To the Editor: Shigella sonnei causes a bacillary dysentery called shigellosis. Shiga toxins 1 (Stx1) and 2 (Stx2) are mainly produced by Shiga toxin–producing Escherichia coli (STEC), but Stx1 can also be produced by S. dysenteriae serotype 1 (1). Compared with STEC-producing Stx1, STEC-producing Stx2 has been reported to be more highly pathogenic and to be associated with hemolytic uremic syndrome (HUS) and hemorrhagic colitis (2); the association with HUS especially has been reported for subtypes Stx2a and Stx2c (3).
Stx-converting bacteriophages play a key role in expression of the stx gene in E. coli and in the lateral gene transfer between the bacteria (4). Although these bacteriophages have not been isolated from S. dysenteriae serotype 1, evidence suggests that the bacteria’s stx gene may be associated with a bacteriophage (5). The stx1 gene, which is located in a bacteriophage, has previously been detected in S. sonnei (6). We describe an S. sonnei isolate with the stx2a gene.
In November 2013, 3 days after her return to Finland from a 2-week visit to relatives in southern Morocco, a 49-year-old woman was admitted to a hospital for bloody diarrhea, fever, and abdominal pain. Her symptoms began with watery diarrhea and fever 5 days before she returned home. The diarrhea ceased after a few days, but symptoms worsened, and diarrhea was visibly bloody after she returned to Finland. On admission, the patient had C-reactive protein and hemoglobin levels of 41 mg/L (reference value <3 mg/L) and 118 g/L (reference value 117–155 g/L), respectively; no thrombocytopenia was observed. No antimicrobial drugs were prescribed because STEC infection was clinically suspected. The patient responded well to intravenous fluid treatment and was discharged from the hospital 3 days after admission. At a follow-up visit 1 week later, she was asymptomatic and had a C-reactive protein level of 19 mg/L.
Shigella spp. was isolated from a feces sample obtained from the patient on illness day 8, and an STEC signal (based on the PCR-positive stx2 gene) was detected from the same sample. A presumptive STEC strain was subsequently isolated. Both isolates were sent for confirmation and further typing to the Bacteriology Unit, National Institute for Health and Welfare, in Helsinki. The first isolate (FE109024) was confirmed as S. sonnei by matching of its biochemical profile to that of the reference strain ATCC 25931 (http://www.atcc.org/Products/All/25931.aspx; Technical Appendix Table) and agglutination by antiserum to S. sonnei (Denka Seiken, Tokyo, Japan). The agglutination by S. sonnei phase I antiserum indicated the smooth form of lipopolysaccharide. Atypical for S. sonnei, the isolate did not ferment mannitol (7). The isolate carried the genes encoding invasion plasmid antigen H, the invasion-associated locus, and invasion gene transcription regulator invE. The second isolate (FE109046) was initially sent to the National Institute for Health and Welfare as an STEC. However, FE109046 showed biochemical reactions in tubes and when using API 20E (bioMérieux, Durham, NC, USA) that were identical to reactions for isolate FE109024 after an overnight and 3-day incubation (Technical Appendix Table). Both isolates were nonmotile. FE109046 also agglutinated by S. sonnei polyvalent antiserum but was negative for S. sonnei phase I antiserum and positive for phase II antiserum, indicating the rough form of lipopolysaccharide. In contrast to FE109024, isolate FE109046 lacked the Shigella/enteroinvasive E. coli–specific invE gene but harbored the STEC-specific stx2 gene. Neither isolate carried other STEC-associated genes. Subtyping of the stx2 gene of isolate FE109046 was performed according to the published protocol (8); the isolate was confirmed to be subtype stx2a.
Genomic comparison of the 2 isolates was performed by using pulsed-field gel electrophoresis according to the standard protocol. The isolates showed 96% similarity. A 3-fragment difference suggests that 1 isolate was a variant of the other (Figure); the variant may have arisen through lysogenization with the phage.
Figure.
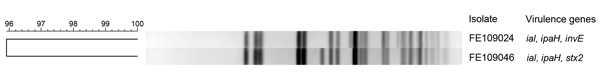
UPGMA dendrogram of XbaI restriction patterns of 2 Shigella sonnei isolates from a patient from Finland who became ill during a visit to Morocco. Genomic comparison of the 2 isolates was performed by using pulsed-field gel electrophoresis according to the standard protocol. The isolates showed 96% similarity, and a 3-fragment difference suggests that 1 isolate was a variant of the other. Scale bar represents % similarity. ial, invasion-associated locus; ipaH, invasion plasmid antigen H; invE, invasion gene transcription regulator invE; stx2, Shiga toxin 2.
The Shiga toxin subtype stx2a identified in isolate FE109046 is linked to severe human disease, including HUS (3). The role of Stx in shigellosis is unclear. Stx is not essential for cell invasion or lysis (9). In the previously reported case of stx1–positive S. sonnei infection, the patient’s symptoms were not severe, and diarrhea lasted 7 days (6).
Shigella spp. can harbor virulence genes found in E. coli because both are genetically defined as members of the same species (10). The stx2–positive S. sonnei may have emerged in the patient initially infected with a mannitol-negative S. sonnei that was subsequently lysogenized by transduction from a STEC co-infection or by a free stx2 phage. S. sonnei isolates are not routinely examined for the production of Stx or stx genes in clinical laboratories, so the properties associated with STEC in S. sonnei isolates from patients remain undetected. S. sonnei with stx2a may have potential to cause severe disease, especially in children. This novel and remarkable virulence characteristic in Shigella spp. would affect diagnostics, infection control, and prevention.
Characteristics of 2 isolates from a patient compared with those of a Shigella sonnei reference strain.
Acknowledgments
We thank the personnel of the Bacteriology Unit at the Finnish National Institute for Health and Welfare for their skillful technical assistance, especially Tarja Heiskanen, who is gratefully acknowledged for the detection of Shigella sonnei with the stx2 gene.
Footnotes
Suggested citation for this article: Nyholm O, Lienemann T, Halkilahti J, Mero S, Rimhanen-Finne R, Lehtinen V, et al. Characterization of Shigella sonnei isolate carrying Shiga toxin 2–producing gene [letter]. Emerg Infect Dis. 2015 May [date cited]. http://dx.doi.org/10.3201/eid2105.140621
References
- 1.Schmidt H. Shiga-toxin-converting bacteriophages. Res Microbiol. 2001;152:687–95. 10.1016/S0923-2508(01)01249-9 [DOI] [PubMed] [Google Scholar]
- 2.Melton-Celsa A, Mohawk K, Teel L, O’Brien A. Pathogenesis of Shiga-toxin producing Escherichia coli. Curr Top Microbiol Immunol. 2012;357:67–103. 10.1007/82_2011_176 [DOI] [PubMed] [Google Scholar]
- 3.Manning SD, Motiwala AS, Springman AC, Qi W, Lacher DW, Ouellette LM, et al. Variation in virulence among clades of Escherichia coli O157:H7 associated with disease outbreaks. Proc Natl Acad Sci U S A. 2008;105:4868–73. 10.1073/pnas.0710834105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martinez-Castillo A, Quirós P, Navarro F, Miró E, Muniesa M. Shiga toxin 2–encoding bacteriophages in human fecal samples from healthy individuals. Appl Environ Microbiol. 2013;79:4862–8. 10.1128/AEM.01158-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McDonough MA, Butterton JR. Spontaneous tandem amplification and deletion of the Shiga toxin operon in Shigella dysenteriae 1. Mol Microbiol. 1999;34:1058–69. 10.1046/j.1365-2958.1999.01669.x [DOI] [PubMed] [Google Scholar]
- 6.Beutin L, Strauch E, Fischer I. Isolation of Shigella sonnei lysogenic for a bacteriophage encoding gene for production of Shiga toxin. Lancet. 1999;353:1498. 10.1016/S0140-6736(99)00961-7 [DOI] [PubMed] [Google Scholar]
- 7.Nataro JP, Bopp CA, Fields PI, Kaper JB, Strockbine NA. Escherichia, Shigella, and Salmonella. In: Murray PR, Baron EJ, Jorgensen JH, Landry ML, Pfaller MA, editors. Manual of clinical microbiology. 9th ed. Washington (DC): ASM Press; 2007. p 670–87. [Google Scholar]
- 8.Statens Serum Institute. Identification of three vtx1 and seven vtx2 subtypes of Verocytotoxin encoding genes of Escherichia coli by conventional PCR amplification. Version 6. 2014. [cited 2014 Mar 10]. http://www.ssi.dk/English/HealthdataandICT/National%20Reference%20Laboratories/Bacteria/~/media/Indhold/EN%20-%20engelsk/Public%20Health/National%20Reference%20Laboratories/vtx%20detection%20%20subtyping%20protocolrev6final.ashx
- 9.Fontaine A, Arondel J, Sansonetti PJ. Role of Shiga toxin in the pathogenesis of bacillary dysentery, studied by using a Tox- mutant of Shigella dysenteriae 1. Infect Immun. 1988;56:3099–109 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pupo GM, Lan R, Reeves PR. Multiple independent origins of Shigella clones of Escherichia coli and convergent evolution of many of their characteristics. Proc Natl Acad Sci U S A. 2000;97:10567–72 and. 10.1073/pnas.180094797 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Characteristics of 2 isolates from a patient compared with those of a Shigella sonnei reference strain.


