Abstract
Patients surviving the acute stages of sepsis develop compromised T cell immunity and increased susceptibility to infection. Little is known about the decreased CD4 T cell function after sepsis. We tracked the loss and recovery of endogenous Ag-specific CD4 T cell populations after cecal-ligation and puncture (CLP)-induced sepsis, and analyzed the CD4 T cell response to heterologous infection during or after recovery. We observed that the sepsis-induced early loss of CD4 T cells was followed by thymic-independent numerical recovery in the total CD4 T cell compartment. Despite this numerical recovery, we detected alterations in the composition of naïve CD4 T cell precursor pools, with sustained quantitative reductions in some populations. Mice that had experienced sepsis and were then challenged with epitope-bearing, heterologous pathogens demonstrated significantly reduced priming of recovery-impaired Ag-specific CD4 T cell responses, both in magnitude of expansion and functional capacity on a per-cell basis, which also correlated with intrinsic changes in Vβ clonotype heterogeneity. Our results demonstrate the recovery of CD4 T cells from sepsis-induced lymphopenia is accompanied by alterations to the composition and function of the Ag-specific CD4 T cell repertoire.
Introduction
CD4 T helper (Th) cells influence the function of a variety of innate and adaptive immune cells critical for the successful generation of a productive and protective immune response (1). For example, effective primary CD8 T cell responses (2, 3), the formation of functional CD8 T cell memory (4-7), efficient isotype switching in primary and memory B cell responses (8, 9), and the effector function of macrophages (10) all develop with the “help” of CD4 T cells. The ability of CD4 T cells to function in such an array of immunological settings is because effector CD4 T cells can take on different phenotypes (i.e., Th1, Th2, Th9, Th17, Tfh (1)), based on the cytokines and costimulatory molecules present at the time of Ag recognition. In turn, this plasticity enables CD4 T cells to drive a response that is best suited for the situation. Due to their importance in a broad variety of immune responses, perturbations in the CD4 T cell compartment can have dramatic consequences on the overall fitness of the immune system.
Sepsis strikes 750,000 Americans every year (11) with ~210,000 of these patients dying (12). Although sepsis has been defined as a systemic inflammatory response syndrome (SIRS) in the presence of a disseminated infection (13-15), it has become clear in the past decade that sepsis is not just the symptoms of a complicated infection. Instead, sepsis is now viewed as a syndrome stemming from the dysregulation of immune responses due to an invasive pathogen – a phenomenon that results in system-wide collateral damage (16). Sepsis-induced immune suppression is intricately related to the process of lymphocyte apoptosis that occurs after a septic event (17, 18). Sepsis-induced lymphopenia transiently creates a reduction in numbers of immune cells, including T cells. While the total T cell compartment recovers numerically after a septic event, it is unknown whether different Ag-specific T cell subpopulations can revert back to the antigenic diversity seen before sepsis, and whether changes in population diversity can affect the functionality of the immune system. Gross quantitation of CD4 T cells reveals that they are severely depleted during the acute stage of sepsis but gradually recover throughout the immunosuppressive phase of sepsis (19). However, there are knowledge gaps regarding the mechanism(s) driving this CD4 T cell recovery, the quality/functionality of the “recovered” CD4 T cell compartment, and the extent to which sepsis impairs Ag-specific CD4 T cell function in surviving animals.
In this study, we used peptide:MHC II (p:I-Ab) tetramer enrichment technology (20) to examine quantitative shifts within the endogenous naïve Ag-specific CD4 T cell repertoire at different time points after sepsis. Our findings suggest that the numerical restoration of the CD4 T cell repertoire after sepsis occurs via a peripherally-driven mechanism that is, in part, independent of Ag availability. And while the total CD4 T cell population recovers numerically, examination of individual Ag-specific populations revealed an asymmetric recovery in different Ag-specific precursor populations. Our results also suggest that, if inadequately recovered, Ag-specific CD4 T cell populations show impairments in expansion and function in response to pathogen challenge after sepsis. The implications of these findings within the context of long-term increased susceptibility to secondary infections (and the associated increased risk of mortality) will be discussed.
Materials and Methods
Mice
Euthymic and thymectomized C57BL/6 (B6) mice were purchased from The National Cancer Institute. Thy1.1/1.1 TCR-transgenic SMARTA (LCMV gp61-77-specific) and SM1 (S.typhimurium FliC447-460-specific) B6 mice were obtained from Drs. David Masopust and Marc Jenkins (University of Minnesota), respectively. All mice were housed in the same facilities for at least 4 weeks, regardless of their source. Animal procedures were performed according to National Institutes of Health guidelines and approved by the University of Minnesota Institutional Animal Care and Use Committee. In all in vivo experiments, groups consisted of four or more animals, and experiments were repeated at least two times with similar results before reporting.
Cecal ligation and puncture
Septic injury was induced by cecal ligation and puncture (CLP) (21). Briefly, mice were anesthetized and the abdomen was shaved, disinfected, and a midline abdominal incision was made. The distal third of the cecum was ligated with 4–0 silk suture and punctured once using a 25-g needle to extrude a small amount of cecal content. The cecum was returned to the abdomen, the peritoneum was closed via continuous suture, and the skin was sealed using surgical glue (Vetbond, 3M, St. Paul, MN). Saline (1 ml) was provided s.c. following the procedure for resuscitation, and Bupivicaine was administered at the incision site for postoperative analgesia. This level of injury was used to create a chronic septic state characterized by the loss of appetite and body weight, ruffled hair, shivering, diarrhea, and/or periorbital exudates, and with a 5-10% mortality rate. Sham-treated mice underwent the same procedure excluding cecal ligation and puncture.
BrdU incorporation and detection
To assess CD4 T cell proliferation, sham- and CLP-treated mice were given a BrdU pulse (2 mg in 0.2 ml/mouse i.p.; Sigma, St. Louis, MO) on d 6 after surgery. Blood was collected at the indicted times, followed by RBC lysis in ACK buffer. Cells were surface stained with PE CD4 (clone GK1.5; BioLegend), after which they were fixed with Cytofix/Cytoperm solution (BD Biosciences; San Diego, CA) and treated with DNase I (300 mg/ml in PBS; Sigma) for 1 h. BrdU was detected by intracellular staining with FITC-conjugated anti-BrdU (clone BU20A) or an IgG1 isotype (clone P3.6.2.8.1) mAb (both from eBioscience; San Diego, CA).
Adoptive cell transfers
SM1 or SMARTA TCR-tg CD4 T cells were obtained from the spleens of naïve SMARTA or SM1 mice. Contaminating memory phenotype (CD44hi CD11ahi CD49hi) TCR-tg cells were consistently <5%. The purified cells were transferred to naïve B6 mice 1 day before sham or CLP surgery.
Experimental pathogens and infections
2W1S (EAWGALANWAVDSA)-expressing or OVA323-339 (ISQAVHAAHAEINEAGR)-expressing, ActAΔ L. monocytogenes (attenuated Lm-2W1S or Lm-OVA, 107 PFU/mouse) was grown and injected i.v. as previously described (22). 2W1S-expressing C.albicans (C. albicans-2W1S) was derived from clinical isolate SC5314 (23). C. albicans-2W1S was grown to log phase (OD600 of 1.5) in YDAP medium, washed, and counted by hemocytometer before being resuspended at a concentration of 5×104 yeasts per i.v. challenge. Recombinant Lm-2W1S and C. albicans-2W1S were obtained from Drs. Marc Jenkins and Daniel Kaplan (University of Minnesota), respectively. For HSV-1 (KOS strain) and influenza A virus (strain ×31) inocula, frozen viral stocks were thawed and 2.5×104 PFU and 3000 EID50 units, respectively, was administered per mouse. Viral stocks were obtained from culture conditions that have been previously described (24-26). Intravenous challenges using 0.1 ml injection volumes were used for all of the inoculants described, except for IAV ×31 given intranasal using 0.02 ml aliquots per nostril. Infected mice were housed under the appropriate biosafety level.
Tetramers and peptides
I-Ab–specific tetramers containing 2W1S (EAWGALANWAVDSA), LCMV glycoprotein (gp)66-77 (DIYKGVYQFKSV), LLO190-201 (NEKYAQAYPNVS), OVA323-339 (ISQAVHAAHAEINEAGR) peptides were obtained from Dr. Marc Jenkins. Biotinylated soluble I-Ab molecules containing HSV glycoprotein D (gD)290-305 (IPPNWHIPSIQDA) or influenza A virus nucleoprotein (NP)311-325 (QVYSLIRPNENPAHK) peptides (27, 28) covalently attached to the I-Ab beta chain were produced with the I-Ab alpha chain in Drosophila melanogaster S2 cells, then purified, and made into tetramers with streptavidin (SA)- phycoerythrin (PE; eBioscience) or SA-allophycocyanin (APC; Biolegend) as previously described (20, 29). Peptides used to elicit cytokine production or expand endogenous Vβ repertoire for quantification were synthesized by Bio-Synthesis (Louisville, TX).
Quantitation of endogenous Ag-specific CD4 T cell populations using p:I-Ab tetramer based-enrichment
To quantify the number of Ag-specific CD4 T cells within the spleens of sham- or CLP-treated mice, a tetramer-based enrichment protocol (29) using p:I-Ab tetramers was employed. Briefly, spleens were harvested for each mouse analyzed, a single-cell suspension was prepared, and APC- and PE-conjugated tetramers were added at a 1:400 dilution in tetramer staining buffer (PBS containing 5% BCS, 2mM EDTA, and 50μM Dasatinib, 1:50 normal mouse serum, and 1:100 anti-CD16/32 mAb). The cells were incubated in the dark at room temperature for 1 h, followed by a wash in 10 ml cold FACS Buffer. The tetramer-stained cells were then resuspended in 0.2 ml FACS Buffer, mixed with 0.05 ml of both anti-APC and –PE mAb-conjugated magnetic microbeads (Miltenyi Biotech), and incubated in the dark on ice for 30 min. The cells were washed and resuspended in 3 ml cold FACS Buffer and passed over a MACS separation column (Miltenyi Biotech) to enrich for the tetramer-specific cells. Columns were washed three times with 3 ml cold FACS buffer, before eluting the bound fraction with 5 ml cold FACS buffer. The resulting enriched fractions were then stained with a cocktail of fluorochrome-labeled mAb (see below). Cell numbers for each sample were determined using AccuCheck Counting Beads (Invitrogen). Samples were then analyzed using an LSR II flow cytometer (BD) and FlowJo software (TreeStar Inc., Ashland, OR). The percentage of tetramer-positive events was multiplied by the total number of cells in the enriched fraction to calculate the total number of Ag-specific CD4 T cells in the spleen.
CD4 T cell assays
In vivo peptide stimulation was used to determine Ag-specific CD4 T cell function by intracellular cytokine production, as previously described (22, 30, 31). Briefly, infected mice were injected i.v. with 100 μg of the appropriate peptide. After 2 h, spleens were harvested in media containing 10 μg/ml brefeldin A. The resulting cell suspensions were fixed, permeabilized, and stained with anti-IFNγ and anti-TNF mAb. To specifically examine the function of Ag-specific Th17 cells sham and CLP-treated mice were infected 30 d after surgery with C. albicans-2W1S epicutaneously (23). On d 7 post-infection, the spleen and skin-draining (inguinal, brachial, axillary and cervical) LN were harvested, and dissociated into a single-cell suspension. The resultant cells were stimulated for 4 h with PMA (50 ng/ml) and ionomycin (1.5 μM) in complete RPMI media supplemented with monensin (1 μM). After stimulation, cell debris was filtered and the samples underwent tetramer enrichment as described. After tetramer enrichment and subsequent staining for cell surface markers, 0.1 ml aliquots from the enriched and flow-through fractions were suspended in fixation/permeabilization buffer (eBioscience) for 20 min at 4°C, and then stained for intracellular IFNγ and IL-17 accumulation overnight in permeabilization buffer (eBioscience). After staining, cells were resuspended in FACS buffer and 0.02 ml of counting beads (eBioscience) were added to each sample immediately before acquisition.
Flow cytometry
To assess the expression of cell surface proteins, cells were incubated with fluorochrome-conjugated mAb at 4°C for 30 min. The cells were then washed with FACS buffer (PBS containing 2% BCS and 0.2% NaN3). For some experiments, the cells were then fixed with PBS containing 2% paraformaldeyhe. In procedures requiring intracellular staining, cells were permeabilized following surface staining using the transcription factor staining kit (eBioscience), stained for 1 h at 4°C with a second set of fluorochrome-conjugated mAb, and suspended in FACS buffer for acquisition. The fluorochrome-conjugated mAb used in both surface and intracellular stainings were as follows: Horizon™ V500 Thy1.2 (clone 53-2.1; BD Biosciences), Brilliant Violet™ (BV) 510 and FITC CD3 (clone 17A2; BioLegend), BV421 and BV605 CD4 (clone GK1.5; BioLegend), BV650 CD8 (clone 53-6.7; BioLegend), AlexaFluor®700 CD44 (clone IM7; BioLegend), APC and BV421 IL17A (clone TC11-18H10.1; BioLegend), APC and BV650 IFNγ (clone XMG1.2; BioLegend), PE-Cy7 IL2 (clone JES6-5H4; BioLegend), AlexaFluor®647 CD49d (clone R1-2; BioLegend), PerCP-Cy5.5 B220 (clone RA3-6B2; eBioscience), PerCP-Cy5.5CD11b (clone M1/70; eBioscience), PerCP-Cy5.5CD11c (clone N418; eBioscience), PerCP-Cy5.5 F4/80 (clone BM8; eBioscience), FITC FoxP3 (clone FJK-15S; eBioscience), FITC CD11a (clone M17/4; eBioscience), FITC TNFα (clone MP6-XT22; eBioscience), and PE-Cy7 CD11a (clone M17/4; eBioscience). FlowJo software (TreeStar) was used for analysis of samples acquired on an LSR II flow cytometer (BD).
Ag-specific TCR Vβ repertoire flow cytometry assay
Assessment of TCR Vβ repertoire diversity performed using a modification of a previously reported method (29, 32). Briefly, sham- or CLP-treated mice were injected with 50 μg of 2W1S peptide and 5 μg LPS on d 30 post-surgery. After another 3 d, splenic T cells were enriched for APC-2W1S:I-Ab tetramer-binding cells. The enriched population was subsequently divided into 2 equal aliquots and stained for surface markers along with 13 available TCR Vβ mAb multiplexed onto four separate flow cytometry detection channels, using directly conjugated antibodies and/or biotinylated mAb detected afterwards with Streptavidin-BV421 (Biolegend; San Diego, CA). The TCR Vβ mAb used were: FITC-conjugated mAb against mouse Vβ 2 (clone B20.6), Vβ 4 (clone KT4), Vβ 6 (clone RR4-7), Vβ 7 (clone TR310), Vβ 8.1/8.2 (clone KJ16-133.18) and Vβ 8.3 (clone 8C1); PE-conjugated mAb against Vβ 5.1/5.2 (clone MR9-4), Vβ 8.1/8.2 (clone MR5-2), Vβ 8.3 (clone 1B3.3), Vβ 9 (clone MR10-2) and Vβ 10 (clone B21.5); PerCP-eFluor®710 conjugated Vβ13 (clone MR12-3); and biotinylated mAb against Vβ 3 (clone KJ25), Vβ 4, V 5.1/5.2, V 6, V 10 and Vβ 14 (clone 14-2). Unless specified, mAb that detected the same TCR Vβ clonotype were from the same clone and vendor. All mAb used to detect Vβ clonotype distribution were purchased from either BD Biosciences, Biolegend, or eBioscience.
Statistical analyses
Data were analyzed using GraphPad Prism® (La Jolla, CA). Specific tests to determine statistical significance are indicated in the figure legends. Statistical signficance is indicated as follows: **** p < 0.001, *** p < 0.005, ** p < 0.01, * p < 0.05, and ns, no significance. Data scatter plots are presented as mean values ± SEM, and data shown as bar graphs are presented as mean ± SEM.
Results
Numerical recovery of CD4 T cells after sepsis occurs by a thymic-independent mechanism
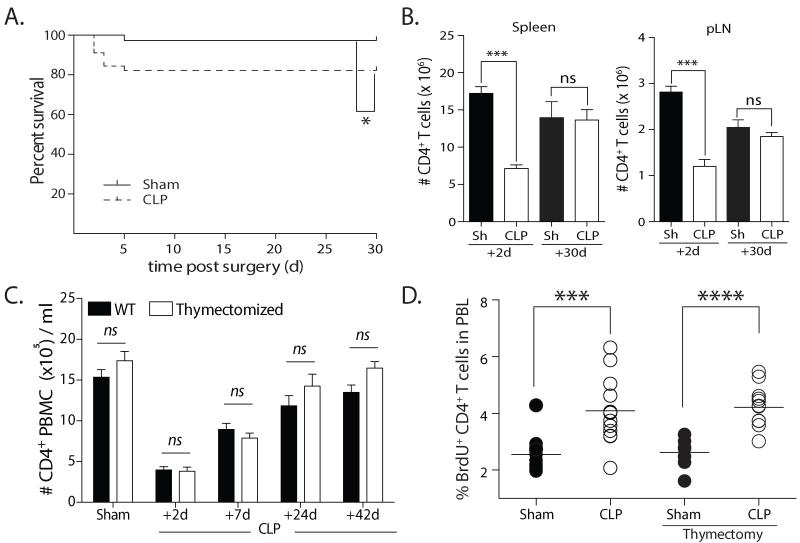
Sepsis can be experimentally investigated using the cecal ligation and puncture (CLP) model (21), which is frequently used to assess the acute complications and mortality associated with severe septic events. The CLP model used in our studies induces a mild septic state resulting in ~10% acute mortality (Fig. 1A). This degree of injury creates immune defects similar to more severe models and permits the long-term study of immune system responses in septic mice (33-35). In addition, mice that experience this milder sepsis demonstrate the same symptomatology characteristic of severe experimental peritonitis, including cachexia, weight loss, piloerection, and lethargy. Consistent with previous data (19), we found a significant decrease in the total number of CD4 T cells in the spleen, inguinal LN, and blood 2 d after septic injury and a numerical recovery apparent by d 30 (Fig. 1B-C). These results led us to conclude that the attenuated CLP procedure that produces a mild septic insult can be used to interrogate the CD4 T cell loss and recovery in the context of sepsis.
FIGURE 1. Numerical recovery of CD4 T cells after cecal ligation and puncture (CLP)-induced sepsis occurs by a thymus-independent mechanism.
A. Kaplan-Meier survival curve of experimental cohorts after undergoing sham or CLP surgery. B. Number of CD4 T cells in spleen and inguinal lymph nodes (pLN) on d 2 and 30 after sham or CLP surgery. C. Thymectomized and euthymic mice underwent CLP surgery, and the number of CD4 T cells in the peripheral blood was measured over time. D. Thymectomized and euthymic mice underwent sham or CLP surgery. BrdU was injected i.p. 6 d later, and the frequency of peripheral blood CD4 T cells incorporating BrdU was determined 24 h later. Statistical significance was determined using Mann-Whitney U test (A) or one-way ANOVA (B-D) with multiple-testing correction using the Holm-Sidak method, and α = 0.05, when deemed appropriate. **** p < 0.001; *** p < 0.005; * p < 0.05; and n.s. – not significant. Data shown are representative of at least 2 independent experiments of 4-5 mice/group in each experiment.
CD4 T cell recovery is not usually dependent on thymic-derived T cells in models of experimentally-induced lymphopenia, since the export rate of naïve T cells from the thymus is not modulated by perturbations in the periphery (36). In contrast, work by Unsinger et al. suggested CD4 T cells did not homeostatically proliferate when transferred into CLP-treated recipients (17). These contradictory findings led us to examine the number of CD4 T cells in the blood of sham- and CLP-treated euthymic (WT) and thymectomized mice. CD4 T cell loss and recovery was similar in WT and thymectomized mice, with no statistical differences at any of the time points analyzed (Fig. 1C). Next, sham- and CLP-treate WT and thymectomized mice were given BrdU on d 6 after surgery to measure proliferation of the peripheral blood CD4 T cells. We found statistically higher frequencies of BrdU+ CD4 T cells in CLP-treated mice versus shams, regardless of thymic presence or absence (Fig. 1D). Together, these results suggest that CD4 T cell recovery after sepsis occurs by a thymus-independent mechanism.
CD4 T cells that numerically recover after septic insult acquire an “Ag-experienced” phenotype
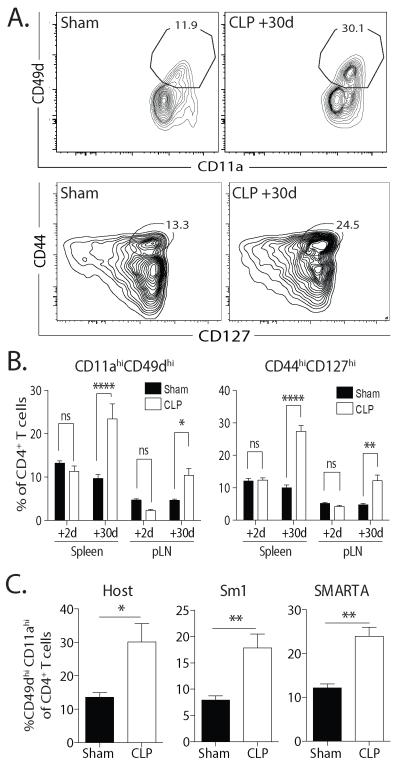
As naïve T cells homeostatically proliferate to fill lymphopenic niches, their surface phenotype changes to resemble T cells that have encountered their cognate Ag (37, 38). Importantly, this change in phenotype can be independent of cognate Ag recognition (39). We recently reported that the numerical recovery of CD8 T cells after septic injury is driven by homeostatic proliferation, indicated by an increased frequency of CD11ahi CD44hi CD8 T cells compared to sham mice (34) – even when cognate Ag was not encountered. Currently there is no canonical phenotype characterizing CD4 T cells that have undergone similar processes, but several phenotypes have been suggested. For example, CD11a and CD49d co-expression indicate an “Ag-experienced” CD4 T cell phenotype (40, 41). We noted that CD4 T cell recovery after sepsis was concomitant with an increased frequency of CD11ahi CD49dhi CD4 T cells among the total CD4 T compartment (Fig. 2A-B). CD44 and CD127 co-expression has also been suggested to be a phenotype for CD4 T cells after Ag-independent expansion (42), and we found CLP-treated mice had an increased frequency of CD44hi CD127hi CD4 T cells (Fig. 2A-B). To determine the extent to which these changes were dependent on TCR interaction with cognate Ag, we transferred TCR-transgenic Sm1 (transgenic epitope: Flic427-441 from S. enterica ser. typhimurium flagellin (43)) and SMARTA (transgenic epitope: gp61-77 from LCMV (44)) CD4 T cells into B6 mice prior to sham or CLP surgery. These are two disparately different pathogens, neither of which is normally found in SPF mice. We observed significantly increased frequencies of CD11ahi CD49dhi Sm1 and SMARTA CD4 T cells in the spleens of CLP-treated mice compared to sham-treated mice after 30 d (Fig. 2C). While Ag cross reactivity cannot be excluded, these phenotypic changes in TCR-transgenic CD4 T cells led us to conclude that the acquisition of an “Ag-experienced” phenotype was occurring without cognate Ag present in the septic host. Together, the data in Fig. 1 and 2 suggest lymphopenia-induced homeostatic proliferation plays a major role in the numerical recovery of the CD4 T cell compartment after sepsis.
FIGURE 2. Numerical recovery of CD4 T cells after sepsis is accompanied by the acquisition of an “Ag-experienced” phenotype in an Ag-independent manner.
A. The phenotype of CD4 T cells in the spleen was assessed 30 d after sham or CLP surgery. Representative flow cytometry plots depicting CD11a/CD49d and CD44/CD127 expression in CD4 T cells 30 d after sham or CLP surgery. B. Frequency of CD11ahi CD49dhi and CD44h CD127hi CD4 T cells 2 and 30 d after sham or CLP surgery. C. Salmonella FliC447-458-specific Sm1 (CD90.1/CD90.1; 5 × 105/mouse) and LCMV gp61-77-specific SMARTA (CD90.1/CD90.2; 106/mouse) CD4 T cells were adoptively transferred together into naïve CD90.2 B6 mice 1 d before sham or CLP surgery. After 30 d, the frequency of CD11ahi CD49dhi endogenous CD90.2/CD90.2 CD4 T cells and adoptively transferred TCR-tg CD4 T cells was determined. Statistical significance was determined using one-way ANOVA with multiple-testing correction using the Holm-Sidak method, and α= 0.05, when deemed appropriate. **** p < 0.001; ** p < 0.01; * p < 0.05; and n.s. – not significant. Data shown are representative of at least 2 independent experiments of 4-5 mice/group in each experiment.
Asymmetric recovery of Ag-specific naïve CD4 T cells after sepsis
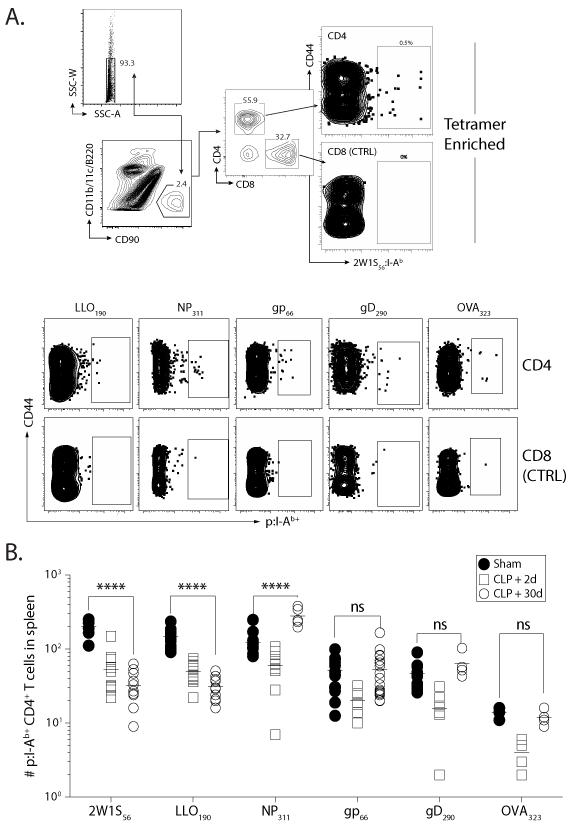
The massive attrition of peripheral CD4 T cells and evidence supporting a peripheral mechanism of CD4 T cell recovery after sepsis led us to question the possibility of discrete changes within individual Ag-specific CD4 T cell populations. We used peptide:MHC II (p:I-Ab) tetramer-based enrichment ((20, 29); Fig. 3A) to quantify 6 different endogenous Ag-specific CD4 T cell populations of varying size, clonotype composition, and immunodominance. CLP-treated mice showed acute reductions on all populations examined on d 2 (consistent with the global lymphopenia) compared to sham-treated mice, but the numerical recovery of the different Ag-specific CD4 T cell populations 30 d post-CLP was asymmetric (Fig. 3B). Quantitatively, the 2W1S- and Listeria LLO190-201-specific CD4 T cell populations were reduced in CLP-versus sham-treated mice, while the number of influenza A virus (IAV) NP311-325-specific CD4 T cells increased in CLP-treated mice over sham mice. In contrast, LCMV gp66-77−, HSV-1 gD290-305−, and OVA323-339–specific CD4 T cell populations recovered (i.e., no statistical difference between sham and d 30 CLP mice). It is important to emphasize that the changes in the individual Ag-specific CD4 T cell populations were not evident when examining bulk CD4 T cells in an Ag-independent manner (Fig. 1). These findings suggest that the loss of peripheral CD4 T cells after sepsis-induced lymphopenia is asymmetric between the individual Ag-specific CD4 T cell populations examined, and that recovery results in the overall changes in the composition of the naïve CD4 T cell pool in sepsis survivors.
FIGURE 3. Numerical recovery of CD4 T cells after sepsis is accompanied by asymmetric changes in Ag-specific repertoire heterogeneity.
Mice underwent sham or CLP surgery, and the number of Ag-specific CD4 T cells specific for 2W1S, L. monocytogenes LLO190-201, influenza A virus NP311-325, LCMV gp66-77, HSV gD290-302, and OVA323-339 was determined 2 and 30 d later using p:I-Ab tetramer enrichment. A. Representative flow plots showing gating strategy used in tetramer-enriched cell fractions to detect the frequency of Ag-specific CD4 T cell populations. Shown is an example used to detect 2W1S:I-Ab-specific CD4+ T cells. Gating for p:I-Ab-specific cells was determined using CD8+ T cells as an internal negative control for tetramer binding. B. Number of Ag-specific, naïve CD4 T cell precursors across the 6 epitopes in sham- and CLP-treated mice 2 or 30 d after surgery. Statistical significance was determined using group-wise, one-way ANOVA with multiple-testing correction using the Holm-Sidak method, and α = 0.05. **** p < 0.001; and n.s. – not significant. Data shown are the combined results from 2-4 independent experiments per population analyzed, with 3-5 mice/group in each experiment.
Incomplete naïve precursor recovery after sepsis correlates with reduced proliferative capacity and cytokine production during Ag-specific CD4 T cell responses
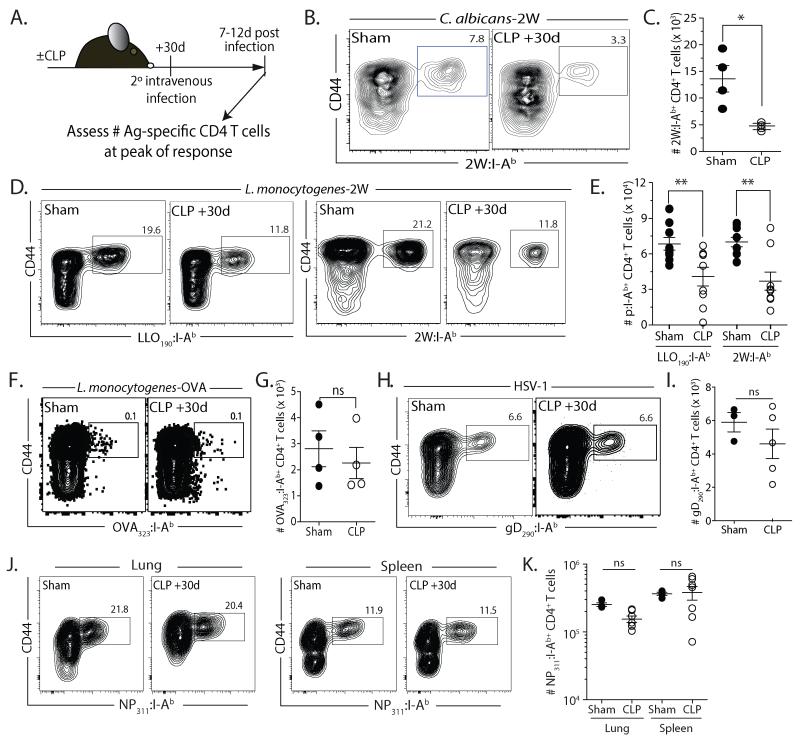
The magnitude of an Ag-specific CD4 T cell response after priming directly correlates with the size of the precursor pool (29, 45). Seeing the numerical changes in Ag-specific CD4 T cell populations in CLP-treated mice, we examined the impact of septic injury on Ag-specific CD4 T cell responses after secondary heterologous pathogen challenge (Fig. 4A). To test this, we used an Ag-specific approach to track the CD4 T cell response to the model antigen 2W1S expressed in Candida albicans (C. albicans-2W1S)(23). This design allowed us to model an opportunistic super-infection that is common for sepsis survivors during convalescence (46). When CLP-treated mice were infected with C. albicans-2W1S on d 2 after surgery, CD4 T cell responses were significantly reduced compared to sham mice (data not shown), which was not surprising given the dramatic reduction in CD4 T cell numbers at this time point. When mice were inoculated with C. albicans-2W1S 30 d after surgery, we still saw a significant reduction in the peak 2W1S-specific CD4 T cell proliferative response in CLP-treated mice compared to sham-treated mice given the same infection (Fig. 4B-C). To see whether the pathogen used influenced the 2W1S-specific CD4 T cell response, sham- and CLP-treated mice were infected with 2W1S expressing attenuated Listeria monocytogenes (Lm-2W1S) 30 d after surgery. Assessing the CD4 T cell response to 2W1S or the endogenous LLO190-201 epitope of Listeria listeriolysin-O revealed significantly reduced expansion for both Ag-specific CD4 T cell populations in CLP-treated mice compared to sham mice (Fig. 4D-E). We next examined the response in Ag-specific CD4 T cell populations that numerically recovered by 30 d after CLP surgery. After infection with recombinant attenuated Lm-OVA (Fig. 4F-G) or HSV-1 (Fig. 4H-I), we found the quantitative expansion of OVA323-339− or HSV gD290-305–specific (47, 48) CD4 T cells was similar regardless of sham or CLP surgery. We also saw no statistical difference in the proliferative capacity of the NP311-specific CD4 T cell population in sham- and CLP-treated mice after intranasal IAV infection (Fig. 4J-K), which was interesting since this Ag-specific CD4 T cell population numerically increased after sepsis-induced lymphopenia (see Fig. 3). However, the majority of the NP311-specific CD4 T cells in uninfected CLP-treated mice adopted a memory phenotype (i.e., CD44hi - data not shown) at d 30 post-surgery. Recent data suggest that naïve CD8 T cells (cognate Ag inexperienced) give rise to more effector CD8 T cells than primary memory CD8 T cells when analyzed on a per-cell-basis after cognate infection (49). While it remains to be tested if the same phenomenon is true for naïve and memory CD4 T cell responses, these results suggest that the phenotype of the CD4 T cells (naïve vs. “memory”-like), in addition to the numbers of cells present, might contribute to the in vivo response to cognate Ag recognition. In summary, the data in Figure 4 shows that the proliferative capacity of an Ag-specific CD4 T cell population after sepsis correlates with the degree (reduced vs. complete or increased) of numerical recovery of its naïve precursor population, and the differences seen are intrinsic to the Ag-specific CD4 T cell populations examined and not due to the pathogen used.
FIGURE 4. Expansion of epitope-specific populations correlates with precursor pool recovery after septic injury.
A. Experimental design. Mice were infected with 2W1S-expressing C. albicans (C. albicans-2W; 5×104 yeasts in 0.1 ml i.v.), attenuated 2W-expressing L. monocytogenes (Lm-2W or Lm-OVA; 107 CFU in 0.1 ml i.v.), HSV-1 (2.5×104 PFU in 0.1 ml i.v.), or influenza A virus (×31; 3000 EID50 in 0.02 ml i.n.) 30 d after sham or CLP surgery. After another 7-12 d, the frequency and number of Ag-specific CD4 T cells was determined in the spleen. B-C. Representative flow plots showing the frequency (B) and number (C) of 2W1S-specific CD4 T cells in the spleens from sham- and CLP-treated mice 7 d after i.v. infection with C. albicans-2W. D-E. Representative flow plots showing the frequency (D) and number (E) of LLO190- and 2W1S-specific CD4 T cells in the spleens from sham- and CLP-treated mice 7 d after i.v. infection with Lm-2W. F-G. Representative flow plots showing the frequency (F) and number (G) of OVA323-specific CD4 T cells in the spleens from sham- and CLP-treated mice 7 d after i.v. infection with Lm-OVA. H-I. Representative flow plots showing the frequency (H) and number (I) of gD290-specific CD4 T cells in the spleens from sham- and CLP-treated mice 9 d after i.v. infection with HSV-1. J-K. Representative flow plots showing the frequency (J) and number (K) of NP311-specific CD4 T cells in the lungs and spleens from sham- and CLP-treated mice 12 d after i.n. infection with ×31. Statistical significance was determined using group-wise, one-way ANOVA analyses followed by multiple-testing correction using the Holm-Sidak method, with α = 0.05. ** p < 0.01; * p < 0.05; and n.s. – not significant. Data shown are the combined results from 2-4 independent experiments per pathogen tested, with 3-5 mice/group in each experiment.
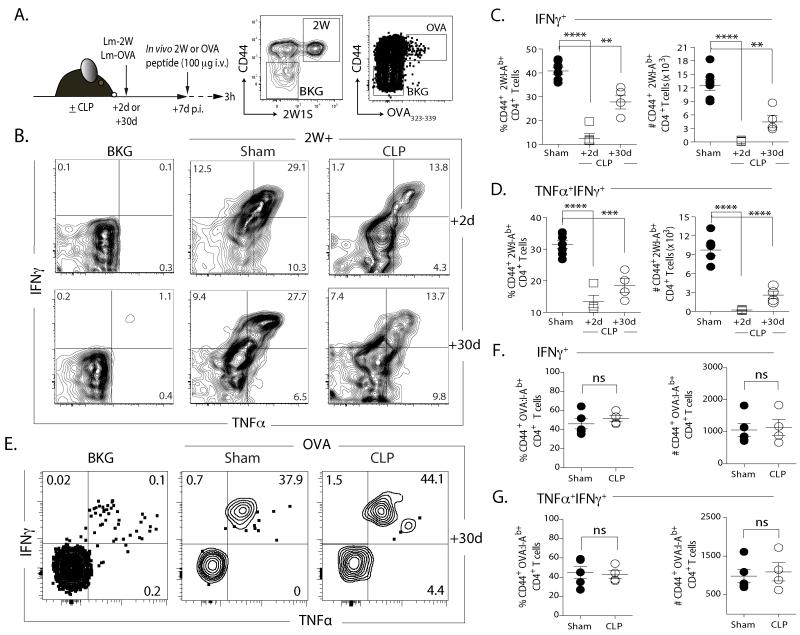
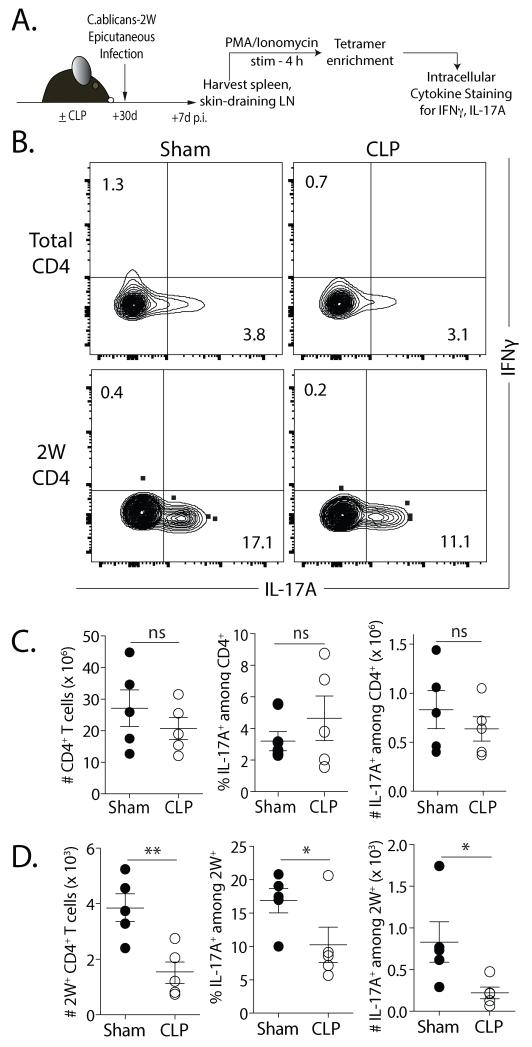
We next examined the function of Ag-specific CD4 T cell populations that underwent a sepsis-induced numerical reduction (2W1S-specfic) or not (OVA323-specific) in CLP-treated mice infected with Lm-2W1S or Lm-OVA 2 or 30 d after surgery via in vivo peptide restimulation (31) (Fig. 5A), which permits evaluation of cytokine production by an Ag-specific CD4 T cell population with almost no background (Fig. 5B). There was a persistent reduction in frequency and number of IFNγ+ (Fig. 5C) or TNFα+ IFNγ+ (Fig. 5D) 2W1S-specific CD4 T cells in CLP-treated mice compared to sham controls. In contrast, there was no difference in frequency or number of IFNγ+ or TNFα+ IFNγ+ OVA323-339-specific CD4 T cells (Fig. 5E-G). Since CD4 T cells can adopt different effector phenotypes based on the pathogen encountered, we examined the function of total and 2W1S-specific CD4 T cells after an epicutaneous C. albicans-2W infection that primes for a Th17 repsonse (Fig. 6A) (23, 50, 51). There was no significant difference in the frequency and number of IL-17A+ CD4 T cells from sham and CLP-treated mice after PMA/ionomycin stimulation (Fig. 6B-C). Significant reductions in the frequency and number of 2W1S-specific CD4 T cells from CLP-treated mice making IL-17A were seen, however, after stimulation (Fig. 6B & D). Together, these data demonstrate that (at least) for 2W1S-specific CD4 T cells, sepsis leads to fewer naïve precursors with a reduced capacity to proliferate and make effector cytokines (on a per cell basis) following antigenic stimulation.
FIGURE 5. Inadequate recovery of precursor population correlates with inadequate CD4 T cell function after pathogen challenge.
A. Experimental design. Mice were infected with attenuated 2W- or OVA-expressing L. monocytogenes (Lm-2W or Lm-OVA; 107 CFU in 100 μl i.v.) 2 or 30 d after sham or CLP surgery. After another 7d, the mice were injected i.v. with 100 μg 2W1S56-68 or OVA323-339 peptide. Spleens were harvested 2 h later, and the frequency and number of IFNγ+ or TNFα+IFNγ+ CD44+2W:I-Ab+ or OVA323:I-Ab+ CD4 T cells was determined. B. Representative flow plots of intracellular IFNγ and TNFα detection in the CD44+2W:I-Ab+ CD4 T cells after in vivo peptide restimulation. Plots show cells gated from internal control populations (CD44lo2W:I-Ab- CD4 T cells) denoted as background (“BKG”) or 2W:I-Ab-enriched CD4 T cells from sham- or CLP-treated mice. C-D. Frequency and number of CD44+2W:I-Ab+-specific CD4 T cells in the spleen producing IFNγ (C) or TNFα and IFNγ (D). E. Representative flow plots of intracellular IFNγ and TNFα detection in the CD44+OVA323:I-Ab+ CD4 T cells after in vivo peptide restimulation. Plots show cells gated from internal control populations (CD44loOVA323:I-Ab− CD4 T cells) denoted as background (“BKG”) or OVA323:I-Ab-enriched CD4 T cells from sham- or CLP-treated mice. F. Frequency and number of CD44+ OVA323:I-Ab+-specific CD4 T cells in the spleen producing TNFα and IFNγ. Statistical significance was determined using group-wise, one-way ANOVA analyses followed by Holm-Sidak correction with α = 0.05. **** p < 0.001; *** p < 0.005; and ** p < 0.01. Data shown are the combined results of 2 independent experiments, with 3-5 mice/group in each experiment.
FIGURE 6. Ag-specific CD4 T cells have functional deficits in Th17-polarized responses.
A. Experimental design. On d 30 after sham or CLP surgery, mice were infected epicutaneously with 2W1S-expressing C. albicans (C. albicans-2W; 108 yeasts in 0.05 ml). After 7 d, lymphocytes obtained from the skin-draining (inguinal, brachial, axillary and cervical) LN of infected mice were stimulated for 4 h with PMA/ionomycin. The stimulated samples were enriched for 2W1S-specific CD4 T cells and production of IFNγ and IL-17A was assayed by flow cytometry. B. Representative flow plots showing the gating strategy to identify IL-17A+IFNγ− cells within bulk and 2W:I-Ab-specific CD4 T cells. C-D. Frequency and number of IL-17A+ in bulk (C) and 2W1S-specific CD4 T cells (D) in infected sham- or CLP-treated mice. Statistical significance was determined using Welch”s t-test. ** p < 0.01; * p < 0.05; and n.s. – not significant. Data shown are representative results from 2 independent experiments, with 5 mice/group in each experiment.
Sepsis alters T cell receptor clonotype composition
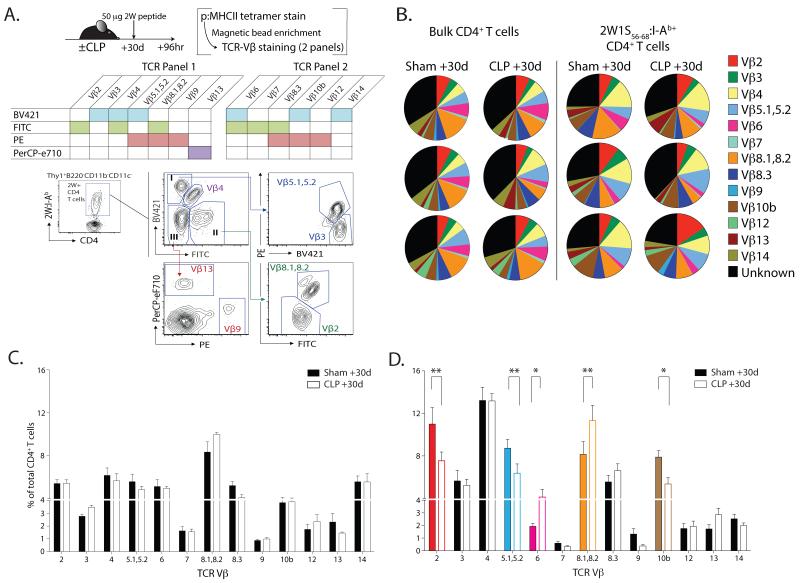
Data in Fig. 1 and 2 suggest one means by which CD4 T cells recover from sepsis-induced lymphopenia is via homeostatic proliferation. However, homeostatic proliferation is limited in that it can only recreate the peripheral T cell pool from the available diversity. Given that changes have been detected in T cell repertoire diversity in septic patients (52), we analyzed TCRβ variable chain isotype (TCR Vβ) frequencies within the total and 2W1S-specific CD4 T cell populations to determine the extent to which sepsis affects clonotype diversity. Due to the technical limitation of analyzing naïve Ag-specific CD4 T cell populations of ≤200 cells, we used a method to approximate clonotype diversity of an Ag-specific CD4 T cell population where excess peptide Ag was injected intravenously (29), and the resultant expansion of Ag-specific CD4 T cells was examined 3 d later via p:MHC II tetramer enrichment. To maximize Vβ identification within the Ag-specific population, we used TCR Vβ-specific mAb multiplexed into 2 flow cytometry panels (Fig. 7A). Similar to previous data (19), we did not detect significant changes in the TCR Vβ clonotypes when examined at the total CD4 T cell population level (Fig. 7B & C). While Vβ distribution of 2W1S-specific CD4 T cells in sham-treated mice was consistent with previous data (29), a skewing in a number of 2W1S-specific CD4 T clonotypes (specifically, Vβ 2, Vβ 5.1/5.2, Vβ 6, Vβ 8.1/8.2, and Vβ 10b) from CLP-treated mice was seen (Fig. 7B & D). These data show that an Ag-specific CD4 T cell population that is numerically truncated in a post-sepsis host after recovery also has altered clonal diversity (based on TCR Vβ usage), which could contribute to the reduction in subsequent function of Ag-specific CD4 T cells.
FIGURE 7. Sepsis alters the T cell receptor clonotype composition of Ag-specific CD4 T cell population.
A. Experimental design. Mice were injected i.v. with 50 μg 2W56-68 peptide (along with LPS) 30 d after sham or CLP surgery. Splenocytes were harvested 4 d later and tetramer-enriched as previously described. The resultant sample was then used to determine the clonotype composition using two multiplexed flow cytometry panels consisting of the indicated murine TCR Vβ mAb. B. Representative flow plots showing the gating strategy to identify Vβ usage on 2W:I-Ab-specific CD4 T cells using TCR panel #1. C. Usage profile for TCR Vβ gene segments in total 2W:I-Ab-specific CD4 T cells from 3 representative individual sham- or CLP-treated mice. D. Averaged frequency of TCR Vβ of 2W:I-Ab-specific CD4 T cells in sham- or CLP-treated mice. Statistical significance was determined using group-wise, one-way ANOVA analyses followed by Holm-Sidak correction with α = 0.05. ** p < 0.01; and * p < 0.05. Data are combined from 2 independent experiments, each having 5 mice/group.
Discussion
Sepsis currently represents an unmet challenge in medicine. Despite modern intensive care practices, mortality from sepsis holds at 30-50% (53). Patients surviving a septic event often have suppressed immune function, a state that is thought to contribute to the increased susceptibility to (and mortality from) secondary nosocomial infections. A number of studies have examined the numerical and functional changes of various immune cell subsets after sepsis, but they have done so at the total population level. The goal of this study was to analyze the quantitative and qualitative changes in CD4 T cells, but at the level of Ag-specific populations, which permits a more rigorous and sensitive analysis of how these cells perform under various immunological settings. With this in mind, we have for the first time (to our knowledge) performed quantitative and qualitative analyses of multiple Ag-specific CD4 T cell populations in septic mice, before and after secondary heterologous infections. Our data demonstrate that a septic event induces deletion within each endogenous Ag-specific CD4 T cell population examined, and that this event is followed by a recovery of the Ag-specific repertoire in an irregular, or asymmetric, fashion. Moreover, our results show that sepsis-induced numerical changes to certain Ag-specific CD4 T cell populations can affect the function of the cells in question during the subsequent response to a pathogenic challenge.
Clearly defining the mechanism by which lymphocyte apoptosis occurs after sepsis is difficult, since no single intrinsic or extrinsic pathway dominates (54, 55). Similarly, there has been limited investigation into the mechanism(s) behind lymphocyte recovery after a septic event. The sole publication examining the process of CD4 T cell recovery following septic injury suggested that CD4 T cells did not undergo homeostatic proliferation during recovery from sepsis (19). This conclusion was largely reached by adoptively transferring a large number of TCR-tg CD4 (OT-II) T cells into septic mice 7 d after surgery. Even though these cells were introduced into a lymphopenic environment, it could be argued that since these CD4 T cells did not “experience” the septic event any T cell-intrinsic changes that occur during sepsis would not be present in these cells. Furthermore, it is clear that adoptive transfer experiments that utilize nonphysiologically large input numbers of TCR-tg T cells do not accurately recapitulate the endogenous Ag-specific T cell response (56). In contrast, the similar recovery of CD4 T cell numbers in thymectomized and euthymic mice, along with the similar rates of BrdU incorporation in CD4 T cells of CLP-treated euthymic and thymectomized mice alike suggest CD4 T cells do indeed undergo homeostatic proliferation after septic injury. Unsinger et al. then went on to show increased frequencies of CD4 T cells in septic mice with “activated” (CD69+) and “memory” (CD44hi CD62Llo) phenotypes (19), leading them to suggest that the majority of activated and memory CD4 T cells arise from endogenous sources. Our data in Fig. 3 are consistent with these findings by Unsigner et al., but extend them to identify cells with phenotypes consistent with “homeostatic proliferation” for both endogenous CD4 T cells and adoptively transferred TCR-tg CD4 T cells (37, 42). It remains to be determined what the driving factor(s) is for the numerical recovery of CD4 T cells after sepsis. In addition to recovery by homeostatic proliferation, it is likely that some CD4 T cell populations respond directly to antigenic epitopes present in the proteins expressed by the various commensal bacterial species within the gut or to self-Ag, leading to a difference in functional potential compared to those CD4 T cells truly undergoing Ag-independent homeostatic proliferation. The cecum contains a high concentration of microbes that are a combination of Gram-positive and Gram-negative bacterial species, and the Ag expressed by these bacteria can be recognized by T cells and can drive effector responses – despite being commensal bacteria (57). It is tempting to speculate that the above-normal numerical recovery in the NP311-specific CD4 T cell population in CLP-treated mice is due to direct antigenic stimulation as a result of cross-reactivity with some yet-to-be defined epitope expressed by the gut commensal bacteria, especially since the majority of NP311-specific CD4 T cells in CLP-treated mice were also CD44hi (data not show). As a result, this population of cells could be considered a “memory” population with different functional characteristic, such as producing fewer effectors after influenza infection (49), over true “naïve” cells. The bacterial constituents of the gut microbiome are unique to each individual (especially humans), and can be strongly influenced by a variety of factors (58). Consequently, the extent of recovery and function of a particular Ag-specific T cell population after a septic event can be easily different as a result of the intestinal “health” of the individual, regardless of possible genetic similarities (e.g., same mouse strain from different vendors). As reagents become available to track CD4 T cell populations specific to Ag expressed by specific gut commensal bacteria, it will be interesting to investigate the potential impact of this component of polymicrobial sepsis on the recovery and function of such Ag-specific CD4 T cell populations in septic mice.
When examined at the bulk CD4 T cell level, our data are consistent with a number of other studies demonstrating sepsis-induced changes in the basic numerical and functional characteristics of CD4 T cells. It is important to emphasize that the sepsis-induced changes in the different Ag-specific CD4 T cell populations would not have been identified had we examined CD4 T cells as a whole. This includes the changes in naïve precursor numbers, proliferative capacity, and cytokine production by the different Ag-specific populations. While we recently showed that sepsis significantly deceases the Ag sensitivity of memory CD8 T cells (35), it remains to be determined to what extent Ag sensitivity is affected in naïve or memory Ag-specific CD4 T cell populations. In addition, TCR Vβ repertoire usage on CD4 T cells from sham- and CLP-treated mice was also investigated previously, where the authors found no skewing of the repertoire toward one particular Vβ subtype (19). However, this conclusion was based on analyzing bulk CD4 T cells. Just as we only observed stochastic changes in the number of naïve CD4 T cells when we examined Ag-specific CD4 T cell populations using p:MHC II tetramers, alterations in Vβ repertoire usage after sepsis were observed when examining the endogenous 2W1S-specific CD4 T cell population. We realize that the method for examining Vβ repertoire usage required in vivo 2W1S56-68 peptide immunization to expand this Ag-specific population of cells, but it is important to emphasize that this technique results in an expanded T cell population that is reflective of the clonotype diversity of the naïve starting population (29). Even after the expansion, 2W1S:I-Ab tetramer enrichment and the multiplexed flow cytometry panel of Vβ-specific mAb were needed to complete the analysis of the endogenous 2W1S-specific CD4 T cell population. These results show the power of using these reagents and techniques to analyze small numbers of endogenous Ag-specific CD4 T cell populations.
Pools of Ag-specific CD4 T cell “precursors” are maintained in the periphery by frequent, low-level signals from self-Ag:MHC II and cytokines (most notably, IL-7 for naïve CD4 T cells (59) and IL-15 for naïve CD8 T cells (60)). The increased availability of these resources turns survival signals into mitogenic stimuli that restores T cell numbers through proliferative expansion in situations where T cell numbers drop acutely (61). With this in mind, there is considerable effort being spent identifying therapeutic strategies designed to enhance T cell recovery and function after sepsis, and the administration of agents that promote lymphocyte proliferation (e.g., IL-2, IL-7, and IL-15 (62-64)) or block the function of inhibitory molecules (e.g., PD-1 (65, 66) and CTLA-4 (67)) are producing encouraging results. For example, administration of IL-7 to septic mice shortly after sepsis induction can prevent T cell apoptosis and restore function (63, 64). Moreover, disruption of the PD-1:PD-L1 signaling pathway improves survival in animal models of sepsis (68, 69), and reverses T cell exhaustion in sepsis patients (66). Additional work is needed to determine the impact of such therapies at the level of Ag-specific T cell populations where, even if apparently subtle, physiologically meaningful changes may actually be present.
In the current study, we have assessed CD4 T cell function after sepsis primarily within the context of a “Th1” response, but it is important to emphasize that sepsis likely affects other CD4 T cell subsets needed for a variety of other immunological responses. For example, Th1 cells also provide necessary signals for B cell isotype switching (70), and ILβ-4 from Th2 cells also facilitates B cell isotype switching to IgG1 and IgE (71). Th17 cells are important in immunity to extracellular fungal and bacterial pathogens (72), and we saw a reduction in the Th17 response when using a secondary epicutaneous C. albicans after sepsis (Fig. 6). Thus, the loss or improper function of CD4 T cell responses is detrimental for immunity to a wide range of pathogens, especially those that frequently cause secondary infections in septic patients. In addition to the different “effector” CD4 Th subsets, CD4+CD25+FoxP3+ regulatory T cells (Treg) have been found at an increased frequency in septic patients, especially early after diagnosis (73-75). It was subsequently determined that the increased frequency of Treg was the consequence of decreases in the effector CD4 T cell populations (76), suggesting that Treg are more resistant to sepsis-induced apoptosis than conventional CD4 T cells (16). Regardless, the role of Treg cells in the immunosuppression after sepsis has not been rigorously investigated and merits study. We also realize that the sepsis-induced alterations in the Ag-specific CD4 T cell populations we examined could be due to CD4 T cell-intrinsic and/or -extrinsic factors. As CD4 T cells stimulation require Ag presentation from professional APC, changes in the number and/or function of DC could contribute to the observed modulation in CD4 T cell function (77-79).
The development and integration of a number of reagents and techniques over the last decade has permitted the characterization of Ag-specific endogenous naïve and memory T cell responses to a handful of experimental bacterial (such as L. monocytogenes) or viral (such as LCMV) pathogens in exquisite detail. These features have given us the ability to track the quantity and quality of endogenous CD4 T cells reactive to antigenic epitopes within these pathogens. Yet, one weakness of these (and other well-characterized) experimental pathogens is that they are typically not seen as nosocomial infection threats for septic patients. Most septic patients potentially face complications arising from secondary infection by the extracellular pathogens Candida, Pseudomonas, and Staphylococcus (46, 80, 81), and these pathogens have been used most often to examine alterations in animal survival in experimental models of sepsis. It is important to keep in mind that our use of Lm-2W for most of the studies was to take advantage of the wealth of information known regarding the infectivity/pathogenicity of this pathogen, the characteristic CD4 T cell response that it elicits, and the availability of reagents to critically investigate different aspects of this response. Moreover, while our analysis of sepsis-induced alterations in the responsiveness of 2W1S-specific CD4 T cells was largely performed after secondary infection with attenuated Lm-2W, we found the similar reductions in proliferative capacity of 2W1S-specific CD4 T cells after infection with C. albicans-2W (Fig. 4). These data serve as a starting point for future studies using pathogens that commonly plague sepsis patients once the reagents are developed to following CD4 T cell populations that are specific for Ag within these pathogens.
In summary, the data we presented here elucidates with detail how CD4 T cells are affected by sepsis. Our results reveal that after a septic event the recovery of individual Ag-specific CD4 T cell populations is skewed, which is an observation that is not evident when examining the bulk CD4 T cell pool. In addition, we show that an incomplete recovery of the Ag-specific T cell repertoire after sepsis stems from the attrition of Ag-specific TCR diversity, and that this phenomenon correlates with altered CD4 T cell responses long after sepsis. Ultimately, this study increases our collective understanding of why septic patients more easily acquire secondary infections.
Acknowledgements
We thank Dr. Jon Linehan (Jenkins Lab, Center for Immunology, University of Minnesota) for assistance in the production of the gD290 and NP311:I-Ab tetramers.
Footnotes
Supported by a U.S. Department of Veterans Affairs Merit Review Award (T.S.G.), an award from American Hearth Association (S.A.C.), and National Institutes of Health Grants AI83286 and AI114543 (V.P.B.).
References
- 1.Pepper M, Jenkins MK. Origins of CD4(+) effector and central memory T cells. Nat. Immunol. 2011;12:467–471. doi: 10.1038/ni.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Green AM, Difazio R, Flynn JL. IFN-gamma from CD4 T cells is essential for host survival and enhances CD8 T cell function during Mycobacterium tuberculosis infection. J. Immunol. 2013;190:270–277. doi: 10.4049/jimmunol.1200061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Phares TW, Stohlman SA, Hinton DR, Bergmann CC. Enhanced CD8 T-cell anti-viral function and clinical disease in B7-H1-deficient mice requires CD4 T cells during encephalomyelitis. J. Neuroinflammation. 2012;9:269. doi: 10.1186/1742-2094-9-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Janssen EM, Droin NM, Lemmens EE, Pinkoski MJ, Bensinger SJ, Ehst BD, Griffith TS, Green DR, Schoenberger SP. CD4+ T-cell help controls CD8+ T-cell memory via TRAIL-mediated activation-induced cell death. Nature. 2005;434:88–93. doi: 10.1038/nature03337. [DOI] [PubMed] [Google Scholar]
- 5.Badovinac VP, Messingham KA, Griffith TS, Harty JT. TRAIL deficiency delays, but does not prevent, erosion in the quality of “helpless” memory CD8 T cells. J. Immunol. 2006;177:999–1006. doi: 10.4049/jimmunol.177.2.999. [DOI] [PubMed] [Google Scholar]
- 6.Sacks JA, Bevan MJ. TRAIL deficiency does not rescue impaired CD8+ T cell memory generated in the absence of CD4+ T cell help. J. Immunol. 2008;180:4570–4576. doi: 10.4049/jimmunol.180.7.4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Church SE, Jensen SM, Antony PA, Restifo NP, Fox BA. Tumor-specific CD4(+) T cells maintain effector and memory tumor-specific CD8(+) T cells. Eur. J. Immunol. 2014;44:69–79. doi: 10.1002/eji.201343718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weinstein JS, Hernandez SG, Craft J. T cells that promote B-Cell maturation in systemic autoimmunity. Immunol. Rev. 2012;247:160–171. doi: 10.1111/j.1600-065X.2012.01122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yates JL, Racine R, McBride KM, Winslow GM. T cell-dependent IgM memory B cells generated during bacterial infection are required for IgG responses to antigen challenge. J. Immunol. 2013;191:1240–1249. doi: 10.4049/jimmunol.1300062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall MJ, Williams SN, DeFrances CJ, Golosinskiy A. Inpatient care for septicemia or sepsis: a challenge for patients and hospitals. NCHS Data Brief. 2011:1–8. [PubMed] [Google Scholar]
- 12.Angus DC. The search for effective therapy for sepsis: back to the drawing board? Jama. 2011;306:2614–2615. doi: 10.1001/jama.2011.1853. [DOI] [PubMed] [Google Scholar]
- 13.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Criti. Care Med. 1992;20:864–874. [PubMed] [Google Scholar]
- 14.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Criti. Care Med. 2003;31:1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 15.Brun-Buisson C. The epidemiology of the systemic inflammatory response. Intensive Care Med. 2000;26(Suppl 1):S64–74. doi: 10.1007/s001340051121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hotchkiss RS, Monneret G, Payen D. Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach. Lancet Infect. Dis. 2013;13:260–268. doi: 10.1016/S1473-3099(13)70001-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Unsinger J, Kazama H, McDonough JS, Griffith TS, Hotchkiss RS, Ferguson TA. Sepsis-induced apoptosis leads to active suppression of delayed-type hypersensitivity by CD8+ regulatory T cells through a TRAIL-dependent mechanism. J. Immunol. 2010;184:6766–6772. doi: 10.4049/jimmunol.0904054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roger PM, Hyvernat H, Ticchioni M, Kumar G, Dellamonica J, Bernardin G. The early phase of human sepsis is characterized by a combination of apoptosis and proliferation of T cells. J. Crit. Care. 2012;27:384–393. doi: 10.1016/j.jcrc.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 19.Unsinger J, Kazama H, McDonough JS, Hotchkiss RS, Ferguson TA. Differential lymphopenia-induced homeostatic proliferation for CD4+ and CD8+ T cells following septic injury. J. Leuk. Biol. 2009;85:382–390. doi: 10.1189/jlb.0808491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moon JJ, Chu HH, Hataye J, Pagan AJ, Pepper M, McLachlan JB, Zell T, Jenkins MK. Tracking epitope-specific T cells. Nat. Prot. 2009;4:565–581. doi: 10.1038/nprot.2009.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rittirsch D, Huber-Lang MS, Flierl MA, Ward PA. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat. Prot. 2009;4:31–36. doi: 10.1038/nprot.2008.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pepper M, Pagan AJ, Igyarto BZ, Taylor JJ, Jenkins MK. Opposing signals from the Bcl6 transcription factor and the interleukin-2 receptor generate T helper 1 central and effector memory cells. Immunity. 2011;35:583–595. doi: 10.1016/j.immuni.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Igyarto BZ, Haley K, Ortner D, Bobr A, Gerami-Nejad M, Edelson BT, Zurawski SM, Malissen B, Zurawski G, Berman J, Kaplan DH. Skin-resident murine dendritic cell subsets promote distinct and opposing antigen-specific T helper cell responses. Immunity. 2011;35:260–272. doi: 10.1016/j.immuni.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Griffith TS, Herndon JM, Lima J, Kahn M, Ferguson TA. The immune response and the eye. TCR alpha-chain related molecules regulate the systemic immunity to antigen presented in the eye. Int. Immunol. 1995;7:1617–1625. doi: 10.1093/intimm/7.10.1617. [DOI] [PubMed] [Google Scholar]
- 25.Brincks EL, Gurung P, Langlois RA, Hemann EA, Legge KL, Griffith TS. The magnitude of the T cell response to a clinically significant dose of influenza virus is regulated by TRAIL. Journal of immunology. 2011;187:4581–4588. doi: 10.4049/jimmunol.1002241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brincks EL, Roberts AD, Cookenham T, Sell S, Kohlmeier JE, Blackman MA, Woodland DL. Antigen-specific memory regulatory CD4+Foxp3+ T cells control memory responses to influenza virus infection. J. Immunol. 2013;190:3438–3446. doi: 10.4049/jimmunol.1203140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.BenMohamed L, Bertrand G, McNamara CD, Gras-Masse H, Hammer J, Wechsler SL, Nesburn AB. Identification of novel immunodominant CD4+ Th1-type T-cell peptide epitopes from herpes simplex virus glycoprotein D that confer protective immunity. J. Virol. 2003;77:9463–9473. doi: 10.1128/JVI.77.17.9463-9473.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crowe SR, Miller SC, Brown DM, Adams PS, Dutton RW, Harmsen AG, Lund FE, Randall TD, Swain SL, Woodland DL. Uneven distribution of MHC class II epitopes within the influenza virus. Vaccine. 2006;24:457–467. doi: 10.1016/j.vaccine.2005.07.096. [DOI] [PubMed] [Google Scholar]
- 29.Moon JJ, Chu HH, Pepper M, McSorley SJ, Jameson SC, Kedl RM, Jenkins MK. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 2007;27:203–213. doi: 10.1016/j.immuni.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pagan AJ, Pepper M, Chu HH, Green JM, Jenkins MK. CD28 promotes CD4+ T cell clonal expansion during infection independently of its YMNM and PYAP motifs. J. Immunol. 2012;189:2909–2917. doi: 10.4049/jimmunol.1103231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nelson RW, McLachlan JB, Kurtz JR, Jenkins MK. CD4+ T cell persistence and function after infection are maintained by low-level peptide:MHC class II presentation. J. Immunol. 2013;190:2828–2834. doi: 10.4049/jimmunol.1202183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chu HH, Moon JJ, Kruse AC, Pepper M, Jenkins MK. Negative selection and peptide chemistry determine the size of naive foreign peptide-MHC class II-specific CD4+ T cell populations. J. Immunol. 2010;185:4705–4713. doi: 10.4049/jimmunol.1002276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gurung P, Rai D, Condotta SA, Babcock JC, Badovinac VP, Griffith TS. Immune unresponsiveness to secondary heterologous bacterial infection after sepsis induction is TRAIL dependent. J. Immunol. 2011;187:2148–2154. doi: 10.4049/jimmunol.1101180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Condotta SA, Rai D, James BR, Griffith TS, Badovinac VP. Sustained and incomplete recovery of naive CD8+ T cell precursors after sepsis contributes to impaired CD8+ T cell responses to infection. J. Immunol. 2013;190:1991–2000. doi: 10.4049/jimmunol.1202379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duong S, Condotta SA, Rai D, Martin MD, Griffith TS, Badovinac VP. Polymicrobial sepsis alters antigen-dependent and -independent memory CD8 T cell functions. J. Immunol. 2014;192:3618–3625. doi: 10.4049/jimmunol.1303460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fink PJ. The biology of recent thymic emigrants. Ann. Rev. Immunol. 2013;31:31–50. doi: 10.1146/annurev-immunol-032712-100010. [DOI] [PubMed] [Google Scholar]
- 37.Sprent J, Surh CD. Normal T cell homeostasis: the conversion of naive cells into memory-phenotype cells. Nat. Immunol. 2011;12:478–484. doi: 10.1038/ni.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cho BK, Rao VP, Ge Q, Eisen HN, Chen J. Homeostasis-stimulated proliferation drives naive T cells to differentiate directly into memory T cells. J. Exp. Med. 2000;192:549–556. doi: 10.1084/jem.192.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ernst B, Lee DS, Chang JM, Sprent J, Surh CD. The peptide ligands mediating positive selection in the thymus control T cell survival and homeostatic proliferation in the periphery. Immunity. 1999;11:173–181. doi: 10.1016/s1074-7613(00)80092-8. [DOI] [PubMed] [Google Scholar]
- 40.McDermott DS, Varga SM. Quantifying antigen-specific CD4 T cells during a viral infection: CD4 T cell responses are larger than we think. J. Immunol. 2011;187:5568–5576. doi: 10.4049/jimmunol.1102104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Butler NS, Moebius J, Pewe LL, Traore B, Doumbo OK, Tygrett LT, Waldschmidt TJ, Crompton PD, Harty JT. Therapeutic blockade of PD-L1 and LAG-3 rapidly clears established blood-stage Plasmodium infection. Nat. Immunol. 2012;13:188–195. doi: 10.1038/ni.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alpdogan O, Muriglan SJ, Eng JM, Willis LM, Greenberg AS, Kappel BJ, van den Brink MR. IL-7 enhances peripheral T cell reconstitution after allogeneic hematopoietic stem cell transplantation. J. Clin. Invest. 2003;112:1095–1107. doi: 10.1172/JCI17865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McSorley SJ, Asch S, Costalonga M, Reinhardt RL, Jenkins MK. Tracking salmonella-specific CD4 T cells in vivo reveals a local mucosal response to a disseminated infection. Immunity. 2002;16:365–377. doi: 10.1016/s1074-7613(02)00289-3. [DOI] [PubMed] [Google Scholar]
- 44.Oxenius A, Bachmann MF, Zinkernagel RM, Hengartner H. Virus- specific MHC-class II-restricted TCR-transgenic mice: effects on humoral and cellular immune responses after viral infection. Eur. J. Immunol. 1998;28:390–400. doi: 10.1002/(SICI)1521-4141(199801)28:01<390::AID-IMMU390>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 45.Jenkins MK, Moon JJ. The role of naive T cell precursor frequency and recruitment in dictating immune response magnitude. J. Immunol. 2012;188:4135–4140. doi: 10.4049/jimmunol.1102661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lipsett PA. Surgical critical care: fungal infections in surgical patients. Crit. Care Med. 2006;34:S215–224. doi: 10.1097/01.CCM.0000231883.93001.E0. [DOI] [PubMed] [Google Scholar]
- 47.Laing KJ, Dong L, Sidney J, Sette A, Koelle DM. Immunology in the Clinic Review Series; focus on host responses: T cell responses to herpes simplex viruses. Clin. Exp. Immunol. 2012;167:47–58. doi: 10.1111/j.1365-2249.2011.04502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gebhardt T, Whitney PG, Zaid A, Mackay LK, Brooks AG, Heath WR, Carbone FR, Mueller SN. Different patterns of peripheral migration by memory CD4+ and CD8+ T cells. Nature. 2011;477:216–219. doi: 10.1038/nature10339. [DOI] [PubMed] [Google Scholar]
- 49.Martin MD, Condotta SA, Harty JT, Badovinac VP. Population dynamics of naive and memory CD8 T cell responses after antigen stimulations in vivo. J. Immunol. 2012;188:1255–1265. doi: 10.4049/jimmunol.1101579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hernandez-Santos N, Gaffen SL. Th17 cells in immunity to Candida albicans. Cell Host Microbe. 2012;11:425–435. doi: 10.1016/j.chom.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hernandez-Santos N, Huppler AR, Peterson AC, Khader SA, McKenna KC, Gaffen SL. Th17 cells confer long-term adaptive immunity to oral mucosal Candida albicans infections. Mucosal Immunol. 2013;6:900–910. doi: 10.1038/mi.2012.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Venet F, Filipe-Santos O, Lepape A, Malcus C, Poitevin-Later F, Grives A, Plantier N, Pasqual N, Monneret G. Decreased T-cell repertoire diversity in sepsis: a preliminary study. Crit. Care Med. 2013;41:111–119. doi: 10.1097/CCM.0b013e3182657948. [DOI] [PubMed] [Google Scholar]
- 53.Iwashyna TJ, Netzer G, Langa KM, Cigolle C. Spurious inferences about long-term outcomes: the case of severe sepsis and geriatric conditions. Am. J. Respir. Crit. Care Med. 2012;185:835–841. doi: 10.1164/rccm.201109-1660OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hotchkiss RS, Tinsley KW, Swanson PE, Schmieg RE, Jr., Hui JJ, Chang KC, Osborne DF, Freeman BD, Cobb JP, Buchman TG, Karl IE. Sepsis- induced apoptosis causes progressive profound depletion of B and CD4+ T lymphocytes in humans. J. Immunol. 2001;166:6952–6963. doi: 10.4049/jimmunol.166.11.6952. [DOI] [PubMed] [Google Scholar]
- 55.Hotchkiss RS, Nicholson DW. Apoptosis and caspases regulate death and inflammation in sepsis. Nat. Rev. Immunol. 2006;6:813–822. doi: 10.1038/nri1943. [DOI] [PubMed] [Google Scholar]
- 56.Badovinac VP, Haring JS, Harty JT. Initial T cell receptor transgenic cell precursor frequency dictates critical aspects of the CD8(+) T cell response to infection. Immunity. 2007;26:827–841. doi: 10.1016/j.immuni.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122:107–118. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 58.Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sportes C, Hakim FT, Memon SA, Zhang H, Chua KS, Brown MR, Fleisher TA, Krumlauf MC, Babb RR, Chow CK, Fry TJ, Engels J, Buffet R, Morre M, Amato RJ, Venzon DJ, Korngold R, Pecora A, Gress RE, Mackall CL. Administration of rhIL-7 in humans increases in vivo TCR repertoire diversity by preferential expansion of naive T cell subsets. J. Exp. Med. 2008;205:1701–1714. doi: 10.1084/jem.20071681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 61.Boyman O, Letourneau S, Krieg C, Sprent J. Homeostatic proliferation and survival of naive and memory T cells. Eur. J. Immunol. 2009;39:2088–2094. doi: 10.1002/eji.200939444. [DOI] [PubMed] [Google Scholar]
- 62.Inoue S, Unsinger J, Davis CG, Muenzer JT, Ferguson TA, Chang K, Osborne DF, Clark AT, Coopersmith CM, McDunn JE, Hotchkiss RS. IL- 15 prevents apoptosis, reverses innate and adaptive immune dysfunction, and improves survival in sepsis. J. Immunol. 2010;184:1401–1409. doi: 10.4049/jimmunol.0902307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Unsinger J, McGlynn M, Kasten KR, Hoekzema AS, Watanabe E, Muenzer JT, McDonough JS, Tschoep J, Ferguson TA, McDunn JE, Morre M, Hildeman DA, Caldwell CC, Hotchkiss RS. IL-7 promotes T cell viability, trafficking, and functionality and improves survival in sepsis. J. Immunol. 2010;184:3768–3779. doi: 10.4049/jimmunol.0903151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Unsinger J, Burnham CA, McDonough J, Morre M, Prakash PS, Caldwell CC, Dunne WM, Jr., Hotchkiss RS. Interleukin-7 ameliorates immune dysfunction and improves survival in a 2-hit model of fungal sepsis. J. Infect. Dis. 2012;206:606–616. doi: 10.1093/infdis/jis383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dirks J, Egli A, Sester U, Sester M, Hirsch HH. Blockade of programmed death receptor-1 signaling restores expression of mostly proinflammatory cytokines in anergic cytomegalovirus-specific T cells. Transplant Infect. Disease. 2013;15:79–89. doi: 10.1111/tid.12025. [DOI] [PubMed] [Google Scholar]
- 66.Chang K, Svabek C, Vazquez-Guillamet C, Sato B, Rasche D, Wilson S, Robbins P, Ulbrandt N, Suzich J, Green J, Patera AC, Blair W, Krishnan S, Hotchkiss R. Targeting the programmed cell death 1: programmed cell death ligand 1 pathway reverses T cell exhaustion in patients with sepsis. Crit. Care. 2014;18:R3. doi: 10.1186/cc13176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Inoue S, Bo L, Bian J, Unsinger J, Chang K, Hotchkiss RS. Dose- dependent effect of anti-CTLA-4 on survival in sepsis. Shock. 2011;36:38–44. doi: 10.1097/SHK.0b013e3182168cce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brahmamdam P, Inoue S, Unsinger J, Chang KC, McDunn JE, Hotchkiss RS. Delayed administration of anti-PD-1 antibody reverses immune dysfunction and improves survival during sepsis. J. Leuk. Biol. 2010;88:233–240. doi: 10.1189/jlb.0110037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang Y, Zhou Y, Lou J, Li J, Bo L, Zhu K, Wan X, Deng X, Cai Z. PD-L1 blockade improves survival in experimental sepsis by inhibiting lymphocyte apoptosis and reversing monocyte dysfunction. Crit. Care. 2010;14:R220. doi: 10.1186/cc9354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mahon BP, Katrak K, Nomoto A, Macadam AJ, Minor PD, Mills KH. Poliovirus-specific CD4+ Th1 clones with both cytotoxic and helper activity mediate protective humoral immunity against a lethal poliovirus infection in transgenic mice expressing the human poliovirus receptor. J. Exp. Med. 1995;181:1285–1292. doi: 10.1084/jem.181.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen F, Liu Z, Wu W, Rozo C, Bowdridge S, Millman A, Van Rooijen N, Urban JF, Jr., Wynn TA, Gause WC. An essential role for TH2-type responses in limiting acute tissue damage during experimental helminth infection. Nat. Med. 2012;18:260–266. doi: 10.1038/nm.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cohen JM, Khandavilli S, Camberlein E, Hyams C, Baxendale HE, Brown JS. Protective contributions against invasive Streptococcus pneumoniae pneumonia of antibody and Th17-cell responses to nasopharyngeal colonisation. PLoS One. 2011;6:e25558. doi: 10.1371/journal.pone.0025558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gouel-Cheron A, Venet F, Allaouchiche B, Monneret G. CD4+ T- lymphocyte alterations in trauma patients. Crit. Care. 2012;16:432. doi: 10.1186/cc11376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Leng FY, Liu JL, Liu ZJ, Yin JY, Qu HP. Increased proportion of CD4(+)CD25(+)Foxp3(+) regulatory T cells during early-stage sepsis in ICU patients. J. Microbiol. Immunol. Infect. 2013;46:338–344. doi: 10.1016/j.jmii.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 75.Monneret G, Debard AL, Venet F, Bohe J, Hequet O, Bienvenu J, Lepape A. Marked elevation of human circulating CD4+CD25+ regulatory T cells in sepsis-induced immunoparalysis. Crit. Care Med. 2003;31:2068–2071. doi: 10.1097/01.CCM.0000069345.78884.0F. [DOI] [PubMed] [Google Scholar]
- 76.Venet F, Pachot A, Debard AL, Bohe J, Bienvenu J, Lepape A, Monneret G. Increased percentage of CD4+CD25+ regulatory T cells during septic shock is due to the decrease of CD4+CD25-lymphocytes. Crit. Care Med. 2004;32:2329–2331. doi: 10.1097/01.ccm.0000145999.42971.4b. [DOI] [PubMed] [Google Scholar]
- 77.Benjamim CF, Lundy SK, Lukacs NW, Hogaboam CM, Kunkel SL. Reversal of long-term sepsis-induced immunosuppression by dendritic cells. Blood. 2005;105:3588–3595. doi: 10.1182/blood-2004-08-3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fazal N, Raziuddin S, Khan M, Al-Ghoul WM. Antigen presenting cells (APCs) from thermally injured and/or septic rats modulate CD4+ T cell responses of naive rat. Biochim Biophys Acta. 2006;1762:46–53. doi: 10.1016/j.bbadis.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 79.Flohe SB, Agrawal H, Schmitz D, Gertz M, Flohe S, Schade FU. Dendritic cells during polymicrobial sepsis rapidly mature but fail to initiate a protective Th1-type immune response. J. Leuk. Biol. 2006;79:473–481. doi: 10.1189/jlb.0705413. [DOI] [PubMed] [Google Scholar]
- 80.Muenzer JT, Davis CG, Dunne BS, Unsinger J, Dunne WM, Hotchkiss RS. Pneumonia after cecal ligation and puncture: a clinically relevant “two-hit” model of sepsis. Shock. 2006;26:565–570. doi: 10.1097/01.shk.0000235130.82363.ed. [DOI] [PubMed] [Google Scholar]
- 81.Opal SM. New perspectives on immunomodulatory therapy for bacteraemia and sepsis. Int. J. Antimicrob. Agents. 2010;36(Suppl 2):S70–73. doi: 10.1016/j.ijantimicag.2010.11.008. [DOI] [PubMed] [Google Scholar]