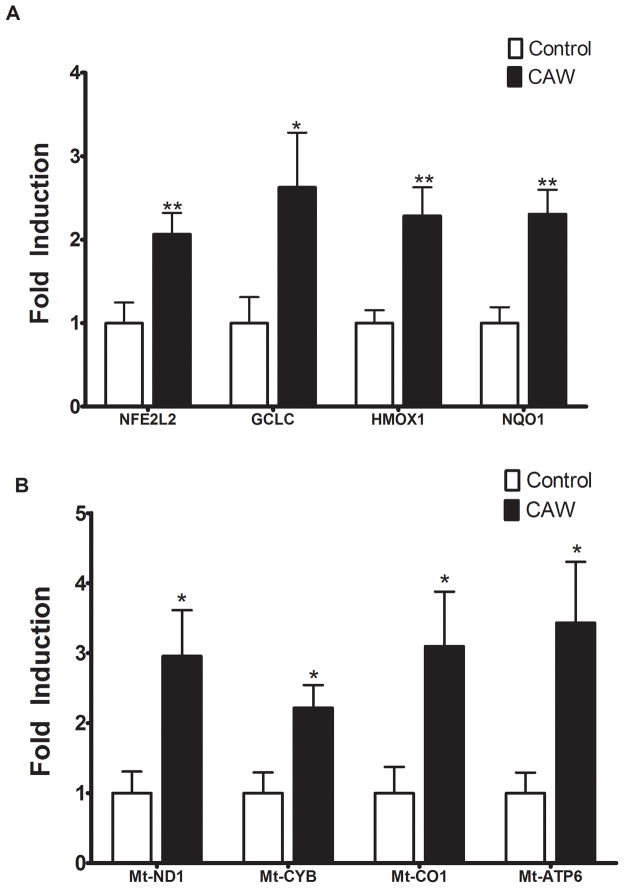
Because metabolism in neuroblastoma cells is different from that of neurons [30] we wanted to evaluate the effects of CAW on antioxidant and mitochondrial gene expression in primary neurons. CAW significantly increased the expression of ETC genes in isolated rat hippocampal primary neurons (Figure 7B). The expression of antioxidant response genes in primary neurons was similarly induced by CAW (Figure 7A).
Figure 7. CAW induces the expression antioxidant and mitochondrial genes in primary hippocampal neurons.
A) CAW treatment (50ug/mL) for 48h significantly increased expression of NFE2L2 and its target genes in primary neurons isolated from rat hippocampus (n=10–11 per treatment condition). B) CAW treatment also robustly induced the expression of ETC genes in rat hippocampal neurons (n=10–11 per treatment condition). *p<0.05, **p<0.01, relative to Control unless otherwise indicated.
Discussion
We previously reported that CAW attenuates cognitive deficits in a mouse model of Aβ toxicity [21] and protects against Aβ-induced cytotoxicity in SH-SY5Y and MC65 cells [22]. Here we characterized the mechanism by which CAW exerts its neuroprotective effects in vitro by demonstrating its ability to reverse Aβ-induced alterations in mitochondrial function, intracellular ROS and calcium levels, three interrelated mechanisms involved in neurodegeneration [31].
In this study, we showed that endogenous Aβ accumulation in MC65 cells as well as exogenous treatment of SH-SY5Y cells with Aβ peptide significantly altered mitochondrial function. In both models, we found that Aβ decreased ATP levels. These findings are consistent with other reports of Aβ-treated SH-SY5Y cells [32] as well as cell lines over-expressing APP [10, 33]. CAW treatment prevented this decrease in ATP. Interestingly, in the absence of Aβ, CAW increased ATP content in both cell types indicating a direct mitochondrial effect, which was confirmed by our experiments in SH-SY5Y cells using the Seahorse XF Analyzer. We found that Aβ treatment reduced both basal and maximal OCR as well as mitochondrial spare capacity. To our knowledge this is the first report of the effects of Aβ on mitochondrial respiration in SH-SY5Y cells. However these results are in line with what has been seen in neurons isolated from mice with Aβ pathology, which demonstrate lower basal and maximal OCR, decreased ATP production and decreased spare capacity [34].
CAW treatment prevented these Aβ-induced decreases in OCR. CAW alone increased basal and maximal OCR as well but did not affect spare capacity, suggesting that CAW may alter mitochondrial biogenesis rather than activity. The up-regulation of mitochondrial proteins (Fig 4C) further supports an effect of CAW on mitochondrial content, although future experiments utilizing microscopic techniques or monitoring the expression of transcription factors that regulate mitochondrial biogenesis would confirm this effect. While an effect of Centella asiatica on mitochondrial biogenesis has not been previously reported, the protective effects of CAW against Aβ-induced mitochondrial dysfunction are consistent with a recent study showing a mitoprotective effect of Centella asiatica in a model of aluminum neurotoxicity [23].
Mitochondria are critical for cell survival because they regulate energy metabolism as well as apoptotic pathways. They are both a target and the major producers of intracellular ROS. Both mitochondrial dysfunction and increased ROS can also disrupt calciuim homeostasis. Changes in all three of these interrelated pathways are observed in AD patients [6, 35, 36] as well as in numerous animal and cell culture models of AD [36–42]. We observed that CAW attenuated Aβ-induced alterations in intracellular ROS and calcium levels in both cell types. However, CAW did not affect ROS levels or calcium influx in the absence of Aβ, suggesting that CAW may indirectly influence these aspects of Aβ toxicity rather than directly scavenging ROS or affecting calcium channels.
Since Aβ is an oxidative insult, it increased both ROS levels, and the expression of the antioxidant response gene NFE2L2 and its target genes in MC65 and SH-SY5Y cells. In both cell types, induction of these genes was higher at 48h than 24h. In SH-SY5Y cells the peak Aβ-induced ROS levels occurred at 24h and were reduced at 48h. By contrast, in MC65 cells Aβ-induced ROS levels were even higher at 48h than 24h. These temporal differences are likely due to the levels of Aβ at each time point. In MC65 cells when tetracycline is withdrawn Aβ begins to accumulate and levels increase over several days. In contrast SH-SY5Y cells are exposed to a uniform concentration of Aβ. The induction of antioxidant response genes was similarly delayed in MC65 cells as compared to SH-SY5Y cells, consistent with the timing of Aβ accumulation.
CAW also induced the expression of the antioxidant response gene NFE2L2 and its target genes with levels increasing from 24h to 48h. The combined treatment of Aβ and CAW produced a greater increase in gene expression than CAW or Aβ alone. The ability of CAW to reduce Aβ-induced ROS levels was likely mediated by its strong induction of an antioxidant response. These results support the previously reported antioxidant properties of Centella asiatica [24, 43].
We observed changes in mitochondrial and antioxidant gene expression in response to CAW in the absence of Aβ treatment as well, indicating that the effects of CAW on these pathways are not specific to an AD-like condition. In fact these antioxidant and mitoprotective effects of CAW are similar to what has been observed with other plant extracts in response to a variety of different insults. An extract of Ginkgo biloba attenuated ATP deficits and decreased oxidative damage following chemically-induced mitochondrial damage in PC12 neuronal cells [44], and stabilized mitochondrial function against nitrosative stress in primary neurons [45]. In SH-SY5Y cells, xanthoceraside, a compound extracted from Chinese flowering chestnuts, prevented Aβ-induced ROS generation, calcium overload and mitochondrial dysfunction [41] and borneol, found in many plants including wormwood and sagebrush, also decreased Aβ-induced ROS production by activating NFE2L2 [46].
CAW is a complex mixture of compounds and it is unknown exactly which are responsible for its many biochemical effects. We previously reported that several CQAs are present in CAW and can protect against Aβ cytotoxicity [22]. CQAs have also been shown to affect mitochondrial function and to possess antioxidant activity in primary neurons as well as neuronal cell lines [47–49]. In this study we evaluated two CQAs, present at the highest concentrations in CAW [22] to determine if they mediate the effects of the complete extract. IsoA was able to increase the expression of antioxidant response genes as well as genes in the ETC in both cell types. These changes in gene expression accompanied an increase in both basal and maximal OCR in response to IsoA in SH-SY5Y cells. There was a consistent trend toward a slight but non-significant effect of 1,5dCQA on gene expression or bioenergetics, with the only significant changes occurring in the ARE genes in SH-SY5Y cells. In both cell types IsoA increased expression of ARE genes to a similar extent as was observed with CAW but the effect on ETC gene expression was less than with CAW suggesting that IsoA alone cannot account for the effects of CAW on mitochondrial gene expression. It may be that the compounds in combination produce a greater effect than in isolation and it’s likely that there are compounds other than the CQAs within CAW that also contribute to the mitochondrial effects of the extract.
The results reported here provide strong evidence for mitochondrial and antioxidant effects of CAW in neuroblastoma cells however the extent to which these mechanisms would contribute to the effects of CAW in a more physiologically relevant experimental systems remains unclear. This especially important because the metabolic profile of neuroblastoma cells is known to vary substantially from differentiated neurons [30, 50] and therefore it is possible CAW would not have the same effects in the two model systems. As an initial step toward addressing this issue we evaluated the effects of CAW on ARE and ETC gene expression in rat primary hippocampal neurons. Our results were consistent with what we observed in the neuroblastoma cell lines. CAW significantly increased the expression of NFE2L2 and its target genes as well as the ETC genes in primary hippocampal neurons. These results suggest that the antioxidant and mitochondrial effects of CAW may be relevant in isolated neurons as well. Experiments are ongoing to fully assess whether CAW modulates other aspects of antioxidant response or the bioenergetics of primary neurons and to determine the effect of Aβ exposure on those endpoints in those cells.
Additional future studies are also needed to confirm that the mechanistic alterations observed in this study are relevant in vivo. MC65 and SH-SY5Y cells provide a robust look at mechanistic pathways in vitro by modeling the intracellular and extracellular responses to Aβ, but efficacy in vivo requires that the active ingredients are both bioavailable and capable of crossing the blood-brain barrier. Our prior study in transgenic mice [21] provides some limited support for this possibility, but the mechanistic studies reported here will need to be replicated in appropriate animal models.
Conclusion
Our findings demonstrate that CAW affects mitochondrial function on its own and appears to counteract the deleterious impact of Aβ in cellular models, suggesting that the effects of CAW are not limited to Aβ toxicity. Because mitochondrial dysfunction is common to many neurodegenerative diseases [51], there are potentially broad implications for the use of CAW. The utility of CAW may extend beyond frank pathological conditions as decreased mitochondrial function is seen in healthy aging as well, and may be linked to age-related cognitive decline [52, 53]. A few limited human studies in healthy adults showed promising cognitive-enhancing effects of Centella asiatica [15, 17] but a rigorous clinical trial of CAW in either healthy or impaired elderly people is still needed to determine if CAW is beneficial in those contexts.
Acknowledgments
This work was funded by NIH-NCCAM grant R01AT008099, a grant awarded to A. Soumyanath from the Oregon Alzheimer’s Disease Center (OADC) 3P30-AG008017 24 S1 and one to N. Gray from the OADC 5 P30 AG008017 24, a T32 grant on which N. Gray was a trainee from NIH-NCCAM AT002688, a K99DK100640 to H. Sampath and by a Department of Veterans Affairs Merit Review grant awarded to J. Quinn. The authors acknowledge Dr. Stephen Lloyd for his assistance with the Seahorse XF Analyzer experiments and Barbara Smoody for her help with the primary neuron experiments. MC65 cells were generously provided by Randy Woltjer, MD, PhD.
Footnotes
Conflict of Interest
The authors declare they have no competing interests.
References
- 1.Hebert LESP, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol. 2003;60:1119–1122. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- 2.Leuner K1MW, Reichert AS. From mitochondrial dysfunction to amyloid beta formation: novel insights into the pathogenesis of Alzheimer’s disease. Mol Neurobiol. 2012;46:186–193. doi: 10.1007/s12035-012-8307-4. [DOI] [PubMed] [Google Scholar]
- 3.Manczak MPB, Jung Y, Reddy PH. Differential expression of oxidative phosphorylation genes in patients with Alzheimer’s disease: implications for early mitochondrial dysfunction and oxidative damage. Neuromolecular Med. 2004;5:147–162. doi: 10.1385/NMM:5:2:147. [DOI] [PubMed] [Google Scholar]
- 4.Manczak MCM, Reddy PH. Impaired mitochondrial dynamics and abnormal interaction of amyloid beta with mitochondrial protein Drp1 in neurons from patients with Alzheimer’s disease: implications for neuronal damage. Hum Mol Genet. 2011;20:2495–2509. doi: 10.1093/hmg/ddr139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saraiva AABM, Madeira MD, Tavares MA, Paula-Barbosa MM. Mitochondrial abnormalities in cortical dendrites from patients with Alzheimer’s disease. J Submicrosc Cytol. 1985;17:459–464. [PubMed] [Google Scholar]
- 6.Rubio-Moscardo FS-SN, Pera M, Bosch-Morató M, Plata C, Belbin O, Gené G, Dols-Icardo O, Ingelsson M, Helisalmi S, Soininen H, Hiltunen M, Giedraitis V, Lannfelt L, Frank A, Bullido MJ, Combarros O, Sánchez-Juan P, Boada M, Tárraga L, Pastor P, Pérez-Tur J, Baquero M, Molinuevo JL, Sánchez-Valle R, Fuentes-Prior P, Fortea J, Blesa R, Muñoz FJ, Lleó A, Valverde MA, Clarimón J. Rare variants in calcium homeostasis modulator 1 (CALHM1) found in early onset Alzheimer’s disease patients alter calcium homeostasis. PLoS One. 2013;8:e74203. doi: 10.1371/journal.pone.0074203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kosenko EAAG, Tikhonova LA, Li Y, Poghosyan AC, Kaminsky YG. Antioxidant status and energy state of erythrocytes in Alzheimer dementia: probing for markers. CNS Neurol Disord Drug Targets. 2012;11:926–932. doi: 10.2174/1871527311201070926. [DOI] [PubMed] [Google Scholar]
- 8.Du HGL, Yan S, Sosunov AA, McKhann GM, Yan SS. Early deficits in synaptic mitochondria in an Alzheimer’s disease mouse model. Proc Natl Acad Sci. 2010;107:18670–18675. doi: 10.1073/pnas.1006586107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hensley KCJ, Mattson MP, Aksenova M, Harris M, Wu JF, Floyd RA, DAB A model for beta-amyloid aggregation and neurotoxicity based on free radical generation by the peptide: relevance to Alzheimer disease. Proc Natl Acad Sci. 1994;91:3270–3274. doi: 10.1073/pnas.91.8.3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rhein VBG, Rao S, Meier F, Bonert A, Müller-Spahn F, Eckert A. Amyloid-beta leads to impaired cellular respiration, energy production and mitochondrial electron chain complex activities in human neuroblastoma cells. Cell Mol Neurobiol. 2009;29:1063–1071. doi: 10.1007/s10571-009-9398-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McManus MJMM, Franklin JL. The mitochondria-targeted antioxidant MitoQ prevents loss of spatial memory retention and early neuropathology in a transgenic mouse model of Alzheimer’s disease. J Neurosci. 2011;31:15703–15715. doi: 10.1523/JNEUROSCI.0552-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cuadrado-Tejedor MCJ, Zamarbide M, Gómez-Isla T, Franco R, Perez-Mediavilla A. Age-related mitochondrial alterations without neuronal loss in the hippocampus of a transgenic model of Alzheimer’s disease. Curr Alzheimer Res. 2013;10:390–405. doi: 10.2174/1567205011310040005. [DOI] [PubMed] [Google Scholar]
- 13.Eckert AHS, Scherping I, Rhein V, Muller-Spahn F, Gotz J, Muller WE. Soluble beta-amyloid leads to mitochondrial defects in amyloid precursor protein and tau transgenic mice. Neurodegener Dis. 2008;5:157–159. doi: 10.1159/000113689. [DOI] [PubMed] [Google Scholar]
- 14.Shinomol GKM, Bharath MM. Exploring the role of “Brahmi” (Bocopa monnieri and Centella asiatica) in brain function and therapy. Recent Pat Endocr Metab Immune Drug Discov. 2011;5:33–49. doi: 10.2174/187221411794351833. [DOI] [PubMed] [Google Scholar]
- 15.Dev RDOMS, Hambali Z, Samah BA. Comparison on cognitive effects of Centella asiatica in healthy middle aged female and male volunteers. Eur J Sci Res. 2009;31:553–565. [Google Scholar]
- 16.Tiwari SSS, Patwardhan K, Gehlot S, Gambhir IS. Effect of Centella asiatica on mild cognitive impairment (MCI) and other common age-related clinical problems. Digest J Nanomat Biostructures. 2008;3:215–220. [Google Scholar]
- 17.Wattanathorn JML, Muchimapura S, Tongun T, Pasuriwong O, Piyawatkul N, Yimtae K, Sripanidkulchai B, Singkhoraard J. Positive modulation of cognition and mood in the healthy elderly volunteer following the administration of Centella asiatica. Neurotoxicology. 2008;29:948–957. doi: 10.1016/j.jep.2007.11.038. [DOI] [PubMed] [Google Scholar]
- 18.Veerendra Kumar MHGY. Effect of Centella asiatica on cognition and oxidative stress in an intracerebroventricular streptozotocin model of Alzheimer’s disease in rats. Clin Exp Pharmacol Physiol. 2003;30:336–342. doi: 10.1046/j.1440-1681.2003.03842.x. [DOI] [PubMed] [Google Scholar]
- 19.Gupta YKVKM, Srivastava AK. Effect of Centella asiatica on pentylenetetrazole-induced kindling, cognition and oxidative stress in rats. Pharmacology Biochemistry and Behavior. 2003;74:579–585. doi: 10.1016/s0091-3057(02)01044-4. [DOI] [PubMed] [Google Scholar]
- 20.Defillipo PPRA, Fedoce AG, Ferreira AS, Polonini HC, Gattaz WF, Raposo NR. Inhibition of cPLA2 and sPLA2 activities in primary cultures of rat cortical neurons by Centella asiatica water extract. Nat Prod Commun. 2012;7:841–843. [PubMed] [Google Scholar]
- 21.Soumyanath AZY, Henson E, Wadsworth T, Bishop J, Gold BG, Quinn JF. Centella asiatica Extract Improves Behavioral Deficits in a Mouse Model of Alzheimer’s Disease: Investigation of a Possible Mechanism of Action. Int J Alzheimers Dis. 2012:381974. doi: 10.1155/2012/381974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gray NEMJ, Kelley J, Maier CS, Stevens JF, Quinn JF, Soumyanath A. Caffeoylquinic Acids in Centella asiatica Protect against Amyloid-β Toxicity. J Alzheimer’s Dis. 2014;40:359–373. doi: 10.3233/JAD-131913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prakash AKA. Mitoprotective effect of Centella asiatica against aluminum-induced neurotoxicity in rats: possible relevance to its anti-oxidant and anti-apoptosis mechanism. Neurol Sci. 2013;34:1403–1409. doi: 10.1007/s10072-012-1252-1. [DOI] [PubMed] [Google Scholar]
- 24.Shinomol GKM. Prophylactic neuroprotective property of Centella asiatica against 3-nitropropionic acid induced oxidative stress and mitochondrial dysfunctions in brain regions of prepubertal mice. Neurotoxicology. 2008;29:948–957. doi: 10.1016/j.neuro.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 25.Sopher BLFK, Kavanagh TJ, Furlong CE, Martin GM. Neurodegenerative mechanisms in Alzheimer disease. A role for oxidative damage in amyloid beta protein precursor-mediated cell death. Mol Chem Neuropathol. 1996;29:153–168. doi: 10.1007/BF02814999. [DOI] [PubMed] [Google Scholar]
- 26.Wagner H. Plant drug analysis. A thin-layer chromatographic atlas. Springer-Verlag; Berlin: 1996. [Google Scholar]
- 27.Yankner BADL, Kirschner DA. Neurotrophic and neurotoxic effects of amyloid beta protein: Reversal by tachykinin neuropeptides. Science. 1990;250:279–282. doi: 10.1126/science.2218531. [DOI] [PubMed] [Google Scholar]
- 28.Kaech SBG. Culturing hippocampal Neurons. Nature Protocols. 2006;1:2406–2415. doi: 10.1038/nprot.2006.356. [DOI] [PubMed] [Google Scholar]
- 29.Wu M1NA, Swift AL, Moran R, Tamagnine J, Parslow D, Armistead S, Lemire K, Orrell J, Teich J, Chomicz S, Ferrick DA. Multiparameter metabolic analysis reveals a close link between attenuated mitochondrial bioenergetic function and enhanced glycolysis dependency in human tumor cells. Am J Physiol Cell Physiol. 2007;292:C125–136. doi: 10.1152/ajpcell.00247.2006. [DOI] [PubMed] [Google Scholar]
- 30.Schneider LGS, Zelickson BR, Johnson SM, Benavides AG, Ouyang X, Fineberg N, Darley-Usmar VM, Zhang J. Differentiation of SH-SY5Y cells to a neuronal phenotype changes cellular bioenergetics and the response to oxidative stress. Free Radic Biol Med. 2011;51:2007–2017. doi: 10.1016/j.freeradbiomed.2011.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brookes PSYY, Robotham JL, Anders MW, Sheu SS. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol. 2004;287:C817–833. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- 32.Kuhla BLC, Garcia De Arriba S, Schinzel R, Huber J, Münch G. Differential effects of “Advanced glycation endproducts” and beta-amyloid peptide on glucose utilization and ATP levels in the neuronal cell line SH-SY5Y. J Neural Transm. 2004;111:427–439. doi: 10.1007/s00702-003-0038-2. [DOI] [PubMed] [Google Scholar]
- 33.Ye XTW, Bao X, Chen X, Zhang D. FLZ inhibited γ-secretase selectively and decreased Aβ mitochondrial production in APP-SH-SY5Ycells. Naunyn Schmiedebergs Arch Pharmacol. 2014;387:75–85. doi: 10.1007/s00210-013-0918-4. [DOI] [PubMed] [Google Scholar]
- 34.Yao JIR, Zhao L, Nilsen J, Hamilton RT, Brinton RD. Mitochondrial bioenergetic deficit precedes Alzheimer’s pathology in female mouse model of Alzheimer’s disease. Proc Natl Acad Sci. 2009;106:14670–14675. doi: 10.1073/pnas.0903563106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hirai KAG, Nunomura A, Fujioka H, Russell RL, Atwood CS, Johnson AB, Kress Y, Vinters HVTM, Shimohama S, Cash AD, Siedlak SL, Harris PL, Jones PK, Petersen RBPG, Smith MA. Mitochondrial abnormalities in Alzheimer’s disease. J Neurosci. 2001;21:3017–3023. doi: 10.1523/JNEUROSCI.21-09-03017.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Correia SC, SR, Cardoso S, Carvalho C, Candeias E, Duarte AI, Plácido AI, Santos MS, Moreira PI. Alzheimer disease as a vascular disorder: Where do mitochondria fit? Exp Gerontol. 2012;11:878–886. doi: 10.1016/j.exger.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 37.Kurokawa TAS, Nishitani S, Hazeki O. Age-related changes in manganese superoxide dismutase activity in the cerebral cortex of senescence-accelerated prone and resistant mouse. Neurosci Lett. 2001;298:135–138. doi: 10.1016/s0304-3940(00)01755-9. [DOI] [PubMed] [Google Scholar]
- 38.Sato EON, Ozaki N, Hashimoto S, Kurokawa T, Ishibashi S. Early and transient increase in oxidative stress in the cerebral cortex of senescence-accelerated mouse. Mech Ageing Dev. 1996;86:105–114. doi: 10.1016/0047-6374(95)01681-3. [DOI] [PubMed] [Google Scholar]
- 39.McLellan MEKS, Hyman BT, Bacskai BJ. In vivo imaging of reactive oxygen species specifically associated with thioflavine S-positive amyloid plaques by multiphoton microscopy. J Neurosci. 2003;23:2212–2217. doi: 10.1523/JNEUROSCI.23-06-02212.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang KZL, Zhu X, Zhang K, Huang B, Zhang J, Zhang Y, Zhu L, Zhou B, Zhou F. Protective Effect of Paeoniflorin on Aβ25–35-Induced SH-SY5Y Cell Injury by Preventing Mitochondrial Dysfunction. Cell Mol Neurobiol. 2014;34:227–234. doi: 10.1007/s10571-013-0006-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chi TYWL, Ji XF, Shen L, Zou LB. Protective effect of xanthoceraside against β-amyloid-induced neurotoxicity in neuroblastoma SH-SY5Y cells. J Asian Nat Prod Res. 2013;15:1013–1022. doi: 10.1080/10286020.2013.821982. [DOI] [PubMed] [Google Scholar]
- 42.Wu HYHE, Hashimoto T, Kuchibhotla K, Rozkalne A, Fan Z, Spires-Jones T, Xie H, Arbel-Ornath M, Grosskreutz CL, Bacskai BJ, Hyman BT. Amyloid beta induces the morphological neurodegenerative triad of spine loss, dendritic simplification, and neuritic dystrophies through calcineurin activation. J Neurosci. 2012;30:2636–2649. doi: 10.1523/JNEUROSCI.4456-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sainath SBMR, Supriya Ch, Reddy KP, Reddy PS. Protective role of Centella asiatica on lead-induced oxidative stress and suppressed reproductive health in male rats. Environ Toxicol Pharmacol. 2011;32:146–154. doi: 10.1016/j.etap.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 44.Eckert AKU, Scherping I, Hauptmann S, Muller WE. Stabilization of mitochondrial membrane potential and improvement of neuronal energy metabolism by Ginkgo biloba. Mol Neurobiol. 2005;46:136–150. doi: 10.1196/annals.1352.023. [DOI] [PubMed] [Google Scholar]
- 45.Abdel-Kader RMHS, Keil U, Scherping I, Leuner K, Eckert A, Muller WE. Stabilization of mitochondrial function by Ginkgo biloba extract (EGb 761) Pharmacol Res. 2007;56:493–502. doi: 10.1016/j.phrs.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 46.Hur JPS, Koo BS, Jeon S. Borneol alleviates oxidative stress via upregulation of Nrf2 and Bcl-2 in SH-SY5Y cells. Pharm Biol. 2013;51:30–35. doi: 10.3109/13880209.2012.700718. [DOI] [PubMed] [Google Scholar]
- 47.Miyamae YHJ, Sasaki K, Terakawa M, Isoda H, Shigemori H. 3,4,5-tri-O-caffeoylquinic acid inhibits amyloid β-mediated cellular toxicity on SH-SY5Y cells through the upregulation of PGAM1 and G3PDH. Cytotechnology. 2011;63:191–200. doi: 10.1007/s10616-011-9341-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee SGLH, Nam TG, Eom SH, Heo HJ, Lee CY, Kim DO. Neuroprotective effect of caffeoylquinic acids from Artemisia princeps Pampanini against oxidative stress-induced toxicity in PC-12 cells. J Food Sci. 2011;76:C250–256. doi: 10.1111/j.1750-3841.2010.02010.x. [DOI] [PubMed] [Google Scholar]
- 49.Volz NBU, Winkler S, Teller N, Schwarz C, Bakuradze T, Eisenbrand G, Haupt L, Griffiths LR, Stiebitz H, Bytof G, Lantz I, Lang R, Hofmann T, Somoza V, Marko D. Effect of coffee combining green coffee bean constituents with typical roasting products on the Nrf2/ARE pathway in vitro and in vivo. J Agric Food Chem. 2012;60:9631–9641. doi: 10.1021/jf302258u. [DOI] [PubMed] [Google Scholar]
- 50.Manczak MMP, Calkins MJ, Cornea A, Reddy AP, Murphy MP, Szeto HH, Park B, Reddy PH. Mitochondria-targeted antioxidants protect against amyloid-beta toxicity in Alzheimer’s disease neurons. J Alzheimer’s Dis. 2010;20:S609–631. doi: 10.3233/JAD-2010-100564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Emerit JEM, Bricaire F. Neurodegenerative diseases and oxidative stress. Biomed Pharmacother. 2004;58:39–46. doi: 10.1016/j.biopha.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 52.Hara YYF, Puri R, Janssen WG, Rapp PR, Morrison JH. Presynaptic mitochondrial morphology in monkey prefrontal cortex correlates with working memory and is improved with estrogen treatment. Proc Natl Acad Sci. 2014;111:486–491. doi: 10.1073/pnas.1311310110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Olsen RHJL, Zuloaga DG, Limoli CL, Raber J. Enhanced hippocampus-dependent memory and reduced anxiety in mice over-expressing human catalase in mitochondria. J Neurochem. 2013;125:303–313. doi: 10.1111/jnc.12187. [DOI] [PMC free article] [PubMed] [Google Scholar]