Abstract
mTOR (mammalian target of rapamycin) signaling plays a key role in the development of many tumor types. Therefore, mTOR is an attractive target for cancer therapeutics. Although mTOR inhibitors are thought to have radiosensitization activity, the molecular bases remain largely unknown. Here we show that treating MCF7 breast cancer cells with rapamycin (an mTOR inhibitor) results in significant suppression of homologous recombination (HR) and nonhomologous end joining (NHEJ), two major mechanisms required for repairing ionizing radiation-induced DNA DSBs. We observed that rapamycin impaired recruitment of BRCA1 and Rad51 to DNA repair foci, both essential for HR. Moreover, consistent with the suppressive role of rapamycin on both HR and NHEJ, persistent radiation-induced DSBs were detected in cells pretreated with rapamycin. Furthermore, the frequency of chromosome and chromatid breaks was increased in cells treated with rapamycin before and after irradiation. Thus our results show that radiosensitization by mTOR inhibitors occurs via disruption of the major two DNA DSB repair pathways.
INTRODUCTION
mTOR, a serine/threonine kinase, plays a central role in regulating cell growth and survival (1–3). The mTOR pathway is believed to largely drive the malignant behavior of many types of tumors. Therefore, mTOR inhibition is considered an attractive target for cancer therapy. Rapamycin is a well-characterized mTOR inhibitor (4), and several rapamycin derivatives have been developed (e.g., CCI-779 and RAD001) (5). As single agents, mTOR inhibitors have interesting but modest antitumor activity (6–8). However, radiosensitizing effects of mTOR inhibitors have been reported in breast and prostate cancer cell models (9–11) and in glioma and HNSCC xenografts in mice (12, 13). Therefore, a combination strategy has significant clinical potential (14). mTOR inhibitors enhance the cytotoxic effects of radiation in breast cancer cell models via attenuation of radiation-induced prosurvival Akt/mTOR signaling (9). However, additional possible mechanisms of rapamycin radiosensitization activity have not been investigated. Therefore, the molecular basis for radiosensitization activity of mTOR inhibitors remains largely unknown.
Radiation therapy is an efficient and widely used modality for cancer treatment. Ionizing radiation damages DNA by both addition and abstraction reactions resulting in base and sugar-derived products, SSBs and DSBs, and DNA-protein crosslinks (15, 16). Of these lesions, DSBs have the greatest potential for cell killing (17, 18), because the radiosensitivity of tumor cells is greatly influenced by the ability to repair DNA DSBs (19, 20).
Homologous recombination (HR) and nonhomologous end joining (NHEJ) are two highly organized mechanisms capable of repairing radiation-induced DSBs (17). HR is a slower and typically error-free repair process and takes place predominantly in S- and G2/M-phase cells (21, 22). In general, HR is triggered when a DSB is processed to a 3′ single-strand DNA tail via resection (23, 24) by Mre11/Rad50/NBS1 complex in mammalian cells (25, 26). Once single-strand DNA is generated, it is rapidly bound by the single-strand DNA binding protein RPA, which is in turn displaced by Rad51. Mediators such as BRCA2 or Rad52 play a role in loading of Rad51 onto RPA-coated single-strand DNA (27). The resultant Rad51 filament facilitates DNA strand invasion and exchange steps. The previous gapped region of damaged DNA has a template of undamaged duplex that then can be repaired by gap repair synthesis and ligation. HR also plays an important role in cell replication. Cells with impaired HR exhibit cell replication defects due to generation of DSBs during replication that are not properly repaired. The essential role of HR in replication is illustrated by the pronounced proliferative defect and embryonic lethality of mice with knockouts of genes required for HR, including the Rad51 recombinase or the breast cancer susceptibility genes BRCA2 or BRCA1 (28). Indeed, the primary purpose of HR may be its role in DNA replication (29).
In contrast, NHEJ is a relatively fast and error-prone process in which nucleotide alterations are tolerated at the sites of rejoining. NHEJ is used during the G0, G1 and early S phases of the cell cycle (30). In mammalian cells, the first step in NHEJ is recognition of DNA termini by the DNA end-binding protein Ku (23). Ku-dependent recruitment of DNA-PKcs to DNA termini stimulates the kinase activity of this protein and promotes the phosphorylation of a number of substrates in vitro. The final step of NHEJ is the rejoining of DSBs by the DNA ligase IV/XRCC4 protein complex (31–33). Many DSB responsive proteins are required for both HR and NHEJ, including BRCA1 and Mre11 complex. Defects in either HR or NHEJ or both lead to the enhanced sensitivity to ionizing radiation (34).
Here we demonstrate that mTOR inhibition by rapamycin results in the inhibition of repair of ionizing radiation-induced DSBs by both HR and NHEJ in breast cancer cells. The decreased DSB repair by HR is correlated with the abrogated recruitment of BRCA1 and Rad51. The suppressive role of rapamycin on both HR and NHEJ is reflected by the persistent DSBs detected by the comet assay and γ-H2AX protein analysis. Moreover, rapamycin-treated cells have increased frequencies of chromatid and chromosome breaks that are enhanced by ionizing radiation. Our studies suggest that the disruption of HR- and NHEJ-mediated DSB repair is a molecular mechanism of radiosensitization by mTOR inhibitors. Our work provides the preclinical rationale for combining mTOR inhibitors with conventional radiotherapy in cancer patients, particularly breast cancer patients.
MATERIALS AND METHODS
Homologous Recombination Assays
HR was measured in MCF7-pDR-GFP cells according to ref. (35). In brief, to determine the chromosomal HR frequency, MCF-7 cells with chromosomal HR reporter DR-GFP integrated were used. The cells with or without rapamycin treatment were transfected with I-SceI expression vector pCMV3xnls-1-Sce-I or a control vector or construct pGFP, containing the full-length GFP cDNA, as a calibration control for GFP-positive cells (36, 37). Forty-eight hours after I-SceI transfection, cells were trypsinized to form a single-cell suspension in Dulbecco’s modified Eagle medium (Sigma) and subjected to flow cytometry. Two-color (green and propidium red) fluorescence analysis revealed the percentage of green fluorescent cells relative to the total cell number. For each analysis, 20,000 cells were processed, and each experiment was repeated three times.
Assay of NHEJ by Circularization of Linear Plasmid Substrate
The assay of NHEJ by circularization of linear plasmid substrate has been described elsewhere (38). pGL3-MCS was cleaved between the promoter and luciferase reporter gene. Cells were cotransfected with a pRL-SV40 internal control (renilla luciferase, Promega) and either cleaved substrate or a circular positive control using LipofectAMINE. All transfections were done in parallel. Cell extracts were prepared 24 h later according to the instructions of the manufacturer and assayed in a luminometer using the Dual-Luciferase Reporter Assay System (Promega). The relative DSB rejoining activity was obtained by comparison of firefly luciferase activity detected in cells transfected with linearized substrate relative to cells transfected with circular plasmid corrected for transfection efficiency and expression levels.
Immunofluorescence Analysis
The cells were treated with 0.5% Triton X-100 in Cytoskeletal buffer, then fixed with 4% formaldehyde (36, 37). The fixed cells were permeabilized using 0.1% Triton X-100 in PBS for 15 min followed by blocking with 10% fetal bovine serum and then incubation with primary antibodies. The bound antibodies were revealed by goat-anti-mouse IgG Alexa Fluor 594. The slides were viewed at 1000× magnification on an Olympus fluorescence microscope (BX40 with Magna-Fire CCD camera). Irradiation was performed using a Pantak pmc1000 X-ray machine with a 0.1-mm copper + 2.5-mm aluminum filter at a dose rate of 1.1 Gy/min.
Cell Cycle Analysis
MCF7 cells were treated with rapamycin for 10 h or 24 h. Then the cells were collected and fixed with cold 70% ethanol and incubated for 30 min with staining solution containing RNase A (250 μg/ml, Sigma), propidium iodide (25 μg/ml, Sigma) and 0.1% Triton X-100. DNA content was measured by flow cytometry (FACSAria, NIH Grant: RR022402). Raw data were analyzed to get the percentages of cells in G1, S and G2/M phases.
Immunoblotting
Cell extracts were prepared in RIPA buffer (50 mM Tris 8.0, 150 mM NaCl, 5 mM EDTA, 0.5% sodium deoxycholate, 0.1% SDS, 1% NP-40); proteins were resolved by SDS-PAGE and transferred to PVDF membranes. Immunoblots were performed using the appropriate antibodies.
Comet Assay
The neutral comet assay was performed using the Comet Assay kit from Trevigen (Gaithersburg, MD) following the manufacturer’s instructions. Lysis occurred at 4°C for 30 min. Comets were analyzed using CometScore software (TriTek, Sumerduck, VA).
Fluorescence In Situ Hybridization (FISH) for Chromosome Aberration Analysis
FISH was performed using pan-telomeric peptide nucleic acid (PNA) probes as described previously (39). Telomere (C3TA2)3-specific probes directly labeled with Cy3 fluorescent dyes were obtained from Applied Biosystems (Foster City, CA). The cells with or without rapamycin treatment were irradiated (2 Gy) and processed for FISH analysis 24 h later as described previously (39).
Antibodies
Mouse anti-BRCA1 (D-9, Santa Cruz) was used at 1:100 for Western blotting and immunofluorescence staining. Monoclonal antibodies RPA1 (Ab-1) and RPA2 (Ab-2) were purchased from Calbiochem and used at 1:100 for Western blotting and at 1:200 for immunofluorescence staining. Monoclonal antibodies for Rad51 (Ab-2, Calbiochem, and 14B4, Abcam) were used at 1:200 for Western blotting and for immunofluorescence staining, respectively. Mouse anti-γ-H2AX (Ser139, clone JBW301, Millipore) was used at 1:1000 for Western blotting and at 1:500 for immunofluorescence staining. Both rabbit anti-p70S6 kinase antibody and rabbit anti-phospho-p70S6 kinase (Thr389) antibody were purchased from Cell Signaling and were used at 1:1000 dilutions for Western blotting. Goat anti-Ku70 antibody, goat anti-XRCC4 antibody, and rabbit anti-53BP1 were purchased from Santa Cruz Biotechnology and used at 1:1000, 1:400 and 1:500, respectively, in immunoblotting. Mouse anti-DNA-PKcs antibody (Thermo) was used at 1:500 dilutions for Western blotting. Rabbit anti-MRE 11 antibody (Novus Biologicals) was used at 1:5000 dilution for Western blotting. Rabbit anti-NBS1 antibody and Rabbit anti-Rad50 antibody (Novus Biologicals) were used at 1:500 and 1:2000 dilution for Western blotting, respectively. Secondary antibodies used were goat-anti-mouse IgG-HRP conjugated, goat-anti-rabbit IgG-HRP conjugated and donkey-anti-goat IgG-HRP conjugated at 1:5000 dilution. The secondary antibody, goat-anti-mouse IgG Alexa Fluor 594, was used at 1:500 concentration.
RESULTS
Rapamycin Mediated Inhibition of DSB Repair by HR in MCF7 Cells
To determine the role of the mTOR inhibitor rapamycin in DSB repair, we first measured HR-mediated DSB repair in the breast cancer cell line MCF7 since it has been suggested that mTOR inhibition sensitizes MCF7 cells to radiation (9, 11). To measure the frequency of HR, we used MCF7 cells that carry the DR-GFP HR reporter substrate (37). Transient expression of the rare cutting restriction enzyme I-SceI produces a DSB in one of the two GFP mutant genes in pDR-GFP (Fig. 1A) (40). The DSB can be repaired by HR between the two GFP mutant genes, resulting in the restoration of a functional GFP gene. Thus the percentage of GFP-positive cells is a readout of the efficiency of HR-mediated DSB repair (Fig. 1A). The I-SceI expression plasmid was transfected into MCF7 cells, and 4 h later rapamycin (25 ng/ml) was added into the medium for an additional 10 or 24 h, then replaced with fresh medium. Forty-eight hours after rapamycin removal, the percentage of GFP-positive cells was measured by flow cytometry (Fig. 1B). Rapamycin treatment significantly decreased the percentage of GFP-positive cells in comparison with nontreated cells (Fig. 1C). A lower dose of rapamycin (10 ng/ml for 10 h) reduced the inhibitory effect on HR (data not shown). Therefore, there is a dose-related effect on I-SceI-induced HR. To rule out the possibility that this severe defect in HR-mediated repair was caused by alteration of cell cycle distribution, we examined the effect of rapamycin on the cell cycle. We found that the fractions of cells in G1, S and G2 phases were virtually identical in cells with or without rapamycin treatment for 10 h (Fig. 1D), whereas cells treated with rapamycin for 24 h accumulated in G1 phase. We conclude that inhibition of mTOR by rapamycin leads to a defect in HR-mediated DSB repair. We chose 25 ng/ml rapamycin for 10 h for use in our subsequent studies, since this treatment did not alter the cell cycle profile (Fig. 1D).
FIG. 1.
Defective HR-mediated DSB repair in rapamycin-treated MCF7 cells. Panel A: HR substrate based on reconstitution of the gene coding for enhanced green fluorescent protein (EGFP) (from M. Jasin). Panel B: Representative flow cytometry analysis of MCF7-pDR-GFP cells treated with vehicle or rapamycin. The top panel shows I-SceI-induced HR event in cells untreated or treated with rapamycin for 10 h. Cells transfected with pGFP plasmid were used as a positive control (bottom panel). The Y axis represents FL1 green and the X axis represents FL2 orange. Rap: rapamycin. Panel C: HR frequency induced by I-SceI expression in cells without rapamycin pretreatment. HR frequency was calculated as the proportion of GFP-positive cells after I-SceI transfection normalized to the proportion of GFP-positive cells after pGFP transfection. Results are the means from three independent experiments; bars, SE. t test, P < 0.01, statistically significant difference between cells treated with vehicle and cells treated with rapamycin for both 10 and 24 h. Panel D: Cell cycle profiles of cells with or without pretreatment with rapamycin (25 ng/ml). Results are from three independent experiments; bars, SE. t test, P > 0.05, no statistically significant difference of cell fraction in G1, S2 and G2/M phase between cells treated with vehicle and cells treated with 10 h rapamycin. P < 0.05, statistically significant difference of cell fraction in G1 phase between cells treated with vehicle and cells treated with 24 h rapamycin in cells. Rap, rapamycin.
Rapamycin Pretreatment Suppresses the Recruitment of Rad51 and BRCA1 before and after Irradiation
To investigate the molecular mechanism by which rapamycin impairs HR, we first examined the formation of Rad51 subnuclear foci, reflecting the assembly of Rad51 at the single-strand DNA tail of resected DSBs (Fig. 2A, lower panel). We found that treatment with rapamycin significantly reduced the percentage of positive cells with Rad51 foci after irradiation (Fig. 2A, upper panel). These data suggest that rapamycin treatment leads to a defect in the recruitment of Rad51 to sites of DSBs. Next, we investigated whether rapamycin affects recruitment of BRCA1, a protein required for Rad51 recruitment (38). We found that rapamycin treatment reduced the percentage of cells with BRCA1 foci both before irradiation and 6 h after irradiation (Fig. 2B, upper and lower panels), indicating that rapamycin treatment leads to a defect in spontaneous and radiation-induced foci of BRCA1. Representative radiation-induced foci of Rad51 and BRCA1 in cells with rapamycin pretreatment are shown in Fig. 2A and B (bottom panel). Again, the defective BRCA1 and Rad51 recruitment in cells pretreated with rapamycin was not due to the alteration of the cell cycle since an identical cell cycle profile was observed (Fig. 1D).
FIG. 2.
Pretreatment with rapamycin impairs ionizing radiation-induced BRCA1 and Rad51 foci. Representative ionizing radiation-induced foci of Rad51 (panel A), BRCA1 (panel B), or RPA2 (panel C) in cells untreated or treated with rapamycin. Data shown are averages from three independent experiments (t test, P < 0.01 for ionizing radiation-induced foci of Rad51 and BRCA1 foci; P > 0.05 for radiation-induced foci of RPA2 foci). IR, ionizing radiation; IRIF, ionizing radiation-induced foci; Rap, rapamycin.
To test whether the decreased proportion of cells with BRCA1 or Rad51 foci was caused by impaired single-strand DNA resection, we examined the effect of rapamycin on radiation-induced foci of RPA2, one subunit of the single-strand DNA binding protein RPA complex. We found that the proportion of cells with radiation-induced foci of RPA2 was not significantly different in cells with or without rapamycin treatment before and after exposure to ionizing radiation (Fig. 2C, upper panel), indicating that single-strand DNA resection and RPA recruitment are intact in cells treated with rapamycin. Representative ionizing radiation-induced foci of RPA2 are shown in Fig. 2C (bottom panel). These results suggest that rapamycin suppresses the recruitment of BRCA1 and Rad51 in response to DSBs and that the inhibition of BRCA1 and Rad51 recruitment by rapamycin cannot be caused by an alteration in single-strand DNA resection and RPA recruitment.
Reduced Efficiency of NHEJ-Mediated DSB Repair by Rapamycin
To study the role of rapamycin on NHEJ, we used a reporter reactivation assay described by Zhuang et al. (41). The plasmid substrate pGL3-MCS was cleaved by the restriction endonuclease in vitro between the promoter and the luciferase reporter gene, thereby preventing expression of the reporter in vivo (Fig. 3A). The linearized plasmid was introduced into cells together with an internal control plasmid. After 4 h, the cells were treated with or without rapamycin for 10 h. Intracellular recircularization of linearized DNA was detected by measuring luciferase activity after 48 h of incubation in the medium without rapamycin. The rejoining of blunt ends (EcoRV) in rapamycin-treated cells was slightly decreased; however, the difference was not statistically significant (Fig. 3B). However, rapamycin treatment led to a significant decrease in rejoining of incompatible ends (ApaI and Pst) compared to cells without rapamycin treatment (Fig. 3C). Thus rapamycin disrupted the proficient rejoining of linearized extra-chromosomal plasmid substrates. These results suggest that the inhibition of mTOR by rapamycin has a significant suppressive effect on NHEJ.
FIG. 3.

Rapamycin treatment results in decreased NHEJ measured by an episomal plasmid assay. Panel A: The luciferase (LUC)-based reporter plasmid pGL3-MCS offers a set of unique restriction enzyme cleavage sites, including EcoRV, between the promoter and the LUC gene. Panel B: The rejoining of EcoRV-cleaved plasmid ends was measured by the LUC activity detected in cells transfected with linear plasmid substrate relative to circular plasmid control. Bars, SE calculated from four independent experiments (P > 0.05). Panel C: The rejoining of incompatible ends created by digestion with ApaI and Pst (pGL3-MCS) enzymes. The cleaved plasmid ends were measured by the LUC activity detected in cells transfected with linear plasmid substrate relative to circular plasmid control. Bars, SE calculated from four independent experiments (P < 0.05). Rap, rapamycin.
It should be noted that the mechanisms of recircularization of “naked” extrachromosomal linearized DNA may be different from the rejoining of chromosomal DSBs, which are packed in highly organized chromatin. Therefore, it would be interesting to assess the effect of mTOR inhibition on NHEJ in an assay based on the rejoining of DSBs generated in the NHEJ substrate integrated in the chromatin in the future.
Rapamycin Pretreatment has no Effect on the Levels of Proteins Required for HR and NHEJ
The inhibition of mTOR may result in a general translational decrease due to the inhibition of downstream factors including ribosomal protein S6 kinase (p70S6K) (activation) and eukaryotic translation initiation factor 4E binding protein (4EBP-1) (inhibition) (1). Therefore, a hypothetical mechanism that could explain the suppressive role of rapamycin on both HR and NHEJ is that the levels of proteins required for DSB repair are down-regulated by rapamycin treatment. To test this idea, we screened the major proteins required for HR- and NHEJ-mediated DSB repair. We found that the levels of BRCA1, Rad51, RPA1, RPA2, Rad52, MRE11, Rad50 and XRCC4 that are required for HR and/or NHEJ are not significantly different between cells with or without rapamycin treatment before and after 8 Gy irradiation. Although a recent study demonstrated that rapamycin treatment leads to increased 53BP1 protein levels (42) in HeLa cells, we did not observe obvious changes in 53BP1 protein levels with or without rapamycin treatment in MCF7 cells (Fig. 4). One exception was that the levels of DNA-PKcs, increased slightly in rapamycin-treated cells (Fig. 4B). Interestingly, we also observed that NBS1 protein levels appear to increase after irradiation in the cells with rapamycin pretreatment (Fig. 4B). BRCA1 and the Mre11/NBS1/Rad50 complex function in both HR and NHEJ. The fact that phosphorylated p70S6K, a readout of rapamycin function on translation initiation, is effectively blocked by a 10-h rapamycin treatment (Fig. 4C) indicates that the levels of the tested proteins are not altered by the translational inhibition of rapamycin. In addition, c-Myc, one of the well-known rapamycin-responsive proteins (43), is down-regulated in cells with rapamycin treatment, suggesting that rapamycin is functioning under our experimental conditions (Fig. 4C). Therefore, the proteins we tested, which are required for HR and NHEJ, are not direct targets of rapamycin. Overall, impairment of HR and NHEJ by rapamycin cannot be explained by decreased levels of the tested proteins. However, we cannot exclude the possibility that rapamycin down-regulates the levels of proteins that were not tested in our study.
FIG. 4.

The levels of proteins required for HR (panel A) and NHEJ (panel B) in MCF7 cells with or without rapamycin pretreatment before and after irradiation. Panel C: p70S6 kinase and c-Myc were detected with phospho-Thr-389-specific p70S6 kinase antisera or anti c-Myc antibody in cells with or without 10 h rapamycin treatment. β-actin was used as a control. IR, ionizing radiation; Rap, rapamycin.
Rapamycin Treatment Leads to Increased DSBs
The presence of DSBs in chromatin causes a prompt initiation of the phosphorylation of the histone H2A variant H2AX at serine 139 to generate γ-H2AX, which can be detected by immunoblotting or immunofluorescence staining. Therefore, this modification can be used as a marker for mechanistic studies of DSB induction and processing (44–46). The defect in DSB repair in cells treated with rapamycin should lead to persistence of γ-H2AX since HR- and NHEJ-mediated DSB repair were blocked by the drug. To test this idea, we first measured γ-H2AX protein levels in untreated cells and cells treated with rapamycin alone, radiation alone, or both. The cells treated with rapamycin and radiation exhibited increased γ-H2AX protein levels (Fig. 5A), indicating that rapamycin causes retention of unrepaired radiation-induced DSBs. We next analyzed the effect of rapamycin on γ-H2AX foci. We found that the proportions of cells with γ-H2AX foci are similar in rapamycin-treated or untreated cells. In unirradiated cells, the proportions were 32% and 35%, respectively, and 80% and 84%, respectively, after irradiation. However, we observed two distinct types of cells with γ-H2AX foci. Type I cells contain smaller foci of γ-H2AX (Fig. 5B, yellow arrow) and type II cells contain larger, bright foci or both larger and small foci (Fig. 5B, white arrowhead). It appears unlikely that the large, bright γ-H2AX foci represent PML bodies since they are not colocalized with PML protein (see Supplementary Fig. S1). We quantified the frequency of type I and type II cells in cells treated with radiation alone or with rapamycin followed by radiation. The frequency of type II cells after treatment with radiation alone was 25% compared to 65% for cells treated with both rapamycin and radiation (Fig. 5C). We speculated that the larger, bright γ-H2AX foci represent the unrepaired DSBs. Cells with representative γ-H2AX foci are shown in Fig. 5B. To verify that the increased γ-H2AX protein levels or percentage of cells with large, bright γ-H2AX foci after rapamycin treatment (Fig. 5A–C) result from an accumulation of DSBs, we next measured DSBs in cells with or without rapamycin treatment by a comet assay under neutral conditions, in which DNA DSBs but not SSBs can be detected. DSBs were analyzed in irradiated MCF7 cells and MCF7 cells pretreated with rapamycin and exposed to radiation. In untreated cells, we found that 55% and 89% of DSBs were repaired in the first 30 min and 6 h after irradiation, respectively, in contrast to 27% and 76% of DSBs for cells pretreated with rapamycin at the same times (Fig. 5D). These values were significantly different (t test, P < 0.01 for 30 min and P < 0.05 at 6 h postirradiation). It has been suggested that the repair of DSBs after irradiation usually follows bimodal kinetics with fast and slow repair phases (47). In addition, NHEJ and HR play a role in the removal of DSBs with fast kinetics and in the slow phase of repair, respectively (48–50). The fact that rapamycin leads to the accumulation of DSBs in both the early and late stages after irradiation is in agreement with the notion that rapamycin treatment blocks both NHEJ- and HR-mediated DSB repair. Taken together, these results suggest that mTOR inhibition leads to the accumulation of radiation-induced DSBs due to a defect in both HR and NHEJ.
FIG. 5.
Rapamycin pretreatment leads to the accumulation of ionizing radiation-induced DSBs. Panel A: The measurement of H2AX phosphorylation by immunoblotting using an antibody raised against ser139 phosphorylated of H2AX. Panel B: Representative spontaneous or radiation-induced γ-H2AX foci in MCF7 cells treated with 25 ng/ml rapamycin for 10 h, then exposed to radiation, or treated with radiation alone. Panel C: The percentages of Type I and Type II cells with or without rapamycin pretreatment. Error bars indicate SE from three independent experiments (t test, P < 0.05). The Y axis represents the percentage of cells with radiation-induced foci of H2AX. Cells presenting with more than 10 foci per nucleus were considered positive. In each experiment, 200 nuclei were counted per time. Panel D: Relative radiation-induced DSBs in cells with or without rapamycin pretreatment. At least 150 images per point were analyzed at the indicated times, and the Olive tail moment (OTM) was determined using CometScore software. Olive tail moment is a measure of both the amount of DNA and the distribution of DNA in the tail. Data are presented as the ratio of the OTM at the indicated times to the OTM immediately after irradiation. The results are from three independent experiments. Note the significant increase of DSBs at the indicated times after irradiation in cells with rapamycin pretreatment compared to the cells without rapamycin pretreatment (t test, P < 0.01 for 30 min postirradiation and P < 0.05 for 6 h postirradiation). Rap, rapamycin; IR, ionizing radiation; IRIF, ionizing radiation-induced foci.
The effect of mTOR inhibition on DNA DSB repair was further confirmed with a second mTOR inhibitor, everolimus (RAD001). Similar to rapamycin, RAD001 treatment led to a decreased HR and impaired radiation-induced fast repair process (Supplementary Fig. S2), which corresponds to NHEJ (48–50). These results further support our finding that mTOR inhibition suppresses DNA DSB repair pathways.
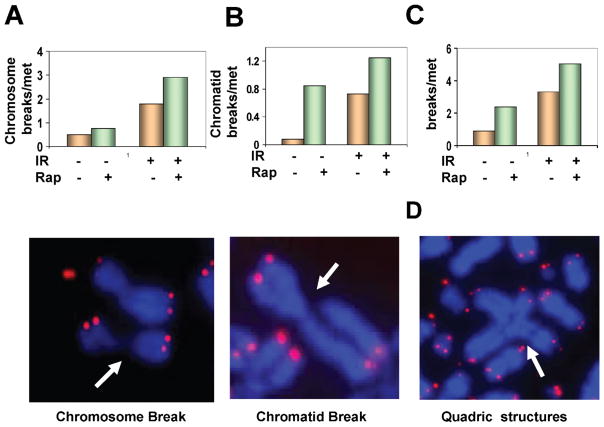
Rapamycin Treatment Results in Increased Chromosome and Chromatid Breaks
Defective HR and NHEJ pathways should cause increased chromatid and chromosome breaks. Chromosome breaks result when a cell is irradiated in G1 phase before the chromosome has been duplicated. In this case, breaks produced by radiation are replicated during DNA replication. This leads to a chromosome aberration that is visible at the next mitosis. If, on the other hand, breaks occur when cells are exposed to radiation after DNA replication, then chromatid aberrations are formed. The suppression of both HR and NHEJ by rapamycin should cause an increase in both types of aberrations. To confirm this, we determined the effect of rapamycin on chromosome and chromatid breaks using fluorescence in situ hybridization (FISH). The cells pretreated with rapamycin displayed higher frequencies of both chromosome breaks (Fig. 6A, upper panel) and chromatid breaks (Fig. 6B, upper panel) before and after irradiation, which is consistent with the increased DSBs detected by γ-H2AX protein levels or comet assay (Fig. 5). Figure 6C shows the overall radiation-induced chromosome abnormalities, including chromosome breaks and chromatid breaks, in cells with or without rapamycin treatment. Representative chromatid and chromosome breaks are shown in Fig. 6A and B (bottom panels). In cells with rapamycin pretreatment, ionizing radiation also induced radial structures, such as quadri-radial aberrations (Fig. 6D), generated by the increased frequency of chromatid breaks and subsequent interchromatid fusions. In contrast, we did not observed any radial structures in cells receiving only radiation treatment. The presence of higher frequencies of radial chromosomes in cells with rapamycin pretreatment further indicates the suppressive role of rapamycin on HR since cells deficient in HR such as those deficient in the FA/BRCA2 pathway often show similar chromosome aberrations (51, 52). mTOR inhibition causes elevated frequencies of chromosome breaks as well as chromatid breaks. The results shown in Fig. 6 agree with the inhibition of both HR- and NHEJ-mediated DSB repair by rapamycin shown in Figs. 1–3.
FIG. 6.
Rapamycin increases the frequency of chromosome and chromatid breaks. Panels A–C: Frequencies of chromosome and chromatid breaks. Thirty to 50 metaphases for each sample were analyzed. The data shown are from one of two independent experiments. Representative metaphase spreads in irradiated MCF7 cells pretreated with rapamycin are shown. FISH using telomeric probe reveals the red/pink color. Chromatid-type and chromosome-type breaks are indicated by arrowheads. Panel D: A typical radial structure induced by radiation from cells pretreated with rapamycin. Star-shaped quadri-radial chromosomes are indicated by arrowheads. IR, ionizing radiation; Rap, rapamycin; met, metaphase.
DISCUSSION
mTOR is a central component of the phosphoinositide 3 kinase/protein kinase B (PI3K)/Akt signaling pathway that mediates cell growth and survival. Recent studies have also linked MAPK/extracellular signal-regulated kinase (ERK) signaling to the activation of the mTOR axis (53, 54). Thus mTOR serves as a convergence point for the PI3K/Akt and MAPK/ERK signaling pathways, which are often hyperactivated in many types of cancers. Although the use of single-agent mTOR inhibitors has not been encouraging in clinical trials (14), mTOR inhibitors have been reported to function as radiation sensitizers in a breast cancer cell model and in other tumor xenografts in mice (9, 10, 12, 13). Therefore, the improvement of our understanding of molecular mechanisms by which mTOR inhibitor sensitize cells to radiation may have a great impact on clinical practice.
Our report provides the first demonstration that the mTOR inhibitor rapamycin disrupts repair of DSBs, a major mechanism determining the efficacy of cell killing by ionizing radiation. We found that rapamycin suppresses both HR and NHEJ. In addition, defects in Rad51 and BRCA1 recruitment to DNA repair foci were observed in cells pretreated with rapamycin, whereas no effect on RPA2 recruitment was observed. In support of the role of rapamycin in HR- and NHEJ-mediated DSB repair, persistent ionizing radiation-induced DSBs in cells treated with rapamycin was observed compared to untreated cells. Our study illustrates that rapamycin functions as a radiation sensitizer via disruption of both major DSB response pathways. The fact that an mTOR inhibitor was also reported to attenuate radiation-induced prosurvival via Akt/mTOR signaling and to enhance the cytotoxic effects of radiation in breast cancer cell models (9) suggests that mTOR sensitizes the cells to radiation via multiple mechanisms.
Consistent with the role of rapamycin in HR suppression (Fig. 1), rapamycin blocked the recruitment of BRCA1 and Rad51 to the DSB sites induced by radiation (Fig. 2). It is possible that the impaired BRCA1 recruitment accounts for the lack of Rad51 foci since BRCA1 is critical for Rad51 recruitment (38). Rapamycin treatment alone also suppresses spontaneous BRCA1 and Rad51 recruitment (Fig. 2) and leads to the persistence of spontaneous DSBs (Fig. 5). HR plays an essential role in cellular proliferation because DSBs generated during DNA replication require HR-mediated repair (55). Thus we suggest that the defect in HR-mediated DNA repair caused by rapamycin inhibitors contributes to the antitumor effect.
We also observed that rapamycin treatment leads to a defect in NHEJ (Fig. 3); this is supported by the observed delay in radiation-induced DSB repair during the first 30 min, since NHEJ is the predominant repair mechanism during this time. However, one of the outstanding questions raised by our finding is how rapamycin blocks both HR and NHEJ. The molecular mechanism by which rapamycin interferes with HR and NHEJ appears to be unrelated to the down-regulation of protein levels since the levels of the major proteins required for HR and NHEJ are either unaffected or slightly increased by rapamycin treatment (Fig. 4). However, we cannot exclude the possibility that other DSB repair proteins that were not tested in our study are down-regulated by mTOR inhibition. We speculate that DSB repair proteins are not direct targets of rapamycin but that their function in DSB repair is blocked by mTOR inhibition. There is ample evidence that modification of chromatin structure plays a central role in the regulation of DSBs (12). Modification of chromatin structure will be important for all pathways used by the cell to repair DSBs, particularly HR (15–17). NHEJ-mediated repair (18) requires a more limited chromatin modification. One potential possibility is that rapamycin may impair chromatin remodeling processes required for the DSB repair process. The DSB repair machinery could not function properly after rapamycin treatment, although the proteins required for HR and NHEJ repair were unchanged or even increased (Fig. 4) (42). A recent report suggested that mTOR inhibition down-regulated several proteins functioning in chromosomal integrity but did not regulate those involved in the DNA damage response (42). Thus the hypothesis that rapamycin impairs HR and NHEJ due to chromatin alterations needs to be tested in the future.
Our data provide evidence that the effectiveness of mTOR inhibition in radiosensitization (9–13) is due to the disruption of the radiation-induced DSB repair pathway. A recent quantitative nuclear proteomics analysis demonstrate that mTOR inhibition activates ATM-induced DNA damage responses (42). The authors proposed that the increased levels of proteins required for DNA damage response upon rapamycin treatment leads to the enhanced resistance to radiation in HeLa cells (42). However, this study did not examine how rapamycin regulates the radiation-induced DNA damage response. The differences in cell types may contribute to the variable effects of mTOR inhibition on radiation-induced cell killing. However, the molecular basis underlying these different effects is not clear. Since radiosensitization by mTOR inhibition is not a universal phenotype, one challenge will be to identify the cancer patients who would benefit from the combination of mTOR inhibition and radiation.
Radiotherapy is an important adjuvant therapy for breast cancer. Therefore, blocking repair of radiation-induced DSBs by rapamycin may be a method for enhancing the cytotoxic effects of radiation in breast cancer patients. In addition, chemotherapy is a powerful modality for breast cancer treatment. Many chemotherapeutic drugs kill tumor cells by directly or indirectly causing DSBs. Our finding that mTOR inhibitors impaired the radiation-induced DSB repair pathway may have implications for a combination of rapamycin with chemotherapeutic drugs in breast cancer treatment. In support of this idea, mTOR inhibitors have been shown to sensitize the cells to cisplatin (56), a common chemotherapeutic drug that causes DNA crosslinkage. DSBs are generated during the repair of cisplatin-induced DNA damage, and HR-mediated repair is a major mechanism to repair cisplatin-induced DNA damage (57, 58). Therefore, our findings are not limited to the understanding of the mechanism of the radiosensitivity of mTOR inhibitor but also explain how mTOR inhibitors sensitize tumor cells to chemotherapy drugs that induce DSBs. In summary, the inhibition of DNA DSB repair by rapamycin is shown to be a molecular mechanism of radiosensitization by mTOR inhibitors. Our study suggests that using mTOR inhibitors in combination with radiation might be a valuable therapeutic approach for treating breast cancer tumors that should be tested in clinical trials.
Supplementary Material
Supplementary Fig. S1. IRIF of γ-H2AX are not colocalized with PML bodies in cells pretreated with rapamycin before and after irradiation. Rapamycin-pretreated MCF7 cells were treated with or without 8 Gy ionizing radiation and 6 h later the cells were fixed. The fixed cells were costained with anti-γ-H2AX (clone JBW301, Millipore) and PML (Abcam, ab53773) antibodies. Supplementary Fig. S2. HR-mediated DSB repair and fast repair of ionizing radiation-induced DSBs are defective in RAD001-treated MCF7 cells. Panel A: The cells treated with RAD001 show a decreased HR frequency induced by I-SceI expression. At 4 h after transfection of the I-SceI expression plasmid, cells were exposed to RAD001 (20 nM) or vehicle, and the treatment was continued for 4 h. HR was analyzed 48 h after removal of RAD001. Results are the means from three independent experiments, bars, SE; t test, P < 0.01, statistically significant difference in I-SceI-induced HR between cells treated with vehicle and cells treated with RAD001. Panel B: Relative ionizing radiation-induced DSBs in cells with or without RAD001 pretreatment. Exponentially growing cells were treated with RAD001 for 4 h and then exposed to 8 Gy. The comet assay was performed under neutral conditions immediately after 30 min after irradiation. At least 150 images per point were analyzed at the indicated times. The results are from three independent experiments. Note the significant increase in DSBs at 30 min after irradiation in cells with RAD001 pretreatment compared to the cells without RAD001 pretreatment (t test, P < 0.05 for 30 min postirradiation). http://dx.doi.org/10.1667/RR2323.1.S1
Acknowledgments
We apologize to those whose work was not cited due to the limited space. We thank M. Jasin for the generous contribution of materials. This work was supported by a seed grant and startup fund from the Department of Radiation Oncology, Washington University School of Medicine, by the Dr. Joseph Roti Roti and Stephanie Pagano Fund for Mesothelioma Research, by a grant from the American Cancer Society (IRG-58-010-51), and by the Wendy Will Case Cancer Fund to J. Zhang.
References
- 1.Bjornsti MA, Houghton PJ. The TOR pathway: a target for cancer therapy. Nat Rev Cancer. 2004;4:335–348. doi: 10.1038/nrc1362. [DOI] [PubMed] [Google Scholar]
- 2.Sawyers CL. Will mTOR inhibitors make it as cancer drugs? Cancer Cell. 2003;4:343–348. doi: 10.1016/s1535-6108(03)00275-7. [DOI] [PubMed] [Google Scholar]
- 3.Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- 4.Sabers CJ, Martin MM, Brunn GJ, Williams JM, Dumont FJ, Wiederrecht G, Abraham RT. Isolation of a protein target of the FKBP12-rapamycin complex in mammalian cells. J Biol Chem. 1995;270:815–822. doi: 10.1074/jbc.270.2.815. [DOI] [PubMed] [Google Scholar]
- 5.Huang S, Houghton PJ. Inhibitors of mammalian target of rapamycin as novel antitumor agents: from bench to clinic. Curr Opin Investig Drugs. 2002;3:295–304. [PubMed] [Google Scholar]
- 6.LoPiccolo J, Blumenthal GM, Bernstein WB, Dennis PA. Targeting the PI3K/Akt/mTOR pathway: effective combinations and clinical considerations. Drug Resist Updat. 2008;11:32–50. doi: 10.1016/j.drup.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiang GG, Abraham RT. Targeting the mTOR signaling network in cancer. Trends Mol Med. 2007;13:433–442. doi: 10.1016/j.molmed.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Abraham RT, Gibbons JJ. The mammalian target of rapamycin signaling pathway: twists and turns in the road to cancer therapy. Clin Cancer Res. 2007;13:3109–3114. doi: 10.1158/1078-0432.CCR-06-2798. [DOI] [PubMed] [Google Scholar]
- 9.Albert JM, Kim KW, Cao C, Lu B. Targeting the Akt/mammalian target of rapamycin pathway for radiosensitization of breast cancer. Mol Cancer Ther. 2006;5:1183–1189. doi: 10.1158/1535-7163.MCT-05-0400. [DOI] [PubMed] [Google Scholar]
- 10.Cao C, Subhawong T, Albert JM, Kim KW, Geng L, Sekhar KR, Gi YJ, Lu B. Inhibition of mammalian target of rapamycin or apoptotic pathway induces autophagy and radiosensitizes PTEN null prostate cancer cells. Cancer Res. 2006;66:10040–10047. doi: 10.1158/0008-5472.CAN-06-0802. [DOI] [PubMed] [Google Scholar]
- 11.Paglin S, Lee NY, Nakar C, Fitzgerald M, Plotkin J, Deuel B, Hackett N, McMahill M, Sphicas E, Yahalom J. Rapamycin-sensitive pathway regulates mitochondrial membrane potential, autophagy, and survival in irradiated MCF-7 cells. Cancer Res. 2005;65:11061–11070. doi: 10.1158/0008-5472.CAN-05-1083. [DOI] [PubMed] [Google Scholar]
- 12.Shinohara ET, Cao C, Niermann K, Mu Y, Zeng F, Hallahan DE, Lu B. Enhanced radiation damage of tumor vasculature by mTOR inhibitors. Oncogene. 2005;24:5414–5422. doi: 10.1038/sj.onc.1208715. [DOI] [PubMed] [Google Scholar]
- 13.Ekshyyan O, Rong Y, Rong X, Pattani KM, Abreo F, Caldito G, Chang JK, Ampil F, Glass J, Nathan CA. Comparison of radiosensitizing effects of the mammalian target of rapamycin inhibitor CCI-779 to cisplatin in experimental models of head and neck squamous cell carcinoma. Mol Cancer Ther. 2009;8:2255–2265. doi: 10.1158/1535-7163.MCT-08-1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang X, Sun SY. Enhancing mTOR-targeted cancer therapy. Expert Opin Ther Targets. 2009;13:1193–1203. doi: 10.1517/14728220903225008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dizdaroglu M, Nackerdien Z, Chao BC, Gajewski E, Rao G. Chemical nature of in vivo DNA base damage in hydrogen peroxide-treated mammalian cells. Arch Biochem Biophys. 1991;285:388–390. doi: 10.1016/0003-9861(91)90378-v. [DOI] [PubMed] [Google Scholar]
- 16.Teoule R, Cadet J. Radiation-induced degradation of the base component in DNA and related substances—final products. Mol Biol Biochem Biophys. 1978;27:171–203. doi: 10.1007/978-3-642-81196-8_9. [DOI] [PubMed] [Google Scholar]
- 17.Jeggo P, Lavin MF. Cellular radiosensitivity: how much better do we understand it? Int J Radiat Biol. 2009;85:1061–1081. doi: 10.3109/09553000903261263. [DOI] [PubMed] [Google Scholar]
- 18.Sakata K, Someya M, Matsumoto Y, Hareyama M. Ability to repair DNA double-strand breaks related to cancer susceptibility and radiosensitivity. Radiat Med. 2007;25:433–438. doi: 10.1007/s11604-007-0161-3. [DOI] [PubMed] [Google Scholar]
- 19.Radford IR. The level of induced DNA double-strand breakage correlates with cell killing after X-irradiation. Int J Radiat Biol. 1985;48:45–54. doi: 10.1080/09553008514551051. [DOI] [PubMed] [Google Scholar]
- 20.Dikomey E, Dahm-Daphi J, Brammer I, Martensen R, Kaina B. Correlation between cellular radiosensitivity and non-repaired double-strand breaks studied in nine mammalian cell lines. Int J Radiat Biol. 1998;73:269–278. doi: 10.1080/095530098142365. [DOI] [PubMed] [Google Scholar]
- 21.Haber JE. Partners and pathways repairing a double-strand break. Trends Genet. 2000;16:259–264. doi: 10.1016/s0168-9525(00)02022-9. [DOI] [PubMed] [Google Scholar]
- 22.Morrison C, Sonoda E, Takao N, Shinohara A, Yamamoto K, Takeda S. The controlling role of ATM in homologous recombinational repair of DNA damage. EMBO J. 2000;19:463–471. doi: 10.1093/emboj/19.3.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jackson SP. Sensing and repairing DNA double-strand breaks. Carcinogenesis. 2002;23:687–696. doi: 10.1093/carcin/23.5.687. [DOI] [PubMed] [Google Scholar]
- 24.Zhang J, Powell SN. The role of the BRCA1 tumor suppressor in DNA double-strand break repair. Mol Cancer Res. 2005;3:531–539. doi: 10.1158/1541-7786.MCR-05-0192. [DOI] [PubMed] [Google Scholar]
- 25.Borde V. The multiple roles of the Mre11 complex for meiotic recombination. Chromosome Res. 2007;15:551–563. doi: 10.1007/s10577-007-1147-9. [DOI] [PubMed] [Google Scholar]
- 26.Assenmacher N, Hopfner KP. MRE11/RAD50/NBS1: complex activities. Chromosoma. 2004;113:157–166. doi: 10.1007/s00412-004-0306-4. [DOI] [PubMed] [Google Scholar]
- 27.Holloman WK, Schirawski J, Holliday R. The homologous recombination system of Ustilago maydis. Fungal Genet Biol. 2008;45(Suppl 1):S31–S39. doi: 10.1016/j.fgb.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Powell SN, Kachnic LA. Roles of BRCA1 and BRCA2 in homologous recombination, DNA replication fidelity and the cellular response to ionizing radiation. Oncogene. 2003;22:5784–5791. doi: 10.1038/sj.onc.1206678. [DOI] [PubMed] [Google Scholar]
- 29.Klein HL, Kreuzer KN. Replication, recombination, and repair: going for the gold. Mol Cell. 2002;9:471–480. doi: 10.1016/s1097-2765(02)00493-8. [DOI] [PubMed] [Google Scholar]
- 30.Lieber MR, Ma Y, Pannicke U, Schwarz K. Mechanism and regulation of human non-homologous DNA end-joining. Nat Rev. 2003;4:712–720. doi: 10.1038/nrm1202. [DOI] [PubMed] [Google Scholar]
- 31.Gao Y, Sun Y, Frank KM, Dikkes P, Fujiwara Y, Seidl KJ, Sekiguchi JM, Rathbun GA, Swat W, Alt FW. A critical role for DNA end-joining proteins in both lymphogenesis and neurogenesis. Cell. 1998;95:891–902. doi: 10.1016/s0092-8674(00)81714-6. [DOI] [PubMed] [Google Scholar]
- 32.Grawunder U, Zimmer D, Fugmann S, Schwarz K, Lieber MR. DNA ligase IV is essential for V(D)J recombination and DNA double-strand break repair in human precursor lymphocytes. Mol Cell. 1998;2:477–484. doi: 10.1016/s1097-2765(00)80147-1. [DOI] [PubMed] [Google Scholar]
- 33.Frank KM, Sekiguchi JM, Seidl KJ, Swat W, Rathbun GA, Cheng HL, Davidson L, Kangaloo L, Alt FW. Late embryonic lethality and impaired V(D)J recombination in mice lacking DNA ligase IV. Nature. 1998;396:173–177. doi: 10.1038/24172. [DOI] [PubMed] [Google Scholar]
- 34.Willers H, Dahm-Daphi J, Powell SN. Repair of radiation damage to DNA. Br J Cancer. 2004;90:1297–1301. doi: 10.1038/sj.bjc.6601729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saintigny Y, Delacote F, Vares G, Petitot F, Lambert S, Averbeck D, Lopez BS. Characterization of homologous recombination induced by replication inhibition in mammalian cells. EMBO J. 2001;20:3861–3870. doi: 10.1093/emboj/20.14.3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shi W, Ma Z, Willers H, Akhtar K, Scott SP, Zhang J, Powell S, Zhang J. Disassembly of MDC1 foci is controlled by ubiquitin-proteasome-dependent degradation. J Biol Chem. 2008;283:31608–31616. doi: 10.1074/jbc.M801082200. [DOI] [PubMed] [Google Scholar]
- 37.Zhang J, Ma Z, Treszezamsky A, Powell SN. MDC1 interacts with Rad51 and facilitates homologous recombination. Nat Struct Mol Biol. 2005;12:902–909. doi: 10.1038/nsmb991. [DOI] [PubMed] [Google Scholar]
- 38.Zhang J, Willers H, Feng Z, Ghosh JC, Kim S, Weaver DT, Chung JH, Powell SN, Xia F. Chk2 phosphorylation of BRCA1 regulates DNA double-strand break repair. Mol Cell Biol. 2004;24:708–718. doi: 10.1128/MCB.24.2.708-718.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shi W, Feng Z, Zhang J, Gonzalez-Suarez I, Vanderwaal RP, Wu X, Powell SN, Roti Roti JL, Gonzalo S, Zhang J. The Role of RPA2 phosphorylation in homologous recombination in response to replication arrest. Carcinogenesis. 2010;31:994–1002. doi: 10.1093/carcin/bgq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pierce AJ, Johnson RD, Thompson LH, Jasin M. XRCC3 promotes homology-directed repair of DNA damage in mammalian cells. Genes Dev. 1999;13:2633–2638. doi: 10.1101/gad.13.20.2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhuang J, Zhang J, Willers H, Wang H, Chung JH, van Gent DC, Hallahan DE, Powell SN, Xia F. Checkpoint kinase 2-mediated phosphorylation of BRCA1 regulates the fidelity of nonhomologous end-joining. Cancer Res. 2006;66:1401–1408. doi: 10.1158/0008-5472.CAN-05-3278. [DOI] [PubMed] [Google Scholar]
- 42.Bandhakavi S, Kim YM, Ro SH, Xie H, Onsongo G, Jun CB, Kim DH, Griffin TJ. Quantitative nuclear proteomics identifies mTOR regulation of DNA damage response. Mol Cell Proteomics. 2010;9:403–414. doi: 10.1074/mcp.M900326-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wall M, Poortinga G, Hannan KM, Pearson RB, Hannan RD, McArthur GA. Translational control of c-MYC by rapamycin promotes terminal myeloid differentiation. Blood. 2008;112:2305–2317. doi: 10.1182/blood-2007-09-111856. [DOI] [PubMed] [Google Scholar]
- 44.Kinner A, Wu W, Staudt C, Iliakis G. Gamma-H2AX in recognition and signaling of DNA double-strand breaks in the context of chromatin. Nucleic Acids Res. 2008;36:5678–5694. doi: 10.1093/nar/gkn550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rogakou EP, Boon C, Redon C, Bonner WM. Megabase chromatin domains involved in DNA double-strand breaks in vivo. J Cell Biol. 1999;146:905–916. doi: 10.1083/jcb.146.5.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem. 1998;273:5858–5868. doi: 10.1074/jbc.273.10.5858. [DOI] [PubMed] [Google Scholar]
- 47.Iliakis G, Wang H, Perrault AR, Boecker W, Rosidi B, Windhofer F, Wu W, Guan J, Terzoudi G, Pantelias G. Mechanisms of DNA double strand break repair and chromosome aberration formation. Cytogenet Genome Res. 2004;104:14–20. doi: 10.1159/000077461. [DOI] [PubMed] [Google Scholar]
- 48.Nevaldine B, Longo JA, Hahn PJ. The scid defect results in much slower repair of DNA double-strand breaks but not high levels of residual breaks. Radiat Res. 1997;147:535–540. [PubMed] [Google Scholar]
- 49.Wang H, Zeng ZC, Bui TA, Sonoda E, Takata M, Takeda S, Iliakis G. Efficient rejoining of radiation-induced DNA double-strand breaks in vertebrate cells deficient in genes of the RAD52 epistasis group. Oncogene. 2001;20:2212–2224. doi: 10.1038/sj.onc.1204350. [DOI] [PubMed] [Google Scholar]
- 50.Wang H, Zeng ZC, Perrault AR, Cheng X, Qin W, Iliakis G. Genetic evidence for the involvement of DNA ligase IV in the DNA-PK-dependent pathway of non-homologous end joining in mammalian cells. Nucleic Acids Res. 2001;29:1653–1660. doi: 10.1093/nar/29.8.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Venkitaraman AR. Tracing the network connecting BRCA and Fanconi anaemia proteins. Nat Rev Cancer. 2004;4:266–276. doi: 10.1038/nrc1321. [DOI] [PubMed] [Google Scholar]
- 52.Niedernhofer LJ, Lalai AS, Hoeijmakers JH. Fanconi anemia (cross)linked to DNA repair. Cell. 2005;123:1191–1198. doi: 10.1016/j.cell.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 53.Ma L, Teruya-Feldstein J, Bonner P, Bernardi R, Franz DN, Witte D, Cordon-Cardo C, Pandolfi PP. Identification of S664 TSC2 phosphorylation as a marker for extracellular signal-regulated kinase mediated mTOR activation in tuberous sclerosis and human cancer. Cancer Res. 2007;67:7106–7112. doi: 10.1158/0008-5472.CAN-06-4798. [DOI] [PubMed] [Google Scholar]
- 54.Ma L, Chen Z, Erdjument-Bromage H, Tempst P, Pandolfi PP. Phosphorylation and functional inactivation of TSC2 by Erk: implications for tuberous sclerosis and cancer pathogenesis. Cell. 2005;121:179–193. doi: 10.1016/j.cell.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 55.Lovett ST. Connecting replication and recombination. Mol Cell. 2003;11:554–556. doi: 10.1016/s1097-2765(03)00110-2. [DOI] [PubMed] [Google Scholar]
- 56.Beuvink I, Boulay A, Fumagalli S, Zilbermann F, Ruetz S, O’Reilly T, Natt F, Hall J, Lane HA, Thomas G. The mTOR inhibitor RAD001 sensitizes tumor cells to DNA-damaged induced apoptosis through inhibition of p21 translation. Cell. 2005;120:747–759. doi: 10.1016/j.cell.2004.12.040. [DOI] [PubMed] [Google Scholar]
- 57.Nakano T, Katafuchi A, Matsubara M, Terato H, Tsuboi T, Masuda T, Tatsumoto T, Pack SP, Makino K, Ide H. Homologous recombination but not nucleotide excision repair plays a pivotal role in tolerance of DNA-protein cross-links in mammalian cells. J Biol Chem. 2009;284:27065–27076. doi: 10.1074/jbc.M109.019174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nojima K, Hochegger H, Saberi A, Fukushima T, Kikuchi K, Yoshimura M, Orelli BJ, Bishop DK, Hirano S, Sonoda E. Multiple repair pathways mediate tolerance to chemotherapeutic cross-linking agents in vertebrate cells. Cancer Res. 2005;65:11704–11711. doi: 10.1158/0008-5472.CAN-05-1214. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. S1. IRIF of γ-H2AX are not colocalized with PML bodies in cells pretreated with rapamycin before and after irradiation. Rapamycin-pretreated MCF7 cells were treated with or without 8 Gy ionizing radiation and 6 h later the cells were fixed. The fixed cells were costained with anti-γ-H2AX (clone JBW301, Millipore) and PML (Abcam, ab53773) antibodies. Supplementary Fig. S2. HR-mediated DSB repair and fast repair of ionizing radiation-induced DSBs are defective in RAD001-treated MCF7 cells. Panel A: The cells treated with RAD001 show a decreased HR frequency induced by I-SceI expression. At 4 h after transfection of the I-SceI expression plasmid, cells were exposed to RAD001 (20 nM) or vehicle, and the treatment was continued for 4 h. HR was analyzed 48 h after removal of RAD001. Results are the means from three independent experiments, bars, SE; t test, P < 0.01, statistically significant difference in I-SceI-induced HR between cells treated with vehicle and cells treated with RAD001. Panel B: Relative ionizing radiation-induced DSBs in cells with or without RAD001 pretreatment. Exponentially growing cells were treated with RAD001 for 4 h and then exposed to 8 Gy. The comet assay was performed under neutral conditions immediately after 30 min after irradiation. At least 150 images per point were analyzed at the indicated times. The results are from three independent experiments. Note the significant increase in DSBs at 30 min after irradiation in cells with RAD001 pretreatment compared to the cells without RAD001 pretreatment (t test, P < 0.05 for 30 min postirradiation). http://dx.doi.org/10.1667/RR2323.1.S1