Abstract
Pentameric ligand-gated ion channels (pLGICs) conduct upon the binding of an agonist and are fundamental to neurotransmission. New insights into the complex mechanisms underlying pLGIC gating, ion selectivity, and modulation have recently been gained via a series of crystal structures in prokaryotes and C .elegans, as well as computational studies relying on these structures. Here we review contributions from a variety of computational approaches, including normal mode analysis, automated docking, and fully atomistic molecular dynamics simulation. Examples from our own research, particularly concerning interactions with general anesthetics and lipids, are used to illustrate predictive results complementary to crystallographic studies.
Keywords: molecular dynamics simulation, neurotransmitter receptor, Cys-loop receptor
1 Introduction
Pentameric ligand-gated ion channels (pLGIC) are a family of integral membrane proteins that increase their permeability to certain ions upon agonist binding. pLGICs can be found in many organisms from bacteria to complex vertebrates; in humans, pLGICs serve as neurotransmitter receptors and are abundant in many locations including central and peripheral nervous systems and neuromuscular junctions. They can be either excitatory (cationic) such as the nicotinic acetylcholine receptor (nAChR) and serotonin receptor (5HT3-R) or inhibitory (anionic) as in γ-aminobutyric acid receptor type A receptor (GABAA-R), glutamate-gated chloride receptor (GluCl) and glycine receptor (Gly-R). Dysfunctional pLGICs have been implicated in serious diseases and conditions such as Alzheimer's disease, [1] Parkinson's disease, [2] epilepsy, [3] schizophrenia, [4–6] myasthenia gravis, [7] smoking addiction [8] and alcohol dependence [9, 10]. They are also likely targets of numerous drugs, including nicotine, alcohol, anesthetics and benzodiazepines. [11–15]
Because of their significance and complexity, there have been extensive experimental, theoretical and computational efforts in the community over the past 30 years to unravel the structural and functional mysteries of pLGICs. These efforts have tremendously improved our understanding of these channels and have been summarized in several excellent reviews. [16–18] In particular, the availability of atomic structures for a number of pLGICs has provided an unprecedented opportunity for many fields, including computational biophysics, to identify molecular mechanisms involved in the pLGIC function and modulation. Here, we briefly review the most recent computational studies regarding various molecular aspects of pLGICs.
2 Structure and Activation
During the past decade several structures of pLGICs with atomic resolution have been reported. These include a medium-resolution cryo-EM structure of Torpedo nAChR in the absence of agonist, [20] a crystal structure of a pLGIC from Erwinia chrysanthemi (ELIC) in the closed state, [21] a presumably open-state crystal structure of a pLGIC from Gloeobacter violaceus (GLIC) [22, 23] and most recently the crystal structure of a glutamate-gated chloride channel from Caenorhabditis elegans (GluCl) in the open-state bound to the potent modulator ivermectin. [19] Although these channels belong to evolutionary distant organisms and share low sequence identity, [24] their three dimensional architecture reveals striking conservation of the overall structural features of pLGICs from bacteria to complex eukaryotes (Figure 1).
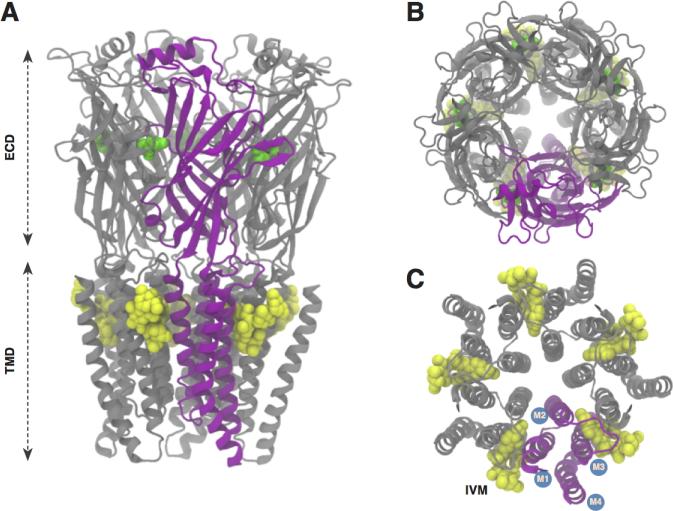
Figure 1.
The general structure of pentameric ligand-gated ion channels. A) View along the axis perpendicular to the membrane, based on GluCl,[19] showing the ECD and TMD, with a single subunit shown in purple. The agonists ivermectin and glutamate are shown in yellow and green respectively, as reported in the GluCl structure. B) View from the extracellular domain, looking toward the membrane. C) View of the transmembrane domain, looking toward the membrane from the extracellular domain, with the M1-M4 helices labeled.
As their name suggests, pentameric LGICs consist of five subunits that are arranged in (pseudo) C5 symmetry around a central pore (Figure 1). Many eukaryotic pLGICs are heteromeric with multiple homologous subunit species labeled α, β, etc, and further divided into subtypes : α1, α2, etc. All subunits contribute to the two main parts of the channel: the extra-cellular domain (ECD) and the transmembrane domain (TMD). The ECD has a beta-sandwich immunoglobulin-like structure while the TMD is composed of four helices (M1 to M4) that span the membrane (Figure 1). Eukaryotic receptors are gated by an agonist that typically binds to the ECD at the interface between adjacent subunits.
Upon agonist binding, a cascade of conformational changes begin from the ECD and propagate about 50Å away in the TMD, where they lead to the opening of the central pore formed by the M2 helices. Channel gating therefore likely involves events on multiple timescales. Various computational techniques, such as molecular dynamics (MD),[25–27] targeted MD (TMD),[28] steered-MD (SMD),[29] elastic-network models,[30] normal mode analysis (NMA)[31–34] or a multiscale combination of them [35] have been employed to elucidate different stages of the transition, mainly from open to closed state. Recent studies have indicated that these conformational changes include rearrangement in the extracellular domain,[27, 36] ECD-TMD interface [27, 30], tilting and rotation of M2 helices [27, 30, 37] and tertiary and quaternary changes. [26, 27, 31, 33] Experimental cysteine cross-linking has identified locally-closed allosteric intermediates for GLIC [38] and α1GlyR [39]; the structure for locally-closed GLIC has been reported and shown to be stable over the course of 100-ns MD simulation.[38]
Analysis of correlated motions of pLGICs using NMA [31, 33, 40] have indicated several low-frequency motions, including a quaternary twist where the ECD and TMD rotate in opposite directions around an axis normal to the membrane. This iris-like motion, first described using models of nAChR, has been proposed to dominate channel opening. [31–34] Comparison of the structures of ELIC (in a closed state) and GLIC (in an open state) later confirmed the presence of such a twist, along with a tertiary motion within the extracellular domain. [22] MD simulations of GLIC and GluCl have also shown the emergence of such a twist during the course of channel closure. [26, 27] Several computational studies have used this motion to generate the open-state of pLGICs. [37, 41]
In a microsecond MD study Nury et al [26] followed the sequence of events during GLIC closure at neutral pH, at which the acid-sensitive channel was expected to be closed. They found that upon adjusting the ionization state of amino acids for pH=7, the channel rapidly closes mainly due the protrusion of the upper part of the M2 helix. [26] However, it has since been shown[42][43] that GLIC closes rapidly (within 50-100 ns) under the CHARMM22 forcefield[44, 45] in recent versions of NAMD even at pH 4.6, for which the channel is expected to remain open; such closure is not observed using the AMBER[46] forcefield with GROMACS.[47] Flat-bottom conformational restraints can be used to maintain an open GLIC conformation in CHARMM. [42]
Yoluk et al [48] observed a rapid closure of GluCl in a microsecond-long study for GluCl when the agonist ivermectin (bound to the TMD) was removed. They observed that upon removal of ivermectin the intersubunit cavities shrink relative to control simulations, which was accompanied by a subtle reduction in pore radius[48]. We have recently[49] proposed that in cholesterol-rich membranes, occupation by cholesterol may offset such shrinkage in some pLGICs. In another study, Calimet et al [27] used the reaction coordinates extracted from GLIC and ELIC structures to monitor the events during the GluCl closure upon removal of ivermectin. They proposed that the sequence of quaternary twist, agonist unbinding and reorientation of the M2-M3 loop causes the closure of the pore.
3 Ion Selectivity
Computational methods have also been used to study the location of the gate and the origin of the ion selectivity in pLGICs. The employed methods range from fast implicit models to full-atomistic simulations and include Poisson-Nernst-Planck (PNP) theory,[50] Brownian dynamics simulations (BD),[37, 50, 51] all-atom MD simulations using various enhanced-sampling techniques, such as umbrella sampling[52–54] or adaptive biasing force,[55, 56] and all-atom unrestrained MD simulations with persistent electrochemical gradient [57] which resembles single-channel electrophysiology.
Computational studies [52, 55] of nAChR based on a medium resolution cryo-EM structure, imaged in the absence of agonist, [20] have supported the presence of a hydrophobic gate around Leu9’ on the M2 helix. The hydrophobic gate is proposed to act by imposing significant dehydration penalty for ions going through the pore so that even when the pore is not physically occluded, the channel is functionally closed. Using MD simulations it has been shown that model hydrophobic pores become functionally closed (i.e. dried) when the radius is below a critical limit,[58] and also possess intrinsic ion selectivity features.[59] Hydrophobic gates in the TMD were also found to be the dominant factor in specifying the ion selectivity in the case of a homology model of the anionic channel GlyRα1, where sodium ions crossed an approximately 6 kcal/mol energy barrier near residue Leu9’ while there was no significant barrier for chloride ions.[55] An experimental chimera of the extracellular domain of GLIC and the transmembrane domain of α1GlyR is a proton-gated anion-selective channel, thus supporting the hypothesis that major components of ion selectivity for GlyRα1 are located in the TMD,[60] while the extracellular domain plays a more dominant role in agonist sensing (in this case protons)
Single-ion potential of mean force (PMF) calculations for GLIC, which was crystallized in lowpH and therefore likely to be in the open state and permeable to cations, showed that in addition to the hydrophobic gate, a ring of five glutamic acid residues near the intracellular opening of the channel form another energy barrier for chloride ion diffusion.[53, 56] Ion conductivity is sensitive to the protonation state of Glu222, and it is likely that some of the five Glu222 residues are in the protonated state, as otherwise the free energy minimum formed by the channel would be too deep for permeation of sodium ions. [56] Similar studies on GluCl, an ion-selective channel crystallized in the open state, showed two sources for ion selectivity: three rings of lysine residues in the ECD, as well as a selectivity filter located at the Pro-2’ residue with a free energy barrier that is about 4 kBT higher for a sodium ion relative to a chloride ion.[37]
Recently, high resolution structures of an open form of GLIC at 2.4Å[61] were solved which revealed two water pentagons (at Ser6’ and Thr2’) in the GLIC channel, with a sodium ion lodged between them. Atomistic MD and steered MD simulations totaling over 2μs were used to further characterize the microscopic interactions underlying conduction, including the evolution of hydration profiles and the critical role of the Ser6’ hydroxyl. [61]
4 Lipid-protein Interactions
Eukaryotic pLGICs can be highly sensitive to the contents of the lipid membrane. Reconstituted nAChRs require cholesterol for native levels of function, with multiple studies indicating specific interactions (for reviews see [62–65]). GABA(A)r function also displays complex effects of cholesterol enrichment or depletion [66] and successful reconstitution procedures for GABA(A) receptors have also included cholesterol.[67, 68]. A recent study[69] reports that the barrier separating conducting and non-conducting states of the nAChR is sensitive to hydrophobic thickness of the surrounding membrane, and suggested a potential mechanism in which upright orientation of the M4 helices is essential to achieving a coupled state[70] capable of conducting ions.
Relatively few computational studies have investigated interactions between pLGICs and cholesterol, however. Baier et al identified potential annular cholesterol sites using recognized cholesterol motifs and automated docking.[71] Cheng et al [72] investigated interactions of α4β2 nAChRs with membrane cholesterol and anionic lipids in simulations lasting over 10 ns, observing penetration of intersubunit cavities by phosphatidic acid acyl chains. Slow mixing equilibration in the membrane, however, precludes atomistic studies of pLGICs with non-random lipid mixtures, which may represent a more realistic configuration for the membrane.
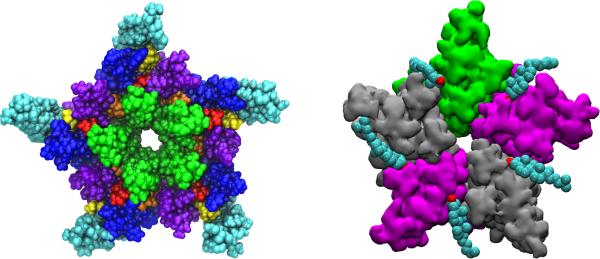
The possibility of more deeply buried sites has been explored as well. The transmembrane domain of the nAChR structure (PDB: 2BG9) shows numerous gaps between helices in the cryo-EM image that have similar density to the membrane.[20, 73] Using docking calculations and molecular dynamics (MD) simulations, we showed that these gaps could represent cholesterol present (but not-resolved) in the cryo-EM structures. [74] Three sites were proposed, labeled A (superficial), B(intersubunit), and C(intrasubunit) (Figure 2). The presence of the cholesterol in the gaps is necessary to maintain the experimentally-determined overall structure during MD simulations, with occupation of all 15 sites yielding the structures most consistent with the cryo-EM structures after 25 ns of simulation. [74]
Figure 2.
Potential embedded sites for cholesterol on nAChR (left) and GABA(A)r (right). Left: nAChR is colored by helix (purple-M1, green-M2, blue-M3, cyan - M4) with cholesterol in proposed sites A (yellow), B (orange) and C(red). Right: Cholesterol (colored by name) orientations after 200 ns of simulation of a GABA(A)r-cholesterol complex, with GABA(A)r colored by subunit (γ- green, α-purple, β-silver).
Using a model of the GABA(A) receptor based on GluCl (with ivermectin removed), we found[49] that automated docking identified the intersubunit (ivermectin) sites as potential cholesterol sites, with orientations consistent with distinct regions of ivermectin. MD simulations lasting 200 ns were run using multiple groups of initial coordinates and multiple models for the GABA(A) receptor. Over the course of the simulations cholesterol was observed to bind in one of two main orientations, with the rough side facing either the M1 or M3 helices; exchange was observed in both directions, as was unbinding and spontaneous rebinding of cholesterol to the sites (Figure 2).
The universality of specific interactions with various lipids across pLGICs is unknown; both of these studies followed similar approaches and yielded distinct predictions for cholesterol sites on the nAChR and GABA(A) receptors, with predicted sites on the GABA(A) receptor less deeply buried and more exposed to the lipid membrane. The two studies used structural templates with numerous differences, including experimental methodology, resolution, lipid environment, presence/absence of agonists, and expected conformational state, in addition to the differences in sequence between nAChR and GABA(A) receptors. However, we note that the location of the backbone helices in the two templates (2BG9 and 3EAW) overlap closely, so that the reduced protein density in the nAChR 2BG9 structure reflects the presence of smaller residues rather than a larger overall footprint of the transmembrane domain. This observation, based entirely on backbone coordinates consistent between the two models as well as known sequence differences, suggests that significant differences in protein density between nAChR and GABA(A)r may yield qualitatively different binding modes for cholesterol.
5 Pharmacology
Numerous drugs have been shown to modulate the function of pLGICs and activate, potentiate, or inhibit the channels. Crystallography has revealed various sites on GLIC, ELIC or GluCl for the competitive acetylcholine antagonist pancurionium [75], the benzodiazapene flurazepam[76], the general anesthetics (GA) propofol, desflurane[77], and bromoform[78], the local anesthetic lidocaine[79], the channel blocker picrotoxin[19], and the anti-parasitic ivermectin[19]. However, the universality of binding sites across pLGICs is generally unknown; for instance, desflurane and propofol are shown to bind to intrasubunit sites in GLIC[77] but evidence from mutagenesis and photoaffinity labeling suggest that general anesthetics may act on GABAArs and nAChRs via intersubunit sites as well. [13–15, 80] Furthermore, a single mutation can switch binding of general anesthetics from intrasubunit to intersubunit sites in GLIC. [81] Consequently, for many modulator/pLGIC combinations the relative a nities of the modulators for various proposed sites are unknown.
Computational studies have primarily focused on interactions of pLGICs with GAs. Modulation of eukaryotic[13–15] and prokaryotic[82] pLGICs by GAs is well established. In general, at clinical concentrations, GAs inhibit excitatory channels (such as nAChR) but potentiate inhibitory channels (such as GABAAR); both effects are consistent with the desired clinical effect. Experimental approaches such as photoa nity labeling and site-directed mutagenesis have yielded three likely classes of sites in the TMD: intra-subunit, inter-subunit, and pore. [80] Although no crystal structures of any vertebrate pLGICs have been reported, several groups have built homology models of the transmembrane domain based on available structures. [41, 49, 83, 84]
5.1 Unbiased searches for binding sites
Computational searches for potential binding sites have largely used automated docking, unrestrained MD simulations including “flooding” simulations, or some combination of the two. Automated docking has been used to provide initial coordinates for further unrestrained simulations[43, 85, 86], which has primarily indicated sites on the surface of the protein or at the interface between the ECD and TMD. The alternative flooding approach[87, 88] begins the simulation with a (usually high) concentration of the anesthetic dispersed randomly in the water and allows it to partition into the membrane and protein sites. Flooding MD simulations are a more expensive alternative to docking with the advantage of being fully atomistic, including water molecules and lipids, and fully flexible. However, they require long simulation times to ensure equilibrium partitioning and su cient phase-space exploration.
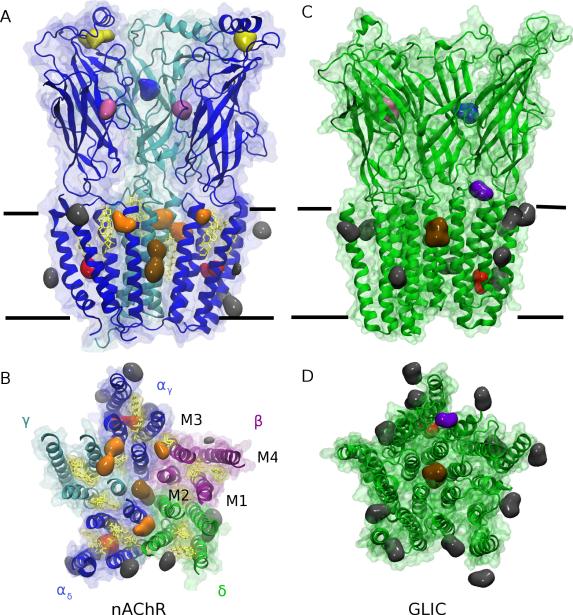
Previous flooding simulations with general anesthetics have used small inhalational anesthetics such as halothane[87] or isoflurane;[88] the approach is less successful with highly insoluble anesthetics such as propofol. We found[88] that 100 isoflurane molecules required 400 ns to fully partition into membrane-pLGIC systems containing torpedo nAChR or GLIC; intrasubunit cavities were found in both receptors, while intersubunit sites were only observed in nAChR (Figure 3). Two isoflurane molecules bound to the pore of both channels, which would have a clear ion-blocking effect. [88] Flooding approaches have also been used for predicting sites for ethanol on GlyR, in which ethanol was observed to bind to both intersubunit and intrasubunit sites, with intersubunit sites having the highest occupancy. [89]
Figure 3.
Regions of persistent occupation by isoflurane. (A and B) Isoflurane binding sites in the nAChR. Protein is colored by subunit: α- blue; β- purple; δ-green; γ- cyan. Embedded cholesterol is yellow. Colored blobs represent an isoflurane density isosurface averaged over the last 100 ns of a 400 ns simulation; large blobs represent occupation over at least most of that period, whereas a few much smaller blobs represent occupation for less than half of that period. (A) Side view of the α and γ subunits, as well as isoflurane sites contacting those subunits. Isoflurane binding sites in the TMD are colored as follows: superficial/annular sites (gray), intrasubunit sites (red), intersubunit sites (orange), and pore site (brown). Isoflurane binding sites in the ECD are pink, blue, and yellow, corresponding with binding to the agonist site, beta sandwiches, and α1 helices, respectively. (B) View of the nAchR TMD, looking down on the membrane from the extracellular region. (C and D) Isoflurane binding sites in GLIC. Protein is green and blobs are colored as in AC, with the addition of isoflurane in the loop site (purple). (C) Side view of three chains of GLIC. (D) TMD of GLIC, with isoflurane in loop site, intrasubunit site, pore site, and annular sites. Figure first printed in [88].
The number of binding sites found using flooding approaches will be highly sensitive to the concentration of the drug present in the surrounding solvent. In order to locate sites within a feasible simulation time, relatively high concentrations of the drug are typically used. Comparison of such concentrations to clinical concentrations used for inducing anesthesia is not straightforward, however, as the equilibrium concentration present in the aqueous phase of the simulation may be significantly lower than that of the lipid phase; for instance, in the flooding simulations of Ref. [88], no isoflurane remained in the water by the simulation's conclusion. A more direct approach to predicting concentration dependence of site occupancy relies on the computational measurement of binding affinity, which is more efficient than running multiple flooding simulations with varying concentrations.
5.2 Binding affinity prediction
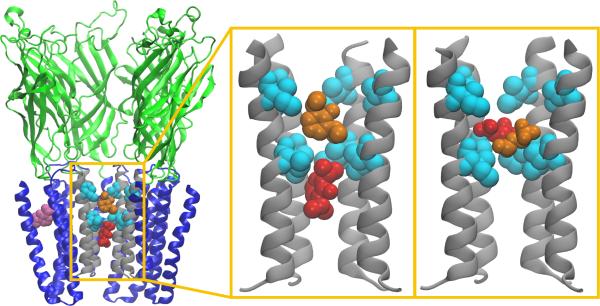
Computational methods for estimating binding a nities range from the inexpensive scoring functions provided by automated docking programs to the sophisticated, thermodynamically exact alchemical free energy perturbation methods (AFEP), but all have the advantage that they can be used to estimate a nities for isolated sites, rather than averages over the entire pLGIC. Although automated docking methods cannot typically be used to measure an absolute binding affinity, Bertaccini et al[90] demonstrated that automated docking scores of propofol analogs display log linear correlation with EC50s for GABAAr potentiation, indicating that such scores can be used to estimate relative a nities of similar compounds. Liu et al [85, 86] used AFEP to estimate absolute binding a nities of halothane for both open and closed conformations of an α4β2 model of a neuronal nAChR, finding that shallow binding sites tested were low-affinity but a deeper intersubunit site indicated by experiments was moderate to high affinity. AFEP calculations of isoflurane and propofol binding to two locations in GLIC (Figure 4), the pore and the allosteric intrasubunit site indicated by crystal structures, predicted that isoflurane has higher affinity for the pore while propofol has similar affinity for the pore and intrasubunit site.[42] Based on the calculated free energies, the GLIC pore was predicted to be blocked by the anesthetics at micromolar concentrations as observed experimentally.[42] Due to occupation of the GLIC pore by detergents, the possibility of GAs binding to the pore has not been addressed using crystallography. Similar methods were used to show that a single-site mutation at the F14’ site in the GLIC transmembrane domain causes desflurane and chloroform to bind with higher affinity to intersubunit sites than intrasubunit sites; the mutation is also associated with a reversal of their effect on GLIC function. [81]
Figure 4.
Binding of propofol and isoflurane to the GLIC pore. Left: View of the GLIC channel with two propofol molecules blocking a pore restrained to be open (shown in red and orange), and one bound in the crystallographic binding site (purple). Center: The two propofol molecules bound to the pore formed by M2 helices (gray). Right: Analogous magnification of two isoflurane molecules in the pore. Isoleucines bounding the hydrophobic gate (I232 and I239) are shown in cyan. To reveal the pore interior, only four of the five GLIC subunits are shown. Figure first printed in [42].
5.3 Effect of binding on protein structure and dynamics
Effects of GA binding are typically subtle over accessible time scales, and efforts to predict effects on function from unrestrained MD simulations can be challenging (with the exception of observed pore block). Flexible docking of the GA halothane to homology models of α4β2 nAChR in open [85] and closed [86] states indicated several binding sites, primarily at the ECD-TMD interface, with subsequent MD simulations showing that halothane has a more pronounced effect on the global structure and dynamics of the open conformation. Since many of the binding sites were low affinity, it was proposed from these calculations that numerous low affinity sites could result in significant effects on dynamics.[86]
Effects of isoflurane on the anesthetic-sensitive GLIC in both the crystallographically identified[77] sites for desflurane/propofol, as well as those suggested by docking and subsequent MD, were investigated[43] using unrestrained MD simulations and principal component analysis; results indicated that interactions at the interface between the ECD and TMD domains were weakened in the presence of isoflurane, and that isoflurane had significant effects on channel dynamics. Further simulations suggested that asymmetric binding of propofol to the crystallographically identified sites caused pore closure, while symmetric binding (five propofol) was observed to have little effect. [91]
GLIC has also been used as a model to study alcohol action on pLGICs. Ethanol was shown to reduce root-mean-squared-deviation (RMSD) of a model of the GlyR relative to a control simulation. [89] Methanol and ethanol weakly potentiate GLIC, while larger alcohols inhibit it. [92] A point mutation at Phe14’ on the M2 helix (to Ala or Cys) converts GLIC to be much more sensitive to ethanol potentiation, comparable to the eukaryotic relatives. [92] MD simulations of the Phe14'Ala mutant in the absence of alcohol showed that the mutation causes an increased kink in the M2 helix at the 9’ position, spreading the subunits away from each other and increasing the volume of the intersubunit cavity and linking tunnel (between the intrasubunit and intersubunit cavities). [92, 93]
6 Conclusion
Pentameric LGICs are an important category of ion channels that are involved in pathogenesis of many diseases (such as Alzheimer's disease and epilepsy) and are the target of many drugs acting on the central nervous system (such as general anesthetics). Detailed molecular understanding of such complex multi-domain membrane proteins is challenging and requires a multidisciplinary approach. Computational simulations can complement experimental findings and in certain cases can be predictive. The emergence of crystal structures of several pLGICs along with advances in computational power has provided an opportunity to study channel gating, ion selectivity factors, and the mechanisms underlying modulation by drugs and lipids. Future increases in accessible time scales as well as improvements to signal-to-noise ratios in finite temperature simulations remain essential for the process of ranking numerous computationally observed interactions in order of functional relevance.
References
- 1.Rissman Robert A, Mobley William C. Implications for treatment: GABAA receptors in aging, Down syndrome and Alzheimer's disease. Journal of neurochemistry. 2011 May;117(4):613–22. doi: 10.1111/j.1471-4159.2011.07237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tanner CM, Goldman SM, Aston DA, Ottman R, Ellenberg J, Mayeux R, Langston JW. Smoking and Parkinson's disease in twins. Neurology. 2002 Feb;58(4):581–8. doi: 10.1212/wnl.58.4.581. [DOI] [PubMed] [Google Scholar]
- 3.Macdonald Robert L, Gallagher Martin J, Feng Hua-Jun, Kang Jingqiong. GABA(A) receptor epilepsy mutations. Biochemical pharmacology. 2004 Oct;68(8):1497–506. doi: 10.1016/j.bcp.2004.07.029. [DOI] [PubMed] [Google Scholar]
- 4.Lewis David A, Volk David W, Hashimoto Takanori. Selective alterations in prefrontal cortical GABA neuro-transmission in schizophrenia: a novel target for the treatment of working memory dysfunction. Psychopharmacology. 2004 Jun;174(1):143–50. doi: 10.1007/s00213-003-1673-x. [DOI] [PubMed] [Google Scholar]
- 5.Lo W-S, Lau C-F, Xuan Z, Chan C-F, Feng G-Y, He L, Cao Z-C, Liu H, Luan Q-M, Xue H. Association of SNPs and haplotypes in GABAA receptor beta2 gene with schizophrenia. Molecular psychiatry. 2004 Jun;9(6):603–8. doi: 10.1038/sj.mp.4001461. [DOI] [PubMed] [Google Scholar]
- 6.Ishikawa Masanori, Mizukami Katsuyoshi, Iwakiri Masahiko, Hidaka Shin, Asada Takashi. GABAA receptor gamma subunits in the prefrontal cortex of patients with schizophrenia and bipolar disorder. Neuroreport. 2004 Aug;15(11):1809–12. doi: 10.1097/01.wnr.0000135695.66366.08. [DOI] [PubMed] [Google Scholar]
- 7.Croxen R, Newland C, Beeson D, Oosterhuis H, Chauplannaz G, Vincent A, Newsom-Davis J. Mutations in different functional domains of the human muscle acetylcholine receptor alpha subunit in patients with the slow-channel congenital myasthenic syndrome. Human molecular genetics. 1997 May;6(5):767–74. doi: 10.1093/hmg/6.5.767. [DOI] [PubMed] [Google Scholar]
- 8.Lippiello PM, Bencherif M, Gray JA, Peters S, Grigoryan G, Hodges H, Collins AC. RJR-2403: a nicotinic agonist with CNS selectivity II. In vivo characterization. The Journal of pharmacology and experimental therapeutics. 1996 Dec;279(3):1422–9. [PubMed] [Google Scholar]
- 9.Porjesz Bernice, Almasy Laura, Edenberg Howard J, Wang Kongming, Chorlian David B, Foroud Tatiana, Goate Alison, Rice John P, O'Connor Sean J, Rohrbaugh John, Kuperman Samuel, Bauer Lance O, Crowe Raymond R, Schuckit Marc A, Hesselbrock Victor, Michael Conneally P, Tischfield Jay A, Li Ting-Kai, Reich Theodore, Begleiter Henri. Linkage disequilibrium between the beta frequency of the human EEG and a GABAA receptor gene locus. Proceedings of the National Academy of Sciences of the United States of America. 2002 Mar;99(6):3729–33. doi: 10.1073/pnas.052716399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edenberg Howard J, Dick Danielle M, Xuei Xiaoling, Tian Huijun, Almasy Laura, Bauer Lance O, Crowe Raymond R, Goate Alison, Hesselbrock Victor, Jones Kevin, Kwon Jennifer, Li Ting-Kai, Nurnberger John I, O'Connor Sean J, Reich Theodore, Rice John, Schuckit Marc A, Porjesz Bernice, Foroud Tatiana, Begleiter Henri. Variations in GABRA2, encoding the alpha 2 subunit of the GABA(A) receptor, are associated with alcohol dependence and with brain oscillations. American journal of human genetics. 2004 Apr;74(4):705–14. doi: 10.1086/383283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Violet JM, Downie DL, Nakisa RC, Lieb WR, Franks NP. Differential sensitivities of mammalian neuronal and muscle nicotinic acetylcholine receptors to general anesthetics. Anesthesiology. 1997 Apr;86(4):866–74. doi: 10.1097/00000542-199704000-00017. [DOI] [PubMed] [Google Scholar]
- 12.Flood P, Ramirez-Latorre J, Role L. Alpha 4 beta 2 neuronal nicotinic acetylcholine receptors in the central nervous system are inhibited by isoflurane and propofol, but alpha 7-type nicotinic acetylcholine receptors are unaffected. Anesthesiology. 1997 Apr;86(4):859–65. doi: 10.1097/00000542-199704000-00016. [DOI] [PubMed] [Google Scholar]
- 13.Krasowski MD, Harrison NL. General anaesthetic actions on ligand-gated ion channels. Cellular and molecular life sciences : CMLS. 1999 Aug;55(10):1278–303. doi: 10.1007/s000180050371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller KW. The nature of sites of general anaesthetic action. British journal of anaesthesia. 2002 Jul;89(1):17–31. doi: 10.1093/bja/aef167. [DOI] [PubMed] [Google Scholar]
- 15.Hemmings Hugh C, Akabas Myles H, Goldstein Peter A, Trudell James R, Orser Beverley A, Harrison Neil L. Emerging molecular mechanisms of general anesthetic action. Trends in pharmacological sciences. 2005 Oct;26(10):503–10. doi: 10.1016/j.tips.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 16.Bouzat Cecilia. New insights into the structural bases of activation of Cys-loop receptors. Journal of physiology, Paris. 2012 Jan;106(1-2):23–33. doi: 10.1016/j.jphysparis.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 17.Corringer Pierre-Jean, Poitevin Frédéric, Prevost Marie S, Sauguet Ludovic, Delarue Marc, Changeux Jean-Pierre. Structure and pharmacology of pentameric receptor channels: from bacteria to brain. Structure (London, England : 1993) 2012 Jun;20(6):941–56. doi: 10.1016/j.str.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 18.Changeux Jean-Pierre. The nicotinic acetylcholine receptor: the founding father of the pentameric ligand-gated ion channel superfamily. The Journal of biological chemistry. 2012 Nov;287(48):40207–15. doi: 10.1074/jbc.R112.407668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hibbs Ryan E, Gouaux Eric. Principles of activation and permeation in an anion-selective Cys-loop receptor. Nature. 2011 Jun;474(7349):54–60. doi: 10.1038/nature10139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Unwin Nigel. Refined structure of the nicotinic acetylcholine receptor at 4A resolution. Journal of molecular biology. 2005 Mar;346(4):967–89. doi: 10.1016/j.jmb.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 21.Hilf Ricarda J C, Dutzler Raimund. X-ray structure of a prokaryotic pentameric ligand-gated ion channel. Nature. 2008 Mar;452(7185):375–9. doi: 10.1038/nature06717. [DOI] [PubMed] [Google Scholar]
- 22.Bocquet Nicolas, Nury Hugues, Baaden Marc, Le Poupon Chantal, Changeux Jean-Pierre, Delarue Marc, Corringer Pierre-Jean. X-ray structure of a pentameric ligand-gated ion channel in an apparently open conformation. Nature. 2009 Jan;457(7225):111–4. doi: 10.1038/nature07462. [DOI] [PubMed] [Google Scholar]
- 23.Hilf Ricarda J C, Dutzler Raimund. Structure of a potentially open state of a proton-activated pentameric ligand-gated ion channel. Nature. 2009 Jan;457(7225):115–8. doi: 10.1038/nature07461. [DOI] [PubMed] [Google Scholar]
- 24.Bocquet Nicolas, de Carvalho Lia Prado, Cartaud Jean, Neyton Jacques, Le Poupon Chantal, Taly Antoine, Grutter Thomas, Changeux Jean-Pierre, Corringer Pierre-Jean. A prokaryotic proton-gated ion channel from the nicotinic acetylcholine receptor family. Nature. 2007 Jan;445(7123):116–9. doi: 10.1038/nature05371. [DOI] [PubMed] [Google Scholar]
- 25.Law Richard J, Henchman Richard H, Andrew McCammon J. A gating mechanism proposed from a simulation of a human alpha7 nicotinic acetylcholine receptor. Proceedings of the National Academy of Sciences of the United States of America. 2005 May;102(19):6813–8. doi: 10.1073/pnas.0407739102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nury Hugues, Poitevin Frédéric, Van Renterghem Catherine, Changeux Jean-Pierre, Corringer Pierre-Jean, Delarue Marc, Baaden Marc. One-microsecond molecular dynamics simulation of channel gating in a nicotinic receptor homologue. Proceedings of the National Academy of Sciences of the United States of America. 2010 Apr;107(14):6275–80. doi: 10.1073/pnas.1001832107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Calimet Nicolas, Simoes Manuel, Changeux Jean-Pierre, Karplus Martin, Taly Antoine, Cecchini Marco. From the Cover: A gating mechanism of pentameric ligand-gated ion channels. Proceedings of the National Academy of Sciences of the United States of America. 2013 Oct;110(42):E3987–96. doi: 10.1073/pnas.1313785110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Hai-Long, Toghraee Reza, Papke David, Cheng Xiao-Lin, Andrew McCammon J, Ravaioli Umberto, Sine Steven M. Single-channel current through nicotinic receptor produced by closure of binding site C-loop. Biophysical journal. 2009 May;96(9):3582–90. doi: 10.1016/j.bpj.2009.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Xinli, Xu Yechun, Li Honglin, Wang Xicheng, Jiang Hualiang, Barrantes Francisco J. Mechanics of channel gating of the nicotinic acetylcholine receptor. PLoS computational biology. 2008 Jan;4(1):e19. doi: 10.1371/journal.pcbi.0040019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng Wenjun, Auerbach Anthony. Decrypting the sequence of structural events during the gating transition of pentameric ligand-gated ion channels based on an interpolated elastic network model. PLoS computational biology. 2011 Jan;7(1):e1001046. doi: 10.1371/journal.pcbi.1001046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taly Antoine, Delarue Marc, Grutter Thomas, Nilges Michael, Le Novère Nicolas, Corringer Pierre-Jean, Changeux Jean-Pierre. Normal mode analysis suggests a quaternary twist model for the nicotinic receptor gating mechanism. Biophysical journal. 2005 Jun;88(6):3954–65. doi: 10.1529/biophysj.104.050229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taly Antoine, Corringer Pierre-Jean, Grutter Thomas, de Carvalho Lia Prado, Karplus Martin, Changeux Jean-Pierre. Implications of the quaternary twist allosteric model for the physiology and pathology of nicotinic acetylcholine receptors. Proceedings of the National Academy of Sciences of the United States of America. 2006 Nov;103(45):16965–70. doi: 10.1073/pnas.0607477103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng Xiaolin, Lu Benzhuo, Grant Barry, Law Richard J, Andrew McCammon J. Channel opening motion of alpha7 nicotinic acetylcholine receptor as suggested by normal mode analysis. Journal of molecular biology. 2006 Jan;355(2):310–24. doi: 10.1016/j.jmb.2005.10.039. [DOI] [PubMed] [Google Scholar]
- 34.Taly Antoine. Opened by a twist: a gating mechanism for the nicotinic acetylcholine receptor. European biophysics journal : EBJ. 2007 Nov;36(8):911–8. doi: 10.1007/s00249-007-0189-4. [DOI] [PubMed] [Google Scholar]
- 35.Zhu Fangqiang, Hummer Gerhard. Pore opening and closing of a pentameric ligand-gated ion channel. Proceedings of the National Academy of Sciences of the United States of America. 2010 Nov;107(46):19814–9. doi: 10.1073/pnas.1009313107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gao Fan, Bren Nina, Burghardt Thomas P, Hansen Scott, Henchman Richard H, Taylor Palmer, Andrew McCammon J, Sine Steven M. Agonist-mediated conformational changes in acetylcholine-binding protein revealed by simulation and intrinsic tryptophan fluorescence. The Journal of biological chemistry. 2005 Mar;280(9):8443–51. doi: 10.1074/jbc.M412389200. [DOI] [PubMed] [Google Scholar]
- 37.Hongying Cheng Mary, Coalson Rob D. Energetics and ion permeation characteristics in a glutamate-gated chloride (GluCl) receptor channel. The journal of physical chemistry. B. 2012 Nov;116(46):13637–43. doi: 10.1021/jp3074915. [DOI] [PubMed] [Google Scholar]
- 38.Prevost Marie S, Sauguet Ludovic, Nury Hugues, Van Renterghem Catherine, Huon Christèle, Poitevin Frederic, Baaden Marc, Delarue Marc, Corringer Pierre-Jean. A locally closed conformation of a bacterial pentameric proton-gated ion channel. Nature structural & molecular biology. 2012 Jun;19(6):642–9. doi: 10.1038/nsmb.2307. [DOI] [PubMed] [Google Scholar]
- 39.Prevost Marie S., Moraga-Cid Gustavo, Van Renterghem Catherine, Edelstein Stuart J., Changeux Jean-Pierre, Corringer Pierre-Jean. Intermediate closed state for glycine receptor function revealed by cysteine cross-linking. Proceedings of the National Academy of Sciences. 2013 doi: 10.1073/pnas.1317009110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bertaccini Edward J., Trudell James R., Lindahl Erik. Normal-mode analysis of the glycine alpha1 receptor by three separate methods. Journal of Chemical Information and Modeling. 2007;47(4):1572–1579. doi: 10.1021/ci600566j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haddadian Esmael J, Hongying Cheng Mary, Coalson Rob D, Xu Yan, Tang Pei. In silico models for the human alpha4beta2 nicotinic acetylcholine receptor. The journal of physical chemistry. B. 2008 Nov;112(44):13981–90. doi: 10.1021/jp804868s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.LeBard David N, Hénin Jérôme, Eckenhoff Roderic G, Klein Michael L, Brannigan Grace. General anesthetics predicted to block the GLIC pore with micromolar affnity. PLoS computational biology. 2012 May;8(5):e1002532. doi: 10.1371/journal.pcbi.1002532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Willenbring Dan, Tian Liu Lu, Mowrey David, Xu Yan, Tang Pei. Isoflurane alters the structure and dynamics of GLIC. Biophysical journal. 2011 Oct;101(8):1905–12. doi: 10.1016/j.bpj.2011.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.MacKerell AD, Jr., Bashford D, Bellott M, Dunbrack RL, Jr., Evanseck JD, Field MJ, Fischer S, Gao J, Guo H, Ha S, Joseph-McCarthy D, Kuchnir L, Kuczera K, Lau FTK, Mattos C, Michnick S, Ngo T, Nguyen DT, Prodhom B, Reiher WE, III, Roux B, Schlenkrich M, Smith JC, Stote R, Straub J, Watanabe M, Wiórkiewicz-Kuczera J, Yin D, Karplus M. All-atom empirical potential for molecular modeling and dynamics studies of proteins. J. Phys. Chem. B. 1998;102:3586–3616. doi: 10.1021/jp973084f. [DOI] [PubMed] [Google Scholar]
- 45.MacKerell Alexander D, Feig Michael, Brooks Charles L. Improved treatment of the protein backbone in empirical force fields. J. Am. Chem. Soc. 2004;126(3):698–9. doi: 10.1021/ja036959e. [DOI] [PubMed] [Google Scholar]
- 46.Duan Yong, Wu Chun, Chowdhury Shibasish, Lee Mathew C., Xiong Guoming, Zhang Wei, Yang Rong, Cieplak Piotr, Luo Ray, Lee Taisung, Caldwell James, Wang Junmei, Kollman Peter. A point-charge force field for molecular mechanics simulations of proteins based on condensed-phase quantum mechanical calculations. J Comput Chem. 2003 Dec;24(16):1999–2012. doi: 10.1002/jcc.10349. [DOI] [PubMed] [Google Scholar]
- 47.Van Der Spoel David, Lindahl Erik, Hess Berk, Groenhof Gerrit, Mark Alan E., Berendsen Herman J C. Gromacs: fast, flexible, and free. J Comput Chem. 2005 Dec;26(16):1701–1718. doi: 10.1002/jcc.20291. [DOI] [PubMed] [Google Scholar]
- 48.Yoluk Ozge, Brömstrup Torben, Bertaccini Edward J, Trudell James R, Lindahl Erik. Stabilization of the GluCl ligand-gated ion channel in the presence and absence of ivermectin. Biophysical journal. 2013 Aug;105(3):640–7. doi: 10.1016/j.bpj.2013.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Henin J, Salari Reza, Murlidaran Sruthi, Brannigan Grace. A predicted binding site for cholesterol on the gaba(a) receptor. 2013 doi: 10.1016/j.bpj.2014.03.024. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chung Shin-Ho, Corry Ben. Three computational methods for studying permeation, selectivity and dynamics in biological ion channels. Soft Matter. 2005;1(6):417. doi: 10.1039/b512455g. [DOI] [PubMed] [Google Scholar]
- 51.Song Chen, Corry Ben. Ion conduction in ligand-gated ion channels: Brownian dynamics studies of four recent crystal structures. Biophysical journal. 2010 Feb;98(3):404–11. doi: 10.1016/j.bpj.2009.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beckstein Oliver, Sansom Mark S P. A hydrophobic gate in an ion channel: the closed state of the nicotinic acetylcholine receptor. Physical biology. 2006 Jun;3(2):147–59. doi: 10.1088/1478-3975/3/2/007. [DOI] [PubMed] [Google Scholar]
- 53.Fritsch Sebastian, Ivanov Ivaylo, Wang Hailong, Cheng Xiaolin. Ion selectivity mechanism in a bacterial pentameric ligand-gated ion channel. Biophysical journal. 2011 Jan;100(2):390–8. doi: 10.1016/j.bpj.2010.11.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhu Fangqiang, Hummer Gerhard. Theory and simulation of ion conduction in the pentameric GLIC channel. Journal of chemical theory and computation. 2012 doi: 10.1021/ct2009279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ivanov Ivaylo, Cheng Xiaolin, Sine Steven M, Andrew McCammon J. Barriers to ion translocation in cationic and anionic receptors from the Cys-loop family. Journal of the American Chemical Society. 2007 Jul;129(26):8217–24. doi: 10.1021/ja070778l. [DOI] [PubMed] [Google Scholar]
- 56.Hongying Cheng Mary, Coalson Rob D, Tang Pei. Molecular dynamics and brownian dynamics investigation of ion permeation and anesthetic halothane effects on a proton-gated ion channel. Journal of the American Chemical Society. 2010 Nov;132(46):16442–9. doi: 10.1021/ja105001a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kutzner Carsten, Grubmüller Helmut, de Groot Bert L, Zachariae Ulrich. Computational electrophysiology: the molecular dynamics of ion channel permeation and selectivity in atomistic detail. Biophysical journal. 2011 Aug;101(4):809–17. doi: 10.1016/j.bpj.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Beckstein Oliver, Biggin Philip C., Sansom Mark S. P. A Hydrophobic Gating Mechanism for Nanopores. The Journal of Physical Chemistry B. 2001 Dec;105(51):12902–12905. [Google Scholar]
- 59.Song Chen, Corry Ben. Intrinsic ion selectivity of narrow hydrophobic pores. The journal of physical chemistry. B. 2009 May;113(21):7642–9. doi: 10.1021/jp810102u. [DOI] [PubMed] [Google Scholar]
- 60.Duret Guillaume, Van Renterghem Catherine, Weng Yun, Prevost Marie, Moraga-Cid Gustavo, Huon Christèle, Sonner James M, Corringer Pierre-Jean. Functional prokaryotic-eukaryotic chimera from the pentameric ligand-gated ion channel family. Proceedings of the National Academy of Sciences of the United States of America. 2011 Jul;108(29):12143–8. doi: 10.1073/pnas.1104494108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sauguet Ludovic, Poitevin Frédéric, Murail Samuel, Van Renterghem Catherine, Moraga-Cid Gustavo, Malherbe Laurie, Thompson Andrew W, Koehl Patrice, Corringer Pierre-Jean, Baaden Marc, Delarue Marc. Structural basis for ion permeation mechanism in pentameric ligand-gated ion channels. The EMBO journal. 2013 Mar;32(5):728–41. doi: 10.1038/emboj.2013.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gimpl G, Burger K, Fahrenholz F. Cholesterol as modulator of receptor function. Biochemistry. 1997 Sep;36(36):10959–10974. doi: 10.1021/bi963138w. [DOI] [PubMed] [Google Scholar]
- 63.Burger K, Gimpl G, Fahrenholz F. Regulation of receptor function by cholesterol. Cell Mol Life Sci. 2000 Oct;57(11):1577–1592. doi: 10.1007/PL00000643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barrantes Francisco J. Cholesterol effects on nicotinic acetylcholine receptor: cellular aspects. Subcell Biochem. 2010;51:467–487. doi: 10.1007/978-90-481-8622-8_17. [DOI] [PubMed] [Google Scholar]
- 65.Barrantes Francisco J. Cholesterol effects on nicotinic acetylcholine receptor. J Neurochem. 2007 Nov;103(Suppl 1):72–80. doi: 10.1111/j.1471-4159.2007.04719.x. [DOI] [PubMed] [Google Scholar]
- 66.Sooksawate T, Simmonds MA. Effects of membrane cholesterol on the sensitivity of the GABA(A) receptor to GABA in acutely dissociated rat hippocampal neurones. Neuropharmacology. 2001;40(2):178–184. doi: 10.1016/s0028-3908(00)00159-3. [DOI] [PubMed] [Google Scholar]
- 67.Bristow DR, Martin IL. Solubilisation of the gamma-aminobutyric acid/benzodiazepine receptor from rat cerebellum: optimal preservation of the modulatory responses by natural brain lipids. J. Neurochem. 1987;49(5):1386–1393. doi: 10.1111/j.1471-4159.1987.tb01004.x. [DOI] [PubMed] [Google Scholar]
- 68.Dunn SM, Martin CR, Agey MW, Miyazaki R. Functional reconstitution of the bovine brain GABAA receptor from solubilized components. Biochemistry. 1989;28(6):2545–2551. doi: 10.1021/bi00432a030. [DOI] [PubMed] [Google Scholar]
- 69.daCosta Corrie J B., Dey Lopamudra, Daniel Therien JP, Baenziger John E. A distinct mechanism for activating uncoupled nicotinic acetylcholine receptors. Nat Chem Biol. 2013;9(11):701–707. doi: 10.1038/nchembio.1338. [DOI] [PubMed] [Google Scholar]
- 70.Baenziger John E., Corringer Pierre-Jean. 3d structure and allosteric modulation of the transmembrane domain of pentameric ligand-gated ion channels. Neuropharmacology. 2011;60(1):116–125. doi: 10.1016/j.neuropharm.2010.08.007. ¡ce:title¿High Resolution¡/ce:title¿. [DOI] [PubMed] [Google Scholar]
- 71.Baier Carlos J., Fantini Jacques, Barrantes Francisco J. Disclosure of cholesterol recognition motifs in transmembrane domains of the human nicotinic acetylcholine receptor. Sci Rep. 2011;1:69. doi: 10.1038/srep00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cheng Mary H., Xu Yan, Tang Pei. Anionic lipid and cholesterol interactions with alpha4beta2 nachr: insights from md simulations. J Phys Chem B. 2009 May;113(19):6964–6970. doi: 10.1021/jp900714b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Miyazawa Atsuo, Fujiyoshi Yoshinori, Unwin Nigel. Structure and gating mechanism of the acetylcholine receptor pore. Nature. 2003 Jun;423(6943):949–55. doi: 10.1038/nature01748. [DOI] [PubMed] [Google Scholar]
- 74.Brannigan Grace, Hénin Jérôme, Law Richard, Eckenhoff Roderic, Klein Michael L. Embedded cholesterol in the nicotinic acetylcholine receptor. Proceedings of the National Academy of Sciences of the United States of America. 2008 Sep;105(38):14418–23. doi: 10.1073/pnas.0803029105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Löwenick CV, Krampfl K, Schneck H, Kochs E, Bufler J. Open channel and competitive block of nicotinic receptors by pancuronium and atracurium. European journal of pharmacology. 2001 Feb;413(1):31–5. doi: 10.1016/s0014-2999(00)00836-0. [DOI] [PubMed] [Google Scholar]
- 76.Spurny Radovan, Ramerstorfer Joachim, Price Kerry, Brams Marijke, Ernst Margot, Nury Hugues, Verheij Mark, Legrand Pierre, Bertrand Daniel, Bertrand Sonia, Dougherty Dennis a, de Esch Iwan J P, Corringer Pierre-Jean, Sieghart Werner, Lummis Sarah C R, Ulens Chris. Pentameric ligand-gated ion channel ELIC is activated by GABA and modulated by benzodiazepines. Proceedings of the National Academy of Sciences of the United States of America. 2012 Oct;109(44):E3028–34. doi: 10.1073/pnas.1208208109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nury Hugues, Van Renterghem Catherine, Weng Yun, Tran Alphonso, Baaden Marc, Dufresne Virginie, Changeux Jean-Pierre, Sonner James M, Delarue Marc, Corringer Pierre-Jean. X-ray structures of general anaesthetics bound to a pentameric ligand-gated ion channel. Nature. 2011 Jan;469(7330):428–31. doi: 10.1038/nature09647. [DOI] [PubMed] [Google Scholar]
- 78.Spurny Radovan, Billen Bert, Howard Rebecca J., Brams Marijke, Debaveye Sarah, Price Kerry L., Weston David A., Strelkov Sergei V., Tytgat Jan, Bertrand Sonia, Bertrand Daniel, Lummis Sarah C R., Ulens Chris. Multisite binding of a general anesthetic to the prokaryotic pentameric erwinia chrysanthemi ligand-gated ion channel (elic). J Biol Chem. 2013 Mar;288(12):8355–8364. doi: 10.1074/jbc.M112.424507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hilf Ricarda J C., Bertozzi Carlo, Zimmermann Iwan, Reiter Alwin, Trauner Dirk, Dutzler Raimund. Structural basis of open channel block in a prokaryotic pentameric ligand-gated ion channel. Nat Struct Mol Biol. 2010 Nov;17(11):1330–1336. doi: 10.1038/nsmb.1933. [DOI] [PubMed] [Google Scholar]
- 80.Forman Stuart a, Miller Keith W. Anesthetic sites and allosteric mechanisms of action on Cys-loop ligand-gated ion channels. Canadian journal of anaesthesia = Journal canadien d'anesthésie. 2011 Feb;58(2):191–205. doi: 10.1007/s12630-010-9419-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brmstrup Torben, Howard Rebecca J., Trudell James R., Adron Harris R, Lindahl Erik. Inhibition versus potentiation of ligand-gated ion channels can be altered by a single mutation that moves ligands between intra- and intersubunit sites. Structure. 2013 Aug;21(8):1307–1316. doi: 10.1016/j.str.2013.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Weng Yun, Yang Liya, Corringer Pierre-Jean, Sonner James M. Anesthetic sensitivity of the Gloeobacter violaceus proton-gated ion channel. Anesthesia and analgesia. 2010 Jan;110(1):59–63. doi: 10.1213/ANE.0b013e3181c4bc69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Saladino Alexander C, Xu Yan, Tang Pei. Homology modeling and molecular dynamics simulations of trans-membrane domain structure of human neuronal nicotinic acetylcholine receptor. Biophysical journal. 2005 Feb;88(2):1009–17. doi: 10.1529/biophysj.104.053421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Law Richard J., Lightstone Felice C. Modeling neuronal nicotinic and gaba receptors: important interface salt-links and protein dynamics. Biophys J. 2009 Sep;97(6):1586–1594. doi: 10.1016/j.bpj.2009.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tian Liu Lu, Willenbring Dan, Xu Yan, Tang Pei. General anesthetic binding to neuronal alpha4beta2 nicotinic acetylcholine receptor and its effects on global dynamics. The journal of physical chemistry. B. 2009 Sep;113(37):12581–9. doi: 10.1021/jp9039513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tian Liu Lu, Haddadian Esmael J, Willenbring Dan, Xu Yan, Tang Pei. Higher susceptibility to halothane modulation in open- than in closed-channel alpha4beta2 nAChR revealed by molecular dynamics simulations. The journal of physical chemistry. B. 2010 Jan;114(1):626–32. doi: 10.1021/jp908944e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vemparala Satyavani, Saiz Leonor, Eckenhoff Roderic G, Klein Michael L. Partitioning of anesthetics into a lipid bilayer and their interaction with membrane-bound peptide bundles. Biophysical journal. 2006 Oct;91(8):2815–25. doi: 10.1529/biophysj.106.085324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Brannigan Grace, LeBard David N, Hénin Jérôme, Eckenhoff Roderic G, Klein Michael L. Multiple binding sites for the general anesthetic isoflurane identified in the nicotinic acetylcholine receptor transmembrane domain. Proceedings of the National Academy of Sciences of the United States of America. 2010 Aug;107(32):14122–7. doi: 10.1073/pnas.1008534107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Murail Samuel, Wallner Björn, Trudell James R, Bertaccini Edward, Lindahl Erik. Microsecond simulations indicate that ethanol binds between subunits and could stabilize an open-state model of a glycine receptor. Biophysical journal. 2011 Apr;100(7):1642–50. doi: 10.1016/j.bpj.2011.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bertaccini Edward J, Yoluk Ozge, Lindahl Erik R, Trudell James R. Assessment of homology templates and an anesthetic binding site within the [gamma]-aminobutyric acid receptor. Anesthesiology. 2013 doi: 10.1097/ALN.0b013e31829e47e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mowrey David, Hongying Cheng Mary, Tian Liu Lu, Willenbring Dan, Lu Xinghua, Wymore Troy, Xu Yan, Tang Pei. Asymmetric ligand binding facilitates conformational transitions in pentameric ligand-gated ion channels. Journal of the American Chemical Society. 2013 Feb;135(6):2172–80. doi: 10.1021/ja307275v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Howard Rebecca J, Murail Samuel, Ondricek Kathryn E, Corringer Pierre-Jean, Lindahl Erik, Trudell James R, Adron Harris R. Structural basis for alcohol modulation of a pentameric ligand-gated ion channel. Proceedings of the National Academy of Sciences of the United States of America. 2011 Jul;108(29):12149–54. doi: 10.1073/pnas.1104480108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Murail Samuel, Howard Rebecca J, Broemstrup Torben, Bertaccini Edward J, Adron Harris R, Trudell James R, Lindahl Erik. Molecular mechanism for the dual alcohol modulation of Cys-loop receptors. PLoS computational biology. 2012 Jan;8(10):e1002710. doi: 10.1371/journal.pcbi.1002710. [DOI] [PMC free article] [PubMed] [Google Scholar]