Abstract
Peripheral blood CD4+ and CD8+ T cells, CD19+/20+ B cells, and serum immunoglobulins (Igs) have been implicated as survival factors for pediatric HIV-1 infection. To determine which of these immune factors might be important in predicting survival, we studied HIV-1 vertically infected (HIV-1+) children over a 5-year period. Peripheral blood lymphocytes and Igs were measured in 298 HIV-1+ children, who were classified as survivors or nonsurvivors, and in 463 HIV-1 vertically exposed and noninfected (HIV-1–) children. Measurements of other possible survival factors were included in this study: albumin, hemoglobin, lactic dehydrogenase (LDH), and HIV-1 RNA levels. Survivors had significantly higher CD4+ T-cell, CD8+ T-cell, and CD19+/CD20+ B-cell counts and serum IgG levels, but lower serum IgA and IgM levels than nonsurvivors. Serum albumin and blood hemoglobin levels were higher, but serum LDH and HIV-1 RNA levels were lower in the survivors compared to non-survivors. In univariable analysis, factors affecting survival were baseline CD4+ T-cell and CD8+ T-cell counts, IgG, albumin, hemoglobin, LDH, and HIV-1 RNA (all p < 0.001). In multivariable analysis, high baseline CD4+ T-cell count, IgG and albumin levels, and low baseline HIV-1 RNA load remained important factors for survival. Serum IgG level has been identified as an immune factor that independently predicts survival, in addition to the already established CD4+ T-cell count. The HIV-1 RNA and serum albumin levels also predicted survival.
INTRODUCTION
A preliminary report of the immune function of the children enrolled in the National Institutes of Health National Heart, Lung and Blood Institute P2C2 HIV-1 Study recorded the measurements of CD4+ (helper) T cells and CD8+ (cytotoxic) T cells in HIV-1+ children followed for less than 24 months of the 60-month study.1 Results of this study showed an early and continuing loss of CD4+ T cells through 17 months of age in HIV-1+ children and an early rise at 2–4 months followed by a decline of CD4+ T cells in HIV-1− children, although at 17 months the mean cell count was higher by 1,200 cells/μl. There was an expansion of the CD8+ T-cell population, beginning as early as 2 months of age in some HIV-1-infected children and rising to 50% of the peripheral blood mononuclear cell population. There was 70% mortality in HIV-1+ children with fewer than 200 CD4+ T cells/μl.1
Several large-scale, multicenter studies have identified the peripheral blood CD4+ T-cell count, and plasma or serum HIV-1 RNA level as important predictors of survival in HIV-1+ children.2–4 Earlier studies of antibody function demonstrated the extraordinarily elevated serum concentrations of IgG, IgA, and IgM.5–7 Measurements of antibody responses to T-cell-dependent recall antigens (e.g., diphtheria and tetanus toxoid) or neoantigen (e.g., bacteriophage φX174) demonstrated mostly weak primary antibody (IgM) responses and severely reduced secondary antibody (IgG) responses.8–11 This failure to switch from an IgM to an IgG antibody (long-lived, high-affinity, memory antibody) is probably due to the lack of CD4+ (helper) T cells, which generate a second signal to B cells upon cognate recognition of antigen.12
This report of the peripheral blood immune cells and serum immunoglobulins in the completed P2C2 HIV Study cohort will evaluate the importance of these immune survival factors identified in studies of HIV-1+ children.13–18
METHODS
Study Population and Informed Consent
The P2C2 HIV Study population has been described fully elsewhere, with explanations of recruitment, examinations, laboratory and clinical tests, quality assessment, and data analysis.19 Briefly, a group of 600 study subjects born to HIV-1+ women were enrolled at birth or by 28 days of life (birth cohort) beginning in 1990 and followed prospectively for up to 6 years. This group comprised 93 HIV-1 +, 463 HIV-1−, and 44 HIV-1-indeterminate infants. Another group of 205 infants and children with HIV-1 infection were enrolled at greater than 28 days of life (older cohort) between 1990 and 1993 and were similarly followed for up to 6 years. Infants and children in both cohorts were examined at regular intervals of 3–6 months.
Definitions of HIV-1 Disease Survival
A survivor was defined as a child who survived for 5 years after enrollment into study or a child who was alive when lost to follow-up. A nonsurvivor was defined as a child who died during the course of this study.
Examinations of Subjects
Study subjects had periodic physical examinations and laboratory tests, including complete blood count, lymphocyte counts (CD4+, CD8+, CD19+/20+ lymphocytes), serum Ig measurements (IgG, IgA, IgM), serum albumin, blood hemoglobin, LDH, and HIV-1 RNA. Laboratory tests and interpretation of physical measurements were quality-controlled.19 Serum Ig data from children who had IgG replacement therapy within 90 days (≥ 4 half-lives of IgG) were excluded from analysis. CD4+ and CD8+ T cells and CD19+/20+ B cells were determined by two- or three-color fluorescence-activated flow cytometry in laboratories certified by the National Institute of Allergy and Infectious Diseases Division of AIDS Quality Assurance Program. Absolute numbers of lymphocyte subsets were determined arithmetically on the basis of complete blood counts performed on the same blood sample. Serum was analyzed for immunoglobulin concentrations by laser nephelometry. Hemoglobin concentrations were measured by local hematology laboratories as part of the complete blood count, and serum albumin and LDH levels were measured by local chemistry laboratories. Serum (81% of the subjects) was frozen at −70°C, stored in a central repository, thawed once, and analyzed for HIV-1 RNA concentration by quantitative HIV-1 RNA polymerase chain reaction (PCR) using the Amplicor HIV-1 Monitor Test (Roche Diagnostic Systems, Branchburg, NJ).2,18
Statistical Analysis
Because the cumulative 5-year survival was similar for the two HIV-1-infected cohorts (65.6% for the older cohort after 5 years of follow-up and 67.3% for the birth cohort at 5 years of age), they were combined across the overlapping ages (1 month to 5 years) for data analysis (168 from the older cohort and 91 from the birth cohort). This was done to ensure reasonable sample sizes across the 5-year period for survivors and nonsurvivors.
Repeated-measures analyses of lymphocyte phenotypes (cube-root transformation of counts), serum Ig (natural log), serum albumin, hemoglobin, LDH (natural log), and HIV-1 RNA (log10) were performed using the SAS mixed linear models procedure, which provided estimates of the mean and 95% confidence intervals at each age by HIV-1 status and by survivorship. Reported p values are two-sided and are considered significant at p ≤ 0.05.
Cumulative survival was estimated with the Kaplan-Meier method. Log-rank tests were used to compare survival according to the baseline measurements of lymphocyte counts, serum Ig levels, serum proteins, and HIV-1 RNA viral burden, with groups defined as above or below the median value for each covariate.
To assess the simultaneous effect of baseline factors on survival time, Cox's proportional-hazards regression model was used. Forward and backward stepwise selections were used to choose variables for the multivariable model. Only factors that were significant at p ≤ 0.05 in the univariable analyses were included in the multivariable analyses. The relative risk and 95% confidence interval were calculated for each factor in the presence of the others in the final model.
RESULTS
Patient Study Groups
The demographic and clinical characteristics of the children in this study are given in Table 1. Most children (87%) were members of minority groups, and the distributions of races in the survival categories were roughly equal. Similarly, the sex distribution of children in the disease categories was approximately equal. Over 60% of the HIV-1+ birth cohort was asymptomatic at 3 months of age, but only 12.2% of the HIV-1+ older cohort was asymptomatic at enrollment (median ages of survivors and nonsurvivors were 22 and 26 months, respectively). By 2 years of age, only 10.5% of the birth cohort remained asymptomatic; cumulative mortality was 16.3%; and 46.8% had died or reached Centers for Disease Control and Prevention category C.20 Virtually all study children (over 90%) took antiretroviral medications (principally zidovudine and dideoxyinosine) at some time during the study period; 29 HIV-1+ children (9.7%) took protease inhibitors (ritonavir, nelfinavir, saquinavir, or indinavir) when they were over 2 years of age; 38% of study subjects received intravenous IgG at some time during the study period.
TABLE 1.
Demographic and clinical characteristics of children born to HIV-1-infected women
| Children enrolled from birth and up to 28 days of age (birth cohort) |
Children enrolled after 28 days of age (older cohort) HIV-1+ (n = 205) |
||||||
|---|---|---|---|---|---|---|---|
| Characteristic | HIV-1+ (n = 93) | HIV-1− (n = 463) | Survivorsa (n = 134) | Nonsurvivorsa (n = 71) | |||
| Race | |||||||
| African-American | 41 | (44.1) | 245 (52.9) | 59 | (44.0) | 30 | (42.3) |
| Hispanic | 32 | (34.4) | 138 (29.8) | 48 | (35.8) | 34 | (47.9) |
| White | 15 | (16.1) | 54 (11.7) | 22 | (16.4) | 6 | (8.5) |
| Other | 5 | (5.4) | 26 (5.6) | 5 | (3.7) | 1 | (1.4) |
| Sex | |||||||
| Male | 44 | (47.3) | 249 (53.8) | 60 | (44.8) | 34 | (47.9) |
| Female | 49 | (52.7) | 214 (46.2) | 74 | (55.2) | 37 | (52.1) |
| CDC Pediatric Disease Classification (1994)b | |||||||
| Asymptomatic | 59 | (63.4) | 20 | (14.9) | 5 | (7.0) | |
| Mild (Category A) | 16 | (17.2) | 31 | (23.1) | 4 | (5.6) | |
| Moderate (Category B) | 11 | (11.8) | 25 | (18.7) | 10 | (14.1) | |
| Severe (Category C) | 7 | (7.5) | 58 | (43.3) | 52 | (73.2) | |
Note: Figures given represent frequency with percent given in parentheses.
Defined at end of 5-year study regardless of age at enrollment.
Most severe symptom status by 3 months of age for HIV-1+ children followed from birth and at the time of enrollment for the older HIV-1+ cohort (median enrollment age = 23 months).20
Lymphocyte Subsets
At all ages the survivors had significantly higher CD4+ T-cell counts compared to the nonsurvivors (p < 0.001) but their CD4+ T-cell counts were lower than those of the HIV-1− controls values (Fig. 1A).
FIGURE 1A,B. Longitudinal changes in mean peripheral blood lymphocyte counts (cells/μl) and serum Igs (mg/dl) in children born to HIV-1-infected women.
The lines represent the model-based means and 95% confidence intervals. The mean age of the infants at the time of first immunological study was 8.6 months (range 1.0–12.0 months). (A) CD4+ T-cell counts (n = 174 survivors, n = 81 nonsurvivors, and 453 HIV-1− controls). (B) CD8+ T-cell counts (n = 174 survivors, n = 81 nonsurvivors, and n = 453 HIV-1− controls).
Mean CD8+ T-cell numbers in survivors were always higher than those of the nonsurvivors (p ≤ 0.001) (Fig. 1B). Nonsurvivors and HIV-1− controls had similar CD8+ T-cell counts at the earliest ages (p = 0.21 at <1 year) but then nonsurvivors began to lose CD8+ T cells as they grew older and demonstrated CD8+ T-cell values lower than those of the HIV-1− controls (all p values ≤ 0.007 except at 2.5–3.0 years [p = 0.10]).
Survivors had significantly higher mean CD19+/20+ B-cell counts than did the nonsurvivors at most ages (< 1 year [p = 0.05], 1.0–1.5 years [p = 0.03], 1.5–2.0 years [p < 0.001], 2.0–2.5 years [p = 0.003], 2.5–3.0 years [p = 0.02], 3.0–3.5 years [p = 0.01], 3.5–4.0 years [p < 0.001], 4.0–4.5 years [p = 0.11] and 4.5–5.0 years [p = 0.01] (Fig. 1C). Both suvivors and nonsurvivors had values well below those of the HIV-1− controls with nonsurvivors being the lowest category.
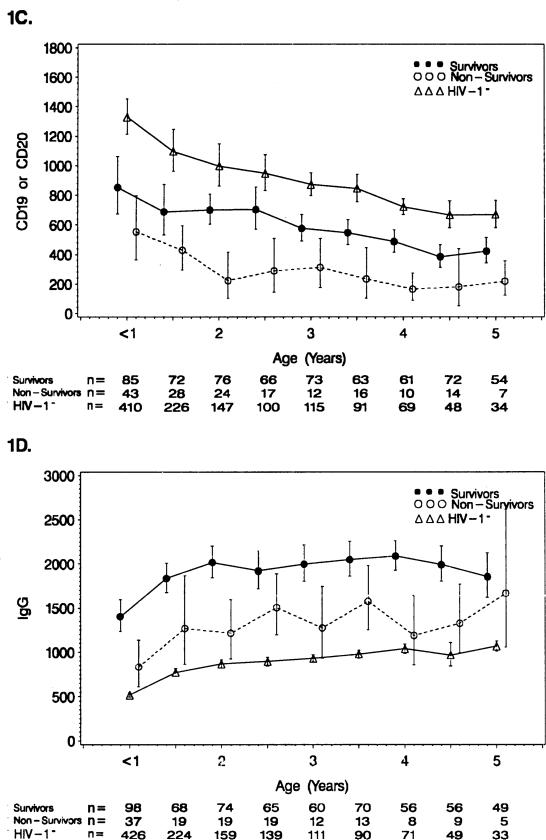
FIGURE 1C,D.
See Figure 1A,B legend. (C) CD19+ or CD20+ B-cell counts (n = 164 survivors, n = 72 nonsurvivors, and n = 435 HIV-1− controls). (D) Serum IgG (n = 159 survivors, n = 63 nonsurvivors, and n = 447 HIV-1− controls).
Serum Immunoglobulin Concentrations
Survivors had higher mean serum IgG values than nonsurvivors (< 1 year [p = 0.003], 1.0–1.5 years [p = 0.07], 1.5–2.0 years [p < 0.001], 2.0–2.5 years [p = 0.06], 2.5–3.0 years [p = 0.008], 3.0–3.5 years [p = 0.04], 3.5–4.0 years [p < 0.001], 4.0–4.5 years [p = 0.009], and 4.5–5.0 years [p = 0.66] (Fig. 1D). Both survivor and nonsurvivor IgG mean values were extremely elevated compared to the HIV-1− controls, with the survivor values being highest.
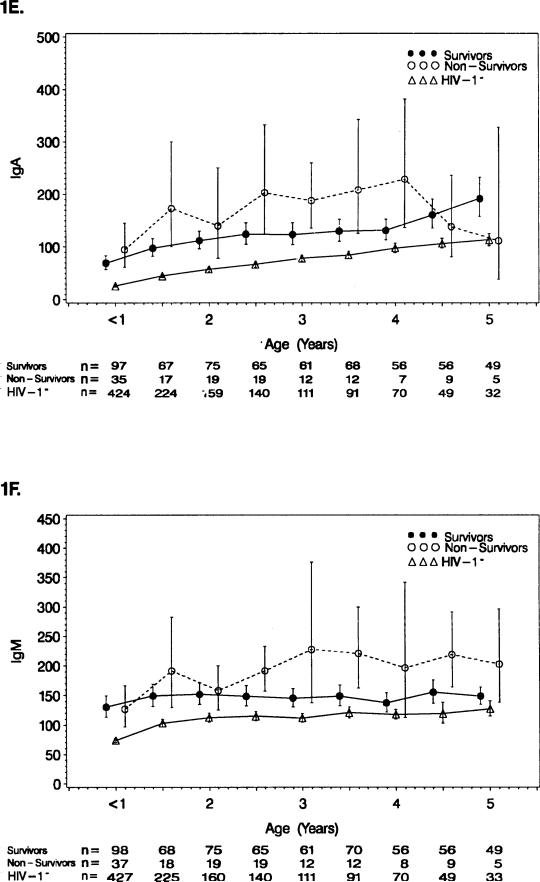
The survivors, in general, had lower mean serum IgA concentrations than the nonsurvivors through 4 years of age (1.0–1.5 years [p = 0.05], 2.5–3.0 years [p = 0.03], and 3.5–4.0 years [p = 0.04]) (Fig. 1E). Both survivors and nonsurvivors had mean values of serum IgA above the HIV-1− controls, with the survivor values being closer to the controls.
FIGURE 1E,F.
See Figure 1A,B legend. (E) Serum IgA (n = 159 survivors, n = 62 non-survivors, and n = 445 HIV-1− controls). (F) Serum IgM (n = 159 survivors, n = 62 nonsurvivors, and n = 445 HIV-1− controls).
Survivors generally had lower mean serum IgM concentrations than the nonsurvivors (2.0–2.5 years [p = 0.03], 3.0–3.5 years [p = 0.02], and 4.0–5.0 years [p = 0.03]) (Fig. 1F). The HIV-1− controls had mean IgM values below both other groups, but were closer to the survivors.
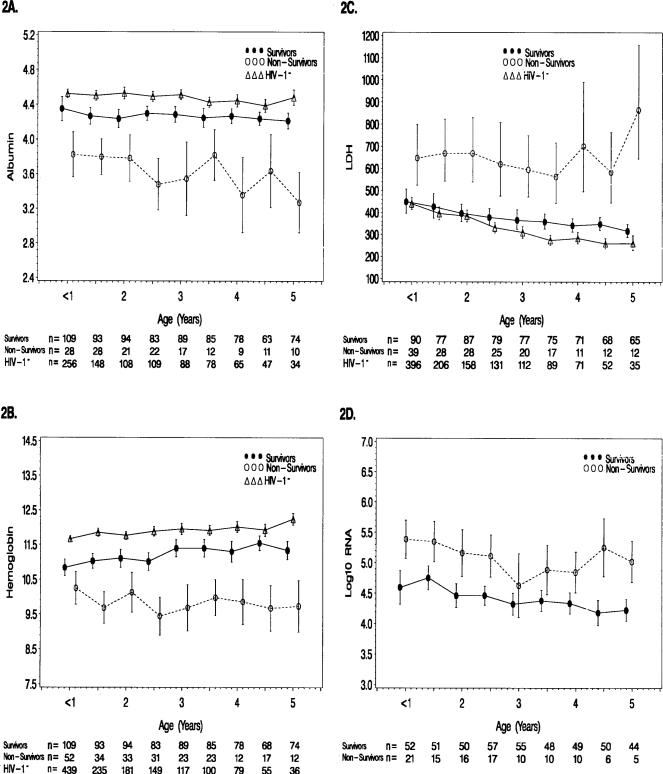
Albumin, Hemoglobin, and LDH
We also studied serum albumin, blood hemoglobin, and serum LDH levels (Fig. 2). Survivors had higher mean albumin (p ≤ 0.007 at all ages, Fig. 2A), higher mean hemoglobin (< 1 year [p = 0.03] and p ≤ 0.002 at all other ages, Fig. 2B), but lower LDH mean levels (p ≤ 0.003 at all ages, Fig. 2C). The HIV-1− controls exhibited mean values of serum albumin and blood hemoglobin that were higher than the other two groups but closest to the survivors, whereas the HIV-1− controls had lower mean LDH levels than both HIV-1+ groups but very close to the survivors before age two.
FIGURE 2. Longitudinal changes in proteins and HIV-1 RNA in children born to HIV-1-infected women.
The lines represent the model-based means and 95% confidence intervals. (A) Albumin (g/dl) (n = 168 survivors, n = 66 nonsurvivors, and n = 323 HIV-1− controls). (B) Hemoglobin (g/dl) (n = 176 survivors, n = 81 nonsurvivors, and n = 449 HIV-1− controls). (C) Lactic dehydrogenase (international units [IU]/l) (n = 170 survivors, n = 76 nonsurvivors, and n = 439 HIV-1− controls). (D) Log10 HIV-1 RNA (copies/ml) (n = 142 survivors and n = 47 nonsurvivors).
HIV-1 RNA Viral Burden of Infected Children
Mean levels of HIV-1 RNA were lower at all but one age for surviving children compared to nonsurvivors (< 1 year–2.5 years [p ≤ 0.002], 2.5–3.0 years [p = 0.28], and 3.0–5.0 years [p ≤ 0.02], Fig. 2D). For infants less than 1 year of age, mean HIV-1 RNA levels were significantly higher in nonsurvivors (geometric mean = 243,355 copies/ml) compared to survivors (geometric mean = 39,196 copies/ml, p < 0.001).
Survival Analysis
Univariable Survival
There was a 5-year cumulative survival rate of 72.3% in the 259 HIV-1+ children (83 deaths). Survival was associated with higher baseline CD4+ T-cell counts and higher baseline CD8+ T-cell counts by univariable analysis (Table 2). Children with higher baseline CD19+ or CD20+ B-cell counts also tended to have higher survival (p = 0.07). Survival was also associated with higher baseline levels of albumin, hemoglobin, and lower LDH. Higher baseline levels of IgG predicted better 5-year survival than lower baseline levels of IgG (89.5% and 61.5%, respectively). Lower baseline HIV-1 RNA levels also predicted 5-year survival.
TABLE 2.
Cumulative survival among 259 HIV-1-infected children according to baseline laboratory measurements
| Total |
Deaths |
5-year cumulative survival ± SE | p Value | |||
|---|---|---|---|---|---|---|
| Measurement | n | n | (%) | |||
| CD4 cell count | ≥ Median | 128 | 13 | (10.2) | 89.8 ± 2.8 | |
| < Median | 127 | 68 | (53.5) | 57.4 ± 4.5 | <0.001 | |
| CD8 cell count | ≥ Median | 127 | 25 | (19.7) | 85.2 ± 3.4 | |
| < Median | 128 | 56 | (43.8) | 61.9 ± 4.4 | <0.001 | |
| CD19 or CD20 cell count | ≥ Median | 118 | 27 | (22.9) | 80.3 ± 3.8 | |
| < Median | 118 | 45 | (38.1) | 69.0 ± 4.4 | 0.07 | |
| IgG | ≥ Median | 111 | 23 | (20.7) | 89.5 ± 3.0 | |
| < Median | 111 | 40 | (36.0) | 61.5 ± 4.9 | <0.001 | |
| IgA | ≥ Median | 112 | 35 | (31.3) | 70.3 ± 4.5 | |
| < Median | 109 | 28 | (25.7) | 82.4 ± 3.8 | 0.07 | |
| IgM | ≥ Median | 108 | 36 | (33.3) | 75.5 ± 4.3 | |
| < Median | 113 | 26 | (23.0) | 76.8 ± 4.2 | 0.47 | |
| Albumin | ≥ Median | 132 | 16 | (12.1) | 91.1 ± 2.6 | |
| < Median | 102 | 50 | (49.0) | 56.4 ± 5.1 | <0.001 | |
| Hemoglobin | ≥ Median | 138 | 31 | (22.5) | 83.1 ± 3.2 | |
| < Median | 119 | 50 | (42.0) | 61.3 ± 4.9 | <0.001 | |
| LDH | ≥ Median | 123 | 51 | (41.5) | 62.8 ± 4.7 | |
| < Median | 123 | 25 | (20.3) | 86.5 ± 3.2 | <0.001 | |
| HIV-1 RNA | ≥ Median | 94 | 32 | (34.0) | 68.5 ± 5.1 | |
| < Median | 95 | 15 | (15.8) | 89.9 ± 3.2 | <0.001 | |
Note: The median age of the children at the first available test was 9.8 months.
Multivariable Modeling of Survival
Multivariable analyses were affected by missing data and the correlation among the laboratory measurements. The sample sizes for the laboratory measurements were 257 for hemoglobin, 255 for CD4+ and CD8+ T-cell counts, 249 for LDH, 236 for CD19+ or CD20+ B-cell counts, 234 for albumin, 222 for IgG, 221 for IgA and IgM, and 189 for HIV-1 RNA. The correlation between CD4+ T-cell counts and CD8+ T-cell counts (Spearman's rho = 0.59, p < 0.001) and between albumin and hemoglobin (Spearman's rho = 0.52, p < 0.001) was high. Based on these relationships and the results from Cox regression analyses, CD4+ T-cell count, IgG, albumin, and HIV-1 RNA appear to be the best predictors of survival among the 10 laboratory measurements.
Table 3 summarizes the Cox regression analyses. In the final model (205 children, 52 deaths, Table 3A), CD4+ T-cell count, IgG, and albumin were independent prognostic factors of survival after adjusting for baseline age. Factors that did not remain significant included C19+ or CD20+ B-cell counts, CD8+ T-cell count, and LDH. Higher baseline levels of both albumin (adjusted relative risk = 1.67 per 0.5 g/dl decrease) and IgG (adjusted relative risk = 1.48 per 500 mg/dl decrease) thus appeared to provide independent markers of survival after adjustment for CD4+ T-cell count and age in HIV-1-infected children that may be clinically useful. In a subset analysis of 160 children for whom HIV-1 RNA copy number was available, CD4+ T-cell count, IgG, and albumin remained associated with survival, as well as HIV-1 RNA copy number (p = 0.03, Table 3B).
TABLE 3.
Multivariable analysis of factors associated with survival for children infected with HIV-1
| Effect | Relative risk of death | 95% CI | p Value |
|---|---|---|---|
| 3A | |||
| CD4 cell count (per 500 cells/μl decrease) | 1.76 | 1.35-2.28 | <0.001 |
| Serum IgG (per 500 mg/dl decrease) | 1.44 | 1.20-1.74 | <0.001 |
| Albumin (per 0.5 g/dl decrease) | 1.53 | 1.25-1.86 | <0.001 |
| 3B | |||
| CD4 cell count (per 500 cells/μl decrease) | 1.45 | 1.11-1.91 | 0.007 |
| Serum IgG (per 500 mg/dl decrease) | 1.52 | 1.20-1.52 | <0.001 |
| Albumin (per 0.5 g/dl decrease) | 2.20 | 1.52-3.19 | <0.001 |
| HIV-1 RNA (per 0.5 log10 unit increase) | 1.27 | 1.02-1.57 | 0.03 |
DISCUSSION
This new analysis of the immune factors in the 5-year P2C2 HIV-1 Study has confirmed the projections of the early report.1 In that report, lower CD4+ T-cell and higher CD8+ T-cell counts appeared to be associated with advanced disease in several age categories, as well as with increased morbidity. Here, we extend these find- ings to demonstrate that the survivor children had a unique age profile of peripheral blood CD4+ T-cells, thus adding useful information about lymphocyte subset counts in various stages of HIV-1 infection. Higher CD4+ T-cell counts were found in survivor children at every age, emphasizing the central role of this T-cell subset in protection from HIV-1 disease progression. At all ages, the CD8+ T-cell counts were higher in survivors than nonsurvivors. At early ages, the numbers of CD8+ T-cells in nonsurvivor children were similar to those of HIV-1− controls but later fell to lower levels, suggesting the protective role these cells play in HIV-1 infection. Polyclonal stimulation by Epstein-Barr virus and possibly cytomegalovirus may be responsible for the increased number of CD8+ T cells in survivor children.22,23 Coinfection with Epstein-Barr virus has been associated with increased survival.13,14,16,17
In addition to observations on T cells, an interesting finding concerning B-cell regulation of IgG has emerged from univariable analysis, indicating that higher concentration of serum IgG was associated with predicted survival of HIV-1+ children. This discovery was Ig class specific, since serum IgA and IgM concentrations of the survivors were generally lower than those of the nonsurvivors. These observations are consistent with the early reports of lack of immunoglobulin class switching from IgM to IgG production in HIV-1+ patients.8–10 Thus, nonsurvivors are likely those that can only mount IgM (primary) antibody responses due to lack of adequate T-cell help to switch from IgM to IgG production. Although this report did not find that higher numbers of B cells were important in 5-year survival, other reports have documented decreases in the following: (a) CD19+/CD5+ B-cell compartment in 7- to 12-month-old HIV-1+ infants,24 (b) CD19+ B-cell numbers in more symptomatic HIV+ children,25 and (c) functionally active B cells in HIV+-1 patients with low p24 antibody serum titers.26 Moreover, increased CD19+ B-cell subsets have been described in pediatric HIV-1+ patients given the protease inhibitor ritonavir.27,28 Both CD19+/CD20+ B-cell counts and serum IgG measurements have been recently shown to be important in the prediction of bacterial infections.29 The mechanism of elevation of serum Igs in HIV-1 infection may have been partially clarified by the in vitro evidence that HIV-1 glycoprotein 120 acts as a superantigen and produces stimulation of several human B-cell functions, including the increased production of Igs.30 All of these observations indicate the need to re-evaluate the role of B cells and IgG in HIV-1 disease progression and survival.
This update of the P2C2 HIV-1 Study has examined the measurements of blood T-cell and B-cell counts, Ig, albumin, hemoglobin, LDH, and HIV-1 RNA concentrations in a 5-year study of 298 HIV-1+ children. Serum IgG was discovered to have predictive power for survival, in addition to CD4+ T-cells, HIV-RNA, and serum albumin.
ACKNOWLEDGMENTS
We are grateful to the investigators, the study staff, and the families who participated in the P2C2 HIV Study. This work was supported by National Heart, Lung, and Blood Institute Grants N01-HR-96037, N01-HR-96038, N01-HR-96039, N01-HR-96040, N01-HR-96041, N01-HR-96042, and N01-HR-960043 and in part by NIH General Clinical Research Center Grants RR-00071, RR-00188, RR-00533, RR-00643, RR-00645, RR-00865, and RR-02172.
Appendix
A complete list of study participants can be found in reference #19.
NATIONAL HEART, LUNG AND BLOOD INSTITUTE
Hannah Peavy, M.D., (Project Officer), Anthony Kalica, Ph.D., Elaine Sloand, M.D., George Sopko, M.D., M.P.H., Margaret Wu, Ph.D.
Chairman of the Steering Committee: Robert Mellins, M.D.
Clinical Centers
Baylor College of Medicine, Houston, TX: William Shearer, M.D., Ph.D.,* Nancy Ayres, M.D., J. Timothy Bricker, M.D., Arthur Garson, Jr., M.D., Peter Hiatt,
*Principal Investigator.
M.D., Debra Kearney, M.D., Howard M. Rosenblatt, M.D., Linda Davis, R.N., B.S.N., Paula Feinman, Mary Beth Mauer, R.N., B.S.N., Ruth McConnell, R.N., B.S.N., Debra Mooneyham, R.N., Teresa Tonsberg, R.N.
The Children's Hospital, Boston/Harvard Medical School, Boston, MA:
Steven Lipshultz, M.D.,* Steven Colan, M.D., Andrew Colin, M.D., Ellen Cooper, M.D., Lisa Hornberger, M.D., Kenneth McIntosh, M.D., Marcy Schwartz, M.D., Suzanne Steinbach, M.D., Mary Ellen Wohl, M.D., Helen Donovan, Janice Hunter, M.S., R.N., Karen Lewis, R.N., Ellen McAuliffe, B.S.N., Patricia Ray, B.S., Sonia Sharma, B.S.
Mount Sinai School of Medicine, New York, NY: Meyer Kattan, M.D.,* Stephen Heaton, M.D., David Hodes, M.D., Wyman Lai, M.D., Andrew Ting, M.D., Debbie Benes, M.S., R.N., Diane Carp, M.S.N., R.N., Donna Lewis, Sue Mone, M.S., Mary Ann Worth, R.N.
Presbyterian Hospital in the City of New York/Columbia University, New York, NY: Robert Mellins, M.D.,* Anastossios Koumbourlis, M.D., Jane Pitt, M.D., Thomas Starc, M.D., Anthony Brown, Margaret Challenger, Kim Geromanos, M.S., R.N.
UCLA School of Medicine, Los Angeles, CA: Samuel Kaplan, M.D.,* Yvonne Bryson, M.D., Joseph Church, M.D., Arno Hohn, M.D., Andrea Kovacs, M.D., Barry Marcus, M.D., Arnold Platzker, M.D., Helene Cohen, P.N.P., R.N., Lynn Fukushima, M.S.N., R.N., Audrey Gardner, B.S., Sharon Golden, R.D.M.S., Lucy Kunzman, R.N., M.S., C.P.N.P., Karen Simandle, R.D.M.S., Ah-Lin Wong, R.D.M.S., Toni Ziolkowski, R.N., M.S.N.
Clinical Coordinating Center
The Cleveland Clinic Foundation, Cleveland, OH: Kirk Easley, M.S.,* Michael Kutner, Ph.D. (through 12/99),* Mark Schluchter, Ph.D. (through 4/98),* Richard Martin, M.D. (Case Western Reserve University), Johanna Goldfarb, M.D., Douglas Moodie, M.D., Cindy Chen, M.S., Scott Husak, B.S., Victoria Konig, ART, Sunil Rao, Ph.D., Paul Sartori, B.S., Lori Schnur, B.S., Amrik Shah, Sc.D., Sharayu Shanbhag, B.Sc, Susan Sunkle, B.A., C.C.R.A.
Policy, Data, and Safety Monitoring Board
Henrique Rigatto, M.D., (Chairman), Edward B. Clark, M.D., Robert B. Cotton, M.D., Vijay V. Joshi, M.D., Paul S. Levy, Sc.D., Norman S. Talner, M.D., Patricia Taylor, Ph.D., Robert Tepper, M.D., Ph.D., Janet Wittes, Ph.D., Robert H. Yolken, M.D., Peter E. Vink, M.D.
REFERENCES
- 1.Shearer WT, Rosenblatt HM, Schluchter MD, et al. Immunologic targets of HIV infection: T-cells. NICHD IVIG Clinical Trial Group, and the NHLBI P2C2. Pediatric Pulmonary and Cardiac Complications of HIV Infection Study Group. Ann. N.Y. Acad. Sci. 1993;693:35–51. doi: 10.1111/j.1749-6632.1993.tb26255.x. [DOI] [PubMed] [Google Scholar]
- 2.Shearer WT, Quinn TC, Larussa P, et al. Women and Infants Transmission Study Group. Viral load and disease progression in infants infected with human immunodeficiency virus type 1. N. Engl. J. Med. 1997;336(19):1337–1342. doi: 10.1056/NEJM199705083361901. [DOI] [PubMed] [Google Scholar]
- 3.Palumbo PE, Raskino C, Fiscus S, et al. Predictive value of quantitative plasma HIV RNA and CD4+ lymphocyte count in HIV-infected infants and children. JAMA. 1998;279:756–761. doi: 10.1001/jama.279.10.756. [DOI] [PubMed] [Google Scholar]
- 4.Mofenson LM, Harris DR, Rich K, et al. Serum HIV-1 p24 antibody, HIV-1 RNA copy number and CD4 lymphocyte percentage are independently associated with risk of mortality in HIV-1 infected children. AIDS. 1996;13:31–39. doi: 10.1097/00002030-199901140-00005. [DOI] [PubMed] [Google Scholar]
- 5.Bernstein LJ, Krieger BZ, Novick B, et al. Bacterial infection in the acquired immunodeficiency syndrome of children. Pediatr. Infect. Dis. 1985;4:472–475. doi: 10.1097/00006454-198509000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Blanche S, Le Deist F, Fischer A, et al. Longitudinal study of 18 children with perinatal LAV/HTLV III infection: attempt at prognostic evaluation. J. Pediatr. 1986;109:965–970. doi: 10.1016/s0022-3476(86)80277-3. [DOI] [PubMed] [Google Scholar]
- 7.Krasinski K, Borkowsky W, BONK S, et al. Bacterial infections in human immunodeficiency virus-infected children. Pediatr. Infect. Dis. J. 1988;7:323–328. doi: 10.1097/00006454-198805000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Bernstein LJ, Ochs HD, Wedgwood RJ, et al. Defective humoral immunity in pediatric acquired immune deficiency syndrome. J. Pediatr. 1985;107:352–357. doi: 10.1016/s0022-3476(85)80505-9. [DOI] [PubMed] [Google Scholar]
- 9.Borkowsky W, Steele CJ, Grubman S, et al. Antibody responses to bacterial toxoids in children infected with human immunodeficiency virus. J. Pediatr. 1987;110:563–566. doi: 10.1016/s0022-3476(87)80549-8. [DOI] [PubMed] [Google Scholar]
- 10.Pahwa S, Fikrig S, Menez R, et al. Pediatric acquired immunodeficiency syndrome: demonstration of B lymphocyte defects in vitro. Diagn. Immunol. 1986;4:24–30. [PubMed] [Google Scholar]
- 11.Rubinstein A, Mizrachi Y, Bernstein L, et al. Progressive specific immune attrition after primary, secondary and tertiary immunizations with bacteriophage φX174 in asymptomatic HIV-1 infected patients. AIDS. 2000;14:F55–F62. doi: 10.1097/00002030-200003100-00004. [DOI] [PubMed] [Google Scholar]
- 12.Fuleihan RL, Geha RS. X-linked hyper IgM. The immunologist. 1997;5:133–136. [Google Scholar]
- 13.Pollack H, Zhan MX, Safrit JT, et al. CD8+ T-cell-mediated suppression of HIV replication in the first year of life: association with lower viral load and favorable early survival. AIDS. 1997;11:F9–F13. doi: 10.1097/00002030-199701000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Krasinski K, Borkowsky W, Holzman RS, et al. Prognosis of human immunodeficiency virus infection in children and adolescents. Pediatr. Infect. Dis. 1989;8:216–220. [PubMed] [Google Scholar]
- 15.Tovo PA, De Martino M, Gabiano C, et al. Prognostic factors and survival in children with perinatal HIV-1 infection. Lancet. 1992;339:1249–1253. doi: 10.1016/0140-6736(92)91592-v. [DOI] [PubMed] [Google Scholar]
- 16.Italian Register for Hiv Infection in Children Features of children perina-tally infected with HIV-1 surviving longer than 5 years. Lancet. 1994;343:191–195. [PubMed] [Google Scholar]
- 17.Kline MW, Paul ME, Bohannon B, et al. Characteristics of children surviving to five years of age or older with vertically acquired human immune deficiency infection. Pediatr. AIDS HIV Infect. 1995;6:350–353. [PubMed] [Google Scholar]
- 18.Shearer WT, Lipshultz SE, Easley KA, et al. for the Pediatric Pulmonary and Cardiovascular Complications of Vertically Transmitted Human Immunodeficiency Virus Study Group Alterations in cardiac and pulmonary function in pediatric rapid HIV-1 disease progressors. Pediatrics. 2000;105:e9. doi: 10.1542/peds.105.1.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The P2C2 HIV Study Group The Pediatric Pulmonary and Cardiovascular Complications of Vertically Transmitted Human Immunodeficiency Virus (P2C2 HIV) Infection Study: design and methods. J. Clin. Epidemiol. 1996;49:1285–1294. doi: 10.1016/s0895-4356(96)00230-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention 1994 revised classification system for human immunodeficiency virus infection in children less than 13 years of age. Morbid. Mortal. Wkly. Rep. 1994;43(RR-12):1–20. [Google Scholar]
- 21.Lipshultz SE, Easley KA, ORAV EJ, et al. for the Pediatric Pulmonary and Cardiac Complications of Vertically Transmitted HIV Infection (P2C2 HIV) Study Group. Cardiac dysfunction and mortality in HIV-infected children: The prospective P2C2 HIV multicenter study. Circulation. 2000;102 doi: 10.1161/01.cir.102.13.1542. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jenson H, McIntosh K, Pitt J, et al. Natural history of primary Epstein-Barr virus infection in children of mothers infected with human immunodeficiency virus type 1. J. Infect. Dis. 1999;179:1395–1404. doi: 10.1086/314764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kovacs A, Schluchter M, Easley K, et al. Cytomegalovirus infection and HIV-1 disease progression in infants born to HIV-1-infected women. Pediatric Pulmonary and Cardiovascular Complications of Vertically Transmitted HIV Infection Study Group. N. Engl. J. Med. 1999;341:77–84. doi: 10.1056/NEJM199907083410203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ibegbu CH, Spira TJ, Nesheim S, et al. Subpopulations of T and B cells in perinatally infected and non-infected age-matched children compared to those in adults. Clin. Immunol. Immunopathol. 1994;71:27–32. doi: 10.1006/clin.1994.1047. [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez C, Stiehm ER, Plaeger-Marshall S. Peripheral B-cell activation and immaturity in HIV-infected children. Ann. NY. Acad. Sci. 1993;693:291–294. doi: 10.1111/j.1749-6632.1993.tb26287.x. [DOI] [PubMed] [Google Scholar]
- 26.Teeuwsen VJ, Lange JM, Keet R, et al. Low number of functionally active B lymphocytes in the peripheral blood of HIV-1-seropositive individuals with low p24-specific serum antibody titers. AIDS. 1991;5:971–979. doi: 10.1097/00002030-199108000-00008. [DOI] [PubMed] [Google Scholar]
- 27.Sleasman JW, Nelson RP, Goodenow MM, et al. Immunoreconstitution after ritonavir therapy in children with human immunodeficiency virus infection involves multiple lymphocyte lineages. Pediatrics. 1999;134:597–606. doi: 10.1016/s0022-3476(99)70247-7. [DOI] [PubMed] [Google Scholar]
- 28.Borkowsky W, Stanley K, Douglas SD, et al. the Pediatric AIDS Clinical Trials Group 338 Study Team Immunologic response to combination nucleoside analogue plus protease inhibitor therapy in stable antiretroviral therapy-experienced HIV-infected children. J. Infec. Dis. 2000;182:96–103. doi: 10.1086/315672. [DOI] [PubMed] [Google Scholar]
- 29.Betensky RA, Calvelli T, Pahwa S. Predictive value of CD19 measurements for bacterial infections in children infected with human immunodeficiency virus. Clin. Diag. Lab. Immunol. 1999;6:247–253. doi: 10.1128/cdli.6.2.247-253.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patke CL, Shearer WT. gp120- and tumor necrosis factor-α-induced modulation of human B-cell function: proliferation, cAMP generation, immunoglobulin production, and B-cell receptor expression. J. Allergy Clin. Immunol. 2000;105:975–982. doi: 10.1067/mai.2000.105315. [DOI] [PubMed] [Google Scholar]